Abstract
Plant roots release about 5% to 20% of all photosynthetically-fixed carbon, and as a result create a carbon-rich environment for numerous rhizosphere organisms, including plant pathogens and symbiotic microbes. Although some characterization of root exudates has been achieved, especially of secondary metabolites and proteins, much less is known about volatile organic compounds (VOCs) released by roots. In this communication, we describe a novel approach to exploring these rhizosphere VOCs and their induction by biotic stresses. The VOC formation of Arabidopsis roots was analyzed using proton-transfer-reaction mass spectrometry (PTR-MS), a new technology that allows rapid and real time analysis of most biogenic VOCs without preconcentration or chromatography. Our studies revealed that the major VOCs released and identified by both PTR-MS and gas chromatography-mass spectrometry were either simple metabolites, ethanol, acetaldehyde, acetic acid, ethyl acetate, 2-butanone, 2,3,-butanedione, and acetone, or the monoterpene, 1,8-cineole. Some VOCs were found to be produced constitutively regardless of the treatment; other VOCs were induced specifically as a result of different compatible and noncompatible interactions between microbes and insects and Arabidopsis roots. Compatible interactions of Pseudomonas syringae DC3000 and Diuraphis noxia with Arabidopsis roots resulted in the rapid release of 1,8-cineole, a monoterpene that has not been previously reported in Arabidopsis. Mechanical injuries to Arabidopsis roots did not produce 1,8-cineole nor any C6 wound-VOCs; compatible interactions between Arabidopsis roots and Diuraphis noxia did not produce any wound compounds. This suggests that Arabidopsis roots respond to wounding differently from above-ground plant organs. Trials with incompatible interactions did not reveal a set of compounds that was significantly different compared to the noninfected roots. The PTR-MS method may open the way for functional root VOC analysis that will complement genomic investigations in Arabidopsis.
The current rise in global atmospheric CO2 concentration reinforces the need to improve our knowledge of the below-ground carbon cycle (Norby and Jackson, 2000; Woodward and Osborne, 2000). An understanding of the mechanisms that regulate the quantity and quality of carbon delivered beneath the ground is an essential prerequisite for predicting the ecosystem response to global climatic changes. Elevated CO2 generally stimulates primary biomass production (Curtis and Wang, 1998; Amthor, 2001), which suggests greater delivery of carbon to the soil through enhanced rhizodeposition (Rogers et al., 1999; Norby and Jackson, 2000). It is becoming clear that through the exudation of a wide variety of compounds, roots may regulate the soil microbial community in their immediate vicinity, cope with herbivores, encourage beneficial symbioses, change the chemical and physical properties of the soil, and inhibit the growth of competing plant species and communicate with other species (Nardi et al., 2000; Bais et al., 2002a, 2002b, 2003; Park et al., 2002). The chemicals released into the soil by roots are broadly referred to as root exudates. It is estimated that 5% to 20% of all photosynthetically fixed carbon is eventually transferred to the rhizosphere in this manner (Barber and Martin, 1976). Exudation represents a significant carbon cost to the plant, but a detailed characterization of these exudates and the mechanisms by which exudation occurs is only beginning to be undertaken. Root exudates include low Mr compounds like amino acids, organic acids, sugars, phenolics, and various secondary metabolites and high Mr compounds like mucilage and proteins (Roshina and Roshina, 1993). Although some chemical characterization of root exudates has been achieved for secondary metabolites, carbohydrates, and proteins (Bais et al., 2001; Knee et al., 2001; Park et al., 2002), much less is known about the volatile organic compounds (VOCs) released by roots.
In contrast, the large variety of VOCs emitted by the aerial parts of green plants has been described and characterized (Guenther et al., 2000; Isidorov and Jdanova, 2002), and recent efforts have revealed that such VOCs are indicators of diverse biological processes (Fall, 1999). For example, the leaves of plants are major emitters of methanol derived from cell wall synthesis, light-dependent emissions of isoprene or methylbutenol originating in chloroplasts, and monoterpenes released from resin ducts or glands. Leaves and stems damaged by herbivory or infectious microbes release a wide array of volatile wound compounds (Fall et al., 1999; Li et al., 2002). In addition, smaller amounts of volatile hormones are released by the above-ground portion of plants; these have been characterized as ethylene and derivatives of salicylic acid or jasmonic acid and serve roles in long-distance communication (Shulaev et al., 1997; Pichersky and Gershenzon, 2002). It is noteworthy that VOCs released by intact plants can be powerful indicators of underlying metabolic processes. For example, Van Poecke et al. (2001) demonstrated that the treatment of Arabidopsis plants with caterpillars leads to the appearance of several green leaf volatiles (i.e. wound compounds derived from hexanal and hexenal), plus volatile nitriles, the monoterpene myrcene, and methyl salicylate. The latter compounds were primarily produced in caterpillar-infested plants and not by physical wounding, and may serve as volatile attractants for parasitic wasps that attack the caterpillars. Numerous other recent examples of complex plant-insect interactions mediated through volatile signals have been reviewed by Gatehouse (2002). It is noteworthy that the sampling and analysis of plant VOCs by gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS) methods can be very time-consuming and until recently it has generally not been feasible to follow the kinetics of formation of VOCs following most kinds of biotic stresses. Here, we propose the use of a new tool to study real-time emission of VOCs in plant biology.
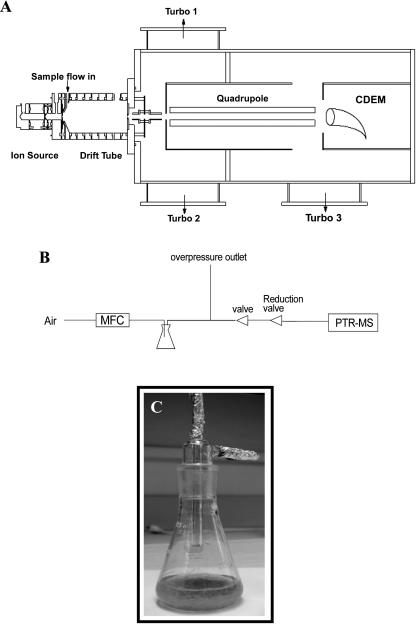
Proton-transfer-reaction mass spectrometry (PTR-MS) is a relatively new technique that has emerged as a useful tool for monitoring VOCs (for review, see Lindinger et al., 1998, 2001). In PTR-MS, the sample air is continuously pumped through a drift reactor, and the VOCs react with H3O+ ions that are added for this purpose from an ion source (Fig. 1, A and B). VOCs with a proton affinity higher than that of water (166.5 kcal mol−1), which includes most unsaturated and almost all oxygenated hydrocarbons, undergo a proton-transfer reaction with H3O+: H3O+ + R → RH+ + H2O. At the end of the drift tube reactor a fraction of the ions is sampled by a quadrupole mass spectrometer, which measures the H3O+ and RH+ ions. The ion signal at a certain mass is linearly dependent on the concentration of the precursor VOC in the sample air. In PTR-MS only the mass of VOCs is determined, which is a valuable but not a unique indicator of the identity of a VOC. Obviously the technique does not allow a separate detection of different VOCs with the same mass, and a further overlap of ion masses is caused by a limited degree of ion fragmentation and ion clustering in the drift tube. A weakness of the technique is therefore the identification of unknown VOCs in a sample. However, the strength of PTR-MS includes the monitoring of VOCs that are known to be present, with a much higher measurement frequency (one measurement per second) than allowed by any of its alternatives such as GC-MS (typically one measurement per 30 min). Also, the lack of sample treatment in PTR-MS allows the detection of species such as organic acids, peroxides, and doubly oxygenated species that are difficult to measure otherwise. PTR-MS is thus a more general detection method than for example GC-MS, in which different columns may be required to target different classes of compounds. The technique thus allows a rapid screening for the presence of VOCs: a single mass scan can indicate the relative abundance of VOCs and can provide important information about the identity, which can then be confirmed by alternative methods.
