Abstract
Triglyceride micro-emulsions such as Intralipid® have been used to reverse cardiac toxicity induced by a number of drugs but reservations about their broad-spectrum applicability remain because of the poorly understood mechanism of action. Herein we report an integrated mechanism of reversal of bupivacaine toxicity that includes both transient drug scavenging and a cardiotonic effect that couple to accelerate movement of the toxin away from sites of toxicity. We thus propose a multi-modal therapeutic paradigm for colloidal bio-detoxification whereby a micro-emulsion both improves cardiac output and rapidly ferries the drug away from organs subject to toxicity. In vivo and in silico models of toxicity were combined to test the contribution of individual mechanisms and reveal the multi-modal role played by the cardiotonic and scavenging actions of the triglyceride suspension. These results suggest a method to predict which drug toxicities are most amenable to treatment and inform the design of next-generation therapeutics for drug overdose.
Keywords: Lipid emulsion, pharmacotoxicity, local anesthetic, bupivacaine, triglyceride, antidote, biodetoxification
Introduction
Micro- and nano-emulsions are frequently studied as drug delivery systems for therapeutic applications [1,2] with the aim of achieving more efficient or targeted delivery. The converse principle of biodetoxification by drug-scavenging-agents is of increasing interest to drug-delivery scientists based on the clinical utility of absorptive properties [3,4]. The need for efficient detoxification agents is clear as drug-overdoses account for thousands of deaths annually in the United-States [5] and around the world, most-often in a young and healthy population. Recently, micro-emulsions of triglycerides and phospholipids (most commonly Intralipid®) have been used clinically to treat pharmacotoxicity from tricyclic-antidepressants, atypical antipsychotics, serotonin antagonist and reuptake inhibitors, selective-serotonin reuptake blockers, calcium channel blockers, cocaine, local anesthetics and other agents [6,7]. However, without a clear understanding of their mechanism of action, some reservations remain as to their specific applicability [8–12]. The primary mechanism is often asserted to be an intravenous partitioning phenomenon, an effect which is supported by in vitro binding studies [13–15], isolated heart experiments [16], small animal experiments [17,18] and clinical reports [19]. This toxin–binding mechanism is both intuitive and relatively simple, and thus has stimulated efforts to optimize therapeutic formulations (e.g. stealth, anionic liposomal preparations) with optimized binding functionality [3,20–22]. Despite the promise of efficient drug scavenging, in vivo experiments demonstrate that tailored liposomes do not reverse cardiovascular toxicity as effectively as triglyceride-based emulsions during acute intoxication [23]. Further, trials in healthy volunteers dispute that a drug-scavenging effect occurs at all [24,25], and computational models assert that a scavenging effect alone cannot account for the rapid recovery from toxicity typically observed following emulsion administration [26]. A larger problem in the field is that the in vivo physiological effects of these delivery agents [27] and their influence on drug pharmacokinetics [28] are not well understood.
We recently demonstrated that a micro-emulsion of triglycerides produces an inotropic effect in the absence of toxicity [29], and further proposed that this effect coupled with the volume load is necessary for full recovery from cardiac pharmacotoxicity [30]. The prominent role of a non-scavenging effect in recovery and the ability of fatty-acid oxidase inhibitors to block emulsion-based recovery from pharmacotoxicity [31] call into question whether scavenging is relevant to recovery. In order to resolve these disparate results we combined in vivo pharmacokinetic experiments with a previously developed physiologically-based pharmacokinetic pharmacodynamic model (in silico) to assess the contributions of a cardiotonic effect, a scavenging effect and metabolic processing to the recovery from toxicity of a cardiotoxic agent with and without an adjuvant triglyceride micro-emulsion. Our results indicated the critical importance of a cardiotonic effect independent of cardiac drug concentration, the undisputable scavenging of drug by the lipid compartment and the acceleration of microsomal (e.g. liver) metabolism to increase processing and excretion of the drug. These long-postulated but previously unproven effects will help inform next-generation treatments for drug detoxification.
Materials and Methods
Reagents
3H-bupivacaine hydrochloride (97.4% pure, specific activity 2.87 Ci/mol) was purchased from Moravek biochemical & radiochemicals (Brea, CA). The intravenous lipid emulsion used was 30% Intralipid®, from Baxter Pharmaceuticals (Deerfield, IL). It is composed of 30% Soybean Oil, 1.2% Egg Yolk Phospholipids, 1.7% Glycerin, and water with pH adjusted to ~8 with sodium hydroxide. The oil is mostly triglycerides primarily composed of linoleic (44-62%) and oleic acid (19-30%) but also with palmitic (7-14%), linolenic (4-11%) and stearic acids (1.4-5.5%). Solvable and Ultima Gold Cocktail were purchased from PerkinElmer (Waltham, MA). NADPH colorimetric tests were purchased from Sigma Aldrich (St Louis, MO) and BioAssay Systems (Hayward, CA). Ketoconazole was also purchased from Sigma Aldrich (St. Louis, MO) and potassium chloride (KCl) was purchased from Fisher Scientific, Pittsburgh, PA). BSA protein assay was purchased from Thermo Scientific (Bradford, IL)
In Vivo protocol
In brief, inhalational anesthesia was induced in a bell jar and rats were maintained on 1.2-1.75% isoflurane for the remainder of the experiment. Sixty-six animals (with two exclusions due to technical errors) received 10mg/kg 3H-bupivacaine hydrochloride into the left internal jugular over 20 seconds to produce a transient asystole followed 30 seconds later by either 10 mL/kg 30% intravenous lipid emulsion over 1 minute or no intervention (Null). At pre-specified time-points (0.5, 1.5, 2.5, 4.5, 5.5, 8, 12, 60 minutes), animals were sacrificed by injecting 0.75 mL 1M KCl solution into the right internal jugular vein. Four non-randomized animals received intraperitoneal injection of 25mg/kg ketoconazole (dissolved in propylene glycol and 0.1M HCl) 30-minutes prior to 3H-bupivacaine challenge in order to inhibit cytochrome P450 3A4.
Tissue Collection and Processing
In animals treated for 60 minutes, 50 microliters of arterial blood were collected at 5, 10, 15, 30, 45 & 60 minutes for subsequent analysis. Arterial blood was taken prior to sacrifice and organs were harvested. Blood was separated into clear plasma, red blood cell and lipid-plasma-component by ultracentrifugation. Organ (10-30mg) and fluid (5-50μL) samples were dissolved overnight in Solvable in scintillation vials at 55°C including heart (apex), lung (left anterior lobes), liver samples (right lateral lobe & left lateral lobe), kidney (apical pole), spleen (anterior edge), brain (frontal lobe), cerebellum (whole), fat (omentum), skeletal muscle (adductor longus), lipid-free plasma, red blood cells, lipid-plasma component, whole blood, and urine. Dissolved tissues were suspended with Ultima Gold Cocktail and radioactivity was quantified (Tri-Carb 1600 TR Liquid Scintillation Analyzer, Packard, Meriden, CT) and reconciled to 3H bupivacaine standards. Tissue bupivacaine content was normalized to tissue weight or fluid volume.
