Abstract
Polyomavirus infections are common and relatively benign in the general human population but can become pathogenic in immunosuppressed patients. Because most treatments for polyomavirus-associated diseases nonspecifically target DNA replication, existing treatments for polyomavirus infection possess undesirable side effects. However, all polyomaviruses express Large Tumor Antigen (T Ag), which is unique to this virus family and may serve as a therapeutic target. Previous screening of pyrimidinone-peptoid hybrid compounds identified MAL2-11B and a MAL2-11B tetrazole derivative as inhibitors of viral replication and T Ag ATPase activity (IC50 of ~20-50μM). To improve upon this scaffold and to develop a structure-activity relationship for this new class of antiviral agents, several iterative series of MAL2-11B derivatives were synthesized. The replacement of a flexible methylene chain linker with a benzyl group or, alternatively, the addition of an ortho-methyl substituent on the biphenyl side chain in MAL2-11B yielded analogs with modestly improved IC50s (~15 μM), which retained antiviral activity. After combining both structural motifs, a new lead compound was identified that inhibited T Ag ATPase activity with an IC50 of ~5 μM. We suggest that the knowledge gained from the structure-activity relationship and a further refinement cycle of the MAL2-11B scaffold will provide a specific, novel therapeutic treatment option for polyomavirus infections and their associated diseases.
1. Introduction
Polyomaviruses are common viral agents in the human population.1 Currently, there are twelve human polyomaviruses, some of which are associated with disease in immunocompromised individuals.2 Polyomavirus infection in humans is usually asymptomatic, and primarily causes pathology in immune-compromised patients. Two human polyomaviruses, Merkel cell virus (MCPyV) and Trichodysplasia Spinulosa virus (TSPyV), have been shown to cause rare forms of cancers in immunocompromised patients. Two other human polyomaviruses, BKV and JCV, are associated with BK Virus Associated Nephropathy and Progressive Multifocal Leukoencephalopathy (PML) respectively.3 Available treatments for polyomavirus infections, such as Cidofovir, non-specifically target viral DNA replication and therefore have off-target and undesirable side effects.4
Beyond the known polyomaviruses that infect humans, new members of the Polyomaviridae family are continuously being identified.5 In the coming years, it is likely that additional human polyomaviruses will be uncovered. Unfortunately, in most cases, new viruses cannot be cultured. Compounding this problem is the fact that the AIDS epidemic, in conjunction with advances in transplant technology, have increased the number of immunocompromised individuals, who are susceptible to polyomavirus infection.6 Accordingly, the identification of specific and effective therapeutic treatment options for polyomavirus infection is imperative.
The ideal therapeutic should target a unique viral protein that is essential for infection or proliferation and for which a human homolog is lacking. In fact, all polyomaviruses express such a protein, the Large Tumor Antigen (T Ag).7-9 This multi-domain, 708 amino acid protein is a valid target for the following reasons. First, T Ag is highly conserved in members of the Polyomaviridae family. Second, the T Ag of Simian Virus 40 (SV40), a rapid-growing and culturable polyomavirus, is well characterized. And third, T Ag contains several domains that are required for viral replication. In theory, any of these domains might be targeted to produce a novel therapeutic.
T Ag encoded domains include an N-terminal J domain that interacts with Hsp70, a molecular chaperone that is encoded by the host.10-14 The stimulation of Hsp70 ATPase activity helps disrupt the pRB-E2F complexes so the virus can replicate in non-dividing cells without activating a DNA damage response.15 T Ag also encodes a DNA binding domain, which recognizes the viral origin of replication, and a AAA+ ATPase domain, which serves as a helicase to facilitate replication of the viral genome.16 Each of these domains is essential for viral replication. As noted above, because polyomaviruses like MCPyV and TSpPyV cause cancers and T Ag J domain is critical for cellular transformation, drugs targeting T Ag could also be beneficial in preventing cellular transformation.
A compound that disrupts the activity of one or more T Ag domains should have therapeutic potential. To this end, we previously screened a collection of pyrimidinone-peptoid hybrid compounds and identified MAL2-11B as a modest inhibitor of T Ag ATPase activity, T Ag-mediated stimulation of Hsp70, and SV40 viral replication with an IC50 of ~50 μM.17 In spite of MAL2-11B’s low potency, limited aqueous solubility, and high (512.6 Da) molecular weight, this study provided a proof-of-concept that the biochemical activity of T antigen could be targeted to isolate an anti-polyomaviral compound. Therefore, we next created MAL2-11B analogs and identified a tetrazole derivative that exhibited somewhat greater potency (IC50 of ~20 μM), improved physicochemical properties, and also inhibited the replication of both SV40 and BK virus in kidney cells.18 Subsequently, our goal has been to refine a structure-activity relationship (SAR) to yield a superior pyrimidinone-peptoid hybrid that might one day be used to prevent polyomavirus infections and their related diseases.
We now report on the synthesis and biochemical characterization of new MAL2-11B derivatives. The compounds were screened for their effects on endogenous T Ag ATPase activity, and select compounds were examined in SV40 replication assays. Two derivative classes were identified that inhibited T Ag ATPase activity and SV40 replication. We then combined the most active structural motifs from members of each class to generate a hybrid compound and discovered that the optimized compound inhibited T Ag ATPase activity with a much-improved IC50 (~5 μM). Unlike its progenitors, this compound also inhibited the endogenous ATPase activity of Hsp70. Together, our data support a continued refinement of the MAL2-11B scaffold and its derivatives as anti-polyomaviral agents.
2. Results and discussion
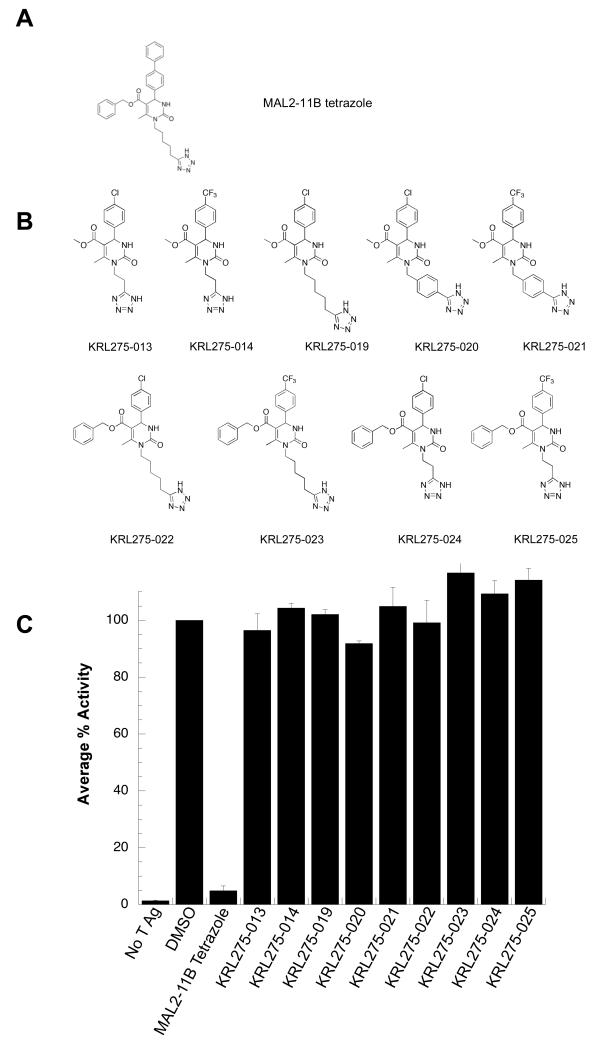
To identify improved inhibitors of SV40 T antigen, we first synthesized nine analogs derived from the MAL2-11B tetrazole (Fig. 1A,B). In this series, the hydrophobic biphenyl side chain was removed to increase solubility and the hydrocarbon linker was altered to test the significance of the distance to the pyrimidinone core as well as the flexibility of the tetrazole moiety. Five of these compounds also lacked the phenyl group at the ester moiety. The nine KRL compounds were then tested in a steady state ATPase assay to examine their effects on endogenous T Ag ATPase activity. None of them displayed a significant inhibition of T Ag ATPase activity, even when used at a final concentration of 100 μM (Fig. 1C). In addition, single turnover assays revealed that the KRL compounds failed to inhibit J domain-stimulated Hsp70 ATPase activity when used at a final concentration of 100 μM (data not shown). These data suggest that the structural changes made to the substituents on the MAL2-11B tetrazole scaffold, namely the replacement of the biphenyl group with substituted phenyl rings, and possibly the modification of the ester side chain, abolished the desired activity. Nevertheless, these negative data proved beneficial for the further refinement of the MAL2-11B scaffold.
Figure 1.
(A) Structure of MAL2-11B tetrazole. (B) Compounds in the KRL series were derived specifically from the MAL2-11B tetrazole. These analogs were designed to determine the significance of the biphenyl moiety as well as the length and flexibility of the hydrocarbon linker to the tetrazole group in inhibiting SV40 T Ag ATPase activity. (C) KRL compounds failed to inhibit SV40 T Ag ATPase activity. Steady state ATPase assays were used to quantitate the percent ATP hydrolyzed by T Ag in the presence of the indicated compounds at a final concentration of 100 μM. The average percent activity of the protein is relative to T Ag in the absence of the inhibitor. Data represent the means of three independent experiments, +/− SD.
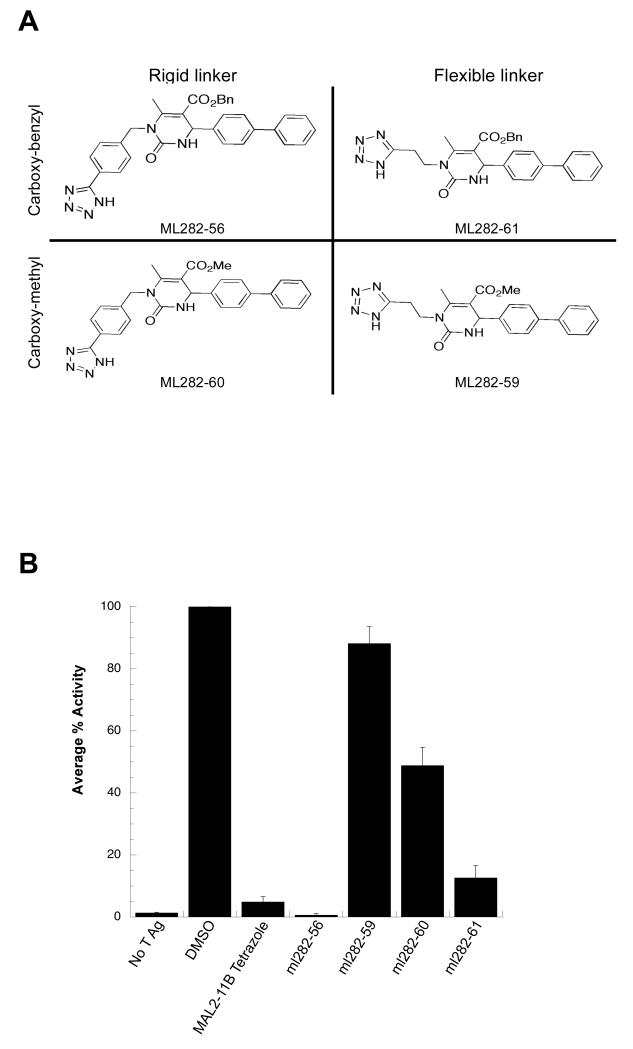
To test the significance of the flexibility in the methylene linker chain connecting the tetrazole heterocycle to the dihydropyrimidine nitrogen, as well as the contribution of the aromatic ester side chain, the ML series was synthesized. In these analogs, the biphenyl group (which is present in MAL2-11B but absent in the KRL series) was reintroduced. Four compounds with either a flexible or rigid linker to the tetrazole and either a benzyl or a methyl ester were prepared for analysis (Fig. 2A) and screened in a steady state ATPase assay with SV40 T Ag (Fig. 2B). At a final concentration of 100 μM, ML282-56 and ML282-61 inhibited SV40 T Ag ATPase activity more effectively than their respective analogs, ML282-59 and ML282-60, which lacked the benzyl ester moiety. Additionally, the data revealed that ML282-56 and ML282-60, which contain a phenyl ring in the tetrazole linker that reduces flexibility, are more potent inhibitors than their respective analogs, ML282-61 and ML282-59, which contain a flexible hydrocarbon chain. It is also worth pointing out that the average C-C bond distance in the phenyl groups is shorter than in alkanes. When taken together with the data in Fig. 1, these observations suggest that all three arene rings on the MAL2-11B scaffold are required for optimal activity and that the replacement of a propylene chain with a phenyl group in the tetrazole linker increases potency.