Figure 1.
A, Prototype of the PTR-MS used in our studies. Air is pumped through a drift tube by a mechanical pump. H3O+ ions are produced in a side arm by electron impact ionization of a He/H2O mixture. Organic trace gases are ionized in the drift tube by proton transfer reaction with H3O+, mass selected with a quadrupole mass detector and detected with a conversion dynode electron multiplier (CDEM). B, Schematic diagram of the gas inlet system for the PTR-MS used in analysis of Arabidopsis root VOCs. C, Prototype of the Erlenmeyer flask used to culture Arabidopsis roots and for the analysis of root-derived VOCs.
Since 1998, PTR–MS has been used in a large number of laboratory and field studies of the biogenic VOCs released from vegetation to the atmosphere. Some recent examples include the fluxes of VOCs from a subalpine forest (Karl et al., 2002a), emissions of VOCs from drying hay crops (Warneke et al., 2002), measurement of the kinetics of VOC release from leaves during light-dark transitions (Karl et al., 2002a), and the 13CO2 labeling of isoprene released from cottonwood and other leaves (Karl et al., 2002b).
The release of VOCs in the root zone of plants has not been the subject of detailed study, and it should be noted that very few studies of insect-induced root volatiles have been reported (Neveu et al., 2002). Similar to the above-ground scenario, we hypothesize that countering a potential challenge, roots may respond by secreting certain chemicals, such as VOCs, that could act as signaling agents in the air spaces in the root and soil zone. It is well known that some metabolic VOCs, such as acetaldehyde and ethanol, are formed in flooded roots (Drew, 1997), but the measurement of volatiles formed in roots subjected to biotic stresses has received very little attention. Our interest in roots in this study was 2-fold. First, the characterization of root volatiles may open new biological frontiers by identifying rhizospheric chemical-ecological interactions mediated by VOCs such as those that occur in the above-ground parts of the plant. Second, we hypothesize that the quantitative and qualitative emission of VOCs by roots, as measured by PTR-MS, may be significantly different than those released from the aerial parts of the plant, thus providing VOC signatures of biotic stresses to roots. In this communication, we have developed a new experimental system using PTR-MS to study the complete set of emitted VOCs from cultured roots of Arabidopsis. Because PTR-MS allows simultaneous real time analysis of most biogenic VOCs, we were able to follow the kinetics of induction of root-derived VOCs and related systemic VOCs after elicitation with biotic stresses.
RESULTS
PTR-MS and GC-MS Analysis of Root Volatiles
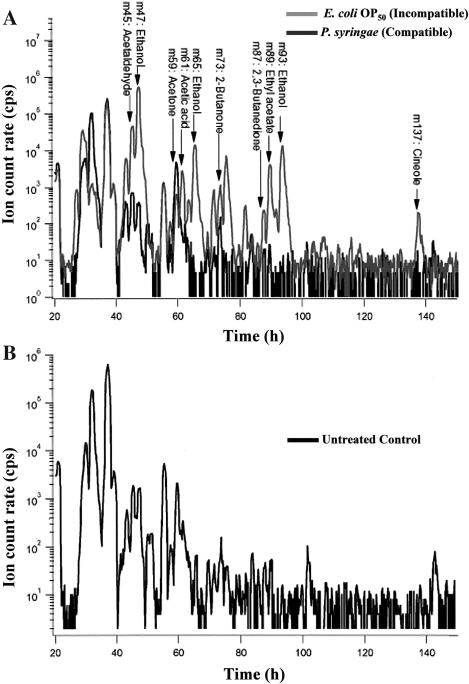
The VOC concentrations in the headspaces of the flasks containing Arabidopsis root cultures were measured on-line using PTR-MS; the experimental set-up is shown in Figure 1. The PTR-MS instrument is capable of rapid mass scans of VOCs in such air samples. Figure 2 shows a typical mass scan result obtained with microbe-infected roots. In order to identify the VOCs corresponding to these signals, GC-MS measurements were performed on headspace samples from parallel root cultures. In Table I, the major compounds induced upon microbial treatments that were identified using both GC-MS and PTR-MS are given together with their corresponding masses in PTR-MS. Most compounds are observed at more than one mass because of fragmentation of the parent ion (RH+) or clusters of the parent ion with water (RH+ × H2O) or their own neutral molecule (R × RH+). Typically, however, there is a unique, characteristic mass for a particular VOC. For Arabidopsis roots such marker masses included methanol (m33) (the quadrupole mass spectrometer formally only determines the mass-to-charge ratio of ions. Because only singly charged ions play a role in PTR-MS, we simply refer to ions by their mass in this paper), acetaldehyde (m45), ethanol (m47), acetone (m59), acetic acid (m61), 2-butanone (m73), 2,3-butanedione (m87), and ethyl acetate (m89). The GC-MS and PTR-MS results were in close agreement, and when treated roots were monitored by both methods, more than 90% of the PTR-MS signals corresponded to compounds identified by GC-MS. Only two peaks in the GC-spectra remained unidentified, while two masses (61 and 75 amu) in the PTR-MS spectra could not be found in the GC-spectra. The signal at mass 61 could not be explained by fragmentation of product ions at a higher mass. This mass is believed to correspond to acetic acid, which was not detectable by the GC-MS set-up used here.
Figure 2.
A typical PTR-MS mass scan of VOCs produced by Arabidopsis roots inoculated with a pathogenic bacterium P. syringae (compatible; A) and a nonpathogen E. coli (incompatible; A) as compared to the untreated control (B). Arabidopsis roots were infected at time zero and samples were taken regularly until 150 h. Some identified VOCs elicited by the pathogen are indicated on the figure.
Table I.
Masses of ions produced and detected by PTR-MS upon in situ interaction of Arabidopsis roots with Pseudomonas syringae (DC3000)a
| Compounds | Primary PTR-MS Masses | Secondary Masses |
|---|---|---|
| (amu) | (amu) | |
| Ethanol | 47 | 65, 93 |
| Ethyl acetate | 89 | 43, 61, 71 |
| 2,3 Butanedione | 87 | 43, 61 |
| Acetaldehyde | 45 | 63 |
| Methyl ethyl ketone | 73 | - |
| Acetic acid | 61 | 43 |
| 1,8- Cineole | 155 | 81, 137 |
| Acetone | 59 | 41 |
Masses were subsequently confirmed by GC-MS.
