Abstract
HIV-mediated neuropathogenesis is a multifaceted process involving several players, including resident brain cells (neurons, astrocytes, and microglia) and infiltrating cells (peripheral blood mononuclear cells (PBMCs)). We evaluated the dynamic interaction between astrocytes and infiltrating PBMCs as it impacts HIV in the CNS. We demonstrate that human primary-derived astrocytes (PDAs) predominantly secrete Wnt 1, 2b, 3, 5b, and 10b. Wnts are small secreted glycoproteins that initiate either β-catenin-dependent or independent signal transduction. The Wnt pathway plays a vital role in the regulation of CNS activities including neurogenesis, neurotransmitter release, synaptic plasticity, and memory consolidation. We show that HIV infection of PDAs altered astrocyte Wnt profile by elevating Wnts 2b and 10b. Astrocyte conditioned media (ACM) inhibited HIV replication in PBMCs by 50%. Removal of Wnts from ACM abrogated its ability to suppress HIV replication in PBMCs. Inversely, PBMCs supernatant activated PDAs, as demonstrated by a 10-fold increase in HLA-DR and a 5- fold increase in IFNγ expression, and enhanced astrocyte susceptibility to HIV by 2-fold, which was mediated by IFNγ in a Stat-3-dependent manner. Collectively, these data demonstrate a dynamic interaction between astrocytes and PBMCs, whereby astrocyte-secreted Wnts exert an anti-HIV effect on infected PBMCs and PBMCs, in turn, secrete IFNγ that enhance astrocyte susceptibility to productive HIV infection and mediate their activation.
Introduction
HIV invades the brain through a “Trojan Horse” whereby infected CD4+ T cells and monocytes cross the blood brain barrier and disseminate HIV into the brain (Williams et al., 2001; Peluso et al., 1985; von Herrath et al., 1995). Microglia/macrophages and astrocytes are a major reservoir for HIV in the brain (Churchill et al., 2009; Churchill et al., 2006; Overholser et al., 2003; Porwit et al., 1989). Astrocytes comprise 40–60% of cells in the brain and are critical in maintaining brain homeostasis. They regulate neuronal development (Stipursky et al., 2012), maintain blood brain barrier integrity (Abbott, 2013), metabolize excess neurotoxic neurotransmitters, secrete neurotrophic factors, and contribute to immune surveillance through secretion of cytokines and chemokines (Hamo et al., 2007; Carpentier et al., 2005; Cornet et al., 2000; Becher et al., 2000; Heaton et al., 2010; Valcour et al., 2012). We previously demonstrated that astrocytes express robust levels of β-catenin (Carroll-Anzinger and Al-Harthi, 2006; Kumar et al., 2008). β-catenin is a central mediator of the Wnt/β-catenin pathway, where it associates with members of T cell factor (TCF) or lymphoid enhancer-binding factor (LEF) transcriptional factors and functions as a transcriptional co-regulator of hundreds of genes. β-catenin interaction with TCF-4 represses HIV transcription in multiple cell types, including CD4+ T cells (Kumar et al., 2008; Schenkel et al., 2010), monocyte/macrophages (Aljawai et al., 2014), and astrocytes (Li et al., 2011; Henderson et al., 2012b; Narasipura et al., 2012).
Wnt ligands initiate the Wnt/β-catenin signaling cascade. The Wnt pathway is involved in many cellular processes including development, proliferation, survival, regeneration, wound healing and stress responses (Coombs et al., 2008; Angers and Moon, 2009; Polakis, 2007). Wnts are a family of 19 small secreted glycoproteins that are evolutionarily conserved. They bind to a seven transmembrane Frizzled receptor and co-receptor LRP5/6, culminating in either a β-catenin-dependent (Canonical) or independent (Non-canonical: i.e. Planar Cell Polarity or Ca2+/Calmodulin) signaling pathway. Historically, Wnts (1, 2, 2b, 3, 3a, 4, 5a, 5b, 6, 7a, 7b, 8a, 8b, 9a, 9b, 10a, 10b, 11, and 16) were categorized on the basis of whether they induce β-catenin dependent or β-catenin independent signaling; however, there is an increasing recognition that this classification is no longer accurate. Wnts, depending on interaction with frizzled receptors, can mediate either pathway. For example, Wnt5a, classically, signals through the non-canonical/β-catenin independent pathway (Mikels and Nusse, 2006). However, by binding to Frizzled 4 and LRP5 receptors, Wnt5a engages the canonical/β-catenin-dependent pathway (Mikels and Nusse, 2006). These studies underscore an emerging paradigm of Wnt signaling, whereby the context of Wnt receptors dictates canonical vs. non-canonical Wnt pathways (Mikels and Nusse, 2006; van Amerongen et al., 2012).
The brain is not an immune privileged site. PBMCs survey the CNS (Kleine and Benes, 2006). However, in the context of an infection or autoimmune disease, there is a higher level of PBMC infiltration and activation in the CNS that can have a detrimental effect on the brain. In the context of HIV, both CD4 and CD8 T cells home to the brain (Petito et al., 2006; Petito et al., 2003; Sadagopal et al., 2008a; Sadagopal et al., 2008b; Marcondes et al., 2007; Marcondes et al., 2003), but their dynamic interaction with resident brain cells is not well defined. We evaluated the interaction between astrocytes and PBMCs as it impacts HIV infection and astrocyte activation. We show that primary human derived astrocytes (PDAs) have a distinct Wnt profile, which is altered by HIV infection. Wnts secreted from PDAs inhibited HIV infection in PBMCs while soluble factor(s) from PBMCs induced IFNγ production, expression of MHCII, and enhanced HIV infection of astrocytes. These findings demonstrate that Wnts function as secreted anti-HIV factors that could regulate extent of HIV load in the CNS and highlight a feedback loop between astrocytes and brain infiltrating PBMCs that impacts extent of HIV dissemination in the brain and astrocyte activation. These interactions are likely to contribute to overall heightened inflammatory responses in the CNS driving HIV-mediated neuropathology.
Materials and Methods
Ethics Statement
Research involving human subjects was conducted in accordance with institutional (IRB-L06080703) and U.S. government guidelines on human research.
Human Progenitor-Derived Astrocytes (PDAs)
PDAs, provided by Dr. Eugene O. Major (National Institute of Neurological Disorders and Stroke, Bethesda, MD) were generated from neural progenitor cells, as previously described (Lamba et al., 2009). Briefly, progenitors were seeded on poly-D-lysine-coated (PDL) T 75 tissue culture flasks at 2 × 106 cells/flask and maintained in progenitor medium consisting of neurobasal media (Life Technologies Invitrogen, Carlsbad, CA) supplemented with 0.5% bovine albumin (Sigma, St. Louis, MO), neurosurvival factor (Lonza BioWhittaker, Walkersville, MD), N2 components (Life Technologies Invitrogen, Carlsbad, CA), 25ng/ml fibroblast growth factor, 20ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN), 50μg/ml gentamycin (Lonza BioWhittaker, Walkersville, MD) and 2mM L-glutamine (Life Technologies Invitrogen, Carlsbad, CA). To induce differentiation, progenitor medium was replaced with PDA medium containing DMEM (Life Technologies Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (Sigma, St. Louis, MO), 2 mM L-glutamine, and 50 μg/ml gentamycin. DMEM referred to for the remainder of this manuscript refers to complete DMEM (containing these components). PDA cultures were 100% GFAP+ and negative for nestin expression after 30 days of differentiation (Lawrence et al., 2004). We further characterized PDA in comparison to commercially purchased primary fetal astrocytes, and showed that they also express EAAT2, glutamine synthetase, and can uptake glutamate (Henderson et al., 2012b). After differentiation PDAs were maintained in PDA medium as described above on PDL-coated plates. Media was changed every three days and cells were split when they reached 80–90% confluency. Two batches of PDAs were used in these experiments and each experiment repeated a minimum of three times. PDAs were plated in PDL-coated 12 well plates at 1 ×106 cells/well. Astrocyte conditioned media (ACM) was harvested from three day cultured PDAs. All ACM used in transfer experiments was taken from PDAs cultured in complete 10% heat-inactivated FBS containing DMEM.
