Abstract
As a complex neuropsychiatric disease with both hereditary and environmental components, schizophrenia must be understood across multiple biological scales, from genes through cells and circuits to behaviors. The key to evaluating candidate explanatory models, therefore, is to establish causal links between disease-related phenomena observed across these scales. To this end, there has been a resurgence of interest in the circuit-level pathophysiology of schizophrenia, which has the potential to link molecular and cellular data from risk factor and post-mortem studies with the behavioral phenomena that plague patients. The demonstration that patients with schizophrenia frequently have deficits in neuronal synchrony, including deficits in local oscillations and long-range functional connectivity, offers a promising opportunity to forge such links across scales.
Introduction
Schizophrenia has long been hypothesized to be a “disconnection syndrome”, resulting from a discoordination of activity within and between brain regions [1]. This hypothesis, based originally on clinical symptomatology, was conceived prior to extensive research on neurophysiology. Nonetheless, over the past two decades a body of physiological evidence has emerged in support of disconnection as a prominent component of schizophrenia. Connectivity in neural systems is commonly assayed with measures of synchrony; temporal covariance in patterned activity is taken as evidence of neural interactions, and changes in synchrony that accompany shifting cognitive and behavioral states are seen as an indication of the relevance of such interactions to these states. There is now a substantial literature reporting schizophrenia-related disturbances in neural synchrony of varying frequency, anatomical regionalization, and cognitive relevance [2]. Broadly, these findings can be divided into deficits in local synchrony, characterized by alterations in the power or amplitude of local oscillations within a brain region, and deficits in long-rage connectivity, characterized by alterations in functional or anatomical connectivity between distant brain regions. Here we review both sets of findings, discuss two candidate mechanisms to explain these disruptions, and review attempts to further define circuit-level explanations using animal models. We then propose ways in which future research could help further link these circuit-level deficits across scales, both downward, to specific molecular and cellular processes, and upward, to behavior.
Disruptions in oscillatory activity, a measure of local synchrony
Disruptions in local synchrony can be detected in oscillatory activity, which is thought to arise during normal brain function from the synchronous activation of large numbers of synapses [3]. Both evoked and spontaneous oscillations can be studied, and these do not necessarily reflect the same underlying circuit dynamics. Evoked oscillations are commonly interrogated by examining steady-state evoked potentials (SSEPs), typically with extracranial EEG electrodes. SSEPs are the responses to trains of sensory stimuli (typically auditory clicks) delivered at varying frequencies; they are typically widespread, and build to steady-state amplitudes over several hundred milliseconds. The gradual emergence, broad spatial extent, resonance, and frequency-dependence of SSEPs (strongest with 40–45Hz stimuli) suggest they reflect reverberant dynamics within recurrent cortical circuitry and not merely the sum of isolated individual responses characteristic of event-related potentials (Figure 1a) [4]. This approach tests the ability of cortical circuitry to support oscillatory activity regardless of the underlying behavioral state, which may differ in patients and controls. Schizophrenia patients show pronounced deficits in the amplitude of SSEPs [4–7] which can be present at the first psychotic episode [8]. The severity of these deficits correlates with the severity of auditory hallucinations, suggesting that the circuit- and behavior-level phenotypes may be related [9]. Inter-trial coherence (the temporal precision of physiological responses across presentations) is also disrupted in patients, and correlates with the severity of schizophrenia-associated working memory deficits [10]. Collectively these data are suggestive of an impaired cortical circuitry that is unable to support normal oscillatory rhythmogenesis in response to appropriately timed stimuli.