In Figure 3A, a total ion count (TIC) chromatogram obtained by GC-MS can be seen (top section). Usually, GC-MS systems are operated in TIC mode, where the whole mass range is scanned continuously. The disadvantage of this is that it only shows strong signals. The picture is blurred by a high oxygen background and high statistical noise because of the high scanning speed. To improve the sensitivity, specific ions are selected to be monitored (5–6 masses per specific time interval). The combination of ions is chosen in such a way that the compounds coming out can still be detected. They can subsequently be identified from a library, according to their retention time and remaining mass spectrum. The result for mass 43 amu, which is a very common fragment among VOCs, is shown in the bottom section (Fig. 3B). Since the fraction at this mass varies considerably among different compounds, no conclusions on relative intensity can be drawn from this picture. But it can be clearly seen that the selection of ions improves the sensitivity enormously. The selected ion mode in GC-MS is particularly suitable for detecting known compounds and determining their concentrations and less suitable for finding (un)known compounds and identifying them. This is reflected by the two unidentified peaks. Unsure identifications of the major compounds have been checked using permeation tubes of the pure compounds. In this way, most compounds could be positively identified and, in general, more than 90% of the PTR-MS signal has been validated by GC-MS.
Figure 3.
A, Total ion chromatogram (TIC) of masses 30 through 150 amu of headspace sample from Arabidopsis roots upon interaction with P.syringae. In this operating mode, only high concentration compounds can be observed and identified. B, Mass 43 from a selected ion monitoring mode chromatogram from same source. Higher sensitivity is achieved by selecting a small number of ions (3–6) to be monitored for short period “windows”. Masses are selected from prior knowledge of compound retention times and electron impact mass spectra. Mass 43, being a very common NMHC fragment, is monitored most of the time (except for a small time period between approximately 6.9–7.5 min). Most compounds that were identified as mediated by the plant-pathogen interaction can be observed in this figure (numbered peaks). The numbers correspond to: (1) ethanol, (2) acetone, (3) 2,3-butadione, (4) 2-butanone, (5) ethyl acetate, and (6) cineole. Depending on the compound, GC-MS can be up to 100 times more sensitive than PTR-MS. The peaks indicated by capitol letters were found by GC-MS at concentrations too low to be reliably observed by PTR-MS. They are: (A) i-propanol, (B) n-propanol, (C) unknown, (D) 2-pentanone, and (E) possibly chloroacetone. Since the relative intensity of the mass 43 fragment varies greatly among the compounds seen, relative peak heights do not represent relative concentrations in the sample.
PTR-MS Analysis of Root VOC Emissions Elicited by Pathogenic Bacteria
Our experiments utilized Arabidopsis roots grown under sterile conditions (Fig. 1C) and challenged with different compatible and incompatible organisms. In this communication, we define compatible interactions as the association between a plant and an organism that results in infection or infestation. The resulting changes in the VOC production patterns were then monitored continuously by PTR-MS. As an example of this approach, roots were separately treated with the compatible bacterial pathogen, Pseudomonas syringae pv tomato DC3000 (Pst DC3000), and the incompatible bacterium, Escherichia coli (OP50), and the resulting PTR-MS mass scans were used to reveal the patterns of VOC elicitation by the microbes. These different treatments were applied to the media solution in which the Arabidopsis roots were submerged, and thus the roots were the only plant organs that sensed the elicitation regimes. A typical VOC spectrogram is reproduced in Figure 2.
The addition of compatible Pst DC3000 to roots resulted in altered emission of numerous VOC masses, as detected by PTR-MS. Qualitatively, addition of the pathogen greatly increased the headspace concentrations of ethanol, which is detected at masses 47 (RH+), 65 (RH+ × H2O) and 93 (RH+ × R) in this experiment. Due to the high ethanol concentration, the signals at 65 and 93 amu, which are only a few percent of the primary detection ion at 47 amu, are also clearly visible in Figure 2A. Also detected in the experiment are an unknown VOC at mass 75, and a VOC at mass 137, which was shown by GC-MS to be 1,8-cineole (it also produces a fragment at m81). Other qualitative changes in VOC concentrations can also be seen in Figure 2; these are discussed in more detail below.
Incompatible interactions with Arabidopsis roots were not extensively studied, but measurements of these interactions showed no significant differences compared to the measurements of untreated control plants.
Kinetics of VOC Concentration Changes Following Treatment of Roots with Pst DC3000
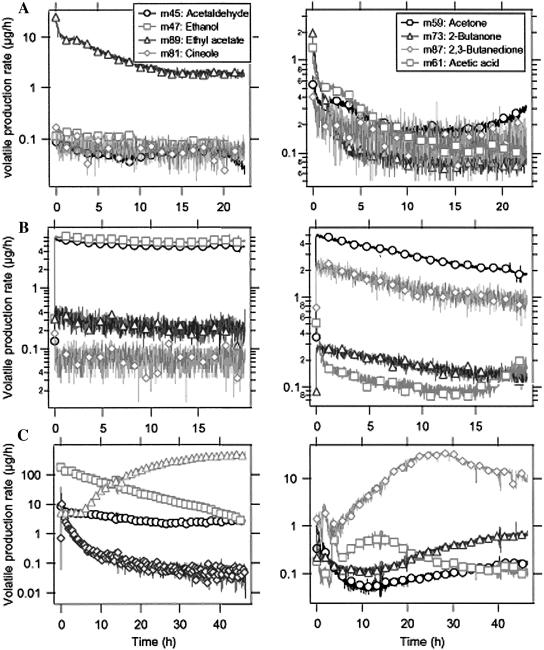
The PTR-MS instrument can be programmed to carry out time scans for selected VOC masses following the administration of a biological stress. A typical PTR-MS time scan of Arabidopsis root head space VOCs following the introduction of Pst DC3000, compared to untreated control roots or media containing no roots, is shown in Figure 4. Two different control experiments have been carried out. In Figure 4A, the emissions by pure cultures of Pst DC3000 added to plant root culture media are shown. Figure 4B depicts the emissions by untreated roots under experimental conditions. The control experiments show relatively low levels of VOCs in the media, primarily ethyl acetate, and reveal that the root culture forms small but significant amounts of acetaldehyde, ethanol, acetone, and 2,3-butanedione (VOC production rates of <1 μg h−1 are considered here as insignificant, background levels). Immediately after the introduction of Pst DC3000, the production of VOCs at rates above 1 μg h−1 at several different masses increased significantly and continued for more than 40 h after infection (Fig. 4C). Most notable were: a rapid increase and then slow decline in ethanol, a lag then large emission of ethyl acetate, a substantial rise in acetic acid levels, and an almost instantaneous spike in concentration of the monoterpene 1,8-cineole followed by its slow decline. It was observed that some VOCs declined after addition of the pathogen; for example, acetone levels were greatly reduced (compare Fig. 4, B and C, right section). Acetaldehyde and ethanol were produced in both the infected and the uninfected root samples; the variation with time of acetaldehyde and ethanol varied by about one order of magnitude over repeated measurements (data not shown). The extensive deterioration associated with cell death would most likely lead to increased VOC release upon whole root colonization by Pst DC3000 (see further evidence below in Fig. 6A).
Figure 4.
This PTR-MS-derived kinetic profile of VOCs of Arabidopsis roots infected with Pst DC3000 was analyzed during a time scan. Immediately after infection with Pst DC3000, the production of positive ions at several different masses increased significantly; this pattern continued until 40 h after infection. Section A shows the control measurement of the pure cultures of microorganisms in plant root culture media, in the absence of roots. Section B shows measurement results versus time of the untreated control Arabidopsis roots. Section C depicts the VOC measurement results versus time of the Pst DC3000-treated Arabidopsis roots. It was observed that some of the breakdown compounds such as ethyl acetate, acetic acid and 2-butanone were elicited for a longer duration post-Pst DC3000 infection. The monoterpene 1,8-cineole showed an instant spike post-Pst DC3000 infection followed by reduced titers until the end of the time scan at 40 h. Note: Eight different masses (compounds) were analyzed in each treatment (A–C) corresponding to the left and right sections.
