Abstract
This pilot study evaluated a reinforcement intervention to improve adherence to antihypertensive therapy. Twenty‐nine participants were randomized to standard care or standard care plus financial reinforcement for 12 weeks. Participants in the reinforcement group received a cell phone to self‐record videos of adherence, for which they earned rewards. These participants sent videos demonstrating on‐time adherence 97.8% of the time. Pill count adherence differed significantly between the groups during treatment, with 98.8%±1.5% of pills taken during treatment in the reinforcement condition vs 92.6%±9.2% in standard care (P<.002). Benefits persisted throughout a 3‐month follow‐up, with 93.8%±9.3% vs 78.0%±18.5% of pills taken (P<.001). Pill counts correlated significantly (P<.001) with self‐reports of adherence, which also differed between groups over time (P<.01). Systolic blood pressure decreased modestly over time in participants overall (P<.01) but without significant time‐by‐group effects. These results suggest that reinforcing medication adherence via cellular phone technology and financial reinforcement holds potential to improve adherence.
Treatment of high blood pressure (BP) depends largely on the use of antihypertensive medications but many patients fail to adhere to treatment.1 Despite decades of research and clinical care guidelines directed toward improving adherence,2 no methods have been found to reliably or markedly improve medication adherence.3, 4
A novel approach to improving adherence involves reinforcement interventions that incorporate principles of behavioral psychology and behavioral economics. Simply put, a behavior that is reinforced will increase in frequency.5 Some trials have found benefits of applying financial reinforcement toward improving medication adherence.6, 7, 8, 9 In a meta‐analyses of all published studies applying financially based reinforcement for medication adherence,10 reinforcement interventions significantly improved adherence relative to control conditions with an overall medium to large effect size of d=0.77 (95% confidence interval, 0.70–0.84). Consistent with behavioral and economic principles, interventions that were longer in duration provided greater financial reinforcers, and reinforced patients more often and more proximally to medication taking resulted in larger effect sizes than those that were shorter, provided lower reinforcement amounts, and reinforced patients less frequently. Typically, in these studies, medical personnel delivered the reinforcement when patients took medication in specialized clinics that treat tuberculosis, hepatitis, psychosis, or substance use disorders.
For medications with once‐ or twice‐daily dosing regimens, such as antihypertensive medications, it is unlikely that patients would be willing or able to travel daily to a clinic to ensure dosing. To assess adherence in the patient's own environment, a few studies have reinforced openings of electronic or medication event monitoring system (MEMS) caps.6, 7, 8 These systems, however, are limited, because electronic caps do not ensure that the participant actually ingests the pill, and participants have disclosed opening the bottle without taking the medication.7
This pilot study employs a novel technique using cell phone technology to monitor and reinforce participants for on‐time medication adherence in their natural environments. Advantages of cell phones are that, similarly to MEMS caps, they provide time‐ and date‐stamped indices of adherence, but, unlike MEMS caps, they allow for observation of medication ingestion. Further, cell phones can reinforce adherence in real time—nearly immediately after ingesting the pill and sending a video. The hypothesis was that participants who were reinforced for video recording on‐time medication ingestion would increase adherence relative to participants who were not reinforced for adherence. Effect sizes of the intervention for reducing systolic BP were estimated, because this pilot project was not powered to detect changes in BP.
Methods
Participants were recruited from advertisements and primary care referrals. Inclusion criteria were age 18 years and older; prescribed antihypertensive medication for ≥3 months and not anticipating changes in doses or medications; self‐reported missing doses in past month; and systolic BP ≥120 mm Hg or diastolic ≥80 mm Hg. Exclusion criteria were uncontrolled psychiatric disorders, significant cognitive impairment, or non–English‐speaking.
