Abstract
Although leaf chloroplast transformation technology was developed more than a decade ago, no reports exist of stable transformation of undeveloped plastids or other specialized plastid types, such as proplastids, etioplasts, or amyloplasts. In this work we report development of a dark-grown tobacco suspension cell model system to investigate the transformation potential of undeveloped plastids. Electron microscope analysis confirmed that the suspension cells carry plastids that are significantly smaller (approximately 50-fold less in volume) and have a very different subcellular localization and developmental state than leaf cell chloroplasts. Using antibiotic selection in the light, we demonstrated that both plastid and nuclear transformation of these cell suspensions is efficient and reproducible, with plastid transformation frequency at least equal to that of leaf chloroplast transformation. Homoplasmic plastid transformants are readily obtained in cell colonies, or in regenerated plants, providing a more consistent and versatile model than the leaf transformation system. Because of the uniformity of the cell suspension model, we could further show that growth rate, selection scheme, particle size, and DNA amount influence the frequency of transformation. Our results indicate that the rate-limiting steps for nuclear and plastid transformation are different, and each must be optimized separately. The suspension cell system will be useful as a model for understanding transformation in those plant species that utilize dark-grown embryogenic cultures and for characterizing the steps that lead to homoplasmic plastid transformation.
Stable chloroplast transformation in higher plants was first achieved in the model crop, tobacco (Nicotiana tabacum), about 10 years ago (Svab et al., 1990; Svab and Maliga, 1993), and today is still used as a tool to answer fundamental questions in plastid biology or for overexpression of recombinant proteins of agronomic importance or potential therapeutic use (Heifetz, 2000; Bock, 2001; Daniell et al., 2002; Staub, 2002; Maliga, 2003). More recently, stable chloroplast transformation has been achieved in additional dicot plant species, including Arabidopsis (Sikdar et al., 1998), potato (Solanum tuberosum; Sidorov et al., 1999), tomato (Lycopersicon esculentum; Ruf et al., 2001), and Lesquerella fendleri (Skarjinskaia et al., 2003). In each case, leaf chloroplasts were used as the target for transformation because of the abundance of the organelle in this tissue and the large plastid genome copy number in chloroplasts. A typical dicot leaf cell contains as many as 100 chloroplasts per cell with up to 100 genome copies per chloroplast (Bendich, 1987).
The most widely used selectable marker for plastid transformation is a chimeric bacterial-derived antibiotic resistance marker, aadA, which confers resistance to spectinomycin and streptomycin (Svab and Maliga, 1993). Selection of transformants is based on greening and enhanced growth of resistant cells while sensitive cells are bleached and growth inhibited (Maliga, 1993). Introduction of foreign DNA into chloroplasts is achieved via the particle bombardment process, and integration of transgenes is mediated by homologous recombination through flanking plastid DNA in the transformation vector. Upon integration of the transgenes, stable transformation is achieved as a result of amplification and sorting of transgenic plastid genome copies with concomitant elimination of wild-type genomes under continued selective pressure. Elimination of wild-type genomes to yield homoplasmic lines is required for stability of the transgenes. In the tobacco leaf chloroplast transformation system, homoplasmic plants or seedlings derived from these are typically the useful endpoint.
In contrast to the success in several dicot species that use leaf chloroplasts as a transformation target, development of plastid transformation technology in nonphotosynthetic plastid types has been more problematic. Currently, the most common monocot plant transformation and regeneration systems use dark-grown embryogenic calli or suspension cultures that carry undeveloped plastids. To date, only rice (Oryza sativa) plastid transformation has been reported, utilizing dark-grown embryogenic suspension cells as the target material. However, the rice plants that were produced were heteroplasmic at the organelle level, chimeric at the cellular level, and did not transmit the plastid transgenes to progeny (Khan and Maliga, 1999). Transient gene expression in chloroplasts (Sporlein et al., 1991; Yoshimoto et al., 2001), including nonphotosynthetic plastid types (Daniell, 1993; Seki et al., 1995; Hibberd et al., 1998), has also been reported. However, direct observation of reporter gene expression in nonphotosynthetic plastids using either light or confocal microscopy indicates only rare occurrences of transformation, and no attempts were made to select stably transformed cells from any of these experiments.
This report demonstrates an efficient and reproducible procedure for plastid transformation of dark-grown tobacco suspension cells. This transformation system has a number of useful advantages, including easy maintenance of stock cultures and the ability for high throughput with less labor and more consistency than observed with leaf material. Importantly, the suspension cells provide a model system that is analogous in many respects to dark-grown embryogenic culture systems of important monocot crop species. Furthermore, our results indicate that plastid size, subcellular localization, and developmental stage are apparently not the rate-limiting factors for successful and efficient plastid transformation.