Pharmacokinetic Analysis
Bupivacaine concentration was calculated in Excel (Microsoft, Redmond WA) and transferred to Prism 6.0 (Graph Pad, La Jolla, CA). Organ data were fit to a one-phase exponential decay with the plateau set to zero and a shared intercept. Fluid data was set to a two-phase exponential decay to account for redistribution and elimination. The x-axis of fluid data-fits is plotted on log2 scale to increase readability. Blood:organ plots were fit using a Boltzmann-sigmoidal function setting the lower limit to zero and setting a shared upper limit. Ratio of drug partitioned into tissue relative to blood was calculated for the V-50 point on the Boltzmann curves.
Statistical Analysis
For inter-group comparisons, time points were grouped into 2, 5, & 10 minutes time points (after confirming no differences between grouped time-points). Data were checked for normality using a D'Agostino & Pearson omnibus normality test, and for equal variances using Bartlett's test. Groups exhibiting normal distribution and equal variations were analyzed with a 1-way ANOVA and post-hoc Bonferroni multiple-comparison tests. For unequal variances, a Welches ANOVA with post-hoc Bonferroni multiple-comparison tests were used (in SAS). For non-normal distributions, groups were compared with a Kruskal-Wallis non-parametric ANOVA and post-hoc Dunn's multiple comparison tests. Total organ content and distribution were analyzed by the same metric.
Continuous Analysis of Physiological Data
Continuous data at 1000 Hz were transferred to MatLab for analysis of the first 8-minutes (n=12 per group). Data were down-sampled to 1 Hz and transferred to Prism 6.0 for plotting. To ascertain the cardiac bupivacaine concentration to flow relationship, one-phase exponential decay fit-curve parameters were identified and time of whole-number concentrations were identified (39-300nmol/g). Using those time-points, corresponding carotid flow values were identified from both null (time = 0-20 minutes, n=4), and ILE treated (0-12 minutes, n=7). Subsequently, differences between groups were calculated using a continuous two-sided Mann-Whitney U-test.
In Silico
The in silico approach is a computational model of the in vivo system that includes representations of the physiological (cardiovascular) and pharmacological (toxin & therapeutic) components. Physiological distribution and metabolism (pharmacokinetics) are coupled with a mathematical model of the toxin and therapeutic's effects on cardiac function (pharmacodynamics). Solution of the resulting differential equations predicts the time course of cardiac failure and lipid-mediated resuscitation as a function of hypothesized mechanism of therapeutic action. The results reported herein were obtained using an augmented version of the model described in reference [30]. Modifications made include: (i) adjustment of the lipid delivery timeline to correspond with the in vivo experiments; and (ii) incorporation of a non-competitive inhibition model for the interplay between bupivacaine toxicity and the cardiotonic effect of the lipid. The consequence of this latter modification is a decrease in the maximal effect constant for lipid inotropy by a factor of (1+Cbup/KI) where the inhibition constant KI is taken to be equal to the ion-channel dissociation constant of bupivacaine as described by Clarkson and Hondeghem [32] (Kd=0.9μM). Cbup is the unbound concentration of bupivacaine in blood plasma. The question of the relevance of hepatic metabolism was addressed by setting the metabolic rate for bupivacaine to zero. Four alternative scenarios were examined: (i) no treatment (null); (ii) a volume & inotropic effect; (iii) volume, inotropic & sink effects; and (iv) volume, inotropy, & sink in the absence of bupivacaine metabolism.
NADPH quantification
Four animals (control, ILE30, Null, ILE only) were injected with bupivacaine (10mg/kg) and sacrificed at 12 minutes to assess NADPH activity with two colorimetric tests. Liver tissue was isolated, homogenized and lysed. Lysate was spun and supernatant transferred into tubes for NADP and NADPH quantification. NADP was degraded by incubating at 60°C for 5-30 minutes. Subsequently, samples were incubated with reaction enzyme and quantified using an iMark microabsorbance plate reader (BioRad, Hercules, CA). NADP/NADPH concentrations were normalized to protein concentration as assessed by Pierce BSA Protein assay and to control to account for variations between kits and preparations.
Results
Triglyceride micro-emulsion improves cardiac output – effect contingent on lowering tissue toxin concentration
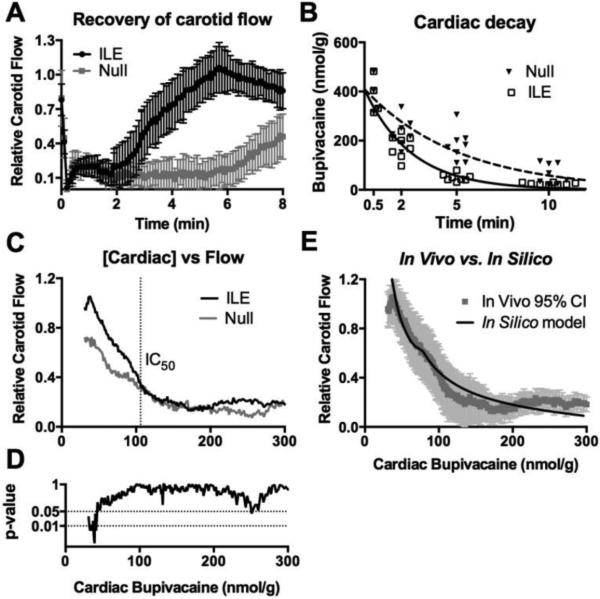
First, we characterized how a triglyceride micro-emulsion (Intralipid®) delivered as an intravenous lipid emulsion (ILE) modifies pharmacokinetic redistribution of a canonical cardiotoxin (bupivacaine) and the associated concentration-dependent effects on hemodynamics. We injected H3-radiolabeled drug intravenously in anesthetized rats at a dose that produces transient asystole recoverable without intervention [30,33]; recovery was monitored with or without ILE intervention (10mL/kg 30% Intralipid® delivered intravenously over 1 minute). Treatment with ILE accelerated cardiovascular recovery (Fig. 1A), consistent with previous reports [30], and reduced cardiac drug tissue concentrations at 2 minutes (Fig. S1A). We fit cardiac bupivacaine concentrations to a first-order exponential decay across time (R = 0.86 for ILE, 0.95 for null) and found that ILE shortened the decay half-life from 3.5 minutes (95%CI: 2.8-4.6 min) to 1.76 minutes (95%CI: 1.4 – 2.3 min; F=28.99, p<0.0001, Fig. 1B). The difference in time-to-recovery (as seen in Figure 1a) presents the potential problem of concentration-dependent effects of the toxin on cardiac function. We therefore removed the confounder of time-to-recovery by comparing carotid flow to cardiac-tissue drug-concentration (Fig. 1C) across all time points and found that carotid-flow substantially improved in both treatment groups only after drug concentration was less than ~100 nmol/g. This threshold is consistent with bupivacaine's IC-50 for blocking cardiac sodium and calcium ion channels [32,34]. Further, it suggests that triglyceride-mediated recovery of cardiac output requires unbinding of the cardiac-toxin from key ionotropic channels. The finding agrees with prior experiments demonstrating that ILE accelerates unbinding of drug from channels [35,36]. Below this threshold, ILE significantly improved carotid flow compared with the null-group at equivalent myocardial drug concentrations (Fig. 1D); this indicates a cardiotonic effect driven by both volume contributions and a positive inotropic effect of the lipid. The dependence of carotid flow on cardiac drug concentration was confirmed with raw data derived at sacrifice in the absence of interpolation (Fig. S1A-C); we used hemodynamic parameters and tissue concentrations recorded immediately at sacrifice instead of data from a fit curve in Figure 1B to produce the continuous curve in Figure 1C.