Figure 2.
(A) The ML library was designed to test the significance of a flexible versus rigid linker between the two heterocycles and the presence of a phenyl substituent at the ester moiety. (B) The benzyl ester and a rigid linker maximize inhibitory activity. The indicated compounds were examined in a steady state ATPase assay with SV40 T Ag to quantitate the percent ATP hydrolyzed by T Ag. The average percent activity of the protein is relative to T Ag in the absence of the inhibitor. Data represent the means of three independent experiments, +/− SD.
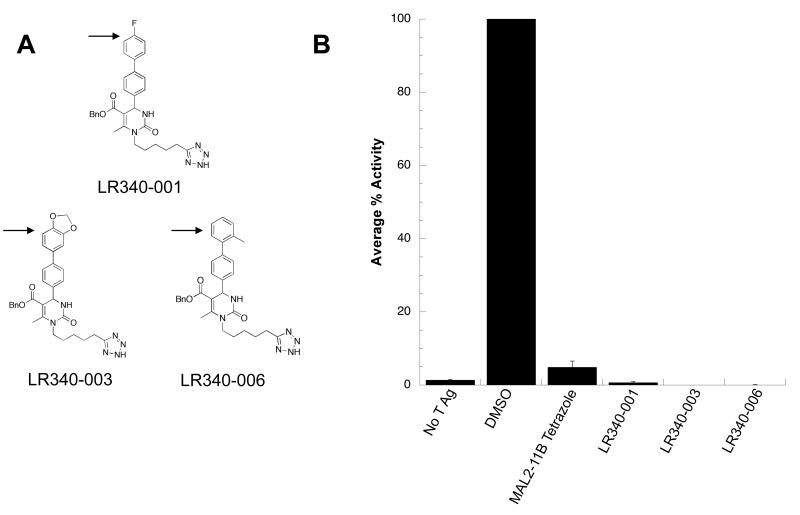
To further refine the scaffold, we then sought to test the effects of substitutions of the biphenyl substituent at C4 of the pyrimidinone. To this end, the LR series was synthesized. Select members of this series are shown in Fig. 3A. At 100 μM, each of the LR compounds inhibited SV40 T Ag ATPase activity in a steady state ATPase assay (Fig. 3B). Interestingly, both halogenated and methylated benzene rings in LR340-001 and LR340-006, respectively, lead to relatively minor changes in the biochemical profile. The effect of adding a dioxolane in LR340-003 suggests that even relatively large and electron-donating substituents at this position are tolerated for inhibitory activity.
Figure 3.
(A) The LR series contains modifications to the terminal benzene ring of the MAL2-11B scaffold. Modifications are denoted with an arrow. (B) The LR analogs inhibit SV40 T Ag ATPase activity. Compounds from the LR series were screened in a steady state ATPase assay with T Ag to quantitate the percent ATP hydrolyzed by T Ag. The average percent activity of the protein is relative to T Ag in the absence of the inhibitor. Data represent the means of three independent experiments, +/− SD.
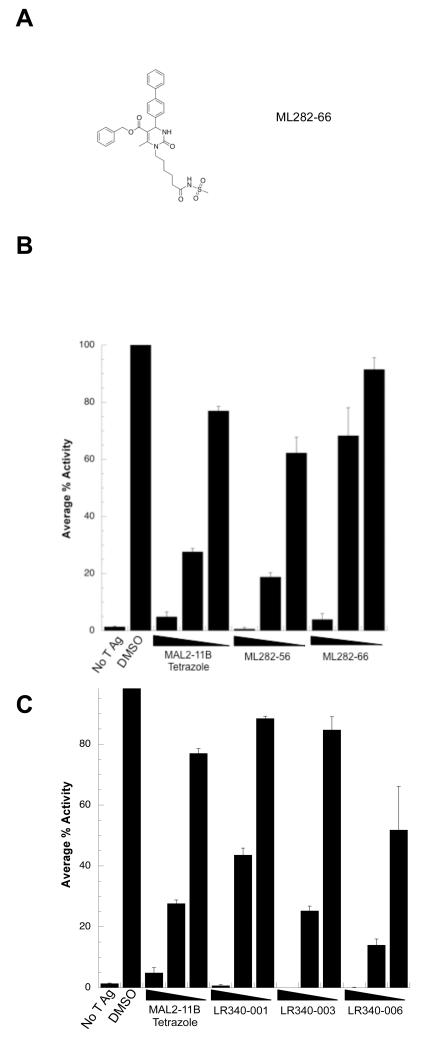
To compare the activities of the ML and LR series, the most active compounds were next titrated into steady state ATPase assays with SV40 T Ag (Fig. 4). Both ML282-56 and ML282-66, an analog with a modified amide group instead of the rigid tetrazole linker, inhibited T Ag ATPase activity at 100 μM; however, inhibition by ML282-66 at 30 μM was reduced compared to ML282-56 (Fig. 4B). In turn, the three LR340 compounds significantly reduced T Ag ATPase activity but LR340-006 was the most potent (Fig. 4C). Based on these data, ML282-56 and LR340-006 proved to be the most effective inhibitors of T Ag ATPase activity amongst the new MAL2-11B tetrazole analogs, inhibiting T Ag ATPase activity with an IC50 of ~50 μM.
Figure 4.
(A) Structure of MAL282-66. (B) Select ML compounds inhibit SV40 T Ag ATPase activtiy. The indicated ML compounds were titrated into a steady state ATPase assay containing T Ag. The average percent activity is relative to T Ag in the absence of inhibitor. Data represent the means of three independent experiments, +/− SD. (C) The LR340 compounds are effective inhibitors of SV40 T Ag ATPase activity. The indicated compounds were titrated into a steady state ATPase assay containing T Ag. The average percent activity of the protein is relative to T Ag in the absence of the inhibitor. Data represent the means of three independent experiments, +/− SD. The final concentrations of the compounds used in these studies were 10, 30, and 100 μM.
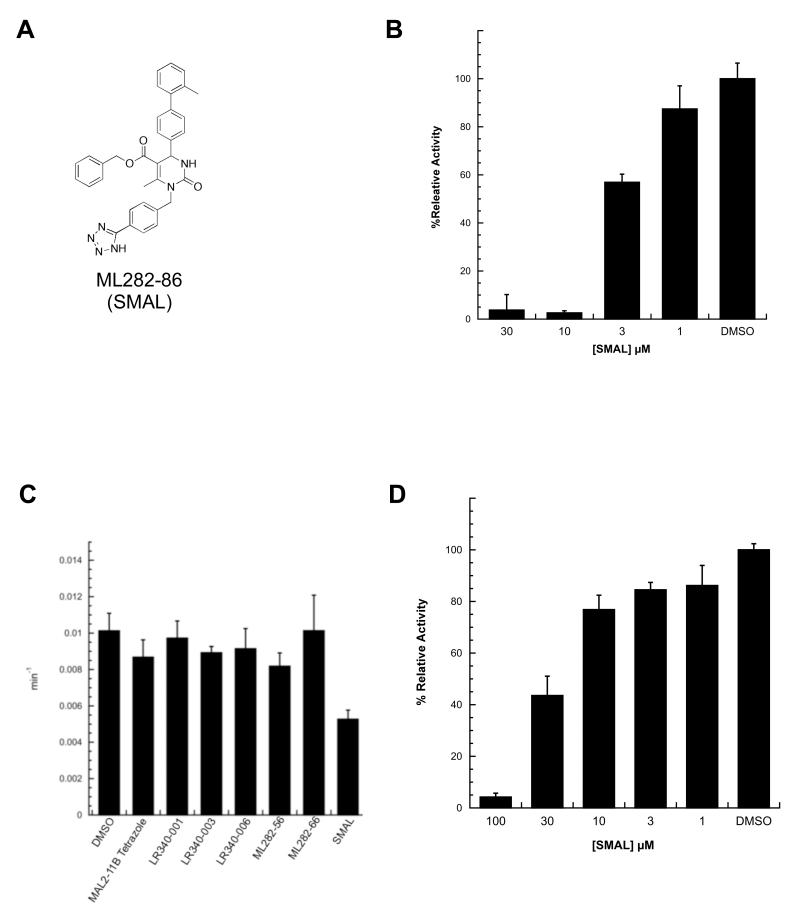
To develop a more potent MAL2-11B tetrazole derivative, we combined the critical structural features in ML282-56 and LR340-006 to obtain a new hybrid molecule (Fig. 5A). The resulting compound, ML282-86, or SMAL, contained both the rigid benzyl-tetrazole linker from the ML series and the methylated terminal phenyl group from the LR series. As hoped, SMAL inhibited T Ag ATPase activity in steady state ATPase assays with a significantly improved IC50 (~5 μM) (Fig. 5B). These results indicate that critical substituents within these analogs can be combined to yield additive inhibitory properties.
Figure 5.
(A) Structure of the optimized hybrid compound, ML282-86 (SMAL), which combines two optimized motifs: the methylated terminal phenyl group was adopted from LR340-006 and the rigid benzyl linker to the tetrazole heterocycle was adopted from ML282-56. (B) SMAL inhibits SV40 T Ag ATPase activity in a dose-dependent manner. SMAL was titrated into a steady state ATPase assay with T Ag. Data represent the means of three independent experiments, +/− SD. (C) SMAL is the only compound amongst the examined MAL2-11B tetrazole derivatives that modulates the ATPase activity of human Hsp70. Steady state ATPase assays were used to quantitate the turnover number (ATP molecules hydrolyzed by Hsp70 per minute) in the presence of the indicated compounds at a final concentration of 30μM versus a DMSO control. Data represent the averages of three independent experiments, +/− SD. (D) SMAL inhibits Hsp70 ATPase activity in a dose-dependent manner. SMAL was titrated into a steady state ATPase assay with human Hsp70. Data represent the means of three independent experiments, +/− SD.