RESULTS
Plastid Morphology in Tobacco Suspension Cells
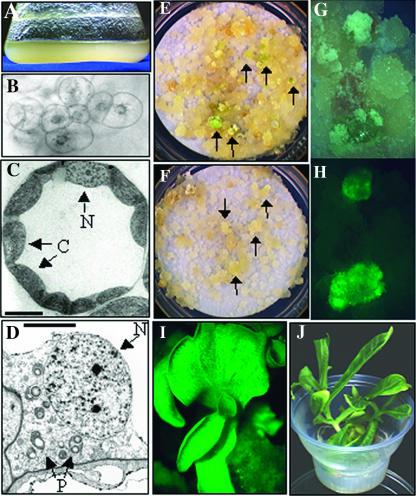
The morphology of the suspension cells can be seen in Figure 1. The dark-grown cells do not accumulate chlorophyll, but do have a yellow appearance presumably due to accumulation of carotenoids or other pigments (Fig. 1A). Under light microscopy, the suspension cells appear to be predominantly small clumps of cells, with the nucleus apparent and large cytoplasm with no obvious vacuole. No other subcellular organelles are visible at this magnification (Fig. 1B). To determine the morphology of plastids in these cells, transmission electron microscopy was performed and compared to chloroplasts found in leaf mesophyll cells of in vitro grown plants. The leaf cell chloroplasts have well-developed membrane structures, are relatively uniform in size, and are localized around the periphery of the cell (Fig. 1C). In contrast, plastids in the tobacco suspension cells are randomly distributed throughout the cell cytoplasm, heterogeneous in size but much smaller than in leaf cells, and have little to no apparent internal membrane structure. The plastids in suspension cells also contain large amounts of starch relative to leaf cell chloroplasts (Fig. 1D).
Figure 1.
Morphology of tobacco suspension cells. A, Dark-grown tobacco suspension cells at 3 d after subculture to fresh medium. B, Light microscope image (at 10× magnification) of suspension cell cluster. Note that the nucleus is visible and centrally located in each cell. C, Transmission electron micrograph of in vitro grown leaf cell. Note that the chloroplasts (C) are similar in size to the nucleus (N) in this cell type. Bar represents 10 μm. D, Transmission electron micrograph of plastids in suspension cells. Note that plastids (P) are significantly smaller than the nucleus (N) and are randomly distributed throughout the cell. Bar represents 5 μm. E, Greening colonies (some indicated by arrows) from nuclear transformation identified after 6 weeks on selective spectinomycin-containing medium. F, Colonies from plastid transformation (arrows) after 9 weeks on selective spectinomycin-containing medium. Note that greening is not obvious at this time point. G, Close-up view of colonies selected during plastid transformation in visible light and (H) the same colonies in fluorescent light for detection of GFP expression. I, GFP fluorescence in tissue culture-derived plantlet regenerating from a plastid-transformed colony. J, Rooted plant derived from plastid transformation.
Confocal scanning microscopy was used to obtain a more precise measure of the diameter and volume of plastids in the different cell types. For this analysis, leaf tissue and suspension cells were derived from homoplasmic plastid transformed lines that express green fluorescent protein (GFP; see below) and measurements were based on GFP fluorescence visualized by the confocal microscope. From this analysis, the undeveloped plastids present in the suspension cells had an average diameter of 2.1 microns and volume of 4.9 cubic microns. In contrast, leaf cell chloroplasts had an average diameter of 7.7 microns and volume of 239 cubic microns (data not shown). Therefore, the average leaf chloroplast diameter is approximately 4-fold and the volume is approximately 50-fold greater than that of undeveloped plastids of suspension cells. Interestingly, plastids in the suspension cells were abundant (45 ± 4; n = 15), suggesting that plastid numbers would not be rate-limiting for transformation.
Selection Conditions for Plastid Transformation
The standard protocol for selection of chloroplast transformants from tobacco leaf employs the antibiotic spectinomycin at selective levels of 500 mg/L. Transformants are selected in the light and typically arise as green shoots on plant regeneration medium. To determine the response of suspension cells to antibiotic treatment and identify selective levels for use in transformation experiments, the dark-grown cell suspensions were plated on spectinomycin levels up to 1,500 mg/L in both the light and the dark (data not shown).
In the light without antibiotic, the tobacco suspension cells turn green within approximately 3 weeks and grow vigorously as large mounds of cells. In the presence of spectinomycin, both greening and growth is delayed. In contrast to leaf cells that bleach completely within a couple of weeks at 500 mg/L spectinomycin, cell suspensions continue to grow and numerous green mounds of cells arise. At 750 mg/L spectinomycin, cell growth was not dramatically inhibited but very few green colonies formed, indicating this concentration may be optimal for selection of plastid transformants. Growth of dark-grown cells was only moderately inhibited at even the highest spectinomycin concentration tested, indicating that selection in the dark would be very difficult using this antibiotic.
Demonstration and Optimization of Stable Plastid Transformation
Preliminary plastid transformation experiments were designed to identify optimal cell growth and antibiotic selection conditions for the cell suspension cultures. To determine if growth rate of the cell suspensions affected the frequency of transformation, cultures were maintained under 2 different subculture regimes: transfers once weekly at a suspended cell to medium ratio (volume to volume) of 1:4 (1 × /wk cells) and transfers twice weekly at 3:4 suspended cell to medium ratio (2 × /wk cells). The 1 × /wk cells were bombarded 3 d post-subculture, whereas the 2 × /wk cells were bombarded 1 d post-subculture.
