Abstract
Objectives
Several reports have demonstrated a relationship between second to fourth digit ratio (2D:4D) and facial shape, suggesting that prenatal sex hormones play a role in the development of the craniofacial complex. Using 3D surface imaging and geometric morphometrics, we test the hypothesis that decreased digit ratio (indicative of increased prenatal androgen exposure) is associated with a more masculine facial phenotype.
Methods
3D facial surface images and digit measures were collected on a sample of 151 adult males. Facial landmarks collected from the images were aligned by Procrustes superimposition and the resulting shape coordinates regressed on 2D:4D. Variations in facial shape related to 2D:4D were visualized with deformable surface warps.
Results
A significant statistical relationship was observed between facial shape variation and 2D:4D (p = 0.0084). Lower 2D:4D ratio in adult males was associated with increased facial width relative to height, increased mandibular prognathism, greater nasal projection, and increased upper and lower lip projection.
Conclusions
A statistical relationship between 2D:4D and facial shape in adult males was observed. Faces tended to look more masculine as 2D:4D decreased, suggesting a biologically plausible link between prenatal androgen exposure and the development of male facial characteristics.
Keywords: digit ratio, face shape, geometric morphometrics, sex hormones
Introduction
Hormones are among the most important factors influencing facial form in humans. The differential action of testosterone and estrogen in males and females throughout the life-span is ultimately responsible for the sexual dimorphism apparent in the craniofacial complex, whether through direct action on bone and cartilage or by affecting the growth of associated soft tissue functional matrices (muscles and organs). Although reduced in humans compared to other primates, sexual dimorphism is still one of the principal sources of facial shape variation in modern humans (1–3). Although most apparent in adults, the influence of sex hormones on facial morphology likely begins early in life; sex differences in mandible shape have been reported in infant osteological samples (4). Such morphological sex differences are typically attributed to the differential effects of endogenous postnatal hormones and/or the action of genes residing on the sex chromosomes. However, prenatal exposure to exogenous sex hormones of maternal origin may also be an important factor. The degree to which prenatal sex hormone exposure influences postnatal patterns of facial growth in humans is still largely unclear.
Because direct access to prenatal sex hormone exposure is impossible in most scenarios, the ratio of second digit to fourth digit length (2D:4D) is often used as a postnatal phenotypic proxy (5). There is a large body of evidence linking 2D:4D to the relative amounts of prenatal testosterone and estrogen available during development (6). Lower 2D:4D, typically observed in males, tends to be associated with a high-testosterone/low-estrogen prenatal environment. The 2D:4D phenomenon can be explained mechanistically by the disproportionately high number of androgen and estrogen receptors present within the developing fourth digit (7); depending on the balance of prenatal sex hormones, chondrocyte proliferation in the fourth digit will be either disproportionately accelerated or decreased relative to other digits. Accordingly, a large number of correlational studies have used 2D:4D to evaluate the influence of prenatal sex hormones on a wide variety of human and non-human traits. To date, only a handful of studies have focused on facial shape.
Using standard frontal photographs, Fink et al. (8) were the first to explore the relationship between 2D:4D and facial shape in a sample of adults. They reported that lower 2D:4D (indicative of a high-testosterone prenatal environment) was associated with a brachycephalic facial configuration, characterized by increased breadth relative to length. This pattern was particularly apparent in the lower portion of the face and was roughly similar in both males and females. In a separate follow-up study, Meindl et al. (9) explored this same relationship in a sample of pre-pubertal boys; they found a pattern similar to adults, with the face becoming more robust and ‘masculine’ as the digit ratio decreased. Conversely, Burriss et al. (10) found relatively no evidence that 2D:4D correlates with specific measurements of the lips, nose, and jaw in adult males; in females, the correlation was limited to nasal width. While these are promising results, all of the aforementioned studies are hampered by data acquisition methods that are incapable of fully capturing the complex 3D geometry of the human face. Two-dimensional photography can significantly distort facial structures and limits the ability to simultaneously quantify facial structures in multiple axes. As a result, a number of facial features suspected to be under the influence of prenatal sex hormones have been incompletely represented in prior studies.