Figure 1. Threshold-based recovery of cardiac output and the cardiotonic effect of ILE.
(A) Treatment with ILE (10mL/kg 30% Intralipid) at t=0.5 over 1 minute (ILE, n=11) improves recovery of carotid flow following a 10mg/kg infusion of bupivacaine ending at t=0, (null, n=9) Mean ± SEM. (B) Treatment with ILE increases removal of bupivacaine from cardiac tissue as assessed by fitting curves to single-phase exponential decay with plateau=0 and shared y-intercept (n=24 animals for ILE, n=28 for null). (C) Carotid flow does not recover until achieving a bupivacaine concentration below ~100nmol/g corresponding to IC50 of bupivacaine inhibition from Clarkson & Hondeghem, 1985. Below 100nmol/g, the curves diverge with a greater improvement in flow at equivalent bupivacaine concentrations for animals treated with ILE (ILE, n=11, mean line) compared to control (null, n=4, mean line). (D) Accompanying Mann-Whitney U-test between Null and ILE for “C” (39-300 nmol/g) demonstrating significant difference in carotid flows for concentrations <56nmol/g. A transient drop at ~250nmol/g corresponds to infusion of lipid. (E) In vivo experimental data (95% confidence intervals) with in silico prediction overlaid demonstrating that incorporating the bupivacaine disassociation constant from the sodium channel produces a robust fit between in vivo and in silico data sets.
We further explored the experimental observations regarding the importance of channel unblocking using a previously described pharmacokinetic-pharmacodynamic computational model [26,30]. Modifying the existing model to fit the timeline and dosing regimens of our in vivo study, we observed a tendency of the simulation to predict an un-physically steep response curve for the restoration of cardiac function. This is due to the independence of the mathematical representations for the cardiotoxic and cardiotonic elements in the prior pharmacodynamic model. In the current implementation, we incorporated the observed threshold effect, postulating that ion channel blocking by cardiotoxin functions as a non-competitive inhibitor of the triglyceride cardiotonic effect (i.e. the heart cannot contract until conduction is restored). Employing an inhibition constant equal to the experimentally derived dissociation constant for bupivacaine binding of sodium channels [32] yielded a response curve that mirrors precisely the observed relationship between cardiac drug concentration and carotid flow (Fig. 1E).
Triglyceride micro-emulsion modifies cardiotoxin pharmacokinetics by transient partitioning into the vascular space
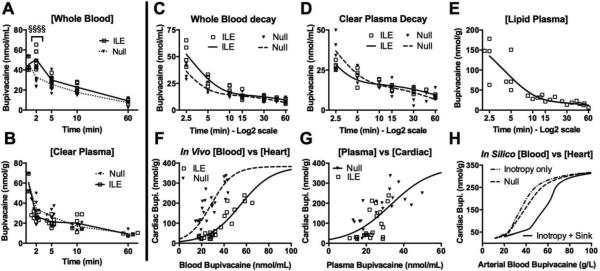
Next, we investigated the contribution of an intravenous partition[37] on hemodynamic recovery. ILE increased the concentration of drug in whole blood at 2 minutes (Fig. 2A), but did not significantly modify concentrations relative to the null-group at later time points. Of interest, at 2 minutes, there was no difference in carotid flows between groups (Fig. S1B). We separated the blood by sequential centrifugation and ultracentrifugation into red blood cell, lipid-free-plasma (clear plasma) and lipid-plasma (lipid-bound) components. Neither the clear plasma (Fig. 2B) nor red blood cells (Fig. S2A) demonstrated differences in drug concentrations at any time between treatment groups. Whole blood, clear plasma, red blood cell and lipid-plasma-component drug concentrations were fit to two-phase exponential decays. No differences were observed in the volume of distribution or elimination constants (K2, t1/2β) in whole blood, but the alpha redistribution half-life (t1/2α) was significantly increased by ILE relative to null (Fig. 2C) The reciprocal effect was observed in the clear plasma (Fig. 2D) and red blood cells (Fig. S2B), resulting in increased redistribution constants (K1). This is consistent with an accelerated redistribution of the drug (i.e. faster equilibration of drug concentrations between highly-perfused organs and less vascularized organs). In contrast, the lipid plasma component had a shorter K1 (Fig. 2E). These results show that ILE increases bupivacaine whole blood redistribution half-life[18] while reducing the redistribution half-life of the drug in clear plasma [38]. This provides the first in vivo demonstration that the lipid-partition [16] produces these inverse effects in the whole blood and plasma. In order to confirm partitioning from organs into blood, we fit whole-blood drug concentrations versus cardiac drug concentration to a Boltzmann curve (Fig. 2F) and assessed partitioning independent of time-to-recovery. Consistent with other reports [39], the cardiotoxin partitioned into the cardiac tissue at a ratio of approximately 6:1 relative to plasma in the untreated condition. ILE treatment shifted the curve rightward, thereby reducing the partitioning of drug into heart relative to blood (p<0.0001) with an effect size of 0.99 at the V50. However, this shift in partitioning was not observed in the clear plasma (Fig. 2G). We then used the in silico model to determine if either increased inotropy or partitioning of toxin (with the accompanying volume increase from the fluid infusion) could in isolation account for such a rightward shift. The computational result indicated that among the putative mechanisms only the sink effect could shift the curve to the same degree as observed experimentally (Fig. 2H).
Figure 2. Intravenous partitioning of toxin.