For selection of plastid transformants, cells were transferred 1 d after bombardment onto selection medium containing spectinomycin (750 mg/L) and incubated in the light. To test the effect of renewed selection medium on transformation frequency, one-half of the selected cells were transferred to fresh spectinomycin medium after 3 weeks. At 6 weeks post-bombardment, all of the cells were transferred to fresh selection medium.
To select plastid transformed tobacco suspension cells, the pMON30125 plastid transformation vector (Fig. 2A) used previously for selection of tobacco and potato plastid transformants was employed. This vector contains a chimeric aadA gene that provides spectinomycin resistance and a GFP reporter gene used for early identification of plastid transformants (Sidorov et al., 1999). Transformation of leaf cells with this vector resulted in approximately 1 transformant per bombardment in tobacco (G.-Y. Ye, C. Langbecker, and J. Staub, unpublished data) and approximately 1 transformant/5 bombardments in potato (Sidorov et al., 1999).
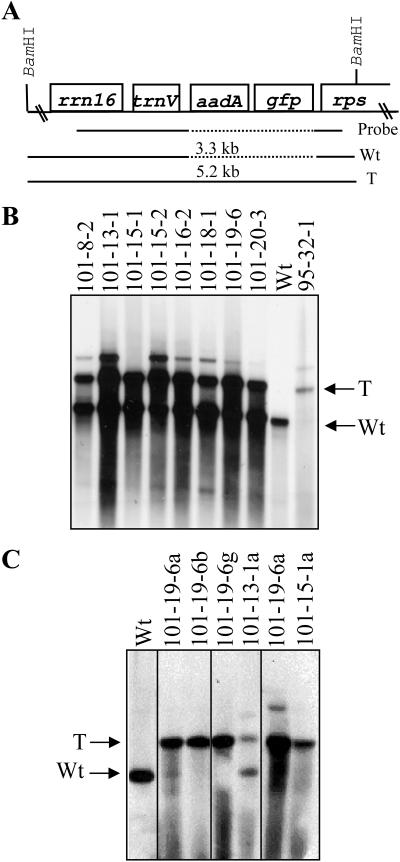
Figure 2.
Southern-blot analysis of plastid-transformed suspension cells. A, The map of wild-type (Wt) and transformed (T) genomes after integration of the aadA and gfp transgenes from plasmid pMON30125 is shown. Neighboring resident plastid genes, rrn16 and the 5′-rps12/rps7 operon (rps), are also shown, along with the DNA probe derived from this region of the genome. In B and C, total cellular DNA from multiple independent lines was digested with BamHI, separated on a 0.8% agarose gel, transferred to nitrocellulose, and probed. B, Note that primary transformed lines are typically heteroplasmic and contain a mixture of wild-type (3.3 kb) and transformed (5.2 kb) genomes, except for line 95-32-1 that appears to be a homoplasmic plastid transformant. C, Analysis of subcultured colonies (lines 101-19-6a, b, and g) and regenerated shoots (lines 101-13-1a, 101-19-6a, and 101-15-1a). Note homoplasmy in both subcultured colonies (101-19-6b and g) and regenerated plants (101-19-6a and 101-15-1a).
GFP fluorescence was used to identify putative plastid transformants on selection plates as early as 6 weeks post-bombardment. As shown in Figure 1, F–H, GFP fluorescence could be used to identify small colonies of transformed cells directly on plates within the background of nontransformed cells. The putative plastid transformed cells were then isolated onto fresh medium for further amplification prior to molecular analysis. Southern-blot analysis was performed on GFP-positive samples to verify plastid transformation. An example of this analysis is shown in Figure 2 and verified that all tested GFP-positive lines were due to insertion of the aadA and GFP transgenes into the plastid genome. As all GFP-positive lines were confirmed to be plastid transformants by this molecular analysis, GFP fluorescence was used as an indicator of plastid transformation in subsequent experiments.
In preliminary experiments to optimize growth and spectinomycin selection, a dramatic effect of subculture frequency and selection conditions on the frequency of plastid transformation was observed. Nearly all of the transgenic events were recovered from 2 × /wk subcultured cells that were allowed to remain on selection plates for 6 weeks prior to transfer to fresh selection medium (data not shown). These cells and selection conditions were therefore used in all subsequent experiments. On average, these optimal parameters routinely resulted in plastid transformation at a minimum of one to two transformants per bombarded plate (see below), a frequency similar to that reported for tobacco leaf transformation (Svab and Maliga, 1993). However, it should be noted that transformation of cell suspensions also routinely resulted in confirmed events on nearly every bombarded plate, while tobacco leaf transformation is inconsistent and highly variable between experiments.
For comparison to plastid transformation frequencies, nuclear transformation of the 2 × /wk cells was also performed at the same time. The pMON38754 nuclear transformation vector (Sidorov et al., 1999) that carries aadA and uidA genes under control of nuclear transcriptional control elements was used for transformation. Based on the greening morphology (Fig. 1E) and β-glucuronidase (GUS) staining (data not shown) in 9- to 12-week-old cell colonies selected using the same parameters as above, nuclear transformation frequency was approximately 25 to 45 transformants per bombarded plate, or about 20 times more frequent than plastid transformation.