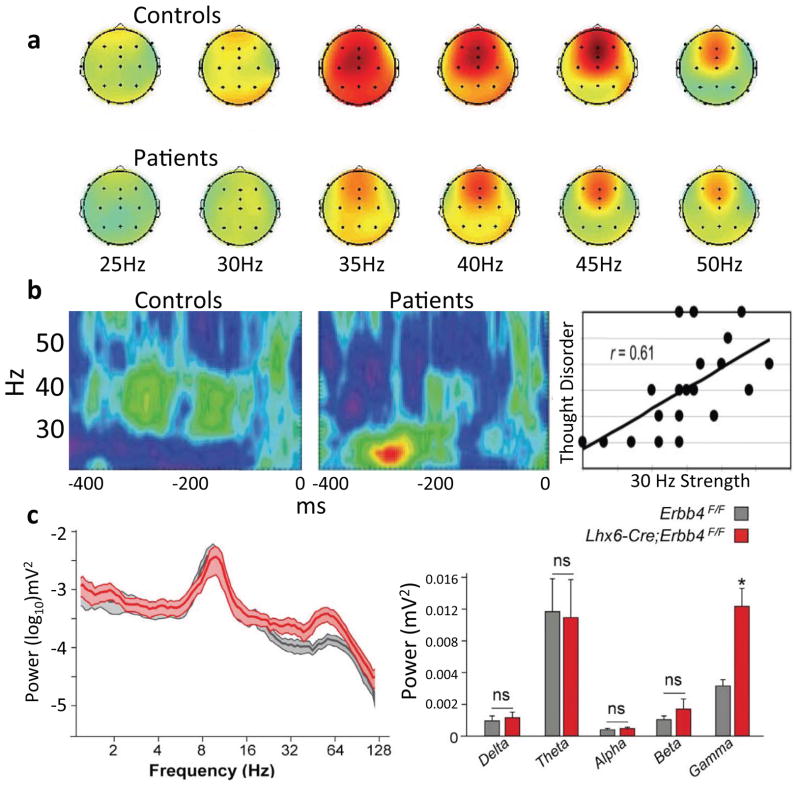
Figure 1. Disruptions in gamma synchrony in schizophrenia patients and in a genetic animal models.
(a) A widespread SSEP response is seen to 35–45Hz stimulus trains; this response is larger in healthy controls compared to patients with schizophrenia [reprinted from 4]. (b) During a visual gestalt task, the characteristic 40 Hz oscillation induced in controls (left) is lower in frequency (middle) and correlates with symptoms in schizophrenia patients [reprinted from 8]. (c) Spectral power in the hippocampus of mice lacking the Erbb4 neuregulin receptor in PV+ interneurons (red) and control mice (grey). Deletion mice show a significant, frequency specific increase in gamma power [reprinted from 54].
Although the finding of impaired steady-state evoked responses is robust, reproducible, and correlated with symptomatology, it constitutes stimulus-locked, rather than intrinsically paced activity and therefore may not engage circuits in a way that mirrors normal functioning. A complementary approach is to measure oscillations evoked by specific tasks, though not time-locked to specific stimuli. For example, during a Gestalt visual stimulus response task in which patients had impaired performance, control subjects display a prominent 40Hz oscillation that corresponds with the initiation of a behavioral response. In schizophrenia patients, this oscillation is lower in frequency, and its strength correlates with symptom severity (Figure 1b) [11]. Similarly, tasks dependent on working memory typically induce increases in oscillation strength in prefrontal cortical circuits in healthy controls [12]. Schizophrenia patients show reduced gamma and theta-frequency oscillatory power in frontal areas during such tasks [13], and this reduction is correlated with impairments in task performance [14]. Using magnetoencephalography, which has improved sensitivity and source localization compared to EEG, impaired oscillatory activity at higher frequencies can be seen. For example, deficits in gamma (60–120Hz) power over visual cortex has been found in patients during a visual face detection task [15]. Together, these studies demonstrate an important link between oscillations and behavior; they are correlated in healthy controls and jointly impaired in patients. However, differences between patients and controls might reflect systematic differences in the way they perform the tasks, rather than fundamental differences in circuit structure or function.