Recent advances in 3D surface imaging technology have now made it feasible to capture and quantify the human face in large numbers of individuals (11). The focus of the current study is to explore the relationship between 2D:4D and facial shape, using a combination of 3D facial surface imaging and geometric morphometrics. We predict, based on prior reports, that low 2D:4D in adult males will be associated with a more euryprosopic (short, wide) facial appearance. We also predict that mandibular prognathism, a trait typically associated with masculine facial appearance, will increase as 2D:4D decreases.
Materials and methods
Study sample
A sample of 151 adult male individuals participated in this study. These participants represent a subset of subjects recruited as part of a larger study of normative craniofacial variation (12). Additional information about the parent study can be found at: https://www.facebase.org/facial_norms. All subjects included in this study were between 18 and 40 years of age, were of recent western European derivation (self-reported), and were unrelated. The mean age of the male sample was 26.55 years (SD = 6.16). Exclusion criteria for participating in the study included any history of craniofacial surgery or head trauma or any personal/family history of any condition that might affect the structure of the head or face. Individuals with conspicuous injuries to the digits under observation and individuals with facial hair were also excluded, as the latter can interfere with 3D facial imaging and the identification of facial landmarks. All aspects of this study received institutional ethics approval.
Digit measurements
The second and fourth digits were measured on the left and right hand of each subject directly with 150-mm sliding calipers (GPM, Switzerland). Each digit measurement was taken in a uniform manner. Subjects were asked to extend their digits until straight, but not over hyperex-tended, in the palm-up position. The tip of one arm of the caliper was then placed at the crease demarcating the metacarpophalangeal joint of the second digit; if there was more than one crease, the proximal-most crease was used. The tip of the other arm of the caliper was then made to contact the central portion of the distal tip of the distal phalanx. Care was taken not to use too much pressure so as to deform the soft tissue. The process was repeated for the 4th digit. The direct measurement of digits has been shown to have a high degree of precision in previous studies (13,14). The 2D:4D ratio was calculated from the raw measurements. Calculated ratios from the left hand were included in the present analysis, as previous reports have shown similar patterns of facial shape both hands (8).
Image acquisition and landmark collection
3D facial surface images were captured using a 3dMD imaging system (3dMD, Atlanta GA), using established protocols (15). The 3dMD system uses digital stereophotogrammetry to capture the geometry and skin texture of faces in less than 2 ms (16). The resulting point cloud is typically comprised of 20 000–40 000 points. Measurements derived from 3dMD facial captures have been independently tested for precision and accuracy (17,18).
Each 3D facial surface was cleaned by removing extraneous surface data from the neck and scalp hair. The 3D surfaces were then landmarked by a single trained investigator using 3dMDvultus software. Twenty-two standard facial landmarks were included (Fig. 1). These landmarks are well defined in the anthropometric literature (19) and have been shown to demonstrate low operator error. The x, y, and z coordinate locations associated with the landmarks were saved for subsequent shape analysis.
Fig. 1.
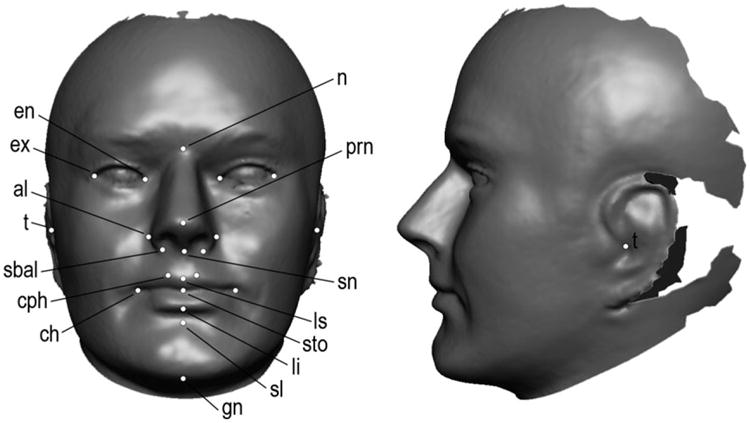
Example of a 3D facial surface with anatomical landmarks identified. Landmark abbreviations: n (nasion); prn (pronasale); sn (subnasale); ls (labiale superius); sto (stomion); li (labiale inferius); sl (sublabiale); gn (gnathion); ch (chelion) cph (crista philtri); sbal (subalare); t (tragion); al (alare); ex (exocanthion); en (endocanthion).