Real time reverse transcriptase (rt)-PCR measurement of Wnts transcripts
Six days after HIV infection in PDAs, total RNA was isolated from PDAs using RNEasy MiniKit (Qiagen, Valencia, CA). A260 was used to measure RNA concentration and 1μg of total RNA was used for further experiments. DNA contamination was removed by DNAse I (Sigma, St. Louis, MO) treatment at room temperature for 15 minutes followed by denaturation of DNAse 1 at 70° C for 10 minutes. cDNA synthesis was performed using qScript cDNA supermix (Qiagen, Valencia, CA) according to manufacturer’s instructions. cDNA mix was diluted so that 1/20 of the original volume was used to perform real time -PCR using Ssofast evagreen supermix with low ROX kit (Bio-Rad, Hercules, CA) in a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) using 7500 software V2.01. The PCR conditions were, hold at 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Melting curve analysis was done to make sure of single and specific product amplification. The following primers were used to amplify for GAPDH- Forward 5′-TGACTTCAACAGCGACACCCACT-3′ and Reverse 5′-ACCACCCTGTTGCTGTAGCCAAAT-3′. The following primers were used to amplify Wnt ligands: Wnt1: Forward 5′-TCTCTGTCGTGGAGCCATTGAACA and Reverse 5′-AACTCGTGGCTCTGTATCCACGTT; Wnt2: Forward 5′-TGTGAAGTCATGTGCTGTGGGAGA and Reverse 5′-ACCAGTGGAACTTACACCCACACT; Wnt2B: Forward 5′-ACAACATCCACTACGGTGTCCGTT and Reverse 5′-TGGCACTTACACTCCAGCTTCAGA; Wnt3: Forward 5′-ATCCTGGACCACATGCACCTCAAA and Reverse 5′-AGGCGCTGTCATACTTGTCCTTGA; Wnt5B: Forward 5′-ATTGTACCAGGAGCACATGGCCTA and Reverse 5′-TGACTCTCCCAAAGACAGATGCGT; Wnt7A: Forward 5′-GGGCGCAAGCATCATCTGTAACAA and Reverse 5′-GCCATTGCGGAACTGAAACTGACA; Wnt7B: Forward 5′-TGGTGTACATTGAGAAGTCGCCCA and Reverse 5′-ACGAAGCAGCACCAGTGGAATTTG; Wnt9B: Forward 5′-AAGAGAAGCAAAGCCTCCTCCCTT and Reverse 5′-TGCTGTGCTCTTGGTCTCCCTTAT; Wnt16: Forward 5′-TCATCTGGTGCTGCTATGTCCGTT and Reverse 5′-AACTTTACTAGCGCTGCACAGGGA; Wnt3A: Forward 5′-GCATCAAGATTGGCATCCAGGAGT and Reverse 5′-TGCACATGAGCGTGTCACTGCAAA; Wnt8A: Forward 5′-GTGCAAGTTCCAGTTTGCTTGGGA and Reverse 5′-TGGTAGCACTTCTCAGCCTGTTGT; Wnt8B: Forward 5′-ATGCCATCAGTTCTGCTGGAGTCA and Reverse 5′-GGGCATCGACAAACTGCTTGGAAA; Wnt6: Forward 5′-CCTTGGCCTCTAGGAGGAAACAGT and Reverse 5′-TACTAACCTCACCCACCATCCTGT; Wnt10A: Forward 5′-ACACAGTGTGCCTAACATTGCCAG and Reverse 5′-ATTGGTGTTGGCATTCGTGGATGG; Wnt10B: Forward 5′-GGCACGAATGCGAATCCACAACAA and Reverse 5′-TGTGCCATGACACTTGCATTTCCG; and Wnt9A: Forward 5′-AGACTGCCTTCCTCTATGCCATCT and Reverse 5′-CCTTGACGAACTTGCTGCTGTACT. Fold change in mRNA expression was calculated by using the comparative CT method with GAPDH as endogenous control. A CT cut-off value of 35 was used to determine if mRNA was expressed by PDAs. After normalization to GAPDH, fold change was determined by normalizing expressed mRNA transcripts to Wnt7b, which was consistently the lowest detected Wnt ligand transcript in PDAs before and after HIV infection.
HIV infection of PDAs
PDAs were pretreated under different conditions as specified by each experiment (e.g. RPMI, 100ng/ml of IFNγ, supernatant from three day activated PBMCs, IFNγ depleted supernatant from three day activated PBMCs, or 1μM STAT3 Inhibitor V (STATtic) (EMD Biomedicals, Gibbstown, NJ)) and propagated in PBMCs conditioned media for 24h prior to HIV infection. PDAs, at 80–90% confluency, were infected with HIVBaL at 10ng of HIVBaL/1×106 cells for 24 hours. PDAs were then washed two times with PBS then returned to pre-treatment culture conditions. Cultures were maintained for 3–6 days as indicated. In some experiments, supernatant was isolated from 6 day post-infection PDA culture and HIVBaL was removed from Astrocyte Conditioned Media (ACM) by ultracentrifugation at 23,000 × g for 1 hour. HIV removal was confirmed using the Roche TaqMan HIV version 2.0 Assay (Roche, Indianapolis, IN). Experiments were performed in triplicate.
Western Blot for qualitative detection of secreted Wnt ligands and IFNγ in supernatant
Wnt or IFNγ-depleted supernatants (20μl) was added to 2X Laemmli buffer (Sigma, St. Louis, MO) and a total of 40μl of supernatant from each sample was separated on a 10% SDS-PAGE gel and transferred onto a 0.45μM nitrocellulose membrane. The membrane was blocked with Pierce SuperBlock in PBS (Thermo Scientific, Waltham, MA) plus 0.5% Tween 20 (Thermo Scientific, Waltham, MA) for 1 hour at room temperature. Primary antibodies used are: anti-Wnt 1(ab15251) at 1:1000; anti-Wnt 2b (ab50575) at 1:2000; anti-Wnt 3 (ab32249) at 1:1000; anti-Wnt 5b (ab94914) at 1:2000; anti-Wnt 10b (ab70816) at 1:2000, or rabbit IgG at 1:1000 or 1:2000. All anti-Wnt antibodies and rabbit IgG were purchase from Abcam (Cambridge, MA). The primary antibody for anti-IFNγ (B27) was used at 1:500 and purchased from BD Biosciences (San Diego, CA). Membranes, after overnight incubation with indicated dilutions of primary antibodies in superblock-T20 (0.1%), were washed three times for 45 minutes with TBS-T and incubated with a 1:1000 dilution of anti-Rabbit-HRP secondary antibody (Cell Signaling, Boston, MA) in superblock-T20 (0.1%) for one hour at room temperature. Membranes were again washed three times for 45 minutes with TBS-T and exposed to SuperSignal Femto ECL substrate reagent (Pierce Thermos Scientific, Waltham, MA) for 3 minutes. Films were exposed and developed on a Konica SLX-101A auto processor. Band densitometry was calculated using ImageJ Software (National Institutes of Health, Bethesda, MD). Experiments are repeated at least 3 times.