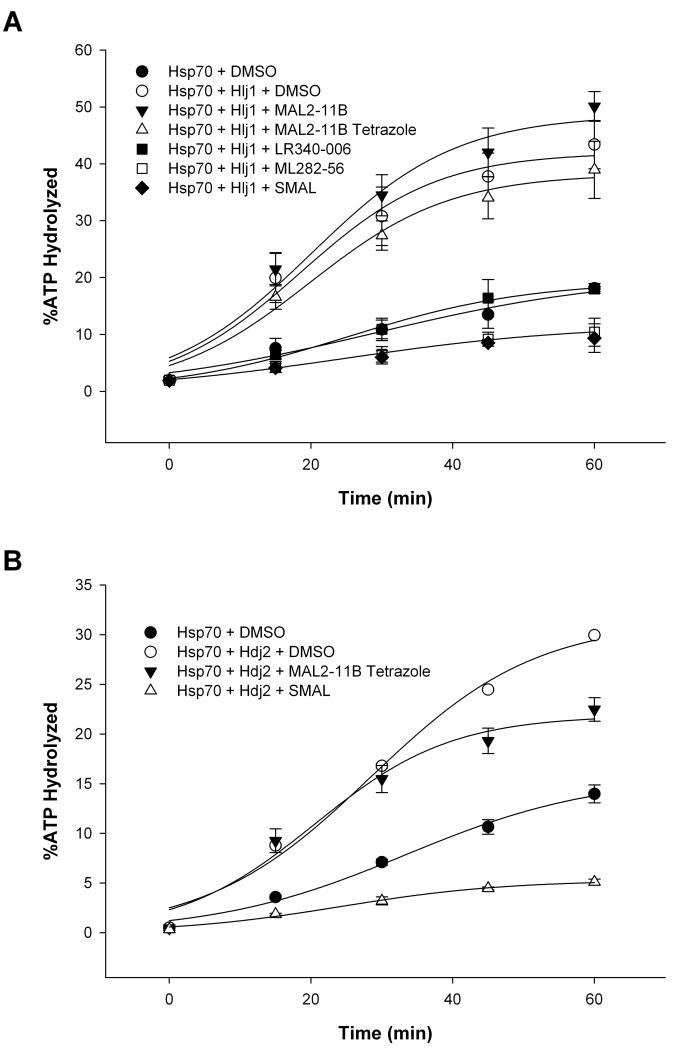
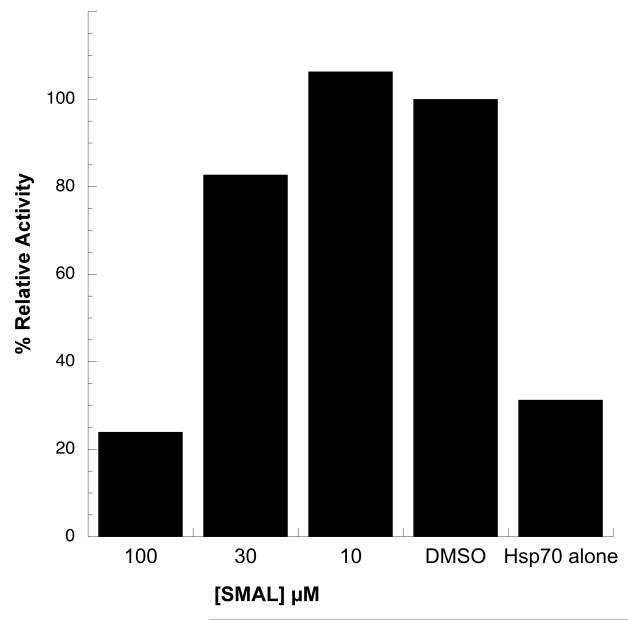
As outlined in the Introduction, the Hsp70 molecular chaperone plays critical roles in cellular physiology by facilitating protein folding, the degradation of misfolded proteins, the formation of protein oligomers, and protein transport. Hsp70 also facilitates polyomavirus replication by associating with the J domain in T Ag and driving the dissolution of the Rb-E2F complex. In addition, Hsp70 catalyzes the assembly of polyomavirus capsids in vitro,19 and more generally inhibits cell death by acting at multiple nodes in the apoptotic pathway. Because other pyrimidinone-peptoid compounds inhibit endogenous or T Ag-stimulated Hsp70 ATPase activity,20 we next asked if some of the compounds examined in this study also affected Hsp70 ATPase activity and J domain-stimulated Hsp70 ATPase activity. As shown in Fig. 5C, only SMAL inhibited the endogenous activity of Hsp70 when used at a final concentration of 30 μM. A titration of the compound in the assay yielded an IC50 of ~24 μM (Fig. 5D). When the effects of selected analogs on the ability of a yeast J domain-containing protein (Hlj1) to activate Hsp70 were examined, we discovered that SMAL and ML282-56 were the two most potent inhibitors, followed by LR340-006, MAL2-11B tetrazole, and MAL2-11B (Fig. 6A). When a human J domain-containing protein (Hdj2) was used, the effect of SMAL was similarly stronger than MAL2-11B tetrazole (Fig 6B). This overall pattern is similar to the relative inhibitory effects of the compounds on SV40 T Ag ATPase activity (see above). By titrating SMAL into this assay, we noted that the IC50 was >30 μM (Fig. 7), which in this preliminary analysis appears to be higher than the effect of SMAL on endogenous T Ag ATPase activity and on Hsp70 ATP hydrolytic activity (see Fig. 5). Together, the data are consistent with the compounds directly affecting the overall conformation and/or functional activities embedded within T Ag.
Figure 6.
Strategic modifications to the MAL2-11B tetrazole scaffold improve inhibition of J domain stimulation of Hsp70-mediated ATPase activity. Steady state ATPase assays were used to quantitate the percent ATP hydrolyzed by human Hsp70 over a 60-min time course in the presence of a J domain containing yeast protein (Hlj1) or a human J domain-containing protein (Hdj2) in parts (A) and (B), respectively, and in the presence of the indicated compounds at a final concentration of 100 μM versus a DMSO control. Data represent the means of three independent experiments, +/− SD, and were fitted using a sigmoidal, 3-parameter line regression. Purified Hlj1 and Hdj2 lacked significant levels of ATP hydrolysis (data not shown).
Figure 7.
SMAL inhibits J domain stimulation of Hsp70 ATPase activity in a dose-dependent manner. SMAL was titrated into a steady state ATPase assay with Hsp70 in the presence of a J domain-containing protein (Hlj1). DMSO indicates that the reaction lacked SMAL but contained the J domain protein, whereas the “Hsp70 alone” result corresponds to a reaction with Hsp70 but lacking compound and the J domain protein. Data represent the means of two independent experiments.
Because T Ag ATPase activity is required for the virus to unwind the viral genome prior to replication,21 we asked if SMAL would inhibit the replication of SV40 in monkey kidney cells. As a positive control we also examined MAL2-11B tetrazole as well as ML282-56 given their similarity to SMAL. We measured the effects of these analogs at a range of concentrations and the amount of viral DNA produced was quantified by qPCR. Each of the compounds inhibited viral replication; however, the potencies of SMAL and ML282-56 were similar (data not shown), even though SMAL was an order of magnitude more potent than ML282-56 when the effects on purified T Ag were measured (see Figs. 4-5, above). In one example, we show that reduced viral DNA was synthesized when the compounds were used at a saturating concentration (see Supporting Information). Based on these data, we next performed replicate metabolic stability assays in rodent microsomes since SMAL might be highly unstable. In contrast to our prediction, ~68% of the compound remained after a 60 min incubation in the presence of NADPH (see “Experimental procedures”). Another possible explanation for the inability of SMAL to inhibit SV40 replication more potently than the progenitor compounds emerged from an analysis of compound toxicities. As shown in Table I, while none of the pyrimidinones were particularly cytotoxic compared to Staurosporine, SMAL exhibited a slightly lower IC50 when the toxicity of the indicated compounds was examined in a human kidney cell line. This characteristic could diminish its effects on viral replication.
Table I.
LD50 (μM) values for select compounds against HEK293 cells with control (staurosporine), cLogD values,35 and aqueous solubilities (μg/mL ± SD).
| Compound | LD50 (μm) | cLogD | Solubility (μg/mL ± SD) |
|---|---|---|---|
| Staurosporine | < 3 | ND | ND |
| MAL2-11B tetrazole |
> 200 | 3.4 | 53.7 ± 1.7 |
| LR340-006 | 166 ± 2 | 3.9 | ND |
| ML282-56 | 162 ± 3 | 3.9 | 14.4 ± 0.5 |
| SMAL | 137 ± 1 | 4.4 | 62.4 ± 3.1 |
Since the in vivo activity of a compound also strongly depends on its pharmacokinetics properties, we calculated the cLogD of MAL2-11B tetrazole, LR340-56, ML282-56 and SMAL (Table I). We found that SMAL was considerably more lipophilic (4.4) than the former compounds (3.4, 3.9, and 3.9, respectively).35 The higher lipophilicity of SMAL will alter its subcellular distribution and could negatively impact its ability to sequester into the cytosol and nucleus. The higher cLogD value did not, however, decrease the aqueous solubility of SMAL, which is at the higher end of the 15 to 60 μg/mL range determined for these relatively closely clustered series (Table I). Overall, the results in the Supporting Information and from our qPCR analysis support the hypothesis that inhibitors of T Ag ATPase activity—and perhaps other T Ag-associated chaperone activities—compromise the replication of a polyomavirus. More generally, this work has allowed us to refine an SAR underlying the ability of pyrimidinones to inhibit the activity of T Ag and thus the replication of a polyomavirus.
3. Conclusions
The efforts described in this report indicate that superior small molecule inhibitors of the large T antigen can be identified by modifying a previously identified heterocyclic scaffold. We identified novel structure-activity relationships and new lead structures that potently inhibit T Ag ATPase activity and the replication of polyomaviruses in culture.17, 22 Specifically, we were able to merge two side chain modifications that rigidified more conformationally flexible precursor to generate a compact new analog, SMAL, that displayed substituent additivity and inhibited T Ag ATPase activity in steady state ATPase assays with an IC50 of ~5 μM.
Based on the improved activity of SMAL in assays with purified T Ag relative to other pyrimidinones, we anticipated that this compound would be a more effective inhibitor of SV40 replication in cell culture. However, the data obtained in the Supporting Information and in other experiments (not shown) indicate that the potency SMAL is similar to the MAL2-11B tetrazole and ML282-56. We determined that this was not due to a reduced stability in biological media, at least not in murine microsomes. In fact, SMAL proved quite stable in liver microsomes. The measurement of cellular toxicity of SMAL and its analogs indicated a slightly lower LD50 (Table I), but overall we found a similarly low level of cytotoxicity in this compound series. Therefore, the mechanism that compromises the ability of SMAL to more potently inhibit viral replication in cell culture remains to be elucidated. Since SMAL is more lipophilic than MAL2-11B tetrazole and ML282-56, it is quite feasible that its cellular distribution is different and that a more hydrophilic analog would match or surpass the viral replication effects of the latter inhibitors.
To date, specific therapeutics for polyomavirus infection do not exist. Contrary to our ongoing efforts, previous work to identify inhibitors of polyomavirus infection has focused on cell-based screens. For example, ellagic acid and spiperone were identified as inhibitors of viral infection in cells.23 Similarly, cidofovir (a known nucleotide analog) and leflunomide24, along with the quinolones25, inhibit polyomaviral replication in culture. Unfortunately, cell-based screens fail to identify the target of viral replication, increasing the likelihood of unwanted polypharmacology and limiting the potential for structural refinement. Perhaps as a consequence, the clinical effectiveness of these compounds is compromised by side effects that may limit their use. In contrast, our work supports the hypothesis that small molecules targeting the AAA+ ATPase domain of T Ag can serve as viral inhibitors.17, 22 In the future, chemical libraries based on the structure of SMAL need to be analyzed for improved inhibitory effects on T Ag and on polyomavirus replication. The successful iterative design and synthesis process reported here based on the MAL2-11B scaffold bodes well for the discovery of new generations of selective and potent polyomavirus inhibitors.