(A) Whole blood bupivacaine concentration at 0.5 2, 5, 10 and 60 minutes (n=4 animals for 0.5min & 60 min; n=8 for others) following infusion for control (null) or ILE (ILE) demonstrating an increase in bupivacaine concentration at 2 minutes (Welch's ANOVA: F = 27.57, p<0.0001; Bonferroni post-test at 2 minutes: §§§§ p<0.0001.) (B) Same as “a” for clear plasma but demonstrating no difference in plasma bupivacaine concentrations. Kruskal-Wallis ANOVA (F=6.789, p=0.0001), all Bonferroni post-test >0.05. (C) Whole blood bupivacaine concentration from sequential samples of null and ILE fit to two-phase exponential decay (R=0.934, 0.947 respectively; n=4 sequential samples) demonstrating no difference in elimination (Extra sum of squares F-test: F=1.286, p=0.2624) but a significant drop in the redistribution constant from 0.5441 - 0.6879 min−1 (95%CI) in null to 0.3431 - 0.4335 min−1 (95%CI) in ILE (Extra sum of squares F-test: F =32.32, p<0.0001) with analogous lengthening of t1/2α in null (1.008-1.274) to ILE (1.599-2.020). (D) Same as “C” but for clear plasma (R = 0.888, 0.924 respectively, n=4 sequential samples) and demonstrating no difference in elimination or t1/2β (Extra sum of squares F-test: F=0.386, p=0.5379) but increase in redistribution constant from 0.3707-0.5746 min−1 in null to 0.5992-0.8076 min−1 in ILE (Extra sum of squares F-test: F=9.062, p=0.0043) and analogous shortening of t1/2α from 1.06-1.870 min in null and 0.8583-1.157 min in ILE. (E) Lipid plasma component from “d”, fit to two-phase decay (R=0.88, n=4 sequential samples) with a significantly smaller redistribution constant = 0.25 ± 0.03 and much longer t1/2α=2.73 (95%CI: 2.227-3.528) than for null. (F) Treatment with ILE shifts the Boltzmann-sigmoidal fit curves (R = 0.844, 0.905 for null and ILE respectively) of blood:cardiac bupivacaine rightward (Extra sum of squares F-test=50.56, p<0.0001) demonstrating an intravenous partitioning effect (n=27 for null; n=23 for ILE). (G) After removing lipid and red blood cells and comparing plasma:cardiac bupivacaine, the partitioning effect of ILE disappears (Extra sum of squares F-test: F=2.973, p=0.0629; n=22 for null; n=21 for ILE). (H) In silico cardiac vs. arterial blood bupivacaine for three models demonstrating that a partitioning effect is required for the rightward shift.
Triglyceride micro-emulsion shifts partitioning of toxin away from central nervous system and other rapidly perfused organs
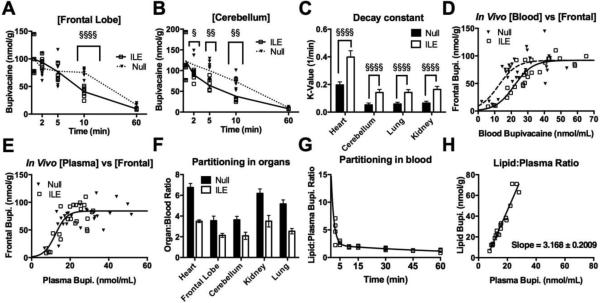
We further analyzed drug concentrations in the rostral pole of the frontal lobe and the cerebellum because central-nervous-system toxicity is a clinical concern in bupivacaine overdose. ILE treatment accelerated removal of drug from both areas compared with the null group (Fig. 3A, B). Treatment also accelerated the removal of drug from the kidney (Fig. S3A) and lung (Fig. S3B). Organ drug concentrations that fit to a one-phase exponential decay included cerebellum (R = 0.8, 0.58 for ILE and null respectively); kidney (R = 0.85, 0.66); lung (R = 0.74, 0.73) and ILE-treated frontal lobe (R=0.81) but not untreated (R=0.14). Decay rates increased with treatment in key organs (p<0.0001 for heart, cerebellum, kidney and lung decay, Fig. 3C) and thereby reduced the corresponding drug tissue half-lives (Fig. S3C). The redistribution half-life in the heart was shorter than for other organs in both treatment groups but was also associated with higher peak concentrations (Fig S3D). Assessing for time-independent partitioning, ILE treatment produced a rightward shift in tissue:blood curves in frontal lobe (Fig. 3D), cerebellum (Fig. S3E), kidney, (Fig. S3F) and lung (Fig. S3G). This shift broke down in the plasma partition (Fig. 3E, for frontal lobe). In sum, treatment with ILE reduced the partitioning of drug into tissue relative to blood in all four organs (Fig. 3F). Consistent with in vitro binding studies[15], the partitioning of drug in lipid relative to plasma dropped exponentially over time as plasma concentrations decreased (Fig. 3G). Further, it fit to a straight-line (R= 0.97, Fig. 3H) with a slope of 3.2 ± 0.2 (unitless) suggesting that ILE partitioning of drug occurs preferentially at higher tissue concentrations and earlier time points. This explains why the increase in blood concentrations is transient and accounts for the failure of previous studies to identify a partitioning effect [38]. Intravascular bulk phase lipid appears to function as a dynamic buffering compartment, confirming the importance of administering ILE early during toxicity when tissue concentrations are high.
Figure 3. ILE treatment partitions toxin out of the central nervous system and other rapidly perfused organs.
(A) ILE treatment accelerates bupivacaine redistribution away from frontal lobe (One-way ANOVA, F=12.96, p<0.0001; Bonferroni post-test at 10 min adjusted p-value<0.0001; §§§§; n=4 animals per group for 0.5 & 60 min; n=8 for all other conditions). (B) ILE treatment accelerates bupivacaine redistribution away from cerebellum. (One-way-ANOVA; F=14.91, p<0.0001; Bonferroni post-test at 2 minutes, adjusted p-value=0.039, §, 5 minutes: p=0.0011, §§, 10 minutes: p=0.0025, 60 minutes: p=0.10; n=4 for 0.5 & 60 min; n=8 for all other conditions). (C) Treatment with ILE increases redistribution time constants (K1) for heart, cerebellum, lung and kidney (§§§§: p<0.0001). Frontal lobe omitted because null condition did not fit to an exponential decay. (D) Treatment with ILE shifts the fit curve for blood:frontal lobe rightward (R = 0.76 and R = 0.89 for null and ILE respectively; Extra-sum-of-squares F-test: F=10.88, p=0.0001) indicating an increased partitioning of bupivacaine into the intravenous compartment (n=30 for Null, n=26 for ILE). (E) After removing lipid and red blood cells, the partitioning effect of ILE disappears (Extra-sum-of-squares F-test: F=0.39, p=0.6784; n=27 for Null, n=23 for ILE). (F) Treatment with ILE significantly reduces drug partitioning into organ relative to whole blood for toxin sensitive organs including heart (Null 6.76 ± 0.38 versus 3.50 ± 0.133 [p<0.001] with ILE), frontal lobe (Null 3.54 ± 0.44 versus 2.14 ± 0.19 [p<0.001] with ILE), cerebellum (Null 3.63 ± 0.34 versus 2.09± 0.35 [p<0.001] with ILE), kidney (Null: 6.18 ± 0.43 to 3.50 ± 0.56 [p<0.001] with ILE) and lung (Null: 5.17 ± 0.38 to 2.5 ± 0.26 [p<0.001] with ILE). (G) Ratio of bupivacaine partitioned into lipid relative to plasma based on ILE data from Fig. 2d & 2e for data from sequential sampling over 60 minutes. Data fit to 2-phase exponential decay (R=0.86). (H) Plasma-bound bupivacaine plotted against lipid bound bupivacaine for data from “g”, but with 2.5 & 5 minutes samples removed to remove confounder of incomplete cardiovascular recovery. Data fit to straight line (R = 0.97): Slope 3.168 ± 0.2009. Y=intercept: -17.91 ± 3.323.