Figure 6.
Disease appearance and symptom formation in Arabidopsis roots upon compatible interactions with (A) Pst DC3000, (B) A. brassicola, and (C) D. noxia. Section A shows CSL microscopy of Arabidopsis roots infected with Pst DC3000 (Note the brackets in sections indicate the observed root length). Section B shows spores and spore germination (inset) of A. brassicola on Arabidopsis root surface with development of necrotic spots (arrows in the section) 4 d post infection. Section C depicts an adult Diuraphis noxia on the Arabidopsis root surface with development of necrotic (arrows in the section) spots 4 d post infection. The bars (A–C) indicate 50 μm.
Root VOCs Resulting from Fungal Pathogens, a Root-Feeding Insect, or Wounding
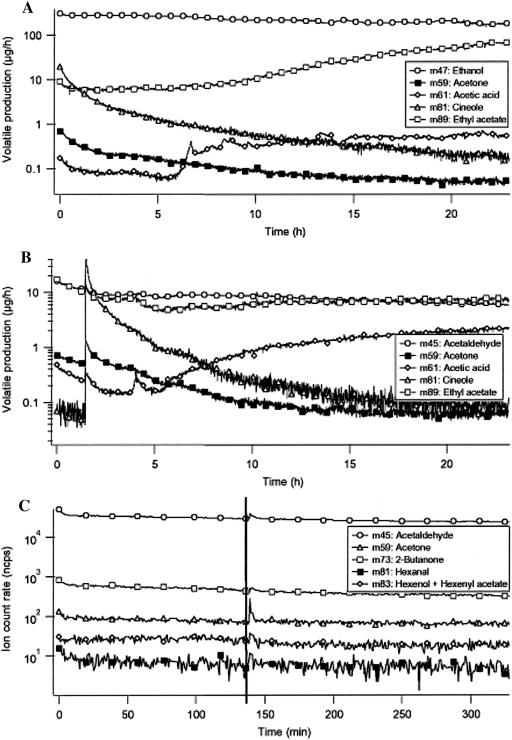
Using the experimental set-up and PTR-MS method described here, we carried out exploratory experiments to determine if other biotic or physical damage to Arabidiopsis roots might lead to the release of sentinel VOCs. These results are briefly summarized here. The compatible interaction between Arabidopsis roots and the pathogenic fungus Alternaria brassicola resulted in a clear change in VOC emission pattern. Figure 5A shows that the ion signals at 47, 59, 61, 81, and 89 amu were enhanced by up to two orders of magnitude (one order of magnitude = factor of 10) as a result of A. brassicola infection. These signals are attributed to ethanol, acetone, acetic acid, 1,8-cineole, and ethyl acetate, respectively. The A. brassicola-induced release of large amounts of ethanol (also seen following infection with Pst DC3000), as observed in the present treatment, suggests that upon addition of a root pathogen the Arabidopsis roots rapidly switch to fermentative metabolism like that seen in anoxic roots (Drew, 1997; Kato-Naguchi, 2003). As seen with the treatment with the bacterial pathogen, 1,8-cineole again showed an instant spike post-A. brassicola infection followed by reduced concentrations of the monoterpene until the end of the time scan at 24 h (Fig. 5A). In contrast to the infection with Pst DC3000, 2-butanone (m73) and 2,3-butanedione (m87) were not found to be produced as a plant response to infection with A. brassicola (compare Figs. 4 and 5A). Acetone and acetic acid, on the other hand, clearly increased about one order of magnitude as a response to infection. No biological explanation exists at present for the release of acetaldehyde, acetone, acetic acid, and ethyl acetate by Arabidopsis roots upon infection with A. brassicola. Notably, treatment of Arabidopsis roots with the incompatible fungi, Pythium ultimum or Phytopthora infestans, did not elicit major changes in VOC formation by treated roots (data not shown).
Figure 5.
A, PTR-MS-derived kinetic profile of VOCs of Arabidopsis roots infected with A. brassicola as analyzed during a time scan. Immediately after infection with A. brassicola, the production of positive ions at several different masses increased significantly; this continued until 20 h after infection. Section B depicts the VOC measurement results versus time of Arabidopsis roots treated with D. noxia. The D. noxia treatments resulted in induction of product ion signals corresponding to ethanol, ethyl acetate, 2-3-butanedione, and acetaldehyde. Titers of 1,8-cineole (m81) started out with a high and instant peak, only to reach the starting value approximately 20 h post-aphid administration. Section C shows the VOC emissions from Arabidopsis roots upon mechanical wounding (at t = 145 min) as measured with the PTR-MS instrument. After wounding, no typical wound compounds were observed.
Our studies revealed that plant roots do respond to aphid interactions by releasing specific VOCs and that a Russian aphid (Diuraphis noxia) may be a compatible pest for Arabidopsis roots. The D. noxia treatments resulted in induction of ethyl acetate (m89) and acetaldehyde (m45) masses (Fig. 5B). Unlike the observed increase of acetone in A. brassicola infection treatments, acetone concentrations remained low during the entire observed aphid infestation. The production rate for acetaldehyde was found to remain at the same level for treated and untreated roots, while the ethanol production was increased by a factor of 10 to 20. Acetone (m59) was found to be emitted in the same amounts from the untreated roots as from the infected roots. Interestingly, concentrations of 1,8-cineole (m81) again started out with a high and instant peak, only to reach the starting value approximately 20 h post-aphid administration (Fig. 5B).
The aerial parts of plants are well known to respond to insect feeding and damage by rapid release of volatile wound compounds, such as the hexanal and hexenal families of C6-VOCs (Fall et al., 1999). In the experiments with D. noxia, we did not see these wound compounds. In order to check the ability of Arabidopsis roots to emit C6 wound VOCs, PTR-MS measurements were done on physically-wounded roots. For this, noninfected root samples were placed in the Erlenmeyer flasks and the air flushed through the headspace was collected and analyzed. After about 2 h of measurements, the flask was opened and the roots were cut in several places with a scalpel. After wounding, the root system was monitored for an additional 3 h, during which time any wound compounds should have come up and decreased again (de Gouw et al., 2000). As seen in Figure 5C, roots did not respond to mechanical injuries by increasing or decreasing any production rate of C6 VOCs. The vertical lines indicate the moment of wounding. The small peaks immediately after the vertical line are caused by pressure changes due to opening of the inlet system.
To confirm if the compatible microbes (Pst DC3000, A. brassicola and D. noxia) caused any disease symptoms in Arabidopsis roots, 2 d post-cocultivation with pathogen roots were viewed by bright field and confocal scanning laser (CSL) microscopy. We observed that Pst DC3000 cells had colonized virtually the entire root surface as revealed by CSL microscopy and bright field microscopy (Fig. 6A), which suggests that Pst DC3000 forms a stable and pathogenic biofilm on these roots. Qualitatively, disease symptoms induced by Pst DC3000 were observed with increased cytoplasmic precipitation and cell death (Fig. 6A). Similarly, compatible fungus A. brassicola interactions with Arabidopsis roots revealed spore germination and necrotic spot development on the root surface (Fig. 6B). Aphid-Arabidopsis root interactions also resulted in similar necrotic spot development as observed before with A. brassicola-root interactions (Fig. 6C). In all cases, root cultures died 4 d post-infection and infestation.