4. Experimental Procedures
4.1 Synthesis of Compounds Used in this Study
Representative procedure for preparation of KRL analogs Methyl 1-(4-(1H-tetrazol-5-yl)benzyl)-6-methyl-2-oxo-4-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (KRL275-021)
A suspension of 1-(4-cyanobenzyl)urea (0.100 g, 0.571 mmol) in THF (1.5 mL) was treated with 4-(trifluoromethyl)benzaldehyde (0.195 mL, 1.43 mmol, 2.5 eq) and methyl acetoacetate (0.154 mL, 1.43 mmol, 2.5 eq). After stirring for 5 min, conc. HCl (1 drop) was added and the reaction mixture was stirred at rt for 48 h, diluted with EtOAc and washed with H2O (3 × 10 mL) and brine (10 mL), dried (Na2SO4), and concentrated to give a dark yellow oil. The oil was dissolved in EtOAc and diluted with cold hexanes until a white precipitate formed. The precipitate was collected by filtration and dried under vacuum to give methyl 1-(4-cyanobenzyl)-6-methyl-2-oxo-4-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.104 g, 0.242 mmol, 42%) as an off white solid: Mp 173-175 °C; ATR IR (neat) 3212, 3093, 2955, 2922, 2232, 1717, 1685, 1634, 1430, 1409, 1322, 1204, 1190, 1160, 1109, 1066, 808, 783, 766 cm−1; 1H NMR (300 MHz, d6-DMSO) δ 8.36 (d, 1 H, J = 3.5 Hz), 7.77-7.72 (m, 4 Hz), 7.47 (d, 2 H, J = 8.0 Hz), 7.25 (d, 2 H, J = 8.0 Hz), 5.34 (d, 1 H, J = 3.5 Hz), 5.09, 4.98 (ABq, 2 H, J = 17.9 Hz), 3.60 (s, 3 H), 2.37 (s, 3 H); 13C NMR (100 MHz, d6-DMSO) δ 165.8, 152.7, 150.1, 148.1, 144.4, 132.4, 128.1 (app d, 2JC-F = 31.0 Hz), 127.0, 125.5 (q, 3JC-F = 3.6 Hz), 124.2 (app d, 1JC-F = 270.0 Hz), 118.7, 109.7, 102.8, 52.0, 51.3, 45.1, 16.1; HRMS (ESI) m/z calcd for C22H19N3O3F3 [M+H]+ 430.1373, found 430.1371.
To a solution of methyl 1-(4-cyanobenzyl)-6-methyl-2-oxo-4-(4-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.100 g, 0.233 mmol) in 1 M TBAF in THF (0.078 mL, 0.078 mmol, 0.3 eq) in a microwave vial was added TMSN3 (0.12 mL, 0.93 mmol, 4 eq). The vial was sealed before heating at 90 °C for 22 h. The solution was diluted with EtOAc and washed with H2O (10 mL), 1 M HCl (10 mL), and brine (10 mL). The combined organic layers were dried (Na2SO4), filtered and concentrated to give a yellow oil that was dissolved in EtOAc and diluted with cold hexanes until a precipitate formed. The precipitate was collected by filtration and dried under vacuum to yield KRL275-021 (0.042 g, 0.088 mmol, 38%) as an off white solid: Mp 245-246 °C; ATR IR (neat) 3370, 2948, 2841, 2735, 2623, 1714, 1652, 1619, 1469, 1421, 1321, 1208, 1164, 1122, 1110, 1066, 923, 868, 758, 667 cm−1; 1H NMR (300 MHz, d6-DMSO) δ 8.34 (d, 1 H, J = 3.9 Hz), 7.95 (d, 2 H, J = 8.1 Hz), 7.73 (d, 2 H, J = 8.4 Hz), 7.48 (d, 2 H, J = 8.1 Hz), 7.27 (d, 2 H, J = 8.1 Hz), 5.34 (d, 1 H, J = 3.6 Hz), 5.12, 4.96 (ABq, 2 H, J = 17.1 Hz), 3.59 (s, 3 H), 2.40 (s, 3 H); 13C NMR (100 MHz, d6-DMSO) δ 165.9, 155.2 (br), 152.8, 150.4, 148.2, 141.9, 128.1 (q, 2JC-F = 31.5 Hz), 127.2, 127.1, 127.0, 125.5 (q, 3JC-F = 3.6 Hz), 124.2 (q, 1JC-F = 270.3 Hz), 122.9, 102.7, 52.0, 51.4, 45.0, 16.2; HRMS (ESI) m/z calcd for C22H20N6O3F3 [M+H]+473.1543, found 473.1541.
Benzyl 1-(4-(1H-tetrazol-5-yl)benzyl)-4-([1,1′-biphenyl]-4-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (ML282-56)
A suspension of 1-(4-cyanobenzyl)urea (0.250 g, 1.43 mmol) in THF (4.8 mL) was treated with 4-biphenylcarboxaldehyde (0.650 g, 3.57 mmol, 2.5 eq) and benzyl acetoacetate (0.635 g, 3.57 mmol, 2.5 eq). After stirring at rt for 5 min, conc. HCl (1 drop) was added, and the resulting solution was stirred for 48 h, concentrated to remove solvent and subsequently purified by chromatography on SiO2 (Hexanes:EtOAc, 1:1) to obtain benzyl 4-([1,1′-biphenyl]-4-yl)-1-(4-cyanobenzyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.574 g, 1.12 mmol, 78%) as a faintly yellow solid: Mp 147-149 °C; 1H NMR (400 MHz, CDCl3) δ 7.59-7.54 (m, 4 H), 7.50-7.44 (m, 4 H), 7.41-7.36 (m, 1 H), 7.30-7.21 (m, 7 H), 7.18-7.14 (m, 2 H), 5.97 (d, 1 H, J = 3.2 Hz), 5.52 (d, 1 H, J = 2.8 Hz), 5.14, 5.07 (ABq, 2 H, J = 12.4 Hz), 5.13, 5.01 (ABq, 2 H, J = 16.8 Hz), 2.43 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 165.7, 153.9, 149.0, 143.5, 141.8, 141.1, 140.5, 135.8, 132.7, 129.1, 128.6, 128.3, 127.7, 127.6, 127.1, 126.9, 118.7, 111.4, 105.1, 66.5, 53.6, 46.0, 16.6; HRMS (ESI) m/z calcd for C33H28N3O3 [M+H]+ 514.2131, found 514.2170.
A mixture of benzyl 4-([1,1′-biphenyl]-4-yl)-1-(4-cyanobenzyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.200 g, 0.389 mmol), 1 M TBAF in THF (0.124 mL, 0.389 mmol, 1 eq), and TMSN3 (0.211 mL, 1.56 mmol, 4 eq) was stirred at 90 °C for 24 h in a sealed vial. The solution was diluted with EtOAc, and the organic layer was washed with H2O (3×) dried (Na2SO4), filtered, and concentrated to obtain a crude residue that was crystalized (EtOAc) and rinses with hexanes and MTBE to yield ML282-56 (0.176 g, 0.316 mmol, 81%) as a colorless solid: Mp 140-142 °C; 1H NMR (400 MHz, d6-DMSO) δ 8.26 (d, 1 H, J = 3.6 Hz), 7.98 (d, 2 H, J = 8.0 Hz), 7.64 (d, 2 H, J = 7.6 Hz), 7.60 (d, 2 H, J = 8.0 Hz), 7.47 (t, 2 H, J = 7.6 Hz), 7.39-7.27 (m, 8 H), 7.22-7.14 (m, 2 H), 5.34 (d, 1 H, J = 3.2 Hz), 5.16, 4.98 (ABq, 2 H, J = 16.8 Hz), 5.12, 5.08 (ABq, 2 H, J = 12.8 Hz), 2.44 (s, 3 H); 13C NMR (100 MHz, d6-DMSO) δ 165.3, 155.2, 152.9, 150.3, 142.8, 142.0, 139.8, 139.5, 136.3, 129.0, 128.3, 127.9, 127.8, 127.5, 127.2, 126.9, 126.7, 122.9, 103.0, 65.4, 52.3, 45.0, 16.2; HRMS (ESI) m/z calcd for C33H29N6O3 [M+H]+ 557.2301, found 557.2299.
Methyl 1-(2-(1H-tetrazol-5-yl)ethyl)-4-([1,1′-biphenyl]-4-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (ML282-59)
A suspension of 1-(2-cyanoethyl)urea (0.200 g, 1.77 mmol) in THF (5.89 mL) was treated with 4-biphenylcarboxaldehyde (0.805 g, 4.42 mmol, 2.5 eq) and methyl acetoacetate (0.477 mL, 4.42 mmol, 2.5 eq). After stirring at rt for 5 min, conc. HCl (1 drop) was added, and the resulting solution was stirred at rt for 48 h, concentrated, and the crude residue was purified by chromatography on SiO2 (Hexanes:EtOAc, 1:1) to yield methyl 4-([1,1′-biphenyl]-4-yl)-1-(2-cyanoethyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.280 g, 0.746 mmol, 42%) as a colorless solid: Mp 153-154 °C; 1H NMR (400 MHz, CDCl3) δ 7.56 (d, 4 H, J = 8.4 Hz), 7.43 (t, 2 H, J = 7.6 Hz), 7.35 (t, 1 H, J = 7.6 Hz), 7.33 (d, 2 H, J = 8.4 Hz), 5.51 (d, 1 H, J = 2.8 Hz), 5.44 (d, 1 H, J = 2.8 Hz), 4.13, 4.01 (t of ABq, 2 H, J = 6.4, 14.4 Hz), 3. 67 (s, 3 H), 2.80, 2.69 (t of ABq, 2 H, J = 7.0, 12.8 Hz), 2.62 (s, 3 H); HRMS (ESI) m/z calcd for C22H22N3O3 [M+H]+ 376.1661, found 376.1638.
A mixture of methyl 4-([1,1′-biphenyl]-4-yl)-1-(2-cyanoethyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.200 g, 0.531 mmol), 1 M TBAF in THF (0.533 mL, 0.533 mmol, 1 eq), and TMSN3 (0.289 mL, 2.13 mmol, 4 eq) was stirred at 90 °C for 24 h in a sealed vial. The solution was diluted with EtOAc and H2O. The aqueous layer was extracted with EtOAc (3×), and the combined organic layers were washed with H2O (3×) and NaHSO4 (1 M, 3×), dried (Na2SO4), filtered, and concentrated to obtain a crude residue that was washed with MTBE (3×) and distilled hexanes (3×), and subsequently crystallized (EtOAc) to obtain ML282-59 (0.0863 g, 0.206 mmol, 39%) as a pale yellow solid: Mp 202-204 °C; ATR IR (neat) 3227, 2983, 2845, 2699, 1715, 1639, 1454, 1238, 1186 cm−1; 1H NMR (300 MHz, d6-DMSO) δ 16.11 (bs, 1 H), 8.08 (d, 1 H, J = 3.6 Hz), 7.63 (d, 2 H, J = 7.2 Hz), 7.56 (d, 2 H, J = 8.1 Hz), 7.46 (t, 2 H, J = 7.5 Hz), 7.37 (d, 1 H, J = 7.2 Hz), 7.18 (d, 2 H, J = 8.1 Hz), 5.19 (d, 1 H, J = 3.3 Hz), 4.21-3.96 (m, 2 H), 3.58 (s, 3 H), 3.34-3.06 (m, 2 H), 2.58 (s, 3 H); 13C NMR (75 MHz,d6-DMSO) δ 166.1, 153.5, 152.5, 149.6, 142.7, 139.9, 139.3, 129.0, 127.5, 126.9, 126.7, 102.9, 59.8, 52.3, 51.3, 39.3, 23.3, 15.8; HRMS (ESI) m/z calcd for C22H23N6O3 [M+H]+ 419.1832, found 419.1862.