Increased liver metabolism observed but not required for accelerated recovery
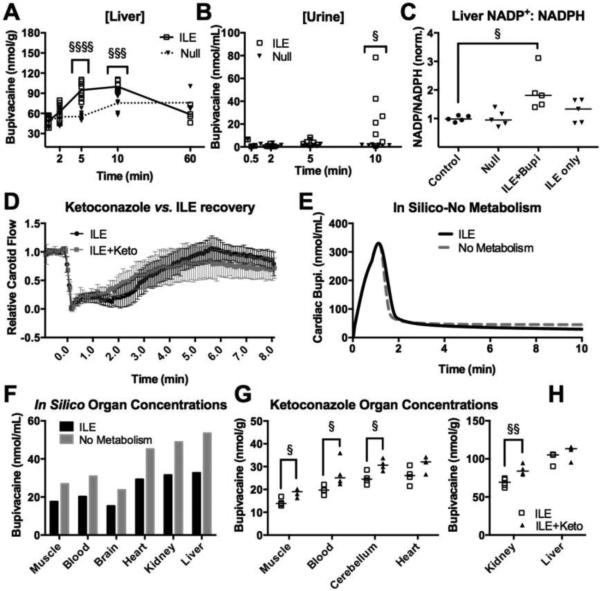
Consistent with previous studies[18], ILE increased drug redistribution to the liver (Fig. 4A) but no difference between groups was observed in skeletal muscle concentrations (Fig. S4A) or adipose tissue (Fig. S4B). Accounting for organ mass, the skeletal muscle contained the largest portion of delivered bupivacaine in both ILE treated and untreated groups at 5-minutes (25 ± 1.5% of total bupivacaine for null; 33 ± 2% for ILE); the muscle of ILE-treated animals contained more drug relative to untreated animals (p<0.0001; Fig. S4C). The elevated liver concentrations could not be accounted for simply by the increased flows seen in ILE treated animals (Fig. S4D), but the in silico model demonstrated that increased liver concentrations are expected (Fig. S4E) without invoking other factors that contribute to the liver-uptake of Intralipid® [40]. Subsequent to increasing liver concentrations, ILE treatment increased excretion of radiolabel into the urine by 10 minutes (Fig. 4B). We confirmed that ILE increased liver metabolism by measuring NADPH activity as a surrogate for Cytochrome P450 (CYP) activity at 10 minutes (Fig. 4C). However, pre-treating with ketoconazole to inhibit metabolizing enzyme CYP3A4 [41] did not affect cardiovascular recovery after ILE. The same conclusion is drawn from the in silico model when the rate constant for hepatic bupivacaine metabolism is set to zero. Drug redistribution from in silico organs is slowed (Fig. 4E) and organ concentrations approach a steady state with concentrations that are elevated at 10 minutes (Fig. 4F). This predicted effect was confirmed in vivo by experiments combining ketoconazole and radiolabel-drug (Fig. 4G).
Figure 4. ILE increases metabolism but the effect is not required for recovery.
(A) Liver bupivacaine concentrations at 0.5, 2, 5, 10 and 60 minutes (n=4 for 0.5 & 60 min; n=8 for all other conditions) following infusion for control (Null) or ILE. One-way ANOVA F=25.62, p<0.0001, with Bonferroni post-test adjusted at 2 minutes (p=0.57), at 5 minutes (p-value<0.0001, §§§§), 10 minutes (p=0.0002, §§§), and 60 minutes (p=0.12). (B) Urine bupivacaine concentrations at 0.5, 2, 5, 10 minutes for control (Null; n=3 at 0.5, n=8 at 2, n=7 at 5 & 10 minutes) and ILE (n=7 at 2 & 10 minutes, n=6 at 5 minutes). Kruskal-Wallis ANOVA (F=18.70, p=0.0022) with Dunn's multiple comparisons post-test at 10 minutes (adjusted p-value=0.0304, §). (C) NADP+/NADPH ratios normalized to control (n=4 for all groups). Friedman paired-values ANOVA (Friedman statistic = 8.938, p=0.019), with Dunn's multiple comparison post-test (adjusted p-value = 0.0429, §). (D) Carotid flow following an infusion bupivacaine and 30% Intralipid with pretreatment of 25mg/kg intraperitoneal Ketoconazole (ILE+Keto, n=6) or without (ILE, n=11). (E) In Silico prediction of cardiac bupivacaine concentration over time with analogous conditions as “d”. Ketoconazole was modeled by setting metabolism to zero (see methods). (F) In silico prediction of organ concentrations at the 10-minute time-point for both the ILE condition and the No metabolism condition. (G) In vivoorgan concentrations for muscle, blood, cerebellum and heart at the 12-minute time-point for ILE (n=4) and ILE+Ketoconazole (n=4); comparisons by one-sided Kruskal-Wallis (§: p<0.05). (H) In vivo organ concentrations for kidney and liver at the 12-minute time-point for ILE (n=4) and ILE+Ketoconazole (n=4); comparisons by one-sided Kruskal-Wallis (§§: p<0.01).
Discussion
Taken together, these results provide a comprehensive mechanism for triglyceride micro emulsion-based recovery from acute pharmacotoxicity (Figure 5). While previous studies have provided piece-meal insight into lipid resuscitation, many have conflicted with one another, and none provided a comprehensive explanation for the rapidity of recovery. We have elucidated an integrated model that can explain the mechanism of lipid resuscitation and provide insight into the need for multi-modal biodetoxification. Like other cardiotoxic drugs, bupivacaine exerts a number of adverse effects that can lead to cardiovascular collapse; these include blockage of key ionotropic channels [32,34], interference with mitochondrial processing [42,43], and blocking intracellular kinase signaling [44] (Fig 5A,B). We have shown that treatment with a triglyceride micro-emulsion provides a rapid scavenging effect, removing drugs from key organs including the heart and brain (Fig 5C). An association between the drug the lipid compartment of the blood achieves this effect, preferentially when tissue concentrations are highest. This association and potential partitioning of drug provides an indirect benefit, producing a partial recovery of cardiovascular parameters (Fig 5D). Once drug concentration in cardiac tissue falls below a certain threshold (coincident with channel disassociation thresholds), the micro-emulsion produces a cardiotonic effect through both volume and direct inotropy (Fig 5E). The inotropic effect is most likely ascribable to lipid metabolism [31] or mitochondrial processing [45], but potentially involves nitric oxide modulation [46]. This increases cardiac output and subsequent flow rates through the vascular system (Fig 5F). The combination of elevated drug concentration in the blood and improved cardiac output accelerates the movement of drug to the liver, with a corresponding elevation of microsomal metabolism (Fig 5G). A transient elevation in drug tissue concentration is also seen in the skeletal muscle (Fig 5G), a finding that comports with the recent clinical observation that diminutive patients are at higher risk of overdose from local-anesthetic toxicity, possibly due to lower skeletal muscle mass [47]. In our experiments, bupivacaine metabolism was not required for cardiovascular recovery; nevertheless, in cases of oral overdose [48,49] when toxicity can persist [50], an accelerated metabolism could be beneficial. In short, ILEs hasten detoxification by directly stimulating flow-mediated redistribution and altering blood-tissue partitioning of the offending compound. The coupling of these mechanisms achieves that dual goal of restoring cardiac function and reducing tissue toxin levels more efficiently than either mechanism alone.