DISCUSSION
For the most part, achieving a clear understanding of the biology and biochemistry of roots has remained difficult due to the underground growth habit of the plant's hidden half. This underground habit poses major technical difficulties for root study and has hindered biochemical research in particular. A recent reincarnation of a classic plant organ culture system, root culture, has proven extremely useful in reinvigorating research on root metabolism. The soil microorganism Agrobacterium rhizogenes causes the hairy root disease, which is characterized by the proliferation of adventitious roots at the infection site. Hairy roots are able to express root-specific biosynthetic pathways such as the synthesis of hyoscyamine, β-carbolines, and rosmarinic acid among other metabolites at levels equal to or greater than in the roots in planta (Flores et al., 1999; Bais et al., 2001, 2002a, 2002b; Vivanco et al., 2002). In addition to secondary metabolites, hairy roots can produce and secrete bioactive proteins (Park et al., 2002). Here we have taken hairy roots of Arabidopsis and used them to characterize the emission of total VOCs by roots in isolation from the whole plant. Our studies revealed that VOCs previously undocumented in the roots of Arabidopsis can be identified and characterized using PTR-MS. PTR-MS has been used primarily in atmospheric studies (Warneke et al., 2001a, 2001b), with only a few studies using this technique to investigate wound responses in detached aerial plant organs (Fall et al., 1999; de Gouw et al., 2000). However, no studies have been undertaken to use PTR-MS in real-time plant biological interactions. We studied the model plant system Arabidopsis because it has been well characterized at the genomic, biochemical, and metabolomic levels (Fiehn et al., 2000; Glassbrook et al., 2003; Trethewey et al., 2001; Walker et al., 2003). Moreover, we have observed that root secondary metabolite secretion in vitro is comparable in many plant systems to such secretion in the soil (Bais et al., 2001, 2002a, 2004), thus validating the broader application of our experimental approach.
The major purpose of this investigation was to study the effect of different stress regimes (compatible and incompatible) on VOC production in the roots of Arabidopsis and to validate the use of PTR-MS in these studies, but we made some important biological observations as well. The quantitative and qualitative variation in the concentrations of certain VOCs within the compatible and incompatible treatments at different time points reconfirms our working hypothesis that roots respond specifically under different stress conditions. Most of the VOCs identified in our studies are known to be emitted by plants, although these VOCs have not been previously detected in roots of Arabidopsis. In our studies, the identified VOCs were produced under different root-microbe regimes, suggesting that the root system may be capable of adapting as the rhizospheric conditions change. This finding also demonstrates the potential usefulness of VOC fingerprinting in functional genomics, since it could lead to the identification of a direct functional link between gene activation (or inactivation) in the various undefined metabolic pathways, production of bioactive volatiles, and response to pathogens in this species.
The compounds observed in this work can be divided into two categories: low Mr metabolic by-products, such as acetaldehyde, ethanol, acetone, ethyl acetate, and 2-butanone, and a monoterpene (1,8-cineole) with possible bioactivity. Of the low Mr metabolites, acetaldehyde and ethanol are well-known components of plant roots, especially in roots subjected to flooding and anoxic stress (for review, see Drew, 1997; Kato-Naguchi, 2003). It is surprising here to see that each biotic stress tested led to a rapid increase in ethanol production and continued formation of high levels of this alcohol by Arabidopsis roots. Perhaps a switch to fermentative metabolism is part of a root stress response. The formation of ethyl acetate seen in infected roots (Figs. 4 and 5) may be similar to processes well known in ripening fruit (Honkanen and Hirvi, 1990). The other oxygenated metabolites, produced in much lower amounts, are known by-products of general plant wounding and are not known to have any particular signaling role in the rhizosphere.
Interaction of Arabidopsis roots with either compatible bacterial or fungal pathogens or root-feeding insects all resulted in the rapid release of 1,8-cineole. Several researchers have shown that 1,8-cineole is a major constituent of plant-insect interactions (Schiestl and Roubik, 2003), and this compound also has antimicrobial effects on a variety of bacteria and fungi (Kalemba et al., 2002). These findings suggest that different types of biotic challenge of Arabidopsis roots lead to the rapid formation and release of a protective monoterpene; it is unlikely that preexisting pools of 1,8-cineole are present in the roots, since this VOC was not released from mechanically wounded roots. Interestingly, compatible interactions, incompatible interactions and mechanical injuries with the Arabidopsis roots did not produce any C6 VOCs (Fig. 4, A–C). The absence of these wound-volatiles after insect-root interactions and mechanical wounding suggests that roots use different mechanisms to inhibit root-feeding herbivores. As mentioned above, the only compounds that were clearly produced as a result of aphid-root interaction were ethanol, acetaldehyde, and 2-butanone. The absence of wound-volatiles might be explained by the fact that aphids are not natural below-ground enemies of plants so a natural defense mechanism is not needed and therefore not developed. Since wound-volatiles are also not seen upon mechanical wounding of the roots, our results suggest that roots cannot respond by releasing a battery of VOCs upon mechanical wounding or insect attacks as the aerial parts of the plant do (Van Poecke et al., 2001) and therefore do not respond in the same way to insect attacks as aerial parts do. The role of primary metabolite VOCs in distance signaling events is yet to be discovered.
The significance of this breakthrough communication is 2-fold. First, we have used a new system (PTR-MS) to analyze volatile formation in plant roots by linking plant biology, environmental biochemistry, and physical sciences. Second, our approach may enable studies to follow the kinetics of formation of VOCs during plant-microbe-insect interactions. Even without a full identification of VOCs, a single mass scan can be used as a chemical fingerprint to distinguish different samples, as shown by Biasioli et al. (2003), who separated heat-treated versus pressure-treated orange juices using PTR-MS analyses of the head space and a principal component analysis of the mass scans. Such a capability, coupled with the tools available in the Arabidopsis system such as genomics, proteomics, metabolomics, and knock-out and transposon-tagged mutatagenesis (Trethewey et al., 2001), may facilitate our understanding of the biological implications of VOC emission by plant roots in response to biotic stresses and help identify essential genes in these processes.
MATERIALS AND METHODS
Plant Material
Seeds of wild-type Arabidopsis ecotype Columbia (Col-0) were obtained from Lehle Seeds (Round Rock, TX). Seeds were surface-sterilized using commercial sodium hypochlorite (0.3% v/v) for 10 to 12 min and then washed 4 times in sterile double distilled water. Surface-sterilized seeds were placed on static Murashige and Skoog (MS, Murashige and Skoog, 1962) basal media in petri dishes for germination and incubated in a growth chamber at 25°C ± 2°C with a light intensity of 80 μmol m−2 s−1.
Root Cultures
Shoot cultures of Arabidopsis were placed separately in Magenta GA-7 vessels containing MS basal medium solidified with 0.3% (w/v) Phytagel (Sigma, St. Louis). Cultures were kept in a light chamber maintained at 24°C with a fluence rate of 100 μmol m−2 s−1. In order to produce hairy root cultures, 1-month-old in vitro plants were infected with a 3-d-old culture of Agrobacterium rhizogenes (ATCC 15834). Briefly, stems and leaves were punctured in several places, inoculated with A. rhizogenes, and then placed in a light chamber. Roots that developed at the infection sites were transferred to petri dishes containing solid MS basal medium supplemented with 250 μg mL−1 Claforan (Hoechst Roussel Pharmaceuticals, Somerville, NJ) and incubated in the dark chamber at 25°C ± 2°C. After 14 d, 1-cm root tips were subcultured twice to eliminate the bacteria before transferring them to fresh medium in the absence of antibiotic. Clonal root lines were established after serial transfers of root tips to fresh MS medium and placed on a gyratory shaker set at 90 rpm in the dark chamber. Root cultures were subcultured on a weekly basis. In all experiments an initial biomass of approximately 500 mg was used for 40 mL of culture medium.