MTS Assay
MTS assay is a colorimetric cell viability assay from Promega (Madison, WI). It is based on the ability of viable cells to reduce a tetrazolium compound [3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and an electron coupling reagent (phenazine methosulfate) PMS into a formazan product which is absorbed at 490nm and detected by an ELISA plate reader. This assay was performed according to the manufacturer’s recommendations. Experiments were performed in triplicate.
Culture and Activation of PBMCs and CD8+ T cells
PBMCs were isolated from four healthy HIV seronegative donors by Ficoll-Hypaque density gradient centrifugation, and each experiment repeated three times. CD8+ T cells were isolated using the untouched CD8+ T cell Isolation Kit II (Miltenyi Biotec, San Diego, CA). CD8+ T cells and bulk PBMCs were suspended in complete RPMI 1640 media (Lonza BioWhittaker, Walkersville, MD) supplemented with 10% FBS (Gemini Bio Products, Calabasas, CA), 1% penicillin/streptomycin (Sigma, St. Louis, MO), and 20 U/mL IL-2 (Sigma, St. Louis, MO). Cultures were stimulated with 1μg/ml soluble α-CD3 and α-CD28 antibodies (BD Biosciences Pharmingen, San Diego, CA) and maintained for 24 hours-6 days; as indicated. Supernatant was collected through centrifugation at 1500rpm for 5 minutes and frozen at −80° C until further use.
HIV Infection and p24 analysis of PBMCs
PBMCs isolated from four healthy HIV seronegative donors by Ficoll-Hypaque density gradient centrifugation were cultured overnight with 20u/mL IL-2 and 1μg/mL αCD3/CD28. PBMCs were then infected with 2ng/1×106 cells/mL of HIVBAL for 4 hours then washed four times with complete RPMI. PBMCs were then maintained in complete RPMI or ACM with 20u/mL IL-2 and αCD3/CD28. Supernatants from PBMCs were collected 6 days post infection and p24 measured using the p24 ELISA kit from SAIC-Frederick (Frederick, MD), performed according to the manufacturer’s protocol.
Depletion of Wnt ligands from PDAs and IFNγ from activated PBMCs supernatant by immunoprecipitation
Pierce Protein A/G Magnetic Beads (Life Technologies Invitrogen, Carlsbad, CA) were washed two times for 1 hour at 4°C with 1XGE Binding Washing Buffer (GE Life Sciences, Pittsburgh, PA). The beads were then coated with 4μg of anti-rabbit Wnt 1, Wnt2b, Wnt 3, Wnt 5b, Wnt 10b, rabbit IgG1 isotype control (Abcam, Cambridge, MA) or 4μg of anti-human IFNγ or mouse IgG1 isotype control (BD Biosciences, San Diego, CA) overnight at 4°C with continuous rotation at 30rpm. One mL of supernatant from PDAs or activated PBMCs was then applied to each of the beads and incubated overnight at 4°C. The supernatant was then collected by separation of magnetic beads under a magnetic field and depletion of Wnt or IFNγ was confirmed by western blot of the supernatant.
Flow Cytometric Analysis
PDAs were washed with 1X PBS before staining with LIVE/DEAD fixable dead aqua fluorescent reactive dye (Life Technologies Invitrogen, Carlsbad, CA). After 30 minutes the cells were washed with 1X PBS and stained with anti-human HLA-DR- PERCP. For intracellular staining, cells were stained according to manufacturer’s instructions using the BD Cytofix/Cytoperm permeabilization and fixation kit (BD Biosciences, San Diego, CA). Cells were then stained with GFAP-APC and IFNγ-FITC. Multicolor flow cytometric analyses were performed using a BD FACSVerse Flow cytometer with FACSuite Software (BD Biosciences, San Diego, CA). Live cells were gated based on negative Aqua Live/Dead staining. Doublets were excluded by FSC-A vs. FSC-H and SSC-A vs. SSC-H gating.
Analysis of HIV integration in PDAs
PDAs were maintained for three days after HIV infection in pre-infection conditions (RPMI, 100ng/mL IFNγ, supernatant from three day activated PBMCs, IFNγ depleted supernatant from three day activated PBMCs, or supernatant from three day activated PBMCs with 1μM STATtic) then genomic DNA was isolated using DNeasy blood and tissue kit (Qiagen, Valencia, CA). DNA was quantified by absorbance at 260nm followed by qPCR for GAPDH using the primers mentioned above. Alu-PCR was performed as previously described in (Narasipura et al., 2014; Szotek et al., 2013). Using equal amounts of gDNA, the first round of Alu-PCR was performed with Alu1, Alu2 and LM667 primers as described in (Brussel and Sonigo, 2003). AmpliTaq Gold polymerase with GeneAmp 10X PCR buffer II was used according to manufacturer’s instructions (Life Technologies, Carlsbad, CA). Reaction conditions were as follows: hold 95°C for 10 min followed by 12 cycles at 95°C for 30 sec, 60°C for 20 sec and 72°C for 170 sec followed by 95°C hold for 10 min. The second round of nested qPCR was performed using F-5′-TCAAGTAGTGTGTGCCCGTCTGTT-3′ and R-5′AGCTCCTCTGGTTTCTCTTTCGCT-3′ primers that amplify ~140bp LTR region essentially as explained in the qRT-PCR section. A minus taq and a minus template controls were included in the first round Alu-PCR.
Statistical Analysis
When the data was distributed normally, ANOVA and post-hoc tests were used. When the data was not normally distributed, nonparametric analysis was performed. All tests assumed a two-sided significance level of 0.05 using GraphPad Instat 3 software (San Diego, CA) for data analysis.
Results
Wnt 2b and Wnt 10b are elevated post-HIV infection of PDAs
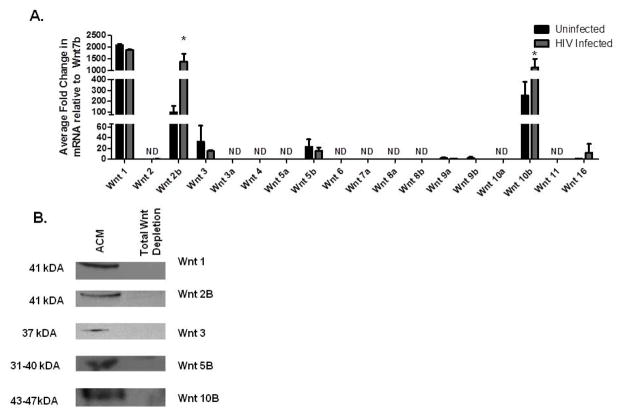
PDAs exhibit prototypical characteristics of astrocytes including expression of GFAP, glutamate transporter 1/EAAT2, glutamine synthetase, and are capable of glutamate uptake in a standard glutamate uptake assay (Henderson et al., 2012b). PDAs express high levels of active β-catenin, which is an intracellular host restriction factor for HIV transcription (Li et al., 2011; Henderson et al., 2012b; Narasipura et al., 2012), but their Wnt ligand expression profile is not known. We evaluated the expression of all 19 Wnts from PDAs by qRT-PCR (Fig. 1A) and subsequently by Western blot (WB) (Fig. 1B). We show that PDAs express Wnts 1, 2b, 3, 5b, 10b, and 16 mRNA (Fig. 1A). Wnt 7b had the lowest detectable CT value of all 19 Wnts and was used to normalize the data. Wnt 1 mRNA was the most abundant, as it was expressed at 2000-fold above Wnt 7b (Fig. 1A). The remaining Wnts (Wnts 2, 3a, 4, 5a, 6, 7a, 8a, 8b, 9a, 9b, 10a, and 11) were below the detection limit of qRT-PCR. Protein expression of Wnt 1, 2b, 3, 5b, and 10b was also detected by WB from PDA-conditioned media (ACM) (Fig. 1B). To determine the impact of HIV infection on Wnt profile of PDAs, PDAs were infected with HIVBaL at 10ng/1×106 cells overnight then washed and cultured for 6 days. Wnt expression was evaluated by qRT-PCR. Wnt 2b mRNA was induced by 14-fold and Wnt 10b was induced by 4- fold post-HIV infection. The remaining Wnts mRNA (1, 3, and 5b) levels were not altered post-HIV infection. These data demonstrate that PDAs have a distinct Wnt ligand profile, which is altered in response to HIV infection.