Geometric Morphometric Analysis
The x, y, and z coordinates were imported into the morphometrics analysis program MorphoJ v1.05f (20). A Procrustes superimposition was performed in order to align the individual landmark configurations to one another, resulting in a new set of coordinates representing shape. The relationship between left digit ratio and facial shape for each sex was assessed using regression, with the shape coordinates as the dependent variable and left 2D:4D ratio as the independent variable. To facilitate visualization, the shape changes associated with the regression vector were imported into the program Landmark v3.6 (UC Davis; http://graphics.idav.ucdavis.edu/research/projects/EvoMorph) to generate surface warps.
Results
The mean left 2D:4D in our adult males sample was 0.98 (SD = 0.04). Regression analysis revealed that 1.7% of facial shape variation was accounted for by left 2D:4D. This relationship was determined to be statistically significant using a nonparametric permutation test (5000 resamples; p-value = 0.0084).
The nature of the facial shape variation associated with 2D:4D is modeled as 3D surface morphs in Fig. 2. In frontal view, lower 2D:4D ratio was associated with an overall increase in facial width relative to height. In profile, lower 2D:4D was associated with increased mandibular prognathism, greater nasal projection, increased upper and lower lip projection, a higher position of the labiomental sulcus, and a greater facial depth (as measured from the central midface to the landmark tragion). The reverse trends were apparent for facial traits associated with higher 2D:4D. Despite the overall tendency toward shorter and wider faces, lower 2D:4D was also associated with a relative lengthening and narrowing of the philtrum; conversely, a shorter and wider philtrum was apparent with higher 2D:4D in combination with the overall narrower and longer face.
Fig. 2.
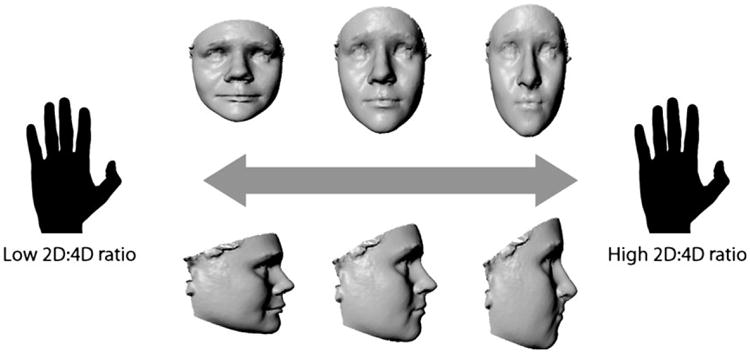
The relationship between 2D:4D and facial shape represented as 3D surface warps. The center image in the top and bottom row represents an average face from our sample. The facial models on either end of the continuum represent deformations based on the regression of 2D:4D on the shape coordinates. The surface warping was created using the program Landmark v3.6 (see text for URL) by applying deformation vectors derived from the shape regression of 22 landmarks to the entire facial surface.
Discussion
The present results suggest that certain features that define the male face are influenced by androgens present during prenatal development. Our results show that within a sample of adult males, digit ratio covaries with specific aspects of facial shape. As predicted, low 2D:4D was associated with an overall tendency toward a relatively euryprosopic facial appearance. This finding is in partial agreement with several prior reports (8,9,21), where the overall shape of the face tended to become broader as 2D:4D decreased. These earlier studies, however, focused much more heavily on the lower third of the face compared with the present analysis, where the breadth increase was observed in the middle and upper portions of the face. Within these individual facial regions, the picture was slightly more complex. For example, at the same time the overall face increased in relative breadth, the philtrum became both narrower and longer as 2D:4D decreased. This suggests that prenatal androgens may influence facial development in a modular manner, with discrete effects on different anatomical components of the face.