Particle Size Influences the Frequency of Plastid and Nuclear Transformation
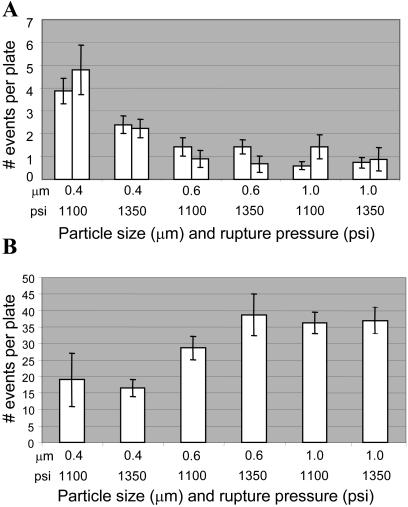
The standard protocol for tobacco leaf chloroplast transformation utilizes 0.6 or 1 micron particles for transformation, using a rupture pressure of 1,100 psi. Because of the random distribution of plastids in suspension cells and significantly smaller volume than leaf cell chloroplasts, we wanted to determine if particle size or bombardment pressure would influence frequency of plastid transformation. Gold particles were chosen for this study because the commercially available bead preparations are very uniform in size as compared to tungsten preparations (Randolph-Anderson et al., 1995).
Figure 3A summarizes the results of these experiments. No significant difference was observed when 0.6 and 1 micron particles were used for transformation, regardless of bombardment pressure used. In contrast, use of 0.4 micron particles resulted in a significantly higher (3- to 4-fold) transformation frequency. Interestingly, at this size of bead particle, the lower rupture-disc pressure (1,100 psi) used was more effective.
Figure 3.
Effect of gold particle size (μm) and rupture pressure (psi) on transformation frequency. Results of (A) two independent experiments for plastid transformation and (B) one experiment for nuclear transformation are shown. Ten plates per treatment were bombarded. The number of events was calculated by counting GFP fluorescence for plastid experiments and GUS histochemical staining for nuclear experiments. Error bars indicate se.
For comparison, the effect of these same particle sizes and bombardment pressures on the frequency of nuclear transformation was also tested. As can be seen in Figure 3B and in contrast to plastid transformation, the frequency of nuclear transformation decreases with smaller particle size independent of rupture pressure.
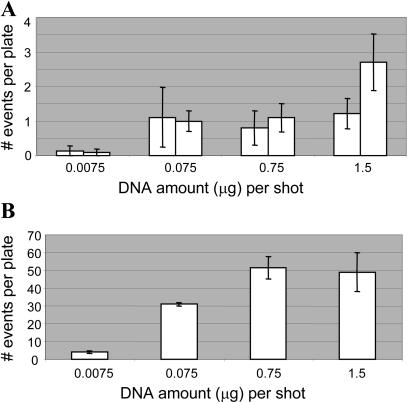
The Effect of DNA Amount on Plastid and Nuclear Transformation
The amount of DNA used for bombardment of the tobacco cell suspensions was also investigated over a 200-fold range, from 7.5 ng to 1.5 μg per bombardment. As shown in Figure 4, no significant difference was observed in plastid transformation frequency when the DNA amount ranged from 75 ng to 1.5 μg per bombardment (Fig. 4A). In contrast, the nuclear transformation frequency was unaffected at high DNA amounts but began to decrease at 75 ng. In both nuclear and plastid systems, the transformation frequency decreased significantly when only 7.5 ng DNA per bombardment was used. Plastid transformation frequency decreased approximately 10-fold while nuclear transformation frequency decreased as much as 30-fold (Fig. 4B).
Figure 4.
Effect of DNA amount (μg) on transformation frequency. Results of (A) two independent experiments for plastid transformation and (B) one experiment for nuclear transformation are shown. Ten plates per treatment were bombarded with 0.6 micron gold particles at 1,100 psi. The number of events was calculated by counting GFP fluorescence for plastid experiments and GUS histochemical staining for nuclear experiments. Error bars indicate se.
Homoplasmy Can Be Achieved in Cell Colonies or Regenerated Plants
The previously reported success with rice plastid transformation utilized cell suspensions as target material, but failed to produce homoplasmic cells (Khan and Maliga, 1999). However, for full utility of the tobacco suspension cell model, obtaining homoplasmic clones was important. Therefore, several clones identified initially by GFP fluorescence and subsequently by Southern-blot analysis (Fig. 2A) were further subcultured on two rounds of selection medium to obtain homoplasmic lines. For subculturing, the transformed colonies were cut into several small pieces (2–3 mm each) and allowed to grow for a further 2 to 3 weeks. These lines grew as cell colonies on solid medium, and shoots subsequently arose from these colonies on the same selection medium (Fig. 1I). Shoots were then transferred to rooting medium containing spectinomycin to form rooted plants (Fig. 1J). In all cases tested, regenerated plants were green and appeared phenotypically normal.