Figure 1. HIV infection upregulates Wnt 2b, 10b, and 16 in PDAs.
(A) PDAs were infected with HIVBaL at 10ng per 1×106 cells for 24 hours or left uninfected. Six days later Wnt ligand mRNA transcript levels of each of the known 19 Wnt ligands were tested by qRT-PCR. Wnt 7b was the lowest detected ligand therefore all of the Wnt ligands were normalized to Wnt 7b. Each experiment was performed twice in triplicate. * indicates significance of p≤0.05 between uninfected and HIV infected groups using one way analysis of variance (ANOVA). (B) ACM was harvested from three day PDA cultures. Wnt ligands (Wnt 1, 2b, 3, 5b, and 10b) were removed by immunoprecipitation. Western blots were performed on 40μl of ACM. Image is representative of three separate experiments.
Astrocyte Conditioned Media (ACM) inhibits HIV infection of PBMCs
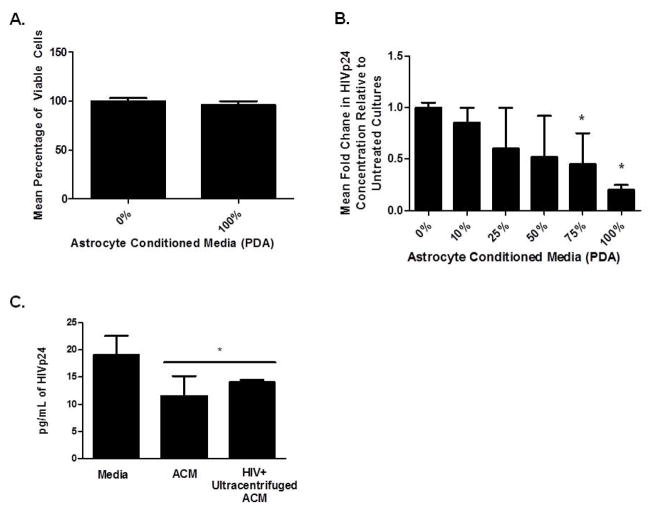
Given that astrocytes release Wnt ligands and that PBMCs enter the brain shortly after infection (Peluso et al., 1985; von Herrath et al., 1995; Porwit et al., 1989; Petito et al., 2006; Petito et al., 2003; Sadagopal et al., 2008a; Sadagopal et al., 2008b) we addressed whether Wnt ligands released by astrocytes could alter HIV replication in PBMCs. PBMCs from four healthy donors were infected with HIVBaL then cultured in presence of ACM from PDAs titrated with DMEM from 10%–100%. We show that ACM had no effect on cell viability, as determined by MTS assay (Fig. 2A) and tryphan blue exclusion (data not shown). At 75% and 100% ACM, HIV replication in PBMCs was reduced by 50% and 75%, respectively (Fig. 2B). ACM from HIV infected PDAs also inhibited HIV replication by approximately 50% in PBMCs, even when residual virus from ACM was removed by ultracentrifugation (Fig. 2C). These data indicate that soluble factors in ACM inhibit HIV replication in PBMCs and that HIV infection of PDAs does not perturb their ability to secrete these soluble factors to suppress HIV.
Figure 2. Astrocytes (PDA) conditioned media (ACM) inhibits HIV infection in PBMCs.
Human PBMCs from four healthy donors were cultured at 2×106 cells/mL overnight with α-CD3/CD28 and IL-2. Cells were infected with HIVBaL at 2ng/1×106 cells for four hours. Infected PBMCs were supplemented with 0–100% ACM. ACM was prepared using supernatant from PDAs cultured for three days in completed DMEM with 10% FBS. ACM was diluted with serum free DMEM media for 0–90% ACM culture conditions. At day 6, MTS assays (A) and HIVp24 ELISA (B) were performed. Data is based on four different healthy donors and experiments were performed in triplicate. *indicates significance of p<0.05 between control and experimental group using one way ANOVA between RPMI control well and 75% and 100% groups. In (C), PBMCs were activated and infected as in (A) then cultured with complete DMEM, ACM, or ACM from HIV infected PDAs. ACM was prepared from 6 day PDA cultures. ACM from HIV infected cultures was ultracentifuged prior to transfer to PBMCs. HIV p24 ELISA from PBMCs was measured at day 6 post-transfer. * indicates significance of p<0.05 between control and experimental group using one way ANOVA between control and experimental groups.
Astrocyte secreted Wnt ligands mediate HIV suppression from PBMCs
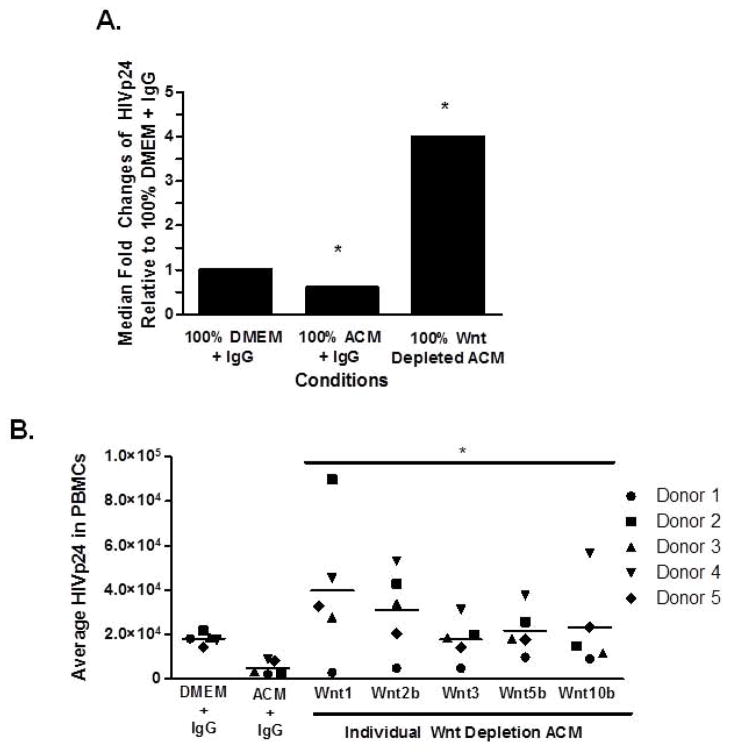
To determine whether Wnt ligands secreted from PDAs are mediating HIV inhibition in PBMCs, ACM was depleted of all Wnt ligands by immunoprecipitation. There are no neutralizing Wnts antibodies and therefore we depleted all Wnts from ACM using a cocktail of antibodies against Wnt 1, 2b, 3, 5b, and 10b followed by magnetic bead immunoprecipitation. We confirmed efficient depletion of Wnts from ACM by Western blot (Fig. 1B). Removal of all Wnts from ACM abrogated its ability to inhibit HIV replication in PBMCs (Fig. 3A). Interestingly, Wnts depletion from ACM led to a 4 fold higher level of HIV replication in PBMCs than IgG treated DMEM, suggesting that removal of Wnts from ACM allows for induction of potential HIV activators, which are under the negative control of β-catenin signaling (Narasipura et al., 2012).