One major advantage of the present study is the use of 3D facial imaging methods, which allow us to simultaneously model shape in more than one axis. When shape was modeled with the face viewed in profile, low 2D:4D was associated with a number of shape changes not described in previous studies, which were limited to 2D frontal photographs. Men with lower 2D:4D tended to have more protrusive mandibles, noses, and lips. These facial features have been associated with more ‘masculine’ appearance (2,22,23), supporting the role of androgens. The co-occurrence of mandibular protrusion with brachycephalic head/face shape is also well documented in the orthodontic literature (24), suggesting underlying changes within the cranial base may be driving this pattern of integration. Unfortunately, in the present study, our data were limited to the facial surface.
Many of the shape features associated with 2D:4D are similar to aspects of facial shape that distinguish males from females. Increased mandibular prominence, nasal projection, and facial breadth are well-known facial feature distinguishing adult males from females (1,2,22–25); that these features are also associated with lower 2D:4D suggests that prenatal androgen exposure may partly explain their sexually dimorphic pattern of expression. In another recent study, Veleminská et al. (23) reported that male faces were characterized by a tendency toward hypotelorism (reduction in the distance between the eyes) once the effect of size was removed. Our data suggest that within males, there was a tendency toward hypertelorism with lower 2D:4D. It is important to remember that sexual dimorphism in facial shape is the net result of multiple factors compounded over the lifespan interacting in complex ways. Prenatal hormone exposure is only one such factor; others include circulating levels of postnatal hormones (most apparent after puberty) and the differential effects of genes residing on sex chromosomes. An appreciation of how all of these factors work in concert to influence variation in facial shape will be required to gain a more complete understanding of the origin of sex differences in the human face.
Clinical relevance.
Understanding the multitude of factors that underlie variation in facial form in humans is of practical interest to clinicians who treat patients with dentofacial deficiencies and malformations. Endocrine factors have long been recognized as exerting an influence on growing craniofacial tissues. We typically think of hormones as playing a significant role during puberty, when craniofacial sex differences become most apparent. This study examines another source of endocrine influence: exposure to exogenous maternal hormones during development. We show in this study that prenatal hormone exposure (as measured by digit ratio) is associated with specific patterns of facial shape in adult males.
Acknowledgments
The authors would like to thank Rebecca DeSensi for help with database management and Carrie Heike, Michael Cunningham, Erik Stuhaug, Linda Peters, Laura Steuckle, Trylla Tuttle, and Eden Palmer for their help with recruitment and data collection. Funding for this project was through a grant from the National Institute of Dental and Craniofacial Research: U01-DE020078.
Contributor Information
S. M. Weinberg, Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Department of Anthropology, Dietrich School of Arts and Sciences, University of Pittsburgh, Pittsburgh, PA, USA
T. E. Parsons, Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA
Z. D. Raffensperger, Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA
M. L. Marazita, Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA
References
- 1.Bulygina E, Mitteroecker P, Aiello L. Ontogeny of facial dimorphism and patterns of individual development within one human population. Am J Phys Anthropol. 2006;131:432–43. doi: 10.1002/ajpa.20317. [DOI] [PubMed] [Google Scholar]
- 2.Toma AM, Zhurov A, Playle R, Richmond S. A three-dimensional look for facial differences between males and females in a British-Caucasian sample aged 151/2 years old. Orthod Craniofacial Res. 2008;11:180–5. doi: 10.1111/j.1601-6343.2008.00428.x. [DOI] [PubMed] [Google Scholar]
- 3.Claes P, Walters M, Shriver MD, Puts D, Gibson G, Clement J, et al. Sexual dimorphism in multiple aspects of 3D facial symmetry and asymmetry defined by spatially dense geometric morphometrics. J Anat. 2012;221:97–114. doi: 10.1111/j.1469-7580.2012.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schutkowski H. Sex determination of infant and juvenile skeletons: 1. Morphognostic features. Am J Phys Anthropol. 1993;90:199–205. doi: 10.1002/ajpa.1330900206. [DOI] [PubMed] [Google Scholar]