Figure 5. Schematic of triglyceride micro-emulsion reversal of acute pharmacotoxicity.
(A) Drug X can cause toxicity by several mechanisms including blocking of ion channels preventing conduction and depolarization in cardiac and nervous tissue, or by interference with mitochondrial energy production. (B) Acute toxicity can result in rapid asystole and cardiovascular collapse. (C) Triglyceride (Tg) micro-emulsion can scavenge drug X out of tissue, thereby alleviating the blockage of key inotropic channels. (D) Below a threshold tissue toxin concentration, cardiac output can recover with return of cardiovascular function. (E) Subsequently, triglycerides from the micro-emulsion can be clipped and processed to fatty-acids to provide energetic substrates for ATP production, thereby improving cardiac performance (F) With the added modulation, cardiovascular function continues to improve above that seen with only a scavenging effect. (G) Drug X is redistributed to the skeletal muscle and to the liver where it is conjugated to permit excretion.
Undoubtedly, the future of detoxification is in toxin-specific emulsions and other suspensions including pH-gradient liposomes [20,21], other specially formulated liposomes [13,51–53] and macromolecular capture systems [54]. However, in the case of acute toxicity with cardiovascular collapse, capture in isolation may not optimize recovery. In the case of a triglyceride micro-emulsion, it is evident that the triglyceride itself provides a positive-effect on the heart [29–31,55]. In contrast to the removal of drug, which indirectly contributes to recovery, the lipid-induced change in contractility directly facilitates cardiac function. Future detoxification agents might likewise function as multi-purpose agents, delivering antidotes— such as acetylcysteine to the liver in the case of acetaminophen overdose—and removing or capturing the harmful drug for redistribution and excretion. In the case of cardiotoxicity, triglyceride emulsions act themselves as metabolic modulators and at the same time remove the cardiotoxic drug for redistribution. Other metabolic modulators could accomplish similar goals; for example, hyperinsulemic euglycemic therapy provides an inotropic benefit during cardiac pharmacotoxicity [56] and could be coupled with low dose liposomes having similar scavenging benefits to ILE at lower total volumes of lipid [21], a significant concern in lipid emulsion therapy. However, the volume effect contributes significantly to recovery and should not be discounted in the design of future agents
Our study has a number of limitations. For the in vivo experiments we labeled only the cardiotoxin and did not track the movement of the component fatty-acids or phospholipids and thus cannot specifically assert how Intralipid® is moderating redistribution. Regarding the role of metabolism, the increased liver bupivacaine concentrations could not be accounted for by a flow effect but the in silico models demonstrated that invocation of reticulo-endothelial cell uptake was not needed to increase liver concentrations. The reticulo-endothelial system might be playing a role in uptake of the ILE [40], but we did not specifically test for this. Also, we tested the effect of inhibiting CYP3A4, the main metabolizer of bupivacaine, but bupivacaine is also processed in small amounts by other CYP's indicating that tests with other inhibitors might yield more information. In regard to the in silico model, we modeled the broader physiological effects of cardioactive species but did not account for all the potential molecular effects of bupivacaine or triglycerides. Likewise, the homeostatic feedback model used to represent autonomic regulation of cardiac output is intended to capture the broader effects of baroreceptor-mediated control and not the multi-variable dynamics of this physiological phenomenon.
Conclusion
In conclusion, we used a physiologically-based, pharmacokinetic, pharmacodynamic model combined with in vivo pharmacokinetic data to identify and evaluate the multiple modes of action of a triglyceride micro-emulsion as it pertains to the treatment of acute pharmacotoxicity. While limited clinical reports assert the effectiveness of ILE to counter toxicity caused many different drugs [6,7], there is strong experimental evidence of efficacy for only a few. The combination of a comprehensive mechanism and a validated in silico model open the door to ascertaining which drug overdoses can be treated with lipid emulsion therapy, a process that could spare unnecessary use of experimental resources while improving effectiveness [57]. Additionally, our results support the possibility of designing more effective detoxification agents by combining scavenging and positive inotropic effects, with the further benefit of directing drugs to muscle and liver for storage and detoxification.
Supplementary Material
Acknowledgements
We would like to thank P. Morgan for his helpful comments and criticisms of the manuscript. Funding was provided by United States Veterans Administration (Washington DC, USA) Merit Review (Weinberg and Rubinstein), NIH CounterACT grant 1U01NS083457-01 (Weinberg and Rubinstein), Department of Anesthesiology, University of Illinois College of Medicine (Chicago, IL, USA) & American Heart Association (Dallas, TX, USA) Predoctoral Fellowship 13PRE16810063 (Fettiplace), and National Science Foundation (Arlington, VA, USA) Grant 1228035 (Akpa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting: Presented, in part at the 2013 Resuscitation Science Symposium (ReSS) Conference, Dallas, TX November 2013
Conflicts of Interest: Guy Weinberg holds a US Patent related to lipid resuscitation. Guy Weinberg & Israel Rubinstein are co-founders of ResQ Pharma, LLC.
Author Contributions: MRF, IR, BSA and GW conceived the experiments. MRF, KL, RR, KK, AP, GW performed physiological experiments, gathered data and performed analysis. BSA developed and executed the in silico model. MRF and AP performed biochemical analyses. MRF assembled figures and drafted the manuscript. All authors contributed to revisions.