Several independent cell colonies and regenerated shoots were analyzed by Southern blot (Fig. 2B) to determine if homoplasmy was achieved. As can be seen in Figure 2C using flanking plastid DNA sequences as a probe, several lines carry exclusively the transgene insert with no apparent wild-type plastid genomes remaining. These results indicate that homoplasmy can be achieved in either cell colonies or shoots regenerated from them and that there is no barrier to achieving homoplasmy in plastid transformants derived from cell suspensions.
DISCUSSION
While tobacco chloroplast transformation technology was developed more than 10 years ago, only recently has this technology been transferred to additional crop species. Technology transfer has been limited to dicot plant species that utilize green leaf tissue as the explant for transformation. Presumably, the presence of abundant, developed chloroplasts has been an important factor for success. Our transmission electron microscopic observations of in vitro-grown tobacco leaf cells suggest that the large size of chloroplasts and their location along the periphery of the cell make them an easy target for transformation. On the other hand, the plastids in dark-grown suspension cells are relatively small, undeveloped, and dispersed throughout the cell. Remarkably, confocal scanning microscopy indicates an approximately 50-fold difference in plastid volume between leaf cell chloroplasts and undeveloped plastids in suspension cells. Despite these obvious differences, we showed that plastid transformation of the suspension cells is at least as efficient as the chloroplast-containing leaf system, with a minimum of one to two plastid transformants per bombardment. Furthermore, this number of transformants was consistently observed on nearly every plate when optimal parameters were used, in contrast to the leaf transformation system that is highly variable.
High-frequency plastid transformation in the suspension cells required optimization of several factors, including growth rate, selection parameters, particle size, and DNA amount. A large increase in plastid transformation frequency was observed using the 2 × /wk cultured cells that were bombarded only 1 d post-transfer, as compared to the 1 × /wk cultured cells bombarded at 3 d post-subculture. Previous analysis of plastid DNA synthesis upon transfer of tobacco suspensions cultures to new media showed a burst of plastid DNA replication shortly after the transfer (Yasuda et al., 1988; Infante and Weissbach, 1990; Suzuki et al., 1992; Takeda et al., 1999). We therefore hypothesize that the predicted burst of plastid DNA replication activity may be responsible for the observed increased transformation frequency, either due to activation of the enzymes required for recombination of the transgenic DNA or by rapid amplification of the inserted selectable marker gene.
A large increase in plastid transformation frequency was also observed when cultures were allowed to remain on antibiotic-containing selection medium rather than being frequently transferred to fresh selection medium. While the reason for this is unclear, we speculate that the effective concentration of antibiotic may decrease during 6 weeks of selection due to inactivation or dilution from new cell growth, thereby contributing to successful recovery of plastid transformants. It should also be noted that our bombardment media included osmotic stabilizers, thought to increase the amount of cell survival after the bombardment process. Previous work showed an improvement in the efficiency of transient gene expression in plastids due to the presence of osmoticum (Ye et al., 1990), although no attempt was made to optimize the concentration in our work.
The suspension cell system was also proven efficient for nuclear transformation, allowing direct comparison of bombardment parameters on the transformation frequency in each case. Interestingly, an approximately 4-fold increase in plastid transformation was observed using the smallest particle (0.4 micron), at either rupture pressure tested. It is tempting to speculate that the smaller particle size may have allowed physical insertion of the transforming DNA into the relatively small plastids without irreversible rupture of the plastid membrane. However, it should be noted that these experiments were done with a constant weight rather than constant number of particles, and therefore there were many more of the smaller particles present per bombardment. Although aggregation of particles in the presence of DNA was not tested, the increased number had no apparent detriment to the cells or to plastid transformation. Interestingly, in Chlamydomonas, chloroplast transformation frequency also increased with decreasing particle size (Randolph-Anderson et al., 1995). In contrast, nuclear transformation was more efficient with the larger sized particles, indicating that the transformation processes for these two organelles are different and must be optimized separately. Also interesting, DNA amount was not critical across a wide range for either nuclear or plastid transformation although a minimal threshold of DNA was required for efficient transformation in both cases.
Plastid transformants were initially identified by GFP fluorescence and subsequently by greening on selective medium. The GFP screen was previously shown to be a reliable indicator of plastid transformation in potato and rice (Khan and Maliga, 1999; Sidorov et al., 1999) and, in this case, by molecular confirmation of all GFP-positive cell colonies (Fig. 2). Further, the GFP screen eliminated spontaneous spectinomycin resistant clones that would normally arise at a low frequency on selective media. In contrast, nuclear transformants were first identified by their greening phenotype and subsequently by histochemical GUS staining. The greening phenotype was consistently observed in nuclear transformants several weeks faster than in plastid transformed lines. This may be due to the strong promoter driving the nuclear-encoded aadA transgene, or due to a delay in greening of plastid transformed lines due to weaker transgene expression in the nongreen plastids during the initial phases of selection, or due to more stringent selection on plastid transformed lines at 750 mg/L. Preliminary experiments using other selectable markers, nptII for kanamycin resistance and epsps for glyphosate resistance (Ye et al., 2003), where selection can be achieved by differential growth rather than on greening, have been successful for nuclear transformation in the tobacco suspension cell system (K. Lutke and C. Langbecker, unpublished data) and are being tested as plastid markers.