Abiotic Material
Arabidopsis roots were subjected to multiple abiotic chemical elicitors and biotic fungal cell wall elicitors by supplementing the 40 mL of liquid MS medium. Initial pilot experiments were performed to determine the final elicitor concentration for the treatments (Bais et al., 2002b). Elicitor concentrations were selected on the basis of inducing maximum root secretion and avoidance of tissue toxicity. Roots were treated with the following: 200 μm jasmonic acid, fungal cell wall elicitors from Rhizoctonia solani (3 mL v v−1). The solution of jasmonic acid was prepared in ethanol and fungal cell wall elicitors were prepared and administered as previously described by McKinley et al. (1993).
Biotic Material
Bacterial and Fungal Strains and Culture Conditions
Pseudomonas syringae pv tomato DC3000 (Pst DC3000) and Alternaria brassicola, wild-type isolates, were obtained from the laboratory of Dr. Christopher Lawrence (Department of Bioagricultural Sciences and Pest Management, Colorado State University); they were maintained and grown on Luria-Bertani (LB) and potato dextrose agar (PDA) media and incubated at 37°C. Phytophthora infestans (US11), Pythium ultimum, and Bacillus subtilis were obtained from the microbial culture collection at Colorado State University. Escherichia coli (OP50) was obtained from the laboratory of Dr. Frederick Ausubel (Department of Molecular Biology, Harvard Medical School). Freshly plated bacterial cells from frozen stock cultures were used for all experiments. All bacterial strains were plated on LB agar and incubated at 37°C. Plated cells were suspended in 5 mL LB broth for overnight growth and shaken at 250 rpm at 37°C.
Insect Cultures
Colonies of Russian aphids (Diuraphis noxia) were obtained from the laboratory of Dr. Nora Lapitan (Department of Soil and Crop Sciences, Colorado State University) and were maintained in large sealed cages on wheat (Triticum aestivum) plants at 25°C under a 12-h photoperiod.
Bacterial, Fungal, and Insect Bioassays
We used bacterial (P. syringae pv tomato DC3000) and fungal (A. brassicola) pathogens that infect Arabidopsis roots for modeling compatible interactions, and P. infestans/P. ultimum (fungi) and B. subtilis/E. coli (OP50; bacterium) for the incompatible interactions. In this manuscript we refer to compatible interactions as the associations between roots and other organisms that lead to infection or infestation. Incompatible interactions are associations that do not produce either infection or infestation. The infections were conducted by independently adding fungal spores and bacterial colonies to the liquid medium in which 15-d-old Arabidopsis hairy roots were grown. The PTR-MS analysis was initiated post infection and continued on-line for several hours; each reading was obtained three times to ensure repeatability. It is possible to run this type of instrument essentially unattended for prolonged periods (up to 24 h) if necessary. We added fungal spores and bacterial colonies to liquid medium without plants and monitored the production of VOCs by the pathogens; this production was used for comparison/subtraction purposes. The disease progression (compatible interactions) and pathogen growth (incompatible interactions) were rated and compared for their ability to produce VOCs.
Both compatible and incompatible bacterial strains were grown to OD600 = 0.2 to 0.4 and added separately to the 40 mL of MS media supporting each root culture to reach an initial OD600 = 0.02. MS basal media without root material was inoculated with the same volume of each bacterial strain tested. A noninfected root control was maintained under the same conditions. All the treatments and controls were incubated at 30°C in an incubator shaker (New Brunswick Scientific, Edison, NJ) set at 30 rpm with a photoperiod of 16 h light and 8 h dark. Each experiment was repeated twice with 5 replicates.
For fungal pathogenesis assays, fungi were harvested for sporangia by rinsing the plates with 5 mL sterile distilled water. Sporangial suspension concentration was estimated using an Ultra Plane Improved Neubauer cell counting chamber (Scientific Products, West Sussex, UK) and adjusted to 1 × 105 sporangia mL−1. The sporangial suspension was then placed in a 4°C cold treatment for 4 h to induce the release of spores (Ali and Reddy, 2000). Light microscopy confirmed the lysis of sporangia by observation of motile spores. Spore suspension (500 mL) was added to each treatment with Arabidopsis root cultures. MS basal media without root material was inoculated with the same volume of each fungal strain tested. A noninfected root control was maintained under the same conditions. All the treatments and controls were incubated at 25°C in a controlled environment incubator shaker (New Brunswick Scientific) in dark conditions with 80% humidity. Each experiment was repeated twice with 5 replicates.
Root-aphid interactions were studied using 20 D. noxia aphids (10 adults, 10 nymphs). Aphids were confined to the floating roots. Adults comprised of only females were allowed to reproduce asexually during the experiments (60 aphids by 72 h). VOC analysis was done from 0-time for several hours' post-aphid inoculation. Each experiment was repeated twice with 5 replicates.
The comparison of measurements of mechanical wounding was begun with a measurement of untreated roots. After a few hours the roots were cut with a scalpel; after wounding, the measurements were continued for 3 to 4 h.
PTR–MS
All analyses of root samples were performed using a custom-built PTR-MS instrument, illustrated in Figure 1A. The PTR-MS technique is described in detail elsewhere (Lindinger et al., 1998); hence, it is only described briefly here. The instrument consists of three parts: an ion source where H3O+ ions are produced, a drift tube section where proton-transfer reactions between H3O+ ions and VOCs take place, and an ion detection section consisting of a quadropole mass spectrometer. The ion source and drift tube were modeled after that of a commercially available PTR-MS instrument (Ionicon, Innsbruck, Austria); the detection end contained an Extrel quadrupole mass filter and a conversion dynode electron multiplier to detect ions.
The product ion mass is a useful but not unique indicator of the identity of a VOC. It is clear that different VOCs with the same mass cannot be separately measured. Moreover, product ion fragmentation, cluster ion formation, and secondary ion-molecule reactions all lead to further mass overlap, which can make the interpretation of the mass spectra a daunting task. A combination of PTR-MS with a gas-chromatographic preseparation of VOCs has been used to identify which VOCs contribute to the signals at different masses (Fall et al., 2001; de Gouw et al., 2003a; Warneke et al., 2003). The results indicate that many VOCs of interest can be measured by PTR-MS without significant interference. GC-MS analyses were performed here to confirm the VOC identities by an alternative method.
In this study, PTR-MS was used for on-line measurements of VOCs in the headspace of Arabidopsis roots. To monitor the emissions from the roots, 1 week before VOC measurement the root cultures (500 mg/40 mL culture) were transferred to 150 mL Erlenmeyer flasks, closed with a glass top possessing a gas inlet and a gas outlet (one-quarter inch; Fig. 1C). Each root culture was transferred in one sample flask at a time, and could be put in the gas-inlet system of our PTR-MS. Here it was continuously flushed with dry air, at a flow rate of 50 to 75 sccm (cm3 min−1 at standard temperature, 273 K, and pressure, 1 atm), depending on the emission rate of the roots. In this way, the headspace of the roots was renewed every 2 to 3 min. The air leaving the sample flask was led toward a reduction valve to reduce the pressure from atmospheric pressure to about 2.5 mbar in the drift tube reaction chamber of the PTR-MS (Fig. 1, A and B). This reduced pressure of 2.5 mbar (1.80 Torr) is the pressure under which the reaction chamber is typically operated. Because the flow through the sample flask is higher than the flow needed in the PTR-MS (25 sccm), part of the flow is split off and channeled out into the laboratory through an overflow-outlet just before the reduction valve. In this way the roots were under atmospheric pressure while being monitored. From the flask to the entrance of the PTR-MS, only Teflon tubing, connectors, and valves were used to reduce memory effects due to surface interactions in the inlet system.