Individuals who appeared to meet criteria were invited to an evaluation, and informed consent was obtained, as approved by the university's institutional review board. Participants brought in pill bottles to the baseline and all subsequent study visits. Pill counts were reconciled with refill histories to determine percentage taken between evaluations.11 The Morisky Medication Adherence Scale12 was administered as a secondary adherence measure at all visits, and BP was measured using a semiautomatic digital device (Omron, Lake Forest, IL). Seated BP was taken in the nondominant arm at least twice, 3 minutes apart, until consecutive readings were within 5 mm Hg; the last two readings were averaged. All participants (regardless of treatment assignment) received $25 for completing the baseline, month 1, and month 2 evaluations, and $50 for the month 3 (post‐treatment) and month 6 (follow‐up) evaluations ($175 total). Completion rates exceeded 98% for each of the four post‐baseline evaluations (Figure 1).
Figure 1.
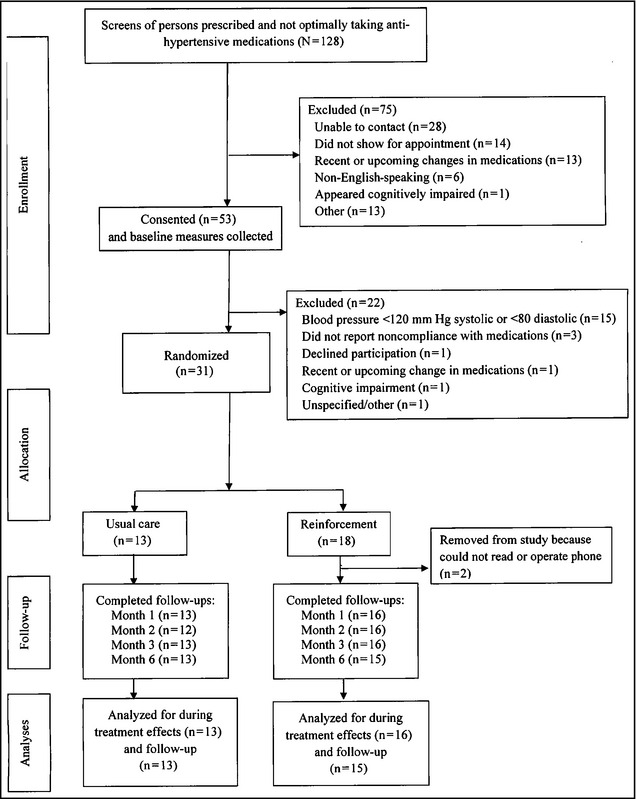
The flow of participants from the point of initial contact through data analysis is presented per Consolidating Standards of Reporting Trials (CONSORT) guidelines.
A computerized program randomly assigned participants to one of two conditions.
Standard care participants (n=13) were instructed to see their physician as usual. Additionally, research assistants conducted a 30‐minute session on improving medication adherence using a structured handout.
Patients in the standard care+reinforcement group received the care above, including the 30‐minute adherence session. They were also provided LGPrime GoPhones (AT&T Inc, Dallas, TX). Research assistants taught participants to press the record button, display bottle(s) and pill(s) to the phone's camera, place pill(s) into mouth, swallow, open mouth, press stop, and send the recording. The process took about 3 minutes. Once comfortable practicing (30–60 minutes), participants were instructed to record taking medication(s). Two individuals could not operate phones (eg, illiterate) and were withdrawn from the study (leaving 16 participants).
For 12 weeks, participants earned $0.50 each time they recorded medication ingestion within their dosing window (±2 hours). For each full day of adherence, they earned bonuses, starting at $1 and increasing by $0.50/d for consecutive days, up to a maximum of $5/d. A missed or late recording reset bonuses to $1. Participants on once‐daily schedules could earn up to $5.50/d and $444 total ($6/d and $468 total for twice‐daily). Most participants (83.8%) were taking one pill per day.
Personnel reviewed and validated recordings at least daily on workdays. For blurry recordings, personnel phoned participants to encourage improved recordings. Occasional problems did not reset earnings, but repeated problems did. Once validated, research assistants sent messages with earnings, eg, “Great job! You earned $___ for taking your med on time today, and $____ to date. You can earn up to $__ for taking meds tomorrow.” Messages were sent within 30 minutes of receiving videos on days 1 to 7 and once daily thereafter.