References
- 1.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 2.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. doi:10.1016/j.addr.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Leroux J-C. Injectable nanocarriers for biodetoxification. Nat. Nanotechnol. 2007;2:679–84. doi: 10.1038/nnano.2007.339. [DOI] [PubMed] [Google Scholar]
- 4.Graham L, Nguyen T, Lee S. Nanodetoxification: emerging role of nanomaterials in drug intoxification treatment. Nanomedicine (Lond) 2011;6:921–928. doi: 10.2217/nnm.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochanek KD, Xu J, Murphy SL, Minin AM, Kung H-C. Deaths: Final Data for 2009. Natl Vital Stat Rep. 2011;60 [PubMed] [Google Scholar]
- 6.Cave G, Harvey M, Willers J, Uncles D, Meek T, Picard J, et al. LIPAEMIC Report: Results of Clinical Use of Intravenous Lipid Emulsion in Drug Toxicity Reported to an Online Lipid Registry. J Med Toxicol. 2014 doi: 10.1007/s13181-013-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cave G, Harvey M. Intravenous Lipid Emulsion as Antidote: Beyond Local Anesthetic Toxicity: A Systematic Review. Acad Emerg Med. 2009;16:815–824. doi: 10.1111/j.1553-2712.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 8.Megarbane B, Jacobs F. Lipid emulsion in acute poisonings: Still no convincing demonstration for its use in non-local anesthetic drug poisoning without life-threatening presentation. Eur Rev Med Pharmacol Sci. 2012;16:990. author reply 991. [PubMed] [Google Scholar]
- 9.a Zausig Y, Zink W, Keil M, Sinner B, Barwing J, Wiese CHR, et al. Lipid emulsion improves recovery from bupivacaine-induced cardiac arrest, but not from ropivacaine-or mepivacaine-induced cardiac arrest. Anesth. Analg. 2009;109:1323–6. doi: 10.1213/ane.0b013e3181af7fb3. [DOI] [PubMed] [Google Scholar]
- 10.Harvey M, Cave G. Lipid rescue: does the sink hold water? And other controversies. Br. J. Anaesth. 2014:22–25. doi: 10.1093/bja/aeu010. [DOI] [PubMed] [Google Scholar]
- 11.Calello DP, Gosselin S. Resuscitative Intravenous Lipid Emulsion Therapy in Pediatrics: Is There a Role? Clin. Pediatr. Emerg. Med. 2012;13:311–316. [Google Scholar]
- 12.Zausig Y, Graf B, Zink W. Is it “lipid sink,” hemodilution, or both? Crit Care Med. 2009;37:2863. doi: 10.1097/CCM.0b013e3181b3a168. [DOI] [PubMed] [Google Scholar]
- 13.Lokajová J, Holopainen JM, Wiedmer SK. Comparison of lipid sinks in sequestering common intoxicating drugs. J Sep Sci. 2012;35:3106–12. doi: 10.1002/jssc.201101038. [DOI] [PubMed] [Google Scholar]
- 14.Laine J, Lokajová J, Parshintsev J, Holopainen JM, Wiedmer SK. Interaction of a commercial lipid dispersion and local anesthetics in human plasma: Implications for drug trapping by “lipid-sinks”. Anal Bioanal Chem. 2010;396:2599–607. doi: 10.1007/s00216-009-3435-z. [DOI] [PubMed] [Google Scholar]
- 15.Mazoit J-X, Le Guen R, Beloeil H, Benhamou D. Binding of long-lasting local anesthetics to lipid emulsions. Anesthesiology. 2009;110:380–6. doi: 10.1097/ALN.0b013e318194b252. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg G, Lin B, Zheng S, Di Gregorio G, Hiller D, Ripper R, et al. Partitioning effect in lipid resuscitation: Further evidence for the lipid sink. Crit Care Med. 2010;38:2268–9. doi: 10.1097/CCM.0b013e3181f17d85. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg GL, Ripper R, Murphy P, Edelman LB, Hoffman W, Strichartz G, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med. 2006;31:296–303. doi: 10.1016/j.rapm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Shi K, Xia Y, Wang Q, Wu Y, Dong X, Chen C, et al. The effect of lipid emulsion on pharmacokinetics and tissue distribution of bupivacaine in rats. Anesth Analg. 2013;116:804–9. doi: 10.1213/ANE.0b013e318284123e. [DOI] [PubMed] [Google Scholar]
- 19.French D, Smollin C, Ruan W, Wong A, Drasner K, Wu AHB. Partition constant and volume of distribution as predictors of clinical efficacy of lipid rescue for toxicological emergencies. Clin Toxicol. 2011;49:801–9. doi: 10.3109/15563650.2011.617308. [DOI] [PubMed] [Google Scholar]
- 20.Bertrand N, Bouvet C, Moreau P, Leroux J-C. Transmembrane pH-gradient liposomes to treat cardiovascular drug intoxication. ACS Nano. 2010;4:7552–8. doi: 10.1021/nn101924a. [DOI] [PubMed] [Google Scholar]
- 21.Forster V, Luciani P, Leroux J-C. Treatment of calcium channel blocker-induced cardiovascular toxicity with drug scavenging liposomes. Biomaterials. 2012;33:3578–85. doi: 10.1016/j.biomaterials.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Dhanikula AB, Khalid NM, Lee SD, Yeung R, Risovic V, Wasan KM, et al. Long circulating lipid nanocapsules for drug detoxification. Biomaterials. 2007;28:1248–57. doi: 10.1016/j.biomaterials.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Cave G, Harvey M, Shaw T, Damitz R, Chauhan A. Comparison of Intravenous Lipid Emulsion, Bicarbonate, and Tailored Liposomes in Rabbit Clomipramine Toxicity. Acad Emerg Med. 2013;20:1076–1079. doi: 10.1111/acem.12224. [DOI] [PubMed] [Google Scholar]
- 24.Litonius ES, Niiya T, Neuvonen PJ, Rosenberg PH. Intravenous lipid emulsion only minimally influences bupivacaine and mepivacaine distribution in plasma and does not enhance recovery from intoxication in pigs. Anesth Analg. 2012;114:901–6. doi: 10.1213/ANE.0b013e3182367a37. [DOI] [PubMed] [Google Scholar]
- 25.Harvey M, Cave G, Ong B. Intravenous lipid emulsion-augmented plasma exchange in a rabbit model of clomipramine toxicity; survival, but no sink. Clin Toxicol. 2014;52:13–9. doi: 10.3109/15563650.2013.866242. [DOI] [PubMed] [Google Scholar]
- 26.Kuo I, Akpa BS. Validity of the lipid sink as a mechanism for the reversal of local anesthetic systemic toxicity: a physiologically based pharmacokinetic model study. Anesthesiology. 2013;118:1350–61. doi: 10.1097/ALN.0b013e31828ce74d. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand N, Leroux J-C. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J Control Release. 2012;161:152–63. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 28.Kupetz E, Bunjes H. Lipid nanoparticles: Drug localization is substance-specific and achievable load depends on the size and physical state of the particles. J Control Release. 2014 doi: 10.1016/j.jconrel.2014.06.007. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Fettiplace MR, Ripper R, Lis K, Lin B, Lang J, Zider B, et al. Rapid Cardiotonic Effects of Lipid Emulsion Infusion. Crit Care Med. 2013;41:e156–e162. doi: 10.1097/CCM.0b013e318287f874. [DOI] [PubMed] [Google Scholar]