Although the cell suspensions were maintained and used for over a year, we saw no loss of greening potential in response to light and no decrease in transformation frequency during the time required to perform the experiments reported here. Once obtained, plastid transformants were readily converted to homoplasmy in either cell colonies or in plants regenerated from those. Plants were green and appeared phenotypically normal with no albino sectors or regenerated plants, as has been observed in plants derived from tissue culture of some cereal species (for example, Day and Ellis, 1984; Harada et al., 1992; Abe et al., 2002). These results indicate that the tobacco suspension cell system can be used for an extended amount of time to optimize transformation parameters and will be useful to further study the fate of transforming DNA. We did not, however, carry the regenerated plants to maturity to assess fertility or somaclonal variation in progeny. Therefore, additional studies should be performed prior to using this transformation system for applications where plant fertility and minimal somaclonal variation in progeny is required.
Our results indicate that there is no fundamental barrier to transformation of plastid types other than chloroplasts. In fact, recent work in rice has suggested that plastid transformation in suspension cells is possible. However, rice plastid transformants were chimeric and heteroplasmic, containing a mixture of cells carrying untransformed plastids and cells carrying partially transformed plastids. The tobacco plastid transformation system described here may prove useful as a model for understanding transformation of those species that utilize embryogenic cultures. With the additional consistency, ease of use and maintenance, and potential to circumvent the need for regenerated plants, the tobacco suspension cell system may also replace tobacco leaf in some cases as the vehicle for study of plastid gene function through transformation.
MATERIALS AND METHODS
Initiation and Maintenance of Tobacco Suspension Cultures
Young, mature leaves from Nicotiana tabacum cv Petit Havana plants grown in sterile tissue culture were used as starting material. Plants were raised from seed on germination medium (Murashige and Skoog salts [Murashige and Skoog, 1962], B-5 vitamins [Gamborg et al., 1968], 3% w/v Suc, and 8 g/L agar [Sigma, St. Louis, catalog no. A-1296]) at 24°C with a 16-h light:8-h dark photoperiod. Light intensity of 35 to 58 μE m−2 s−1 was delivered by cool-white fluorescent bulbs. Mature plants were propagated by placing nodal cuttings onto fresh germination medium. Mature leaves were removed from plants about 4 to 6 weeks after propagation, cut into 0.5- to 1.0-cm squares, and placed onto callus induction medium (Murashige and Skoog salts, B-5 vitamins, 4 mg/L p-chlorophenoxyacetic acid, 5 μg/L kinetin, 3% Suc, and 2.5 g/L Schweizerhall gelling agent) and cultured at 25°C in continuous dark for callus initiation. Translucent friable callus was separated from the leaf segments and transferred to fresh medium and subcultured every 3 to 4 weeks. Callus that had been propagated for 2 to 5 months was used to initiate liquid suspension cultures.
Suspension cell cultures were initiated by placing 20 g of callus into 40 mL of filter sterilized suspension medium (Murashige and Skoog salts, MS vitamins, 4 mg/L p-chlorophenoxyacetic acid, 5 μg/L kinetin, 0.2 g/L myo-inositol, 0.15 g/L l-Asn, and 3% w/v Suc) in a 250-mL Erlenmeyer flask. Suspensions were cultured in the dark at 25°C with shaking at 140 to 160 rpm. Subcultures of 1:1 with fresh medium were performed at weekly intervals for a period of 5 to 8 weeks with large clumps removed each week until a consistent suspension was obtained. Cultures were used for plastid transformation experiments by subculturing 10 mL of suspended cells into 40 mL of fresh medium weekly (1 × /wk cells). The suspensions were also transferred at a ratio of 30 mL of suspended cells into 40 mL of fresh medium 2 times per week (2 × /wk cells). As before, culture density became consistent after approximately 5 weeks of subcultures at which time transformation experiments could be initiated.
Tobacco Suspension Transformation
The 2 × /wk cells were used for bombardment experiments 24 h post-subculture while the 1 × /wk cells were used 3 d post-subculture. Settled cell volume was determined by allowing 10 mL of the cell suspension to settle for 20 min in a 15-mL conical tube and then recording the amount of cells. The remaining suspension culture was then diluted to 0.5 mL cells/10 mL of medium, using suspension medium.