Methyl 1-(4-(1H-tetrazol-5-yl)benzyl)-4-([1,1′-biphenyl]-4-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (ML282-60)
A suspension of 1-(4-cyanobenzyl)urea (0.250 g, 1.43 mmol) in THF (4.76 mL) was treated with 4-biphenylcarboxaldehyde (0.650 g, 3.57 mmol, 2.5 eq) and methyl acetoacetate (0.385 mL, 3.57 mmol, 2.5 eq). After stirring at rt for 5 min, conc. HCl (1 drop) was added, and the resulting solution was stirred at rt for 45 h, and filtered. The filtered white solid was was re-suspended in THF (4.76 mL), treated with conc. HCl (2 drops), stirred at 50 °C for 1 h, and at rt for an additional 3 h. The solvent was evaporated, and the crude residue was purified by chromatography on SiO2 (Hexanes:EtOAc, 3:2) to yield methyl 4-([1,1′-biphenyl]-4-yl)-1-(4-cyanobenzyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.560 g, 1.28 mmol, 90%) as a colorless solid: Mp 180-181 °C; 1H NMR (400 MHz, CDCl3) δ 7.60-7.52 (m, 6 H), 7.46 (t, 2 H, J = 7.6 Hz), 7.37 (t, 1 H, J = 7.2 Hz), 7.32 (d, 2 H, J = 8.0 Hz), 7.23 (d, 2 H, J = 8.0 Hz), 5.69 (d, 1 H, J = 2.8 Hz), 5.52 (d, 1 H, J = 2.8 Hz), 5.17, 5.01 (ABq, 2 H, J = 16.8 Hz), 3.69 (s, 3 H), 2.42 (s, 3 H); HRMS (ESI) m/z calcd for C27H24N3O3 [M+H]+ 438.1818, found 438.1830.
A mixture of methyl 4-([1,1′-biphenyl]-4-yl)-1-(4-cyanobenzyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.200 g, 0.457 mmol), 1 M TBAF in THF (0.457 mL, 0.457 mmol, 1 eq), and TMSN3 (0.248 mL, 1.83 mmol, 4 eq) was stirred at 90 °C for 24 h in a sealed vial. The solution was diluted with EtOAc and H2O, and the aqueous layer was extracted with EtOAc (3×). The combined organic layers were washed with H2O (3×) and NaHSO4 (1 M, 3×), dried (Na2SO4), filtered, and concentrated to obtain a crude residue that was washed with MTBE (3×), distilled hexanes (3×), and recrystallized (EtOAc) to obtain ML282-60 (0.153 g, 0.319 mmol, 70%) as a colorless solid: Mp 193-195 °C; ATR IR (neat) 3208, 3057, 2731, 1700, 1692, 1642, 1501, 1486, 1447, 1430, 1419, 1380, 1366, 1325, 1311, 1204, 1186 cm−1; 1H NMR (300 MHz, d6-DMSO) δ 8.27 (d, 1 H, J = 3.6 Hz), 7.97 (d, 2 H, J = 8.1 Hz), 7.63 (dd, 4 H, J = 7.8, 3.6 Hz), 7.46 (t, 2 H, J =7.5 Hz), 7.39-7.31 (m, 5 H), 5.31 (d, 1 H, J = 3.3 Hz), 5.16, 4.97 (ABq, 1 H, J = 16.8 Hz), 3.60 (s, 3 H), 2.41 (s, 3 H); 13C NMR (75 MHz, d6-DMSO) δ 166.1, 155.1, 153.0, 149.7, 142.8, 142.1, 139.8, 139.5, 128.9, 127.5, 127.2, 126.9, 126.7, 122.8, 103.3, 52.1, 51.3, 45.0, 16.2; HRMS (ESI) m/z calcd for C27H25N6O3 [M+H]+ 481.1988, found 481.1949.
Benzyl 1-(2-(1H-tetrazol-5-yl)ethyl)-4-([1,1′-biphenyl]-4-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (ML282-61)
A suspension of 1-(2-cyanoethyl)urea (0.200 g, 1.77 mmol) in THF (5.89 mL) was treated with 4-biphenylcarboxaldehyde (0.805 g, 4.42 mmol, 2.5 eq) and benzyl acetoacetate (0.789 mL, 4.43 mmol, 2.5 eq) and stirred at rt for 5 min. After addition of conc. HCl (1 drop), the resulting solution was stirred at rt for 48 h, concentrated to remove solvent and subsequently purified by chromatography on SiO2 (Hexanes:EtOAc, 3:2 to 1:1) to obtain benzyl 4-([1,1′-biphenyl]-4-yl)-1-(2-cyanoethyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.365 g, 0.808 mmol, 46%) as a colorless solid: 1H NMR (400 MHz, CDCl3) δ 7.57-7.55 (m, 2 H), 7.52-7.50 (m, 2 H), 7.45 (t, 2 H, J = 7.4 Hz), 7.36 (t, 1 H, J = 7.4 Hz), 7.28-7.25 (m, 5 H), 7.14-7.12 (m, 2 H), 5.44 (d, 1 H, J = 2.4 Hz), 5.38 (bs, 1 H), 5.12, 5.06 (ABq, 2 H, J = 12.4 Hz), 4.12, 4.02 (t of ABq, 2 H, J = 7.0, 14.4 Hz), 2.81, 2.70 (t of ABq, 2 H, J = 7.2, 16.8 Hz), 2.64 (s, 3 H); HRMS (ESI) m/z calcd for C28H25N3O3Na [M+Na]+ 474.1794, found 474.1801.
A mixture of benzyl 4-([1,1′-biphenyl]-4-yl)-1-(2-cyanoethyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.200 g, 0.443 mmol), 1 M TBAF in THF (0.443 mL, 0.443 mmol), and TMSN3 (0.240 mL, 1.77 mmol, 4 eq) was stirred at 90 °C for 23 h in a sealed vial. The solution was diluted with EtOAc and H2O. The aqueous layer was extracted with EtOAc (3×), and the combined organic layers were washed with H2O (3×), 1 M NaHSO4 (3×), dried (Na2SO4), filtered, and concentrated to obtain a crude residue that was washed with distilled hexanes (3×), MTBE (3×), and recrystallized (EtOAc) to obtain ML282-61 (0.0161 g, 0.0326 mmol, 7%) as a solid: 1H NMR (400 MHz,d6-DMSO) δ 8.06 (d, 1 H, J = 3.6 Hz), 7.64 (d, 2 H, J = 7.2 Hz), 7.54 (d, 2 H, J = 8.4 Hz), 7.48 (t, 2 H, J = 7.6 Hz), 7.38 (t, 1 H, J = 7.2 Hz), 7.29-7.27 (m, 3 H), 7.18-7.14 (m, 4 H), 5.22 (d, 1 H, J = 3.2 Hz), 5.11, 5.06 (ABq, 2 H, J =12.8 Hz), 4.20-4.12 (m, 1 H), 4.10-4.00 (m, 1 H), 3.40-3.30 (m, 1 H), 3.18-3.10 (m, 1 H), 2.60 (s, 3 H); 13C NMR (100 MHz, d6-DMSO) δ 165.3, 152.3, 150.2, 142.8, 139.9, 139.4, 136.3, 129.0, 128.3, 127.8, 127.7, 127.5, 126.9, 126.8, 123.7, 102.5, 65.3, 52.5, 39.9, 23.3, 15.8; HRMS (ESI) m/z calcd for C28H26N6O3Na [M+Na]+ 517.1964, found 517.1974.
Benzyl 1-(5-(1H-tetrazol-5-yl)pentyl)-4-(4′-fluoro-[1,1′-biphenyl]-4-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (LR340-001)
To a suspension of 1-(5-cyanopentyl)urea (0.520 g, 3.05 mmol) and 4-bromobenzaldehyde (0.837 g, 4.52 mmol, 1.5 eq) in THF (4.0 mL) were added benzyl acetoacetate (0.81 mL, 4.52 mmol, 1.5 eq) and conc. HCl (0.070 mL, 0.603 mmol). The suspension was stirred at rt for 18 h, diluted with ether and the precipitate was collected by filtration and dried under vacuum to give benzyl 4-(4-bromophenyl)-1-(5-cyanopentyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (1.03 g, 2.08 mmol, 69%) as a white solid: Mp 156-157 °C; 1H NMR (400 MHz, CDCl3) δ 7.37 (d, 2 H, J = 8.4 Hz), 7.32-7.30 (m, 3 H), 7.16-7.13 (m, 2 H), 7.03 (d, 2 H, J = 8.4 Hz), 5.35-5.33 (m, 1 H), 5.15, 5.01 (ABq, 2 H, J = 12.4 Hz), 3.86 (ddd, 1 H, J = 14.8, 9.2, 5.6 Hz), 3.62 (ddd, 1 H, J = 14.6, 9.2, 5.4 Hz), 2.54 (s, 3 H), 2.32 (t, 2 H, J = 7.0 Hz), 1.70-1.49 (m, 4 H), 1.44-1.36 (m, 2 H); 13C NMR (100 MHz, CDCl3) δ 165.7, 153.2, 149.6, 142.4, 135.9, 131.9, 128.7, 128.7, 128.4, 121.2, 121.9, 119.6, 103.9, 66.4, 53.6, 42.5, 29.1, 25.9, 25.1, 17.2, 16.4; HRMS (ESI) m/z calcd for C25H26N3O3BrNa [M+Na]+ 518.1055, found 518.1094.
To a suspension of benzyl 4-(4-bromophenyl)-1-(5-cyanopentyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (1.00 g, 2.01 mmol) and di-N-butyltin oxide (51.2 mg, 0.201 mmol, 0.1 eq) in toluene (4 mL) was added TMSN3 (0.56 mL, 4.03 mmol, 2 eq). The mixture was heated in a sealed vial to 110 °C for 24 h. Additional TMSN3 (250 μL, 1.89 mmol) was added and heating was continued at 110 °C for 18 h. After addition of hexanes, the precipitate was collected by filtration, washed several times with hexanes and recrystallized from a MeOH/H2O solution to provide benzyl 1-(5-(1H-tetrazol-5-yl)pentyl)-4-(4-bromophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.650 g, 1.21 mmol, 60%) as a white solid: Mp 174-176 °C; ATR IR (neat) 3059, 2934, 1705, 1681, 1604, 1420, 1216, 1192, 1128, 1094, 839, 798, 768, 734 cm−1; 1H NMR (300 MHz, d6-DMSO) δ 7.98 (d, 1 H, J = 3.6 Hz), 7.48 (d, 2 H, J = 8.4 Hz), 7.30-7.23 (m, 3 H), 7.17-7.13 (m, 2 H), 7.11 (d, 2 H, J = 8.4 Hz), 5.15-5.10 (m, 1 H), 5.11, 5.01 (ABq, 2 H, J = 12.6 Hz), 3.84-3.74 (m, 1 H), 3.50-3.41 (m, 1 H), 2.78 (t, 2 H, J = 7.4 Hz), 2.49 (s, 3 H), 1.67-1.58 (m, 2 H), 1.50-1.34 (m, 2 H), 1.17-1.20 (m, 2 H); 13C NMR (100 MHz, d6-DMSO) δ 165.2, 155.8, 152.4, 150.9, 143.2, 136.3, 131.3, 128.4, 128.3, 127.9, 127.7, 120.5, 102.0, 65.2, 51.8, 41.6, 28.8, 26.6, 25.5, 22.6, 15.7; HRMS (ESI) m/z calcd for C25H28N6O3Br [M+H]+ 539.1406, found 539.1437.