During week 1, a research assistant phoned participants to remind them of dosing windows and reinforcement possibilities, if a video had not been received within an hour of their dosing time. No reminders were sent thereafter.
Beyond the reinforcers, other costs associated with the intervention included 30 minutes of personnel time to review medication adherence (both groups), plus an average of 45 minutes of personnel time to train these participants on how to record and send videos of medication adherence. Personnel spent an average of about 2 hours in week 1 communicating with participants regarding videos, and thereafter <10 minutes per day reviewing videos and sending reinforcement texts (total personnel time is estimated at 15 hours per participant over 12 weeks, or $300 in personnel salary to provide this intervention to each participant). Phones incurred a $40/mo charge for usage ($120 over 12 weeks).
Data Analyses
Pill counts were the primary adherence measure. Repeated‐measures analysis of variance evaluated group effects during treatment (months 1, 2, and 3) and through month 6 for follow‐up. For missing data during treatment, data from most proximal evaluations were averaged; missing 6‐month data were dropped from follow‐up analyses. Self‐reported proportions of doses from the past month were compared with baseline throughout treatment and follow‐up, examining time and time‐by‐group interaction effects. Similar analyses evaluated changes in BP. Cohen's d effect sizes were calculated for change from baseline during treatment and at follow‐up.
Results
Participants, on average, were aged 50.4±11.0 years, 55.2% were women, and members of racial/ethnic minority groups comprised 62.1% of the sample. Although the sample size precludes detection of all but large between‐group differences, groups had similar baseline characteristics (Table). Pill counts in the month before study participation could not be calculated at baseline, but self‐reported adherence did not differ between groups (P=.32).
Table 1.
Demographics and Baseline Characteristics by Treatment Groups
| Variable | Standard Care (n=13) | Reinforcement (n=16) | Statistic (df) | P Value |
|---|---|---|---|---|
| Age, y | 52.1±9.4 | 49.0±12.3 | t (27)=−0.74 | .46 |
| Years of education | 13.6±2.6 | 13.8±2.6 | t (27)=0.14 | .89 |
| Income | $18,770±$20,552 | $18,338±$23,285 | t (27)=−0.05 | .96 |
| Systolic blood pressure | 131.1±10.1 | 133.2±12.5 | t (27)=0.50 | .62 |
| Diastolic blood pressure | 84.4±7.7 | 83.2±8.0 | t (27)=−0.40 | .70 |
| Past month adherence | 80.2%±21.5% | 72.3%±19.7% | t (27)=−1.02 | .32 |
| Past month on‐time adherence | 64.5%±27.2% | 60.5%±26.3% | t (27)=−0.40 | .69 |
| Female, % (No.) | 53.8 (7) | 56.3 (9) | χ2 (1)=0.02 | .90 |
| Race/ethnicity, % (No.) | ||||
| Hispanic American | 7.7 (1) | 12.5 (2) | χ2 (4)=2.22 | .70 |
| African American | 46.2 (6) | 43.8 (7) | ||
| European American | 46.2 (6) | 31.3 (5) | ||
| Native American | 0.0 (0) | 6.3 (1) | ||
| More than one race | 0.0 (0) | 6.3 (1) | ||
| Other medical conditions, % (No.) | ||||
| Arthritis | 38.5 (5) | 18.8 (3) | χ2 (1)=1.40 | .24 |
| Asthma | 7.7 (1) | 18.8 (3) | χ2 (1)=0.74 | .39 |
| Diabetes | 23.1 (3) | 18.8 (3) | χ2 (1)=0.08 | .78 |
Values are expressed as means±standard deviations unless otherwise indicated.