- 30.Fettiplace MR, Akpa B, Ripper R, Zider B, Lang J, Rubinstein I, et al. Resuscitation with Lipid Emulsion: Dose-dependent Recovery from Cardiac Pharmacotoxicity Requires a Cardiotonic Effect. Anesthesiology. 2014;120:915–925. doi: 10.1097/ALN.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partownavid P, Umar S, Li J, Rahman S, Eghbali M. Fatty-acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine-induced cardiotoxicity by lipid emulsion in rats. Crit Care Med. 2012;40:2431–2437. doi: 10.1097/CCM.0b013e3182544f48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarkson C, Hondeghem L. Mechanism for Bupivacaine Depression of Cardiac Conduction: Fast Block of Sodium Channels during the Action Potential with Slow Recovery from Block during Diastole. Anesthesiology. 1985;62:396–405. [PubMed] [Google Scholar]
- 33.Fettiplace MR, Ripper R, Lis K, Feinstein DL, Rubinstein I, Weinberg G. Intraosseous Lipid Emulsion: An Effective Alternative to IV Delivery in Emergency Situations. Crit Care Med. 2014;42:e157–160. doi: 10.1097/01.ccm.0000435677.76058.15. [DOI] [PubMed] [Google Scholar]
- 34.Coyle DE, Sperelakis N. Bupivacaine and lidocaine blockade of calcium-mediated slow action potentials in guinea pig ventricular muscle. J Pharmacol Exp Ther. 1987;242:1001–5. [PubMed] [Google Scholar]
- 35.Mottram AR, Valdivia CR, Makielski JC. Fatty acids antagonize bupivacaine-induced I(Na) blockade. Clin Toxicol. 2011;49:729–733. doi: 10.3109/15563650.2011.613399. doi:10.3109/15563650.2011.613399. [DOI] [PubMed] [Google Scholar]
- 36.Wagner M, Zausig Y, Ruf S, Rudakova E, Gruber M, Graf BM, et al. Lipid Rescue Reverses the Bupivacaine-induced Block of the Fast Na+ Current (INa) in Cardiomyocytes of the Rat Left Ventricle. Anesthesiology. 2014;121:724–36. doi: 10.1097/ALN.0b013e3182a66d4d. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg GL, VadeBoncouer T, a Ramaraju G, Garcia-Amaro MF, Cwik MJ. Pretreatment or Resuscitation with a Lipid Infusion Shifts the Dose-Response to Bupivacaine-induced Asystole in Rats. Anesthesiology. 1998;88:1071–5. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Litonius E, Tarkkila P, Neuvonen PJ, Rosenberg PH. Effect of intravenous lipid emulsion on bupivacaine plasma concentration in humans. Anaesthesia. 2012;67:600–605. doi: 10.1111/j.1365-2044.2012.07056.x. [DOI] [PubMed] [Google Scholar]
- 39.Hiller N, Mirtschink P, Merkel C, Knels L, Oertel R, Christ T, et al. Myocardial accumulation of bupivacaine and ropivacaine is associated with reversible effects on mitochondria and reduced myocardial function. Anesth. Analg. 2013;116:83–92. doi: 10.1213/ANE.0b013e31826c8095. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Hitchens TK, Ye Q, Wu Y, Barbe B, Prior DE, et al. Decreased reticuloendothelial system clearance and increased blood half-life and immune cell labeling for nano- and micron-sized superparamagnetic iron-oxide particles upon pre-treatment with Intralipid. Biochim Biophys Acta. 2013;1830:3447–53. doi: 10.1016/j.bbagen.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gantenbein M, Attolini L, Bruguerolle B, Villard P, Puyoou F, Durand A, et al. Oxidative Metabolism of Bupivacaine into Pipecolylxylidine in Humans Is Mainly Catalyzed by CYP3A. Drug Metab Dispos. 2000;28:383–385. [PubMed] [Google Scholar]
- 42.Weinberg GL, Palmer JW, VadeBoncouer TR, Zuechner MB, Edelman G, Hoppel CL. Bupivacaine Inhibits Acylcarnitine Exchange in Cardiac Mitochondria. Anesthesiology. 2000;92:523–8. doi: 10.1097/00000542-200002000-00036. [DOI] [PubMed] [Google Scholar]
- 43.Sztark F, Nouette-Gaulain K, Malgat M, Dabadie P, Mazat JP. Absence of stereospecific effects of bupivacaine isomers on heart mitochondrial bioenergetics. Anesthesiology. 2000;93:456–62. doi: 10.1097/00000542-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 44.Piegeler T, Votta-Vellis G, Bakhshi FR, Mao M, Carnegie G, Bonini MG, et al. Endothelial Barrier Protection by Local Anesthetics: Ropivacaine and Lidocaine Block Tumor Necrosis Tactor-α-Induced Endothelial Cell Src Activation. Anesthesiology. 2014;120:1414–28. doi: 10.1097/ALN.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lou P-H, Lucchinetti E, Zhang L, Affolter A, Schaub MC, Gandhi M, et al. The mechanism of Intralipid®-mediated cardioprotection complex IV inhibition by the active metabolite, palmitoylcarnitine, generates reactive oxygen species and activates reperfusion injury salvage kinases. PLoS One. 2014;9:e87205. doi: 10.1371/journal.pone.0087205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin I-W, Hah Y-S, Kim C, Park J, Shin H, Park K-E, et al. Systemic Blockage of Nitric Oxide Synthase by L-NAME Increases Left Ventricular Systolic Pressure, Which Is Not Augmented Further by Intralipid(®). Int J Biol Sci. 2014;10:367–76. doi: 10.7150/ijbs.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med. 2013;38:289–97. doi: 10.1097/AAP.0b013e318292669b. [DOI] [PubMed] [Google Scholar]
- 48.Finn SDH, Uncles DR, Willers J, Sable N. Early treatment of a quetiapine and sertraline overdose with Intralipid. Anaesthesia. 2009;64:191–194. doi: 10.1111/j.1365-2044.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 49.Blaber MS, Khan JN, Brebner JA, McColm R. “Lipid Rescue” for Tricyclic Antidepressant Cardiotoxicity. J Emerg Med. 2012;43:465–7. doi: 10.1016/j.jemermed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Wilson BJ, Cruikshank JS, Wiebe KL, Dias VC, Yarema MC. Intravenous lipid emulsion therapy for sustained release diltiazem poisoning: a case report. J Popul Clin Pharmcol. 2012;19:e218–22. [PubMed] [Google Scholar]
- 51.Fallon MS, Chauhan A. Sequestration of amitriptyline by liposomes. J. Colloid Interface Sci. 2006;300:7–19. doi: 10.1016/j.jcis.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 52.Muhonen J, Holopainen JM, Wiedmer SK. Interactions between local anesthetics and lipid dispersions studied with liposome electrokinetic capillary chromatography. J Chromatogr A. 2009;1216:3392–7. doi: 10.1016/j.chroma.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 53.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. doi:10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 54.Bertrand N, a Gauthier M, Bouvet C, Moreau P, Petitjean A, Leroux J-C, et al. New pharmaceutical applications for macromolecular binders. J Control Release. 2011;155:200–10. doi: 10.1016/j.jconrel.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Fettiplace MR, Chen S-J, Steinhorn B, Shao Z, Zhu X, et al. Lipid Emulsion Rapidly Restores Contractility in Stunned Mouse Cardiomyocytes: A Comparison With Therapeutic Hypothermia. Crit Care Med. 2015 doi: 10.1097/CCM.0000000000000656. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stehr SN, Pexa a, Hannack S, Heintz a, Heller a R., Deussen a, et al. Insulin effects on myocardial function and bioenergetics in L-bupivacaine toxicity in the isolated rat heart. Eur. J. Anaesthesiol. 2007;24:340–6. doi: 10.1017/S0265021506002109. [DOI] [PubMed] [Google Scholar]
- 57.Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Ann Rev Pharm Toxicol. 2011;51:45–73. doi: 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.