Figure 3. Wnts secreted from PDAs mediate HIV inhibition in PBMCs.
(A) Human PBMCs from four healthy donors were cultured at 2×106 cells/mL overnight with αCD3/CD28 and IL-2. PBMCs were then infected with 2ng/1×106 cells HIVBaL for 4 hours and cultured for 6 days with IL-2 and DMEM, ACM, or ACM depleted of all five Wnt ligands (Wnts 1, 2b, 3, 5b, and 10b) by immunoprecipitation. At day 6 of culture HIVp24 levels were measured by ELISA. * indicates p≤0.05 between control and experimental groups using student’s t test. (B) PBMCs were activated and infected as in (A), then cultured in DMEM, complete ACM, or each individual Wnt depleted ACM (either Wnt 1, 2b, 3, 5b, or 10b) for 6 days. HIVp24 was determined by ELISA. * indicates p≤0.05 between ACM and Wnt depleted ACM using one ANOVA between control treated supernatant and each group.
To assess which specific Wnt ligand in ACM is mediating HIV inhibition in PBMCs, ACMs were depleted of individual Wnts (either Wnt 1, 2b, 3, 5b, or 10b) by magnetic bead immunoprecipitation. Wnt depleted ACMs were added to HIV infected PBMCs. Depletion of Wnt 1, 2b, 3, 5b, or 10b removed the anti-HIV effect from PDAs but the extent to which varied (Fig. 3B). ACM depletion of Wnt 1 or Wnt 2b led to greater HIV replication in PBMCs than Wnt 3, 5b, or 10b (Fig. 3B). These data indicate that while all Wnts in ACM can mediate HIV inhibition in PBMCs, Wnt1 and Wnt2b exert the highest inhibitory effect.
Soluble factors secreted from CD8+ T cells induces HLA-DR and IFNγ in astrocytes
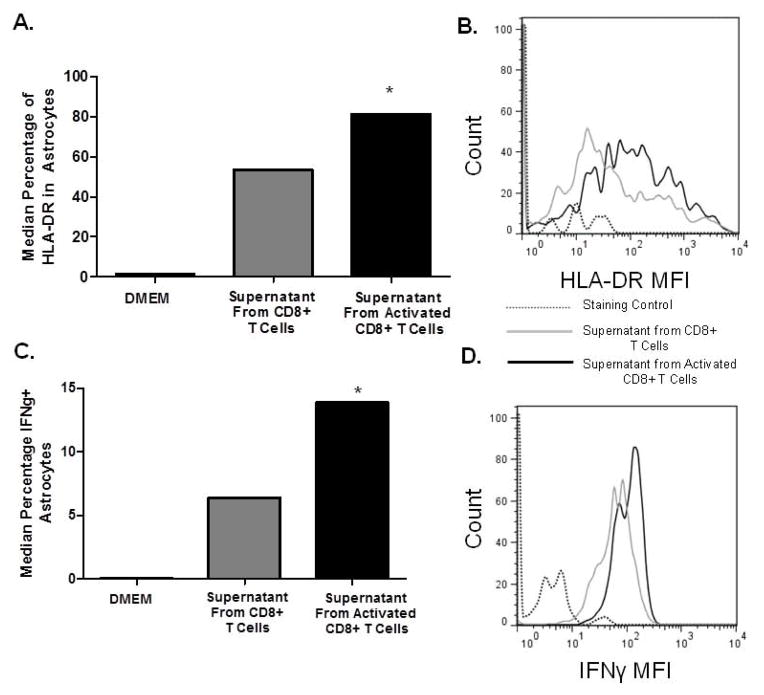
Astrogliosis is a hallmark of HIV infection in the CNS. Because CD8+ T cells are found in post-mortem tissue of HIV infected brains as well as in SIV macaque model of neuroAIDS (von Herrath et al., 1995; Petito et al., 2006; Petito et al., 2003; Sadagopal et al., 2008a; Sadagopal et al., 2008b), we assessed the impact of CD8+ T cells on astrocytes. CD8+ T cells from healthy individuals were activated by α-CD3/CD28 co-stimulation for three days or left unstimulated and their respective supernatant was added to PDAs. On day three, we evaluated percent expression of HLA-DR+ (Fig. 4A&B) and IFNγ+ (Fig. 4C&D) on PDAs by flow cytometry. PDAs cultured in CD8+ T cell conditioned media showed a median 50% increase in HLA-DR expression (Fig. 4A) and a median 15% increase in IFNγ+ cells (Fig. 4C). These data indicate that activated CD8+ T cells impact the phenotype of PDAs by promoting their induction of HLA-DR and IFNγ. IFNγ is particularly interesting because it also leads to HLA-DR induction (Carpentier et al., 2005), which can set up a positive feed-back loop in relation to enhanced HLA-DR expression on PDAs.
Figure 4. Conditioned Media from CD8+ T cells activates astrocytes.
CD8+ T cells were isolated from PBMCs from healthy seronegative donors by magnetic bead isolation and left untreated or treated with 1 μg/ml α-CD3/CD28 co-stimulation and 20U/mL IL-2. At day 3, supernatant from un-activated or activated CD8+ T cells was added to PDAs for three days. PDAs were analyzed by FACS analysis for surface expression of HLA-DR (A & B) and intracellular IFNγ (C & D). Data is representative of three experiments *p≤0.05 between DMEM media and treated groups, as measured by student’s t test.
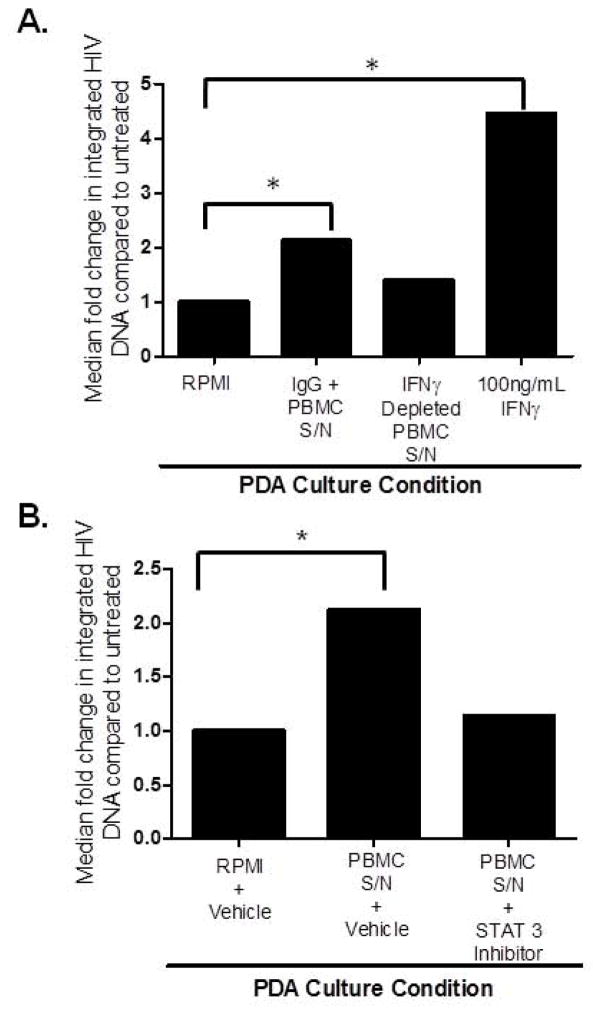
Supernatant from activated PBMCs enhances HIV infection in PDAs in an IFNγ/Stat-3-dependent manner
Given our finding that soluble factors from CD8+ T cells induce IFNγ in PDAs and that IFNγ itself leads to induction of HIV replication in astrocytes by antagonizing Wnt signaling in Stat-3-dependent manner (Li et al., 2011), we assessed whether IFNγ secreted from PBMCs could be driving higher level of HIV infection in PDAs. PDAs were pre-treated with supernatant from activated PBMCs depleted of IFNγ or an isotype control (IgG). An additional control, treating PDAs with IFNγ at 100ng/ml was also included. At 24 hours, PDAs were infected with HIVBaL and propagated under pre-infection treatment groups for three days. As expected, PDAs treated with IFNγ showed a 4.5 fold increase in HIV infection, as determined by Alu-PCR for HIV-LTR (Fig. 5A). PDAs treated with supernatant from activated PBMCs showed a 2-fold increase in integrated HIV (Fig. 5A). Depletion of IFNγ from PBMCs conditioned media, which was confirmed by WB (data not shown), abrogated the ability of this conditioned media to enhance HIV infection in PDAs (Fig. 5A). To further evaluate the role of IFNγ secreted from PBMCs to modulate HIV infection in astrocytes, PDAs were treated with Stat-3 inhibitor or a vehicle control in presence of PBMC supernatant. Stat-3 inhibitor abrogated the effect of PBMC supernatant to enhance HIV infection in astrocytes (Fig. 5B). Together, removing IFNγ from PBMCs conditioned media (Fig. 5A) and treating astrocytes with Stat-3 inhibitor (Fig. 5B) indicates that IFNγ secreted from PBMCs drives enhanced HIV infection of astrocytes in a Stat-3 dependent mechanism.