- 5.Manning JT. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc Natl Acad Sci U S A. 2011;108:16143–4. doi: 10.1073/pnas.1113312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breedlove SM. Organizational hypothesis: instances of the fingerpost. Endocrinology. 2010;151:4116–22. doi: 10.1210/en.2010-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci U S A. 2011;108:16289–94. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink B, Grammer K, Mitteroecker P, Gunz P, Schaefer K, Bookstein FL, et al. Second to fourth digit ratio and face shape. Proc R Soc Lond B Biol Sci. 2005;272:1995–2001. doi: 10.1098/rspb.2005.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meindl K, Windhager S, Wallner B, Schaefer K. Second-to-fourth digit ratio and facial shape in boys: the lower the digit ratio, the more robust the face. Proc R Soc Lond B Biol Sci. 2012;279:2457–63. doi: 10.1098/rspb.2011.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burriss RP, Little AC, Nelson EC. 2D:4D and sexually dimorphic facial characteristics. Arch Sex Behav. 2007;36:377–84. doi: 10.1007/s10508-006-9136-1. [DOI] [PubMed] [Google Scholar]
- 11.Hammond P, Suttie M. Large-scale objective phenotyping of 3D facial morphology. Hum Mutat. 2012;33:817–25. doi: 10.1002/humu.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg SM, Marazita ML, Raffensperger ZD, Maher TW, Cuenco KT, Gandhi PM, et al. The 3D Facial Norms Project: a phenotypic and genotypic repository. J Dent Res. 2012;91(Spec Iss A):593. [Google Scholar]
- 13.Weinberg SM, Scott NM, Neiswanger K, Marazita ML. Intraobserver error associated with measurements of the hand. Am J Hum Biol. 2005;17:368–71. doi: 10.1002/ajhb.20129. [DOI] [PubMed] [Google Scholar]
- 14.Allaway HC, Bloski TG, Pierson RA, Lujan M. Digit ratios (2D:4D) determined by computer-assisted analysis are more reliable than those using physical measurements, photocopies, and printed scans. Am J Hum Biol. 2009;21:365–70. doi: 10.1002/ajhb.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heike CL, Upson K, Stuhaug E, Weinberg SM. 3D digital stereophotogrammetry: a practical guide to facial image acquisition. Head Face Med. 2010;6:18. doi: 10.1186/1746-160X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane C, Harrell W. Completing the 3-dimensional picture. Am J Orthod Dentofacial Orthop. 2008;133:612–20. doi: 10.1016/j.ajodo.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Aldridge K, Boyadjiev SA, Capone GT, DeLeon VB, Richtsmeier JT. Precision and error of three-dimensional phenotypic measures acquired from 3dMD photogram-metric images. Am J Med Genet Part A. 2005;138A:247–53. doi: 10.1002/ajmg.a.30959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg SM, Naidoo S, Govier DP, Martin RA, Kane AA, Marazita ML. Anthropometric precision and accuracy of digital three-dimensional photogrammetry: comparing the Genex and 3dMD imaging systems to one another and to direct anthropometry. J Craniofac Surg. 2006;17:477–83. doi: 10.1097/00001665-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Kolar JC, Salter EM. Craniofacial Anthropometry: Practical Measurement of the Head and Face for Clinical, Surgical and Research Use. Springfield: Charles C. Thomas; 1997. [Google Scholar]
- 20.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–7. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer K, Fink B, Mitteroecker P, Neave N, Bookstein FL. Visualizing facial shape regression upon 2nd to 4th digit ratio and testosterone. Coll Antropol. 2005;29:415–9. [PubMed] [Google Scholar]
- 22.Kau CH, Zhurov A, Richmond S, Cronin A, Savio C, Mallorie C. Facial templates: a new perspective in three dimensions. Orthod Craniofacial Res. 2006;9:10–7. doi: 10.1111/j.1601-6343.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 23.Velemínská J, Bigoni L, Krajíček V, Borský J, Šmahelová D, Cagáňová V, et al. Surface facial modelling and allometry in relation to sexual dimorphism. Homo. 2012;63:81–93. doi: 10.1016/j.jchb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Enlow DH. Facial Growth. Philadelphia: W.B. Saunders Company; 1990. [Google Scholar]
- 25.Bigoni L, Velemínská J, Brůzek J. Three-dimensional geometric morphometric analysis of cranio-facial sexual dimorphism in a Central European sample of known sex. Homo. 2010;61:16–32. doi: 10.1016/j.jchb.2009.09.004. [DOI] [PubMed] [Google Scholar]