Participants in the reinforcement condition sent in 99.3%±1.6% of expected videos, and 97.8%±2.7% were recorded on time (±2 hours). Seven participants received ≥1 reminder in week 1 only. Of >1300 videos received, just nine (<0.7%) were of poor quality (blurry, could not see inside mouth to confirm swallowing of pill), and only two reset bonuses because of recurrent issues. On average, participants earned $408±$43 during the 12‐week reinforcement period.
Pill count adherence is shown in Figure 2 (top). Pill count adherence was higher in the reinforcement condition during treatment (F 1,25=11.57, P<.002) and follow‐up (F 1,20=15.36, P<.001) with effect sizes d=0.94 and 1.08, respectively, reflecting that >82% of control participants adhered below the average of participants in the reinforcement group.
Figure 2.
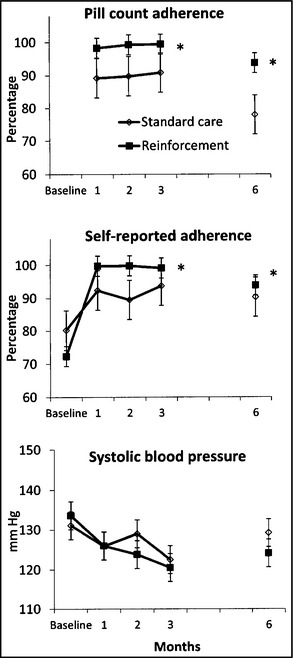
(Top). Pill counts of adherence to antihypertensive medication in the past 30 days during the 3‐month treatment period and prior to the 6‐month follow‐up. Values represent group means and standard errors. The * indicates that the group effect is significant (P<.01) throughout treatment and follow‐up. (Middle). Self‐reported proportion of days adherent to antihypertensive medication in the past 30 days from baseline throughout treatment and at follow‐up. Self‐reported adherence was assessed by the Morisky Medication Adherence Scale.12 Values represent group means and standard errors. The * indicates that the time‐by‐group effect is significant (P<.01) throughout treatment and follow‐up. (Bottom). Systolic blood pressure (mm Hg) measured at baseline, throughout treatment, and at follow‐up. Values represent group means and standard errors.
Correlations between past‐month pill counts and self‐reported adherence ranged from r=0.63 to 0.73 (P<.001), and Figure 2 (middle) shows proportions of self‐reported doses monthly. Relative to baseline, self‐reported adherence increased during treatment (F 1,27=23.57, P<.001) and follow‐up (F 1,26=19.90, P<.001) in the sample as a whole. The time‐by‐group effect was significant throughout treatment (F 1,27=11.31, P<.01) and follow‐up (F 1,26=9.97, P<.01), with a very large effect size (d=1.12) during treatment and small‐to‐medium at follow‐up (d=0.37). These effect sizes indicate that >86% of controls adhered less than the average of reinforcement participants during treatment and >66% at follow‐up.
Relative to baseline, reductions in BP occurred during treatment (Figure 2, bottom panel), (F 1,27=9.97, P<.01) and follow‐up (F 1,25=7.08, P<.01) in the sample as a whole with no significant differences between groups (F 1,27=0.50, P=.48 and F 1,25=2.16, P=.15, respectively). Effect sizes were d=0.29 and d=0.66, reflecting a mean reduction from baseline of 8.1±10.0 mm Hg during treatment and throughout follow‐up in reinforced participants vs a mean decrease relative to baseline of 5.1±11.1 mm Hg during treatment and 1.8±10.6 mm Hg at follow‐up in control participants.
Discussion
This reinforcement intervention increased verified on‐time medication adherence to >97% throughout 3 months of treatment, with benefits persisting throughout follow‐up. These data suggest that reinforcement can quickly boost adherence. Once patients learn to integrate medication taking into daily routines, many maintain adherence, even without reinforcement.
Although effective in improving adherence, the intervention did not significantly impact BP. Some participants may not have been on optimal therapeutic regimens, thus blunting the impact of improved adherence on BP. Nevertheless, the effect size at follow‐up suggests that reinforcement interventions may lead to health benefits, as shown in larger trials reinforcing adherence to other medication types.7 Similar benefits with increased adherence to antihypertensive therapy cannot be assumed unless larger trials reveal clinically significant reductions in BP in participants with reinforced vs non‐reinforced treatment.