Figure 5. IFNγ released by activated PBMCs drives HIV infection of PDAs.
PBMCs from four healthy donors were stimulated with 1μg/mL α-CD3/CD28 and 20U/mL IL-2 for 3 days. (A) PBMC Supernatant was then treated with either 1μg/mL rabbit IgG isotype or anti-IFNγ coated magnetic beads to deplete IFNγ by immunoprecipitation overnight. PDAs were cultured in RPMI, pre-treated with IFNγ-depleted supernatant (S/N) from activated PBMCs, pre-treated with S/N from IgG-depleted activated PBMCs, or pre-treated with IFNγ (100ng/ml) for 24 hrs. PDAs were then infected with 10ng of HIVBaL per 1×106 cells overnight. PDAs were washed then placed in identical treatment conditions (PBMC supernatant, IFNγ depleted supernatant or 100ng/mL IFNγ) as was used for pre-treatment for three days. Genomic DNA was isolated and Alu-PCR for HIV-LTR was performed to determine level of HIV integration in PDAs. (B) PDAs were pre-treated with RPMI plus 1μM DMSO, pre-treated with supernatant from activated PBMCs with 1μM DMSO, or pre-treated with supernatant from activated PBMCs and 1μM Stat-3 Inhibitor (STATtic) for 24 hrs. PDAs were then infected with 10ng of HIVBaL per 1×106 cells overnight, washed, and placed in the same pre-treatment condition. At day three, genomic DNA was isolated and Alu-PCR for HIV-LTR was performed to determine level of HIV integration in PDAs. Data is representative of three donors, * indicates significance of p≤0.05 between RPMI group and IFNγ treated or IgG + S/N from PBMCs groups as measured by student’s t test.
Discussion
We evaluated here whether Wnts secreted from astrocytes could impact HIV replication in PBMCs and the impact of activated PBMCs on astrocytes. We show that astrocytes secrete a distinct profile of Wnt ligands. This profile includes abundant expression of Wnt 1 and expression of Wnt 2b, 3, 5b, 10b, 16, with low level of Wnt 7b. HIV infection of astrocytes perturbs some of these Wnt ligands by elevating Wnt 2b and 10b expression. The impact of HIV on astrocyte Wnt ligand profile is not clear, although it does not seem to alter the ability of astrocytes to suppress HIV replication in PBMCs through these Wnts. This is the first evidence to indicate that astrocytes have anti-viral activity not only to restrict HIV replication in astrocytes but that it is released and can inhibit HIV infection in susceptible cells. As such infiltrating T cells in the brain could be subject to restricted HIV replication driven by Wnts released from astrocytes.
Interestingly, the effect of astrocytes on PBMCs is bi-directional. Both CD4 and CD8+ T cells infiltrate the brain (Williams et al., 2001; von Herrath et al., 1995; Petito et al., 2006; Petito et al., 2003; Sadagopal et al., 2008a; Sadagopal et al., 2008b). CD4+ T cells contribute to dissemination of HIV in the CNS and they likely die in response to HIV infection in the CNS (Ho et al., 2013). CD8+ T cells are detected in post-mortem tissue although the role of CD8+ T cells, whether neuroprotective or pathogenic is unclear (Williams et al., 2001; von Herrath et al., 1995; Petito et al., 2006; Petito et al., 2003; Sadagopal et al., 2008a; Sadagopal et al., 2008b). CD8+ T cells are critical players in anti-viral responses. However, within the CNS, due to their effector responses, they can drive neuroinflammatory processes and mediate damage in the CNS (Getts et al., 2010). The most well studied pathogenic role of CD8+ T cells is in the context of myelin-specific CD8+ T cells and their role in multiple sclerosis (McDole et al., 2006). While extensive studies have probed the cellular and molecular mechanisms driving HIV-mediated neuropathogenesis, little is known about the role of T cells and their interaction with resident brain cells, such as astrocytes, in the context of HIV. Given that CD8+ T cells release a number of inflammatory mediators to kill infected cells, these mediators may have an impact on astrocytes as well as impact HIV replication in the CNS. Here we demonstrate that supernatant from activated CD8+ T cells activated astrocytes, as indicated by elevation in HLA-DR expression and IFNγ. MHCII expression has been identified as a marker of cell activation on T cells, macrophages, dendritic cells, B cells, stromal cells (Ko et al., 1979; Evans et al., 1978; Quade and Roth, 1999; Chang CH et al., 1995; Pieters 2000) and astrocytes (Takiguchi and Frelinger, 1986; Shrikant and Beneveniste, 1996; Traugott and Raines 1985; Krogsgard et al. 2000). The ability of astrocytes to induce HLA-DR, a MHC-II molecule involved in antigen presentation, is intriguing however the ability of astrocytes to process and present antigens is controversial (Hamo et al., 2007; Carpentier et al., 2005; Cornet et al., 2000; Becher et al., 2000). The ability of astrocytes, in response to soluble factors produced by PBMCs, to express IFNγ also has wider implications. IFNγ is elevated in the CNS of patients infected with HIV (Shapshak et al., 2004; Wesselingh et al., 1997) and is a potent antiviral cytokine produced by macrophages, dendritic cells, NK cells, NKT cells, CD4+ T cells and CD8+ T cells during an immune response and by astrocytes after activation (Lau and Yu, 2001). Most importantly, IFNγ induces DKK1, an antagonist of Wnt signaling, in a Stat-3 dependent manner, which leads to enhanced HIV infection of astrocytes (Li et al., 2011). Interestingly, IFNγ released from PBMCs functions in a paracrine manner to also inhibit HIV in astrocytes through Stat-3, which in turn inhibits β-catenin signaling. Based on our findings, a model emerges of the interaction between PBMCs and astrocytes in the CNS in the context of HIV (Fig. 6). Astrocytes inhibit HIV replication in PBMCs through secretion of Wnts while activated PBMCs secrete IFNγ that we show enhances HIV infection in astrocytes. Collectively, these findings highlight Wnt ligands as powerful mediators of anti-HIV activity of astrocytes.