Initially, the root samples were inoculated with various organisms; after a few hours, mass spectra were measured to determine which masses should be monitored on-line. Eventually 39 masses, including primary ions, were chosen and monitored simultaneously with an integration time of 1.1 s per mass. This system resulted in one data point every 45 s. Some of these masses corresponded to a cluster or a fragment of the parent ion, and some corresponded to the same compound containing one 13C- or 18O-isotope. Measurements were extended over either a 24 or a 40 h period. The sample was placed in the inlet system of the PTR-MS and after about 1.5 h the roots were infected with the treatments and monitored for the prolonged period (>24 h).
Signals of identified compounds are given as production rates in μg h−1, based on their concentrations in the sample air and calculated according to the equations in Lindinger et al. (1998). Reaction constants were also taken from this study, where for unknown reaction constants the average value of 2.0 × 10−9 cm3 s−1 was used. These assumptions were verified by comparing the calculated concentrations with the results from calibrations (Warneke et al., 2003). In addition, the stability of the instrument's response was shown to be constant within a few percent by de Gouw et al. (2003b) using calibrations over a 4-week period. Volatiles are mainly emitted into the liquid in which the roots are submerged, out of which the gases subsequently partition into the sample air. Since there are major differences in Henry constants (indication of equilibrium ratio between gas phase and liquid phase concentrations) between different compounds (Sander, 2003), ratios between compound concentrations in the gas phase do not necessarily correspond to the ratios of the actual emission rates from the roots. Production rates in this work are given disregarding partitioning effects from liquid to gas phase. Therefore, the values of the production rates should be used with some caution. Each experiment was repeated thrice with 5 replicates.
GC-MS Verification of VOCs
The GC-MS used was developed and operated at the NOAA Aeronomy Laboratory. It is described in detail by P.D. Goldan (unpublished data) and is only briefly outlined here. Two 5-min samples are automatically acquired simultaneously from the same source, are cryogenically concentrated in separate sample loops and analyzed in parallel on 2 different analytical columns: a 30-m × 0.35-mm Al2O3 column (Chrompack, Middleburg, The Netherlands), carrier gas H2 at 1.6 sccm, equipped with a Flame Ionization Detector and a 30-m × 0.25-mm DB624 column (J&W Scientific, Folsom, CA), and carrier gas He at 2 sccm, equipped with an Agilent 5973 mass selective detector, using electron impact ionization. The Al2O3/FID column and detector combination provides quantitative analysis of C2 through C5 alkanes and alkenes and acetylene. The DB624/MSD column and detector combination provides quantitative analysis of C5 through C10 alkanes, C5 through C7 alkenes, C1 through C7 alchols, C3 through C7 ketones, C2 through C7 aldehydes, C6 through C9 aromatics, and C10 monoterpenes. This system is capable of 1 complete sample acquisition and analysis cycles each 30 min. All compound identifications and quantitations are supported by gravimetrically prepared calibration standards of the pure compounds.
Flasks under study were flushed with dry VOC free zero air at 180 sccm and samples for analysis were drawn from this stream after a 25 min flushing time. To avoid detector saturation from the high ethanol concentrations observed to be in the sample gas, flush air from the flasks under study was accumulated for only 10 s out of the 5 min automated sample acquisition period; the remainder of the sample (i.e. 96.7%) was zero air.
Once the observed compounds have been unambiguously identified by GC-MS, PTR-MS is an ideal tool to investigate temporal changes of VOC production in the root system and can give valuable information on the underlying metabolic processes.
Acknowledgments
The authors are grateful to David Fahey and Ru-Shan Gao, whose instrument was used for the experiments in this manuscript, and to Carsten Warneke for useful discussions. Marco Steeghs is especially grateful to Frans Harren and Dave Parker from the University of Nijmegen and the Dutch Foundation for Research on Matter (FOM) for giving him the opportunity to participate in this work.
This work was supported by grants from the Colorado State University Agricultural Experiment Station (to J.M.V.), NSF-SGER (grant no. MCB–0314255 to J.M.V.), NSF-CAREER (grant no. MCB–0093014 to J.M.V.), the Invasive Weeds Initiative of the State of Colorado (to J.M.V.), DOE (grant no. DE–FG02–03ER15400 to R.F.), and NSF (grant no. ATM–0207587 to R.F. and J.d.G).
References
- Ali GS, Reddy ASN (2000) Inhibition of fungal and bacterial plant pathogens by synthetic peptides: in vitro growth inhibition, interaction between peptides and inhibition of disease progression. Mol Plant Microbe Interact 13: 847–859 [DOI] [PubMed] [Google Scholar]
- Amthor J (2001) Effects of atmospheric CO2 concentration on wheat yield: review of results from experiments using various approaches to control CO2 concentration. Field Crops Res 73: 1–34 [Google Scholar]
- Bais HP, Loyola-Vargas VM, Flores HE, Vivanco JM (2001) Root-specific metabolism: the biology and biochemistry of underground organs. In Vitro Cell Dev-Pl 37: 730–741 [Google Scholar]
- Bais HP, Park S-W, Halligan KM, Stermitz R, Vivanco JM (2002. b) Exudation of fluorescent β-carbolines from Oxalis tuberosa L. roots. Phytochemistry 61: 539–543 [DOI] [PubMed] [Google Scholar]
- Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301: 1377–1380 [DOI] [PubMed] [Google Scholar]
- Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9: 26–32 [DOI] [PubMed] [Google Scholar]
- Bais HP, Walker TS, Schweizer H, Vivanco JM (2002. a) Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of sweet basil (Ocimum basilicum L.). Plant Physiol Biochem 40: 983–995 [Google Scholar]
- Barber DA, Martin JK (1976) The release of organic substances by cereal roots into soil. New Phytol 76: 69–80 [Google Scholar]
- Biasioli F, Gasperi F, Aprea E, Colato L, Boscaini E, Märk TD (2003) Fingerprinting mass spectrometry by PTR-MS: heat treatment vs. pressure treatment of red orange juice–a case study. Int J Mass Spectrom 223–224: 343–353 [Google Scholar]
- Curtis P, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form and physiology. Oecologia 113: 299–313 [DOI] [PubMed] [Google Scholar]
- de Gouw JA, Carleton CJ, Custer TG, Baker BM, Fall R (2000) Proton-transfer chemical-ionization mass spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environ Sci Technol 34: 2640–2648 [Google Scholar]
- de Gouw JA, Goldan PD, Warneke C, Kuster WC, Roberts JM, Marchewka M, Bertman SB, Pszenny AAP, Keene WC (2003. b) Validation of proton-transfer-reaction mass spectrometry (PTR-MS) measurements of gas-phase organic compounds in the atmosphere during the New England Air Quality Study (NEAQS) in 2002. J Geophys Res 108: 4682 [Google Scholar]