A suspension of benzyl 1-(5-(1H-tetrazol-5-yl)pentyl)-4-(4-bromophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.050 g, 0.093 mmol), 4-fluorophenylboronic acid (0.016 g, 0.111 mmol, 1.2 eq), Pd(dppf)2 (0.014 g, 0.014 mmol, 0.015 eq) and Cs2CO3 (0.151 g, 0.463 mmol, 5 eq) was heated to 110 °C in a sealed vial for 18 h. The mixture was then diluted with EtOAc, filtered through a cotton plug, washed with 0.1 N HCl (2x) and brine, dried (Na2SO4), filtered, and concentrated. Purification by chromatography on SiO2 (MeOH:CH2Cl2, 0:100 to 10:90) provided LR340-001 (0.035 g, 0.063 mmol, 68%) as an amorphous solid: ATR IR (neat) 3059, 2934, 2975, 1663, 1629, 1500, 1389, 1215, 1193, 1077, 839, 798, 768, 734 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.50-7.46 (m, 2 H), 7.33 (d, 2 H, J = 8.4 Hz), 7.28-7.26 (m, 2 H), 7.16-7.09 (m, 5 H), 5.88 (d, 1 H, J = 2.8 Hz), 5.44 (d, 1 H, J = 3.2 Hz), 5.13, 5.06 (ABq, 2 H, J = 12.4 Hz), 4.08-4.00 (m, 1 H), 3.75-3.68 (m, 1 H), 3.00-2.96 (m, 2 H), 2.58 (s, 3 H), 2.04-1.95 (m, 1 H), 1.85-1.78 (m, 2 H), 1.68-1.57 (m, 2 H), 1.30-1.20 (m, 1 H), 1.03-0.91 (m, 1 H); HRMS (ESI) m/z calcd for C31H31N6O3FNa [M+Na]+ 577.2339, found 577.2380.
Benzyl 1-(5-(1H-tetrazol-5-yl)pentyl)-4-(4-(benzo[d][1,3]dioxol-5-yl)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (LR340-003)
A suspension of benzyl 1-(5-(1H-tetrazol-5-yl)pentyl)-4-(4-bromophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.050 g, 0.093 mmol), 3,4-methylenedioxyphenylboronic acid (0.018 g, 0.111 mmol, 1.2 eq), Pd(dppf)2 (0.010 g, 0.014 mmol, 0.15 eq) and Cs2CO3 (0.151 g, 0.463 mmol, 5 eq) was heated to 110 °C. The reaction mixture became homogeneous and was stirred in a sealed vial overnight, filtered through a cotton plug, then diluted with EtOAc and washed with 0.1 N HCl, dried (Na2SO4), filtered, and concentrated. The residue was purified by chromatography on SiO2 (MeOH:CH2Cl2, 0:100 to 1:9) to provide LR340-003 (0.031 g, 0.053 mmol, 58%) as an amorphous solid: ATR IR (neat) 3059, 2934, 2816, 1674, 1629,1450, 1420, 1359, 1232, 1193, 1163, 1077, 1029, 798, 768, 734, 692 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32 (d, 2 H, J = 8.4 Hz), 7.28-7.26 (m, 3 H), 7.15-7.10 (m, 4 H), 7.01-6.98 (m, 2 H), 6.87 (d, 1 H, J = 8.4 Hz), 6.00 (s, 2 H), 5.83 (br s, 1 H), 5.43 (d, 1 H, J = 2.8 Hz), 5.13, 5.05 (ABq, 2 H, J = 12.2 Hz), 4.04-3.97 (m, 1 H), 3.75-3.68 (m, 1 H), 2.98 (t, 2 H, J = 6.2 Hz), 2.58 (s, 3 H), 2.00-1.90 (m, 1 H), 1.90-1.80 (m, 1 H), 1.70-1.50 (m, 2 H), 1.30-1.20 (m,1 H), 1.05-0.95 (m, 1 H); 13C NMR (125 MHz, CDCl3) δ 165.8, 156.0, 154.4, 148.8, 148.3, 147.4, 141.5, 140.8, 135.9, 134.8, 128.6, 128.3, 128.2, 127.2, 126.9, 120.7, 108.8, 107.6, 104.9, 101.3, 66.4, 53.8, 41.8, 29.1, 25.9, 25.1, 23.1, 16.3; HRMS (ESI) m/z calcd for C32H33N6O5 [M+H]+ 581.2512, found 581.2642.
Benzyl 1-(5-(1H-tetrazol-5-yl)pentyl)-6-methyl-4-(2′-methyl-[1,1′-biphenyl]-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (LR340-006)
A suspension of 1-(5-cyanopentyl)urea (0.150 g, 0.966 mmol) in THF (3.0 mL) was treated with 2′-methylbiphenyl-4-carbaldehyde (0.383 g, 1.93 mmol, 2 eq) and benzyl acetoacetate (0.34 mL, 1.9 mmol, 2 eq). After stirring for 5 min, conc. HCl (1 drop) was added and the resulting solution was stirred at rt for 3 d and concentrated. The residual oil was purified by chromatography on SiO2 (EtOAc:Hexanes, 0:100 to 100:0) to provide benzyl 1-(5-cyanopentyl)-6-methyl-4-(2′-methyl-[1,1′-biphenyl]-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.439 g, 0.778 mmol, 81%) as an off-white foam: Mp 46-48 °C; ATR IR (neat) 3327, 3234, 3063, 2935, 2866, 2245, 1681, 1618, 1388, 1195, 1158, 1117, 1085, 762, 731, 699 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.34-7.14 (m, 13 H), 5.61 (d, 1 H, J = 3.0 Hz), 5.43 (d, 1 H, J = 2.7 Hz), 5.16, 5.08 (ABq, 2 H, J = 12.5 Hz), 3.90 (ddd, 1 H, J = 15.0, 9.6, 6.0 Hz), 3.66 (ddd, 1 H, J = 14.7, 9.3, 5.6 Hz), 2.58 (s, 3 H), 2.31 (t, 2 H, J = 7.1 Hz), 2.52 (s, 3 H), 1.73-1.58 (m, 4 H), 1.57-1.40 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ 165.9, 153.4, 149.3, 141.8, 141.7, 141.4, 136.0, 135.4, 130.5, 129.8, 129.7, 128.6, 128.2, 127.5, 126.2, 125.9, 119.5, 104.3, 66.3, 54.0, 42.5, 29.1, 25.9, 25.1, 20.6, 17.2, 16.4; HRMS (ESI) m/z calcd for C32H34O3N3 [M+H]+ 508.2595, found 508.2597.
To a solution of benzyl 1-(5-cyanopentyl)-6-methyl-4-(2′-methyl-[1,1′-biphenyl]-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.350 g, 0.621 mmol) in THF (0.3 mL) in a 5 mL microwave vial was added 1 M TBAF in THF (0.807 mL, 0.807 mmol 1.3 eq) and TMSN3 (0.34 mL, 2.48 mmol, 4 eq). The vial was flushed with Ar and sealed before heating at 70-75 °C for 14 h. As the reaction was still incomplete, the reaction mixture was concentrated and further TMSN3 (0.34 mL, 2.48 mmol, 4 eq) was added before stirring at 75-80 °C for 6 d. The reaction mixture was concentrated and purified by chromatography on by chromatography on SiO2 (MeOH:CH2Cl2, 0:100 to 5:95 with 0.1% formic acid) to yield a foam containing product with some TBAF. The foam was dissolved in CH2Cl2 and washed with 0.1 M aq. HCl (x 2) and H2O (x 1). The organic layer was dried (Na2SO4), filtered and concentrated to provide LR340-006 (0.248 g, 0.448 mmol, 72%) as a yellow foam: ATR IR (neat) 3235, 3031, 2930, 2866, 1672, 1622, 1455, 1388, 1196, 1159, 1083, 910, 761, 731, 698 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.29-7.13 (m, 13 H), 6.06 (d, 1 H, J = 2.7 Hz), 5.44 (d, 1 H, J = 3.0 Hz), 5.14, 5.07 (ABq, 2 H, J = 12.5 Hz), 4.00-3.91 (m, 1 H), 3.75-3.66 (m, 1 H), 2.97 (app. t, 2 H, J = 7.1 Hz), 2.58 (s, 3 H), 2.20 (s, 3 H), 2.00-1.78 (m, 2 H), 1.77-1.56 (m, 2 H), 1.36-1.25 (m, 1 H), 1.25-1.10 (m, 1 H); 13C NMR (75 MHz, d6-DMSO) δ 165.8, 156.1, 154.4, 148.9, 141.9, 141.3, 141.2, 135.9, 135.3, 130.5, 129.8, 129.7, 128.6, 128.3, 128.2, 127.6, 126.2, 126.0, 104.9, 66.5, 53.9, 41.9, 29.1, 25.9, 25.2, 23.2, 20.6, 16.3; HRMS (ESI) m/z calcd for C32H35O3N6 [M+H]+ 551.2765, found 551.2764.
Benzyl 4-([1,1′-biphenyl]-4-yl)-6-methyl-1-(6-(methylsulfonamido)-6-oxohexyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (ML282-66)
A suspension of 6-ureidohexanoic acid (1.00 g, 5.74 mmol) and 4-biphenylcarboxaldehyde (1.58 g, 8.61 mmol, 1.5 eq) in THF (10 mL) at rt was treated with benzyl acetoacetate (1.53 mL, 8.61 mmol, 1.5 eq), followed by addition of conc. HCl (1 drop). The suspension was stirred at rt for 20 h, and concentrated in vacuo to give a yellow oil that was suspended several times in hexanes over 3 h to obtain a yellow solid. The yellow solid was collected by filtration, then dissolved in hot EtOAc and precipitated with hexanes. The precipitate was collected by filtration and dried in vacuo to give 6-(4-([1,1′-biphenyl]-4-yl)-5-((benzyloxy)carbonyl)-6-methyl-2-oxo-3,4-dihydropyrimidin-1(2H)-yl)hexanoic acid (1.48 g, 2.89 mmol, 50%) as a light yellow solid that was used without further purification: 1H NMR (400 MHz, CDCl3) δ 7.55 (d, 2 H, J = 8.0 Hz), 7.48 (d, 2 H, J = 8.0 Hz), 7.43 (t, 2 H, J = 8.0 Hz), 7.34 (t, 1 H, J = 7.2 Hz), 7.29-7.23 (m, 5 H), 7.17-7.15 (m, 2 H), 5.98 (d, 1 H, J = 2.4 Hz), 5.42 (d, 1 H, J = 2.1 Hz), 5.12, 5.08 (ABq, 1 H, J = 12.4 Hz), 3.94-3.86 (m, 1 H), 3.64-3.57 (m, 1 H), 2.54 (s, 3 H), 2.30 (t, 2 H, J = 5.4 Hz), 1.70-1.51 (m, 4 H), 1.35-1.24 (m, 2 H).