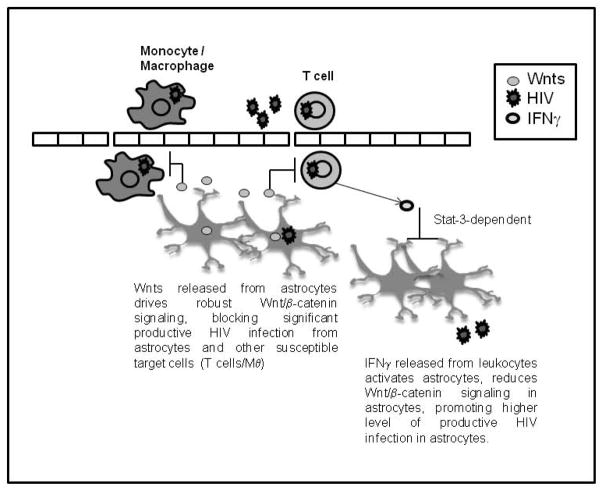
Figure 6. Crosstalk between astrocytes and infiltrating PBMCs is mediated by Wnt ligands.
Robust endogenous Wnt/β-catenin signaling, largely driven by Wnts released from astrocytes in an autocrine manner, blocks HIV replication in astrocytes. Under inflammatory conditions, leukocytes infiltrate the brain and release IFNγ, which activates astrocytes, down regulates β-catenin signaling in astrocytes, and consequently removes the block to HIV replication. Astrocytes then release a higher level of virions which in turn infect other susceptible targets in the CNS. Astrocytes also release a low-level of virions, even in presence of Wnt/β-catenin signaling, that can transmit virus to susceptible cells. The proposed consequence of reduced Wnt/β-catenin signaling in astrocytes under such inflammatory conditions is a compromise in the astrocyte/neuronal communication network, contributing to pathology associated with HAND.
Astrocytes are conditionally permissive to HIV infection and under inflammatory conditions can be productively infected with HIV. Particularly, high endogenous expression of β-catenin in astrocytes restricts HIV productive replication in astrocytes and PBMCs (Kumar et al., 2008; Li et al., 2011; Carroll-Anzinger et al., 2007; Henderson et al., 2012a; Wortman et al., 2002). Signals that diminish β-catenin signaling in astrocytes such as IFNγ lead to a higher level of productive HIV replication in astrocytes (Li et al., 2011). β-catenin, a central mediator of Wnt/β-catenin signaling, associates with TCF-4 and SMAR-1 to tether on the HIV LTR at −143 site from transcription initiation site to lead to repression of HIV transcription. Signals that disrupt β-catenin signaling also disrupt this association, promoting higher level of HIV transcription (Henderson et al., 2012a). Of note, in a previous publication we tested cytokines for their ability to modulate Wnt/β-catenin signaling in astrocytes (TNFα, IL-1β, IL-7, IFNγ, and GM-CSF). Only IFNγ and GM-CSF down regulate β-catenin. The effect of IFNγ is more robust than GM-CSF and, unlike GM-CSF, is consistent between primary astrocytes and astrocytoma cell lines (Carroll-Anzinger et al., 2006).
Our study adds to a growing body of evidence indicating that Wnt signaling, whether through direct effects on viruses or effects on anti-viral immune responses, regulate viral replication and/or pathogenesis. Our lab demonstrated this relationship for HIV (Kumar et al., 2008; Li et al., 2011; Carroll-Anzinger et al., 2007; Henderson et al., 2012a; Wortman et al., 2002). Emerging data now demonstrates that human CMV suppresses Wnt-induced transcriptional activity of β-catenin in fibroblasts (Angelova et al., 2012). Hepatitis C Virus (HCV) also modulates Wnt signaling to regulate its replication (Liu et al., 2011). HCV core protein is able to work synergistically with Wnt 3A, leading to enhanced Wnt/β-catenin signaling which promotes hepatocyte proliferation and carcinogenesis (Liu et al., 2011). Wnts through β-catenin, were also recognized to suppress type I interferon responses, which would have a wider impact on inhibiting a number of viruses, especially those that do not have mechanisms to evade type 1 interferon responses (Baril et al., 2013). Wnt 2b has been identified as a negative regulator for type I IFN signaling in Sendai-Virus and increased following Sendai Viral infection (Baril et al., 2013). Collectively, our studies demonstrate a dynamic interaction between astrocytes and infiltrating PBMCs through these small secreted Wnt glycoproteins that, on one hand, can control HIV-mediated CNS inflammation, partly, by limiting extent of HIV in the CNS, but on the other hand, infiltration of PBMCs into the CNS could drive astrocyte activation and subsequently contribute to neuronal injury.
Main Points.
Human primary-derived astrocytes (PDAs) secret a specific Wnt ligand profile which suppress HIV replication in PBMCs
PBMCs in turn induce astrocytes to express IFNγ and HLA-DR and enhance HIV replication in astrocytes in an IFNγ/Stat-3-dependent manner
Acknowledgments
We thank Dr. Eugene O. Major (NINDS, NIH) for providing PDAs used in this study. We thank blood donors for consenting to donate their blood for research purposes. This work was funded by R01 NIMH100628 (LA); 2R01NS06032 (LA) and 1F32NS080657-01A (MHR).