Aliquots of 0.25 mL of settled suspended cells were transferred onto a 70-mm Whatman number 1 filter using a Corning 500-mL 0.45-micron vacuum filter system. Small holes were introduced into the 0.45-micron filter to allow for more rapid adherence of the cells to the Whatman filter. The filter paper including the cells was then placed onto bombardment medium (Murashige and Skoog salts, B-5 vitamins, 18.2 g/L mannitol, 18.2 g/L sorbitol, 0.1 mg/L 1-naphthaleneacetic acid, 1 mg/L 6-benzylaminopurine (BAP), 3% w/v Suc, and 8 g/L TC agar) for 4 h prior to bombardment. Bombardment was performed using the Bio-Rad PDS-1000/He Delivery System (Hercules, CA; Sanford et al., 1993) under a vacuum of 28 inches of mercury with the target shelf located 9 cm from the stopping screen. Particle size and rupture pressures were as outlined in the text. Gold particles were purchased from Degussa, Precious Metals Division (South Plainfield, NJ). Approximately 20 h after bombardment filters were transferred to selection medium (Murashige and Skoog salts, B-5 vitamins, 3% w/v Suc, 0.1 mg/L naphthylacetic acid, 1 mg/L BAP, 750 mg/L spectinomycin, and 8 g/L TC agar) and cultured at 25°C with a 16:8-h photoperiod using cool-white fluorescent lamps. A minimum of 10 bombarded plates was used for each treatment and experiments were repeated at least twice, except where noted in the text. Plastid and nuclear transformation experiments were performed side-by-side. Transformations were scored for reporter gene expression (see below) at 11 to 14 weeks after bombardment.
Construction of Transformation Vectors
pMON30125 has been described previously (Sidorov et al., 1999) and was used for plastid transformation. This vector carries the aadA selectable marker driven by psbA gene expression signals and the gfp gene driven by the Prrn promoter and Trps16 expression cassette. For nuclear transformation, pMON38754 (Sidorov et al., 1999) was used. This vector carries the aadA gene driven by the figwort mosaic virus promoter and the uidA gene driven by the cauliflower mosaic virus 35S promoter.
Selection of Tobacco Suspension Cells and Regeneration of Plants
After 3 or 6 weeks on selection medium the filters were transferred to fresh medium. By 6 weeks post-bombardment, putative plastid transformants could be identified by GFP fluorescence and by 9 weeks putative plastid transformants could be identified by the green color of growing calli. Resistant calli were isolated at this time and transferred onto fresh selection medium without filter papers. Once the colonies had grown to 2 to 3 cm in diameter they were sampled for Southern-blot analysis or cut into 3- to 4-mm slices and transferred flat-side down onto selection medium to stimulate shoot formation. Small shoots typically formed as soon as 2 weeks after cutting. Shoots were removed and placed onto rooting medium (same as selection medium but without BAP and naphthylacetic acid).
DNA Gel Blots and Probing of Total Cellular DNA
Total cellular DNA was extracted and analyzed by Southern probing as previously described (Sidorov et al., 1999). The DNA was digested with BamHI, electrophoresed on 0.8% agarose gels, transferred to nylon membrane, and hybridized overnight at 65°C in Rapid Hybridization buffer (Amersham, Piscataway, NJ). After hybridization, blots were washed in 0.1% sodium chloride/sodium phosphate/EDTA plus 0.1% SDS and then exposed to x-ray film. Radioactively labeled probes were generated by random priming (Boehringer Mannheim, Basel).
GFP Fluorescence Microscopy and Confocal Scanning Microscopy
Fluorescence from plastid transformants was observed using a Leica MZ80 dissecting microscope (Wetzlar, Germany; equipped with a filter set [HQ480/40 excitation filter, HQ535/50 emission filter, and Q505LP dichroic mirror]). Photographs were taken with 35-mm Fujichrome Provia1600 slide film (Fuji, Tokyo) or a digital imaging system (Leica DC200).
Confocal scanning microscopy was performed using a Bio-Rad MRC 1024 confocal laser scanning microscope. A 60× oil immersion Zeiss (Jena, Germany) lens was used to image the specimens. The laser was operated with an excitation wavelength of 488 nm and an emission wavelength of 522 nm. A z-series was typically collected with z axis steps of between 2 and 5 μm. Images were viewed and processed using the Confocal Assistant v4.02 software package (Todd Clark Brelje).
As a tool for the confocal microscopy analysis, a homoplasmic plastid transformed line derived from pMON30125 transformation was used to generate a suspension culture as outlined above and described in the text. In vitro grown mature leaves and dark-grown suspension cultures from the pMON30125-derived plants were used for the analysis.
Histochemical GUS Assay
Nuclear transformants were detected by 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (X-gluc assay; Jefferson et al., 1987). Samples were added to one-half-strength X-gluc solution at room temperature for 24 h. After color development, the X-gluc solution was removed and 70% ethanol was added to preserve the samples. Positive cell colonies were counted manually.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Cindy Brobst for technical assistance in pilot experiments, Jeanne Layton for assistance with suspension culture maintenance, and Kevin W. Lutke for contributions to selection strategies. We also thank Drs. Ken Barton and Steve Padgette for support during this work.