To a solution of 6-(4-([1,1′-biphenyl]-4-yl)-5-((benzyloxy)carbonyl)-6-methyl-2-oxo-3,4-dihydropyrimidin-1(2H)-yl)hexanoic acid (49.6 mg, 0.0968 mmol) in CH2Cl2 (0.49 mL) was added oxalyl chloride (15.0 mg, 0.116 mmol) and DMF (1 drop) at rt. The solution was stirred at rt for 1 h, and treated with methanesulfonamide (11.2 mg, 0.116 mmol) and DBU (44.3 mg, 0.290 mmol). The brown solution was stirred at rt for 16 h, diluted with EtOAc, and acidified with 1 M HCl. The organic layer was washed with sat. NaHCO3 solution (3×), dried (Na2SO4), filtered, and concentrated. The crude residue was purified by chromatography on SiO2 (CH2Cl2:MeOH, 97:3) to obtain crude product that was dissolved in EtOAc and washed with aqueous NaHSO4 (3×) to remove DMF, H2O (3×), dried (Na2SO4), filtered, and concentrated to obtain ML282-66 (24.9 mg, 0.0422 mmol, 44%) as a pale yellow oily solid: 1H NMR (400 MHz, CDCl3) δ 10.99 (bs, 1 H), 7.55 (d, 2 H, J = 7.6 Hz), 7.50 (d, 2 H, J = 8.0 Hz), 7.43 (t, 2 H, J = 7.4 Hz), 7.34 (t, 1 H, J = 7.4 Hz), 7.27-7.22 (m, 4 H), 7.13-7.11 (m, 2 H), 6.45 (d, 1 H, J = 3.2 Hz), 5.42 (d, 1 H, J = 2.8 Hz), 5.11, 5.06 (ABq, 2 H, J = 12.4 Hz), 3.90-3.80 (m, 1 H), 3.65-3.55 (m, 1 H), 3.11 (s, 3 H), 2.56 (s, 3 H), 2.29 (t, 2 H, J = 7.6 Hz), 1.72-1.51 (m, 5 H), 1.32-1.22 (m, 2 H); 13C NMR (100 MHz, CDCl3) δ 172.8, 165.7, 154.4, 149.0, 142.0, 141.0, 140.5, 135.9, 129.0, 128.6, 128.2, 127.6, 127.1, 126.9, 104.5, 66.4, 53.6, 42.5, 41.3, 36.6, 29.8, 29.7, 26.4, 24.4, 16.2; HRMS (ESI) m/z calcd for C32H35N3O6NaS [M+Na]+ 612.2144, found 612.2162.
Benzyl 1-(4-(1H-tetrazol-5-yl)benzyl)-6-methyl-4-(2′-methyl-[1,1′-biphenyl]-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (ML282-86, SMAL)
A suspension of 1-(4-cyanobenzyl)urea (0.15 g, 0.86 mmol) in THF (3.0 mL) was treated with 2′-methylbiphenyl-4-carbaldehyde (0.336 g, 1.71 mmol, 2 eq) and benzyl acetoacetate (0.34 mL, 1.7 mmol, 2 eq). After stirring for 5 min, conc. HCl (1 drop) was added and the reaction mixture was stirred at rt for 3 d and concentrated. The residual oil was purified by chromatography on SiO2 (EtOAc:Hexanes, 0:100 to 100:0) to provide benzyl 1-(4-cyanobenzyl)-6-methyl-4-(2′-methyl-[1,1′-biphenyl]-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.369 g, 0.686 mmol, 80%) as an off-white foam: Mp 74-76 °C; ATR IR (neat) 3332, 3232, 3063, 2951, 2229, 1684, 1623, 1385, 1204, 1163, 1104, 910, 761, 732, 698 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.58 (d, 2 H, J = 8.1 Hz), 7.30-7.15 (m, 15 H), 5.77 (d, 1 H, J = 3.3 Hz), 5.53 (d, 1 H, J = 3.0 Hz), 5.19-5.05 (m, 4 H), 2.45 (s, 3 H), 2.27 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 165.7, 153.7, 149.0, 143.5, 142.0, 141.3, 135.8, 135.4, 132.7, 130.6, 129.8, 128.8, 128.6, 128.4, 128.3, 127.6, 127.1, 126.2, 126.0, 118.6, 111.4, 105.1, 66.5, 53.9, 46.1, 20.6, 16.7; HRMS (ESI) m/z calcd for C34H30O3N3 [M+H]+ 528.2282, found 528.2281.
To a solution of benzyl 1-(4-cyanobenzyl)-6-methyl-4-(2′-methyl-[1,1′-biphenyl]-4-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (0.250 g, 0.464 mmol) in 1 M TBAF in THF (0.46 mL, 0.464 mmol 1.3 eq) in a 5 mL microwave vial was added TMSN3 (0.25 mL, 1.9 mmol, 4 eq). The vial was flushed with Ar and sealed before heating at 70-75 °C for 15 h. The solution was diluted with EtOAc and washed with H2O (1 × 2 mL). The combined organic layers were dried (Na2SO4), filtered and concentrated. The residual oil was purified by chromatography on SiO2 (MeOH:CH2Cl2, 0:100 to 5:95 with 0.1% formic acid) to obtain ML282-86 (SMAL, 0.206 g, 0.361 mmol, 78%) as a yellow foam: Mp 123-125 °C; ATR IR (neat) 3301, 3084, 3073, 3068, 3031, 2969, 2876, 1676, 1619, 1455, 1385, 1206, 1164, 1104, 949, 757, 698 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.81 (d, 2 H, J = 8.1 Hz), 7.30-7.14 (m, 15 H), 6.14 (d, 1 H, J = 3.0 Hz), 5.54 (d, 1 H, J = 2.7 Hz), 5.16, 5.08 (ABq, 2 H, J = 12.3 Hz), 5.10 (d, 1 H, J = 17.4 Hz), 4.90 (d, 1 H, J = 17.1 Hz), 2.48 (s, 3 H), 2.24 (s, 3 H); 13C NMR (125 MHz, CDCl3) δ 165.6, 154.5, 148.6, 142.1, 141.2, 140.9, 135.7, 135.4, 130.6, 129.9, 128.7, 128.4, 128.4, 127.9, 127.7, 126.9, 126.3, 126.0, 123.2, 105.4, 66.7, 54.2, 46.7, 20.6, 16.7; HRMS (ESI) m/z calcd for C34H31O3N6 [M+H]+ 571.2452, found 571.2459.
4.2 Solubility Measurements
General Protocol A stock solution of analyte (1.0 mg) in DMSO/distilled water (1:9) was further diluted in a serial fashion to obtain the following concentrations: 75, 50, 25, 10, 5, and 1 μg/mL. A solution of caffeine (10 μg/mL) in DMSO/pH 7.4 phosphate buffer (1:9) was used as an external standard, and aliquots (1.0 μL) were analyzed at the beginning and after every 2 samples. A calibration curve was generated by plotting the integrated TIC peak area versus solution concentration to determine the linear dynamic range. An aliquot (1 μL) of each analyte solution was sampled in duplicate by LC-HRMS using an Accela HPLC/Exactive orbitrap mass spectrometer system and a linear gradient from 3 to 95% acetonitrile and water with 0.1% formic acid and constant 5% methanol at 0.5 mL/min with a Thermo Hypersil Gold C18 column (2.1 × 50 mm, 3 μm particle size) utilizing electrospray ionization. Data analysis was carried out on a single-ion peak for each analyte. To determine the solubility of each analyte, an LCMS vial containing a precisely measured 1-2 mg of compound in distilled water (1.0 mL) was vortexed for 10 s, followed by rotation of the vial at 30 °C for 24 h. An aliquot (200 μL) was removed, filtered through a nylon membrane (0.45 μm), and the filtered solution (1.0 μL) was analyzed by LC-HRMS. The caffeine standard solution (10 μg/mL, 1.0 μL) was analyzed in duplicate at the start of each analysis and after every 2 samples. The solubility of each analyte was determined by integration of the TIC peak area and calculated from a linear regression plot of the calibration curve for each respective analyte.
4.3 Protein Purifications
The large T antigen from SV40 was expressed in baculovirus infected cells and isolated using antibody affinity chromatography as previously described.26
The human Hsp70 protein, encoded by HSPA1A, was isolated using a modified protocol previously developed in the laboratory.27 A 25 mL culture of E. coli containing pMSHSP70 BL21(DE3) (kindly provided by R. Morimoto, Northwestern University) in LB-ampicillin media was used to inoculate a 1 L culture. When the 1 L culture reached an OD600 of ~0.9, expression was induced with IPTG. After 3 h of shaking at 26°C, cells were harvested and washed in TEK50 (20 mM Tris pH 8, 0.1 mM EDTA, 50 mM KCl), and the cell pellet was frozen at −80°C. Thawed cells were resuspended in TEK50 containing 1 mg/mL lysozyme, 0.5 M PMSF, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, and 1% Triton X-100. Cells were lysed by sonication and cell debris was removed by centrifugation using a SS34 rotor at 13,000 rpm for 10 min at 4°C.
Next, cleared lysate was chromatographed on a DEAE column (GE Healthcare) equilibrated with TEK50. The column was washed with TEK50 containing 0.5 M PMSF, 1 μg/mL leupeptin, and 1 μg/mL pepstatin A. Bound proteins were eluted over a linear gradient of TEK50 to TEK50 supplemented with 500 mM NaCl. Fractions containing Hsp70, as confirmed by SDS-PAGE and staining with Coomassie Brilliant Blue (1% solution), were pooled and incubated with rocking overnight with an ATP-agarose (Sigma-Aldrich) slurry equilibrated with ATP-agarose buffer (20 mM Tris pH 7.2, 100 mM NaCl, 10 mM KCl, 5 mM MgCl2). The ATP-agarose slurry was then poured into a column and washed with ATP-agarose buffer. Bound proteins were eluted with ATP-agarose buffer supplemented with 7 mM ATP, and fractions containing Hsp70, as confirmed by SDS-PAGE and staining with Coomassie Brilliant Blue, were diluted 1:1 with 20 mM KPO4 buffer at pH 6.8. The pooled peak fractions were loaded onto a hydroxyapatite column (BioRad) equilibrated with 20 mM KPO4 buffer at pH 6.8. The column was washed with the same buffer and bound proteins were eluted with a linear gradient containing 20 mM to 400 mM KPO4 buffer at pH 6.8. Peak fractions containing Hsp70, as determined above, were pooled and dialyzed overnight against 20 mM Tris pH 7.2, 50 mM NaCl, 10% glycerol. The protein concentration was determined by carrying out a Bradford assay and was then concentrated using an Amicon Ultra Centrifugal Filter (Millipore). Aliquots were flash-frozen in liquid nitrogen and stored at −80°C.
A glutathione-S-transferase (GST) fusion to the J domain of Hlj1, a yeast J domain-containing protein, was purified as previously published28 and used in assays containing human Hsp70 based on its promiscuity for Hsp70 isolates from various sources.27 Hdj2 was purified as previously described using a bacterial expression plasmid kindly provided by D. Cyr (University of North Carolina School of Medicine)29.
4.4 Biochemical Assays
Chemical derivatives were screened for their effects on SV40 T Ag ATPase activity and Hsp70 ATPase activity using a steady state ATPase assay. This assay was performed as previously published.17,30 In brief, purified SV40 T Ag (1-3 μg) or Hsp70 (0.5 μg) and either a compound dissolved in dimethyl sulfoxide (DMSO) or an equivalent volume of DMSO were preincubated on ice for 15 min in 50mM HEPES pH 7.4, 50 mM NaCl, 2 mM MgCl2, and 10 mM DTT. (Where indicated, Hlj1 was also included in this assay to determine the effect of a co-chaperone in ATPase activity and was used at a final concentration of 0.56 μg, or a 2:1 molar ratio of the J-domain to Hsp70). The compound was added last to ensure solubility. In a separate tube, 1 nmol ATP and 0.2 μCi [α32P]ATP were combined on ice. The ATPase reaction was started by adding the T Ag or Hsp70 solution to the ATP mixture and placing the tube in a 30°C water bath. When the impact of a J domain-containing protein was examined, the cochaperone was also included during the 15 min preincubation on ice. Over a 60-min time course, 3 μL aliquots were removed at 0, 15, 30, 45, and 60 min and quenched with 1μL of 2M LiCl, 4 M formic acid, and 36 mM ATP. The quenched reactions were spotted in duplicate on a thin layer chromatography plate (TLC PEI Cellulose F, EMD Chemicals). Chromatography was performed on plates for 10 min in a 0.5M LiCl/1M formic acid solution to resolve the ATP and hydrolyzed ADP species. TLC plates were exposed to phosphoimager plates and data were analyzed on a Fujifilm BAS-2500 phosphoimager and quantified using ImageGuage software (Fuji Film Science Lab). All reactions were performed in triplicate and established statistical methods were used. Where indicated, results are shown as percent activity, relative to the DMSO control, which is calculated as 100%. Normalization to this standard was used to ensure assay consistency.