References
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- Aljawai Y, Richards MH, Seaton MS, Narasipura SD, Al-Harthi L. b-catenin/TCF-4 signaling regulates susceptibility of macrophages and resistance of monocytes to HIV productive infection. Current HIV Research. 2014;12:164–173. doi: 10.2174/1570162x12666140526122249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova M, Zwezdaryk K, Ferris M, Shan B, Morris CA, Sullivan DE. Human cytomegalovirus infection dysregulates the canonical Wnt/beta-catenin signaling pathway. PLoS Pathog. 2012;8:e1002959. doi: 10.1371/journal.ppat.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Baril M, Es-Saad S, Chatel-Chaix L, Fink K, Pham T, Raymond VA, Audette K, Guenier AS, Duchaine J, Servant M, Bilodeau M, Cohen E, Grandvaux N, Lamarre D. Genome-wide RNAi screen reveals a new role of a WNT/CTNNB1 signaling pathway as negative regulator of virus-induced innate immune responses. PLoS Pathog. 2013;9:e1003416. doi: 10.1371/journal.ppat.1003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- Brussel A, Sonigo P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J Virol. 2003;77:10119–10124. doi: 10.1128/JVI.77.18.10119-10124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol. 2006;80:541–544. doi: 10.1128/JVI.80.1.541-544.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol. 2007;81:5864–5871. doi: 10.1128/JVI.02234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Hong SC, Hughes CC, Janeway CA, Jr, Flavell RA. CIITA activates the expression of MHC class II genes in mouse T cells. Int Immunol. 1995;9:1515–1518. doi: 10.1093/intimm/7.9.1515. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Coombs GS, Covey TM, Virshup DM. Wnt signaling in development, disease and translational medicine. Curr Drug Targets. 2008;9:513–531. doi: 10.2174/138945008784911796. [DOI] [PubMed] [Google Scholar]
- Cornet A, Bettelli E, Oukka M, Cambouris C, Avellana-Adalid V, Kosmatopoulos K, Liblau RS. Role of astrocytes in antigen presentation and naive T-cell activation. J Neuroimmunol. 2000;106:69–77. doi: 10.1016/s0165-5728(99)00215-5. [DOI] [PubMed] [Google Scholar]
- Evans RL, Faldetta TJ, Humphreys RE, Pratt DM, Yunis EJ, Schossman SF. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978;148:1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts MT, Richards MH, Miller SD. A critical role for virus-specific CD8(+) CTLs in protection from Theiler’s virus-induced demyelination in disease-susceptible SJL mice. Virology. 2010;402:102–111. doi: 10.1016/j.virol.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamo L, Stohlman SA, Otto-Duessel M, Bergmann CC. Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia. 2007;55:1169–1177. doi: 10.1002/glia.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Narasipura SD, Adarichev V, Kashanchi F, Al-Harthi L. Identification of novel T cell factor 4 (TCF-4) binding sites on the HIV long terminal repeat which associate with TCF-4, beta-catenin, and SMAR1 to repress HIV transcription. J Virol. 2012a;86:9495–9503. doi: 10.1128/JVI.00486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Sharma A, Monaco MCG, Major EO, Al-Harthi L. Human immunodeficiency virus type 1 (HIV-1) transactivator of transcription through its intact core and cysteine-rich domains inhibits wnt/β-catenin signaling in astrocytes: Relevance to HIV neuropathogenesis. J Neurosci. 2012b;32:16306–16313. doi: 10.1523/JNEUROSCI.3145-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E. Cellular Composition of Cerebrospinal Fluid in HIV-1 Infected and Uninfected Subjects. PLoS One. 2013;8:e66188. doi: 10.1371/journal.pone.0066188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine TO, Benes L. Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A. 2006;69:147–151. doi: 10.1002/cyto.a.20225. [DOI] [PubMed] [Google Scholar]
- Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia Determinants on stimulated human T lymphocytes. Occurrence on mitogen and antigen-activated T cells. J Exp Med. 1979;150:246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgard M, Wucherpfennig KW, Cannella B, Hansen BE, Svejgaard A, Pyrdol J, Ditzel H, Riane C, Engberg J, Fugger L. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85-99 complex. J Exp Med. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zloza A, Moon RT, Watts J, Tenorio AR, Al-Harthi L. Active β-catenin signaling is an inhibitory pathway for human immunodeficiency virus replication in peripheral blood mononuclear cells. J Virol. 2008;82:2813–2820. doi: 10.1128/JVI.02498-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba S, Ravichandran V, Major EO. Glial cell type-specific subcellular localization of 14-3-3 zeta: an implication for JCV tropism. Glia. 2009;57:971–977. doi: 10.1002/glia.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human Immunodeficiency Virus Type I Infection of Human Brain-Derived Progenitor Cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-γ mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the β-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, Shan X, Cai X, Chen Q, Ling N, Zhang B, Bi Y, Chen K, Ren H, Huang A, He TC, Tang N. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS One. 2011;6:e27496. doi: 10.1371/journal.pone.0027496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, Flynn C, Zandonatti M, Fox HS. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol. 2007;178:5812–5819. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Phillipson CA, Fox HS. Distinct clonal repertoire of brain CD8+ cells in simian immunodeficiency virus infection. AIDS. 2003;17:1605–1611. doi: 10.1097/00002030-200307250-00004. [DOI] [PubMed] [Google Scholar]
- McDole J, Johnson AJ, Pirko I. The role of CD8+ T-cells in lesion formation and axonal dysfunction in multiple sclerosis. Neurol Res. 2006;28:256–261. doi: 10.1179/016164106X98125. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasipura SD, Henderson LJ, Fu SW, Chen L, Kashanchi F, Al-Harthi L. Role of β-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. J Virol. 2012;86:1911–1921. doi: 10.1128/JVI.06266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasipura SD, Kim S, Al-Harthi L. Epigenetic Regulation of HIV-1 Latency in Astrocytes. J Virol. 2014;88:3031–3038. doi: 10.1128/JVI.03333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77:6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Petito CK, Adkins B, McCarthy M, Roberts B, Khamis I. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. J Neurovirol. 2003;9:36–44. doi: 10.1080/13550280390173391. [DOI] [PubMed] [Google Scholar]
- Petito CK, Torres-Munoz JE, Zielger F, McCarthy M. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol. 2006;12:272–283. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- Pieters J. MHC class II-restricted antigen processing and presentation. Adv Immunol. 2000;75:159–208. doi: 10.1016/s0065-2776(00)75004-8. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Porwit A, Parravicini C, Petren AL, Barkhem T, Costanzi G, Josephs S, Biberfeld P. Cell association of HIV in AIDS-related encephalopathy and dementia. APMIS. 1989;97:79–90. doi: 10.1111/j.1699-0463.1989.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Quade MJ, Roth JA. Dual-color clow cytometric analysis of phenotype, activation marker expression, and proliferation on mitogen-stimulated bovine lymphocyte subsets. Vet Immunol Immunopathol. 1999;67:33–45. doi: 10.1016/s0165-2427(98)00209-8. [DOI] [PubMed] [Google Scholar]
- Sadagopal S, Amara RR, Kannanganat S, Sharma S, Chennareddi L, Robinson HL. Expansion and exhaustion of T-cell responses during mutational escape from long-term viral control in two DNA/modified vaccinia virus Ankara-vaccinated and simian-human immunodeficiency virus SHIV-89.6P-challenged macaques. J Virol. 2008a;82:4149–4153. doi: 10.1128/JVI.02242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopal S, Lorey SL, Barnett L, Basham R, Lebo L, Erdem H, Haman K, Avison M, Waddell K, Haas DW, Kalams SA. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol. 2008b;82:10418–10428. doi: 10.1128/JVI.01190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Zloza A, Li W, Narasipura SD, Al-Harthi L. β-Catenin signaling mediates CD4 expression on mature CD8+ T cells. J Immunol. 2010;185:2013–2019. doi: 10.4049/jimmunol.0902572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapshak P, Duncan R, Minagar A, Rodriguez de la Vega P, Stewart RV, Goodkin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Front Biosci. 2004;9:1073–1081. doi: 10.2741/1271. [DOI] [PubMed] [Google Scholar]
- Shrikant P, Beneveniste EN. The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol. 1996;157:1819–1822. [PubMed] [Google Scholar]
- Stipursky J, Spohr TC, Sousa VO, Gomes FC. Neuron-astroglial interactions in cell-fate commitment and maturation in the central nervous system. Neurochem Res. 2012;37:2402–2418. doi: 10.1007/s11064-012-0798-x. [DOI] [PubMed] [Google Scholar]
- Szotek EL, Narasipura SD, Al-Harthi L. 17beta-Estradiol inhibits HIV-1 by inducing a complex formation between beta-catenin and estrogen receptor alpha on the HIV promoter to suppress HIV transcription. Virology. 2013;443:375–383. doi: 10.1016/j.virol.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi M, Frelinger JA. Induction of antigen presentation ability in purified cultures of astroglia by interferon-gamma. J Mol Cel Immunol. 1986;2:269–280. [PubMed] [Google Scholar]
- Trauggott U, Raines CS. Multuple Sclerosis. Evidence for antigen presentation in situ by endothelial cells and astrocytes. J Neurol Sci. 1985;69:365–370. doi: 10.1016/0022-510x(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J RV254/SEARCH 010 Study Group. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrath M, Oldstone MB, Fox HS. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol. 1995;154:5582–5589. [PubMed] [Google Scholar]
- Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortman B, Darbinian N, Sawaya BE, Khalili K, Amini S. Evidence for regulation of long terminal repeat transcription by Wnt transcription factor TCF-4 in human astrocytic cells. J Virol. 2002;76:11159–11165. doi: 10.1128/JVI.76.21.11159-11165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]