- de Gouw JA, Warneke C, Karl T, Eerdekens G, van der Veen C, Fall R (2003. a) Sensitivity and specificity of atmospheric trace gas detection by proton-transfer-reaction mass spectrometry. Int J Mass Spectrom 223–224: 365–382 [Google Scholar]
- Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol 48: 223–250 [DOI] [PubMed] [Google Scholar]
- Fall R (1999) Biogenic emissions of VOCs from higher plants. In CN Hewitt, ed, Reactive Hydrocarbons in the Atmosphere. Academic Press, San Diego, pp 43–96
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W (1999) Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. J Geophys Res 104: 15963–15974 [Google Scholar]
- Fall R, Karl T, Jordan A, Lindinger W (2001) Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmos Environ 35: 3905–3916 [Google Scholar]
- Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L (2000) Metabolite profiling for plant functional genomics. Nat Biotechnol 18: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Flores HE, Vivanco JM, Loyola-Vargas V (1999) ‘Radicle’ biochemistry: the biology of root-specific metabolism. Trends Plant Sci 4: 220–226 [DOI] [PubMed] [Google Scholar]
- Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156: 145–169 [DOI] [PubMed] [Google Scholar]
- Glassbrook N, Beecher C, Ryals J (2003) Metabolic profiling on the right path. Nat Biotechnol 18: 1142–1153 [DOI] [PubMed] [Google Scholar]
- Guenther A, Geron C, Pierce T, Lamb B, Harley P, Fall R (2000) Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos Environ 34: 2205–2230 [Google Scholar]
- Honkanen E, Hirvi T (1990) The flavour of berries. In ID Morton, AJ Macleod, eds, Food Flavours. Elsevier, Amsterdam; pp 125–193
- Isidorov V, Jdanova M (2002) Volatile organic compounds from leaves litter. Chemosphere 48: 975–999 [DOI] [PubMed] [Google Scholar]
- Kalemba D, Kusewicz D, Swiader K (2002) Antimicrobial properties of the essential oil of Artemisia asiatica Nakai. Phytother Res 16: 288–291 [DOI] [PubMed] [Google Scholar]
- Karl T, Curtis A, Rosenstiel T, Monson R, Fall R (2002. a) Transient releases of acetaldehyde from tree leaves—products of a pyruvate overflow mechanism? Plant Cell Environ 25: 1121–1131 [Google Scholar]
- Karl T, Fall R, Rosenstiel T, Prazeller P, Larsen B, Seufert G, Lindinger W (2002. b) On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple sub-cellular origins of isoprene precursors. Planta 215: 894–905 [DOI] [PubMed] [Google Scholar]
- Kato-Naguchi H (2003) Anoxia tolerance in rice roots accelerated by small periods of hypoxia. J Plant Physiol 160: 565–568 [DOI] [PubMed] [Google Scholar]
- Knee EM, Gong FC, Gao M, Teplitski M, Jones AR, Foxworthy A, Mort AJ, Bauer WD (2001) Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol Plant Microbe Interact 14: 775–784 [DOI] [PubMed] [Google Scholar]
- Li X, Schuler MA, Berenbaum MR (2002) Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature 419: 712–715 [DOI] [PubMed] [Google Scholar]
- Lindinger W, Fall R, Karl T (2001) Environmental, food and medical applications of proton-transfer-reaction mass spectrometry (PTR-MS). Advances in Gas Phase Ion Chemistry 4: 1–48 [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998) On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS): medical applications, food control, and environmental research. Int J Mass Spectrom Ion Process 173: 191–241 [Google Scholar]
- McKinley TC, Michaels PJ, Flores HE (1993) Is lipooxygenase involved in polyacetylene biosynthesis in Asteraceae? Plant Physiol Biochem 31: 835–843 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Nardi S, Concheri G, Pizzeghello D, Sturaro A, Rella R, Parvoli G (2000) Soil organic matter mobilization by root exudates. Chemosphere 5: 653–658 [DOI] [PubMed] [Google Scholar]
- Neveu N, Grandgirard J, Nenon JP, Cortesero AM (2002) Systemic release of herbivore-induced plant volatiles by turnips infested by concealed root-feeding larvae Delia radicum L. J Chem Ecol 28: 1717–1732 [DOI] [PubMed] [Google Scholar]
- Norby R, Jackson RB (2000) Root dynamics and global change: seeking an ecosystem perspective. New Phytol 147: 1–12 [Google Scholar]
- Park S-W, Lawrence CB, Linden JC, Vivanco JM (2002) Characterization of a novel ethylene-inducible ribosome-inactivating protein exuded from root cultures of Phytolacca americana. Plant Physiol 130: 164–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Rogers H, Runion B, Prior SA, Torbert HA (1999) Response of plants to elevated atmospheric CO2: root growth, mineral nutrition, and soil carbon. In Y Luo, HA Mooney, eds, Carbon Dioxide and Environmental Stress. Academic Press, San Diego, pp 215-244
- Roshina VV, Roshina VD (1993) The excretory functions of higher plants. Springer-Verlag, Berlin
- Sander R (2003) Compilation of Henry's Law constants for inorganic and organic species of potential importance in environmental chemistry, version 3. http://www.pmch-mainz.mpg.de/∼sander/res/henry.html
- Schiestl FP, Roubik DW (2003) Odor compound detection in male euglossine bees. J Chem Ecol 29: 253–257 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I (1997) Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 385: 718–721 [Google Scholar]
- Trethewey RN, Krotzky AJ, Willmitzer L (2001) Metabolic profiling: a rosetta stone for genomics? Curr Opin Plant Biol 2: 83–85 [DOI] [PubMed] [Google Scholar]
- Van Poecke RM, Posthumus MA, Dicke M (2001) Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol 27: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Vivanco JM, Guimaraes R, Flores HE (2002) Underground plant metabolism: the biosynthetic potential of plant roots. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots: the Hidden Half. Marcel Dekker Press, New York, pp 1045–1070
- Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM (2003) Metabolic profiling of root exudates of Arabidopsis thaliana. J Agric Food Chem 51: 2548–2554 [DOI] [PubMed] [Google Scholar]
- Warneke C, de Gouw J, Kuster WC, Goldan GD, Fall R (2003) Validation of atmospheric VOC measurements by proton-transfer-reaction mass spectrometry using a gas-chromatographic preseparation method. Environ Sci Technol 37: 2494–2501 [DOI] [PubMed] [Google Scholar]
- Warneke C, Holzinger R, Hansel A, Lindinger W, Williams J, Poschl U, Crutzen P (2001. a) Isoprene and its oxidation products methyl vinyl ketone, methacrolein, and isoprene related peroxides measured online over the tropical rainforest of Surinam in March 1998. J Atmos Chem 38: 167–185 [Google Scholar]
- Warneke C, Luxembourg S, de Gouw J, Rinne J, Guenther A, Fall R (2002) Disjunct eddy covariance measurements of oxygenated VOC fluxes from an alfalfa field before and after cutting. J Geophys Res 107: ACH6-1 to ACH6–11 [Google Scholar]
- Warneke C, van der Veen C, Luxembourg S, de Gouw JA, Kok A (2001. b) Measurements of benzene and toluene in ambient air using proton-transfer-reaction mass spectrometry: calibration, humidity dependence, and field intercomparison. Int J Mass Spectrom 207: 167–182 [Google Scholar]
- Woodward FI, Osborne CP (2000) The representation of root processes in models addressing the responses of vegetation to global change. New Phytol 147: 223–232 [Google Scholar]