To determine whether inhibitors of SV40 T Ag ATPase activity inhibited J-domain mediated stimulation of Hsp70 ATPase activity, either the protocol described above was employed or a single turnover assay was used where indicated. This assay was performed as previously published.31 In brief, Hsp70-[α32P]ATP was prepared by incubating 25 μg Hsp70 with 100 μCi of [α32P]ATP in 10 mM HEPES-KOH (pH 7.5), 30 mM KCl, and 8 mM magnesium acetate on ice for 30 min. The complex was purified from free [α32P]ATP on a NICK G-50 column (Amersham Biosciences) at 4°C. Glycerol (10% final concentration) was added to fractions enriched for Hsp70-[α32P]ATP complexes. Aliquots were flash frozen in liquid nitrogen. ATPase activity was assayed by quick-thawing the complex and adding the solution to 2 μg SV40 T Ag (as a J-domain-containing protein) at 30°C. Over a 10-min time course, 6 μL aliquots were removed at the indicated times and added into 2 μL of the same quench solution used above. When included, test compounds were added at the 60-sec time point. The quenched reaction was resolved by TLC, the plates were exposed to phosphoimager plates overnight, and the data were analyzed on a Fujifilm BAS-2500 phosphoimager and quantified using ImageGuage software, as above. All reactions were performed in triplicate and established statistical methods were used.
4.5 Viral replication assays
To identify inhibitors of SV40 DNA replication, a viral DNA replication assay was performed that quantifies the amount of SV40 DNA in infected CV1 cells, a monkey kidney cell line. Cells were grown at 37°C in 5% CO2 in DMEM media to 90-95% confluency. The viral DNA replication assay was performed as previously published.18, 32, 33 In brief, CV1 cells were infected with SV40 at an MOI of either 0.1 (when DNA levels were detected by agarose gel electrophoresis) or 6 (when qPCR was used) for 2 h. After infection, the media containing non-adhered viral particles was replaced with media containing a test compound or an equivalent volume of DMSO. This media was replaced at 24 h post infection. At 48 h post infection, the media was aspirated from the CV1 cells. SV40 DNA was harvested using a modified Qiagen mini-prep in which the aspirated cells were first washed in PBS before the addition of 250 μL each of Qiagen P1 and P2. This mixture was allowed to incubate for 5 min before the lysate was collected and 50 μL (10μg/μL) of Proteinase K was added. After a 55°C incubation for 2 h, the standard Qiagen protocol was followed. The isolated SV40 viral DNA was then digested with BamHI and visualized on an ethidium bromide-stained agarose gel.
Where indicated, qPCR was also performed to quantitate the amount of SV40 DNA replicated. The DNA stocks, prepared as described above, were serial diluted for proper analysis as reported elsewhere.18 A reference plasmid (pSVB334) allowed for absolute quantification. The plasmid contained the entire viral genome cloned into a BamHI site. A mix of primers and Taqman probe were used to detect SV40. The primers and probe used for the qPCR were as follows: forward, 5′-GATGAACACTGACCACAAGG-3′; reverse, 5′-GCACATTTTCCCCACCT-3′; probe, (FAM)/5′-ATCAGGAACCCAGCACTCCACT-3′/(IOWA BLACK). Three technical replicates were examined for each biological replicate and data were compared to the amount of replicated viral DNA with a DMSO control.
4.6 HEK293 cell viability and compound stability assays
To assay for the effects of select compounds on cell viability, HEK293 cells were cultured in DMEM with 10% FBS at 37°C in 5% CO2. Cells (100 μL of 1 × 105 cells) were seeded into 96-well plates and grown for 5 h at 37°C in 5% CO2 prior to addition of compounds (33 μL) to reach final concentrations of 200, 150, 100, 75, 50 and 25 μM or 10, 5, 1, 0.1, 0.01 and 0.001 μM for staurosporine (as a positive control). The plates were then incubated for a further 20 h prior to room temperature equilibration for 30 min, addition of Cell Titer-Glo (67 μL; Promega, Madison, WI, USA), and assessment of cell viability according to the manufacturer’s protocol. Data represent the means of two independent experiments each run in triplicate. A sigmoidal, 3-parameter line regression of the resulting data was performed using SigmaPlot (v. 11.0, Systat Software, San Jose, CA).
Compound stability measurements in male mouse liver microsomes were performed in the presence of NADPH in parallel by Sai Life Sciences Ltd. (Pune, India) and by Pharmaron (Louisville, KY) in order to obtain independent measurements. After a 1 h incubation, the compounds were analyzed by LC-MS/MS. Controls included Imipramine (24% remaining after 1 h) and verapamil (5% remaining after 1 h), which were used respectively at Sai Life Sciences Ltd. and Pharmaron.
Supplementary Material
Acknowledgements
This work was supported by grant DK79307 (The Pittsburgh Center for Kidney Research) and the National Institutes of Health P50 CMLD Program (GM067082). P.W. and J.L.B. also acknowledge support from Actelion Pharmaceuticals, Ltd. A.M.-T. was funded by an American Australian Association Merck Company Foundation Fellowship. A.W.I. was supported by a fellowship from the Beckman foundation and T.A.G. acknowledges support from a Howard Hughes Medical Institute undergraduate research fellowship. The authors thank Ms. Kayla Lloyd for technical assistance in the preparation of the KRL analogs, Ms. Celeste Alverez for solubility measurements, and Mr. Peter G. Chambers and Ms. Taber Lewis for QC and sample preparations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kean JM, Rao S, Wang M, Garcea RL. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeCaprio JA, Imperiale MJ, Major EO. In: Fields Virology. 6 th Ed Knipe DM, Howley PM, editors. Wolters Kluwer; Philadelphia: 2013. p. 1633. [Google Scholar]
- 3.Berger JR, Major EO. Semin Neurol. 1999;19:193. doi: 10.1055/s-2008-1040837. [DOI] [PubMed] [Google Scholar]
- 4.Josephson MA, Williams JW, Chandraker A, Randhawa PS. Transplant Infect Dis. 2006;8:95. doi: 10.1111/j.1399-3062.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 5.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Cell Host Micr. 2010;7:509. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunisaki KM, Janoff EN. Lancet Infect Dis. 2009;9:493. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan CS, Pipas JM. Microbiol Molec Biol Rev. 2002;66:179. doi: 10.1128/MMBR.66.2.179-202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantalupo P, Doering A, Sullivan CS, Pal A, Peden KW, Lewis AM, Pipas JM. J Virol. 2005;79:13094. doi: 10.1128/JVI.79.20.13094-13104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, DeCaprio JA, Fluck MM, Schaffhausen BS. Semin Cancer Biol. 2009;19:218. doi: 10.1016/j.semcancer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell KS, Mullane KP, Aksoy IA, Stubdal H, Zalvide J, Pipas JM, Silver PA, Roberts TM, Schaffhausen BS, DeCaprio JA. Genes & Dev. 1997;11:1098. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 11.Fewell SW, Pipas JM, Brodsky JL. Proc Natl Acad Sci USA. 2002;99:2002. doi: 10.1073/pnas.042670999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley WL, Georgopoulos C. Proc Natl Acad Sci USA. 1997;94:3679. doi: 10.1073/pnas.94.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan A, McClellan AJ, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky JL, Pipas JM. Mol Cell Biol. 1997;17:4761. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stubdal H, Zalvide J, Campbell KS, Schweitzer C, Roberts TM, DeCaprio JA. Mol Cell Biol. 1997;17:4979. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahuja D, Sáenz-Robles MT, Pipas JM. Oncogene. 2005;24:7729. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 16.Smelkova NV, Borowiec JA. J Virol. 1997;71:8766. doi: 10.1128/jvi.71.11.8766-8773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright CM, Seguin SP, Fewell SW, Zhang H, Ishwad C, Vats A, Lingwood CA, Wipf P, Fanning E, Pipas JM, Brodsky JL. Virus Res. 2009;141:71. doi: 10.1016/j.virusres.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huryn DM, Brodsky JL, Brummond KM, Chambers PG, Eyer B, Ireland AW, Kawasumi M, LaPorte MG, Lloyd K, Manteau B, Nghiem P, Quade B, Seguin SP, Wipf P. Proc Natl Acad Sci USA. 2011;108:6757. doi: 10.1073/pnas.1015251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chromy LR, Pipas JM, Garcea RL. Proc Natl Acad Sci USA. 2003;100:10477. doi: 10.1073/pnas.1832245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodsky JL, Chiosis G. Curr Top Med Chem. 2006;6:1215. doi: 10.2174/156802606777811997. [DOI] [PubMed] [Google Scholar]
- 21.An P, Sáenz Robles MT, Pipas JM. Annu Rev Microbiol. 2012;66:213. doi: 10.1146/annurev-micro-092611-150154. [DOI] [PubMed] [Google Scholar]
- 22.Seguin SP, Evans CW, Nebane-Akah M, McKellip S, Ananthan S, Tower NA, Sosa M, Rasmussen L, White EL, Maki BE, Matharu DS, Golden JE, Aube J, Brodsky JL, Noah JW. J Biomol Screen. 2012;17:194. doi: 10.1177/1087057111421630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin EC, Atwood WJ, DiMaio D. J Virol. 2009;83:5630. doi: 10.1128/JVI.00203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farasati NA, Shapiro R, Vats A, Randhawa P. Transplantation. 2005;79:116. doi: 10.1097/01.tp.0000149338.97084.5f. [DOI] [PubMed] [Google Scholar]
- 25.Abed Y, D W, Boivin G. Emerg Infect Dis. 2007;13:1939. doi: 10.3201/eid1312.070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantalupo P, Saenz-Robles MT, Pipas JM. In: Methods in Enzymology. Joseph C, Glorioso MCS, editors. Vol. 306. Academic Press; 1999. p. 297. [DOI] [PubMed] [Google Scholar]
- 27.Chiang AN, Valderramos J-C, Balachandran R, Chovatiya RJ, Mead BP, Schneider C, Bell SL, Klein MG, Huryn DM, Chen XS, Day BW, Fidock DA, Wipf P, Brodsky JL. Bioorg Med Chem. 2009;17:1527. doi: 10.1016/j.bmc.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. Mol Biol Cell. 2004;15:4787. doi: 10.1091/mbc.E04-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meacham GC, Lu Z, King SK, Sorscher E, Toussen A, Cyr DM. EMBO J. 1999;18:1492. doi: 10.1093/emboj/18.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cyr DM, Lu X, Douglas MG. J. Biol. Chem. 1992;267:20927. [PubMed] [Google Scholar]
- 31.Fewell SW, Smith CM, Lyon MA, Dumitrescu TP, Wipf P, Day BW, Brodsky JL. J. Biol. Chem. 2004;279:51131. doi: 10.1074/jbc.M404857200. [DOI] [PubMed] [Google Scholar]
- 32.Li JJ, Kelly TJ. Proc Natl Acad Sci USA. 1984;81:6973. [Google Scholar]
- 33.Randhawa P, Ron S, Vats A. J Infect Dis. 2005;192:504. doi: 10.1086/431522. [DOI] [PubMed] [Google Scholar]
- 34.Pipas JM, Peden KW, Nathans D. Mol Cell Biol. 1983;203 doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.cLogD values were calculated with Instant JChem 5.3.1. ChemAxon. 2010 http://www.chemaxon.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.