References
- Abe T, Ii N, Togashi A, Sasahara T (2002) Large deletions in chloroplast DNA of rice calli after long-term culture. J Plant Physiol 159: 917–923 [Google Scholar]
- Bendich AJ (1987) Why do chloroplasts and mitochondria contain so many copies of their genome? Bioessays 6: 279–282 [DOI] [PubMed] [Google Scholar]
- Bock R (2001) Transgenic plastids in basic research and plant biotechnology. J Mol Biol 312: 425–438 [DOI] [PubMed] [Google Scholar]
- Daniell H (1993) Foreign gene expression in chloroplasts of higher plants mediated by tungsten particle bombardment. Methods Enzymol 217: 536–556 [DOI] [PubMed] [Google Scholar]
- Daniell H, Khan MS, Allison LA (2002) Milestones in chloroplast genetic engineering: an environmentally friendly era in biotechnology. Trends Plant Sci 7: 84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A, Ellis TH (1984) Chloroplast DNA deletions associated with wheat plants regenerated from pollen: possible basis for maternal inheritance of chloroplasts. Cell 39: 359–368 [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 [DOI] [PubMed] [Google Scholar]
- Harada T, Ishikawa R, Niizeki M, Saito K (1992) Pollen-derived rice calli that have large deletions in plastid DNA do not require protein synthesis in plastids for growth. Mol Gen Genet 233: 145–150 [DOI] [PubMed] [Google Scholar]
- Heifetz P (2000) Genetic engineering of the chloroplast. Biochimie 82: 655–666 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Linley PJ, Khan MS, Gray JC (1998) Transient expression of green fluorescent protein in various plastid types following microprojectile bombardment. Plant J 16: 627–632 [Google Scholar]
- Infante D, Weissbach A (1990) Organellar DNA replication in Nicotiana tabacum cultured cells. Plant Mol Biol 14: 891–897 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MS, Maliga P (1999) Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat Biotechnol 17: 910–915 [DOI] [PubMed] [Google Scholar]
- Maliga P (1993) Towards plastid transformation in flowering plants. Trends Biotechnol 11: 101–106 [Google Scholar]
- Maliga P (2003) Progress towards commercialization of plastid transformation technology. Trends Biotechnol 21: 20–28 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for the growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Randolph-Anderson B, Boynton JE, Dawson J, Dunder E, Eskes R, Gillham NW, Johnson A, Perlman PS, Suttie J, Heiser WC (1995) Sub-micron gold particles are superior to larger particles for efficient biolistic transformation of organelles and some cell types. BioRad Technical Bulletin no. 2015
- Ruf S, Herrmann M, Berger IJ, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol 19: 870–875 [DOI] [PubMed] [Google Scholar]
- Sanford JC, Smith FD, Russell JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217: 483–509 [DOI] [PubMed] [Google Scholar]
- Seki M, Shigemoto N, Sugita M, Sugiura M, Koop H-U, Irifune K, Morikawa H (1995) Transient expression of β-glucuronidase in plastids of various plant cells and tissues delivered by a pneumatic particle gun. J Plant Res 108: 235–240 [Google Scholar]
- Sidorov VA, Kasten D, Pang S-Z, Hajdukiewicz PTJ, Staub JM, Nehra N (1999) Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J 19: 209–216 [DOI] [PubMed] [Google Scholar]
- Sikdar SR, Serino G, Chaudhuri S, Maliga P (1998) Plastid transformation in Arabidopsis thaliana. Plant Cell Rep 18: 20–24 [Google Scholar]
- Skarjinskaia M, Svab Z, Maliga P (2003) Plastid transformation in Lesquerella fendleri, an oilseed Brassicacea. Transgenic Res 12: 115–122 [DOI] [PubMed] [Google Scholar]
- Sporlein B, Streubel M, Dahlfeld G, Westhoff P, Koop H-U (1991) PEG-mediated plastid transformation: a new system for transient gene expression assays in chloroplasts. Theor Appl Genet 82: 717–722 [DOI] [PubMed] [Google Scholar]
- Staub JM (2002) Expression of recombinant proteins via the plastid genome. In VA Vinci and SR Parekh, eds, Handbook of Industrial Cell Culture: Mammalian, Microbial and Plant Cells. Humana Press, Totowa, NJ
- Suzuki T, Kawano S, Sakai A, Fujie M, Kuroiwa H, Nakamura H, Kuroiwa T (1992) Preferential mitochondrial and plastid DNA synthesis before multiple cell divisions in Nicotiana tabacum. J Cell Sci 130: 831–837 [Google Scholar]
- Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87: 8526–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90: 913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Kaneko Y, Matsushima H, Yamada Y, Sato F (1999) Cultured green cells of tobacco as a useful material for the study of chloroplast replication. Methods Cell Sci 21: 149–154 [DOI] [PubMed] [Google Scholar]
- Yasuda T, Kuroiwa T, Nagata T (1988) Preferential synthesis of plastid DNA and increased replication of plastids in cultured tobacco cells following medium renewal. Planta 174: 235–241 [DOI] [PubMed] [Google Scholar]
- Ye G-N, Colburn S, Xu CW, Hajdukiewicz PTJ, Staub JM (2003) Persistence of unselected transgenic DNA during a plastid transformation and segregation approach to herbicide resistance. Plant Physiol 133: 402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G-N, Daniell H, Sanford JC (1990) Optimization of delivery of foreign DNA into higher plant chloroplasts. Plant Mol Biol 15: 809–819 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Sakaiya M, Isono K, Kobayashi H (2001) Comparison of strength of endogenous and exogenous gene promoters in Arabidopsis chloroplasts. Plant Biotechnology 18: 135–142 [Google Scholar]