Study Limitations and Strengths
Findings from this study should be interpreted in light of its limitations. This was a pilot study, including only a small number of participants that precluded the ability to detect all but large group differences. Although multiple adherence indices were included and correlated, MEMS caps might have provided an additional index. Some benefits persisted after removal of the reinforcers, but the follow‐up period was only 3 months. Further, this study arranged for about $450 in reinforcers. Although this amount may be considered large, it is less than half used in other studies successfully reinforcing antiretroviral medication adherence,7, 8 but those medications have more significant side‐effect profiles, perhaps requiring greater financial reinforcement to boost adherence. Given the benefits achieved in this study, lower amounts or probabilistic earnings9, 13 may also improve adherence for antihypertensive medications.
Cellular phones allowed for immediate reinforcement of adherence, a critical feature of successful reinforcement interventions,13 and the video function on standard cell phones has advantages over other technology in these regards. Perhaps because of these features, this study produced nearly perfect adherence in all individuals randomly assigned to the reinforcement condition. However, adherence also increased in the standard intervention, and attention differed between groups. Thus, specific aspects of the reinforcement intervention that engendered benefits require greater study.
Reinforcing patients is highly controversial. Opponents note that these interventions may label individuals as noncompliant, hold potential to be applied unfairly, and unintentionally encourage individuals with healthy behavior patterns to adopt unhealthy ones to access reinforcers. Reinforcers may be perceived as coercive in low‐income and disadvantaged populations, and the converse of reinforcement may be unfair, ie, penalizing individuals for not taking medications. Concerns also relate to paying people to “do what they should do anyway.” However, the behavioral economics literature finds that even highly motivated patients have difficulty in making decisions in the short‐term that represent their long‐term best interests.14 Reinforcement can “jump start” optimal decisions and create an ingrained behavior pattern of adherence. Providing reinforcers to individuals who are perceived as not responsible for their condition are viewed most favorably,15 as may be the case for hypertension. Further, small increases in the effectiveness of reinforcement interventions can substantially increase their public acceptability.16 Clearly, ethical and practical concerns require careful consideration prior to wide‐scale implementation, but data from this study suggest that a cell phone reinforcement intervention holds promise for improving antihypertensive medication adherence.
Future research with larger samples is needed to determine whether reinforcing adherence is cost‐effective. Reinforcing adherence may be particularly appropriate for high‐risk patients who are initiating pharmacotherapy for the first time. If adherence is ensured initially, doses and medications can be better titrated or adjusted to achieve optimal clinical benefits. Reinforcing medication adherence may also be a method to distinguish patients with truly resistant hypertension from those who are nonadherent. Reinforcing adherence may even be cost‐effective on a long‐term basis. Poor adherence increases the cost of treating hypertension by 15% to 20%, and it is associated with more frequent hospitalization, use of emergency services, and admission to intensive care.17 Thus, from the standpoint of payors, especially in closed healthcare systems,18 the costs of treating complications associated with nonadherence could outweigh those associated with reinforcing adherence.
For these reasons, the Veterans Administration has begun applying reinforcing interventions in the treatment of substance use disorders nationwide,19 suggesting potential for large‐scale dissemination of these interventions in other contexts as well. Ultimately, insurers or society may be willing to pay for interventions that reinforce adherence to medications for which nonadherence is associated with significant medical costs or public health consequences, as is the case for tuberculosis, hepatitis, and HIV treatment.10, 20 Reinforcing preventive efforts may be preferable in other contexts.21, 22, 23 The National Business Group on Health24 has reported that 85% of employers use incentives to encourage health behavior, and section 2705 of the Affordable Care Act allows employers to use up to 50% of total premiums for penalties or rewards tied to outcome‐based incentives in areas including smoking, obesity, cholesterol, and BP. These policies and procedures suggest that ultimately there will be adoption of interventions reinforcing adherence to antihypertensive medications or to preventive efforts such as increasing ambulatory activities21 or weight loss.22, 23
Conclusions
Larger‐scale studies are needed to replicate and extend these findings and better elucidate their costs‐benefits. The reasons for poor medication adherence are multifactorial and complex,25 but this study provides one of the first demonstrations of an intervention with a robust effect in improving medication adherence to antihypertensive medications.
Financial Disclosures
The research and preparation for this report was supported in part by National Institutes of Health grants P30‐DA023918, R01‐HD075630, R01‐DA024667, R01‐DA027615, P50‐DA09241, R01‐AG022092, and T32‐AA07290. The authors report no conflicts of interest to disclose relative to this research.
J Clin Hypertens (Greenwich). 2015;17:33–38. DOI: 10.1111/jch.12441. © 2014 Wiley Periodicals, Inc.
References
- 1. Sabate E. Adherence to Long‐Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 2. Hill MN, Miller NH, DeGeest S; American Society of Hypertension Writing Group . ASH position paper: adherence and persistence with taking medication to control high blood pressure. J Clin Hypertens (Greenwich). 2010;12:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. [DOI] [PubMed] [Google Scholar]
- 5. Ferster CB, Skinner BF. Schedules of Reinforcement. New York, NY: Appleton‐Century Crofts; 1957. [Google Scholar]
- 6. Rigsby MO, Rosen MI, Beauvais J, et al. Cue‐dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosen MI, Dieckhaus K, McMahon T, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21:30–40. [DOI] [PubMed] [Google Scholar]
- 8. Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV‐positive methadone patients. Drug Alcohol Depend. 2007;88:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: findings from a meta‐analysis. Am J Med. 2012;125:888–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. [DOI] [PubMed] [Google Scholar]
- 12. Morisky DE, Ang A, Krousel‐Wood M. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Petry NM. Contingency Management: A Guide to Implementing This Evidence‐Based Practice. New York, NY: Routledge; 2012. [Google Scholar]
- 14. Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298:2415–2417. [DOI] [PubMed] [Google Scholar]
- 15. Promberger M, Brown RC, Ashcroft RE, Marteau T. Acceptability of financial incentives to improve health outcomes in UK and US samples. J Med Ethics. 2011;37:682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Promberger M, Dolan P, Marteau TM. “Pay them if it works”: discrete choice experiments on the acceptability of financial incentives to change health related behaviour. Soc Sci Med. 2012;75:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. [DOI] [PubMed] [Google Scholar]
- 18. Naito NA, Higgins ST. Controlling health care costs in the military: the case for using financial incentives to improve beneficiary personal health indicators. Prev Med. 2012;55:S113–S115. [DOI] [PubMed] [Google Scholar]
- 19. Petry NM, DePhilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: the Veterans Administration initiative. Am J Addict. 2014;23:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weaver T, Metrebian N, Hellier J, et al. Use of contingency management incentives to improve completion of hepatitis B vaccination in people undergoing treatment for heroin dependence: a cluster randomised trial. Lancet. 2014;384:153–163. [DOI] [PubMed] [Google Scholar]
- 21. Petry NM, Andrade LF, Barry D, Byrne S. A randomized study of reinforcing ambulatory exercise in older adults. Psychol Aging. 2013;28:1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petry NM, Barry D, Pescatello L, White WB. A low‐cost reinforcement procedure improves short‐term weight loss outcomes. Am J Med. 2011;124:1082–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volpp KG, John LK, Troxel AB, et al. Financial incentive‐based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Business Group on Health . (2013). http://www.businessgrouphealth.org/resources/library/index.cfm. Accessed September 12, 2014.
- 25. AlGhurair SA, Hughes CA, Simpson SH, Guirguis LM. A systematic review of patient self‐reported barriers of adherence to antihypertensive medications using the World Health Organization multidimensional adherence model. J Clin Hypertens (Greenwich). 2012;14:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]