By contrast, data on oscillations in a “resting state”, absent a stimulus or task, has been used to demonstrate disease-related functional abnormalities absent any particular behavioral demands. Of course, the interpretation of resting state date is still challenging, most notably because it is unclear whether patients and controls are in equivalent behavioral states when asked simply to “rest.” Perhaps as a result, there is some disagreement as to whether the disorder affects resting state oscillatory synchrony. Some studies [16] suggest an increase in “baseline” or “resting state” gamma-frequency (30–80 Hz) activity in patients. However, one study found that resting-state activity in gamma-frequency oscillatory power was impaired in schizophrenia patients, as well as in their unaffected siblings, suggesting a relationship to genetic risk rather than disease state per se [17]. Notably, this report localized the impairments in gamma to cortical areas corresponding to the “default-network,” which is more active in a resting or internally-oriented state. Thus the impairment could reflect engagement in some alternative, active state (such as the experiencing of hallucinations or other positive symptoms), resulting in an inability to maintain a resting state. However, this is perhaps unlikely given that gamma oscillations have been shown to increase, not decrease, during hallucinations [18].
Deficits in long-range synchrony
The strength of a locally generated oscillation is at best an indirect measure of synchrony, a conflation of the size of the rhythmically active neural population and the simultaneity of its firing. Synchrony across brain regions provides a direct measure of the functional connectivity of distant neural populations. While lacking in temporal resolution and therefore unable to speak to oscillatory dynamics, functional magnetic resonance imaging (fMRI) can be used to measure the covariance in localized BOLD signals from multiple brain regions more or less simultaneously. This allows one to examine synchronous activation of far-flung brain areas during tasks or in the resting state, albeit on a slower time scale than with EEGs. Results from fMRI studies of functional connectivity in specific circuits in schizophrenia patients are varied, and have included both increases and decreases relative to controls. For example, the examination of connectivity with medial prefrontal cortex (mPFC) found a reliable anticorrelation between mPFC and dorsolateral prefrontal cortex (dlPFC) in healthy controls that was absent in schizophrenia patients [19]. Meanwhile, a study of connectivity within and between two large brain networks – the default-mode and fronto-parietal networks – suggested that patients had decreased synchrony within parts of the default mode network, but increased synchrony between the default-mode and fronto-parietal networks [20].
A less directed approach to examining long-range synchrony is to measure global connectivity rather than analyzing specific networks of interest. Groups employing this approach have generally found lower connectivity in schizophrenia. For instance, a recent resting state fMRI study of schizophrenia and bipolar patients found lower global connectivity in both patient groups compared to healthy controls; connectivity correlated with symptom severity [21]. Another recent whole-brain examination found increased fronto-parietal connectivity and decreased parietal-temporal and bilateral temporo-temporal connectivity. The increase in front-parietal connectivity correlated with positive symptom severity, while the decreases in connectivity correlated with negative symptom severity [22]. Yet another study demonstrated that pairwise functional connectivity throughout the brain is globally and significantly decreased in schizophrenia, while variance is increased; the brain-wide organization of weak connections in schizophrenia patients correlated with negative symptom scores, allowing for a diagnostic accuracy of 75% [23]. The relative consistency of these whole-brain connectivity studies suggest that long-range connectivity is indeed globally disrupted in schizophrenia.
Possible neurobiological mechanisms of altered synchrony
As described above, studies of local synchrony with EEG or MEG suggest an inability of local circuits to support normal oscillations in schizophrenia, and examination of BOLD signal covariance indicates a global reduction in functional connectivity as well as specific disruptions in coordinated activity in critical schizophrenia-associated pathways. These two sets of findings are related in that they both suggest disrupted synchrony. But do they follow from the same underlying pathophysiology? Does either finding provide a causal link between schizophrenia risk and disease symptomology? Addressing these questions requires finding plausible mechanisms by which disruptions of cellular function produce the observed circuit and systems-level abnormalities. Two strong candidate mechanisms are hypofunction of fast-spiking parvalbumin-positive (PV+) interneurons, and disruption of myelination.
The critical role of GABAergic interneurons, particularly fast-spiking PV+ interneurons, in the generation of cortical oscillations, particularly gamma-frequency oscillations, is well established by direct manipulation of these cells in vivo; from such studies we have learned that optogenetically silencing PV+ interneurons impairs gamma [24] and that optogenetically driving them induces gamma [25]. The literature on impairments in GABAergic interneurons in schizophrenia is long-standing and sizable [26]. Post-mortem studies have demonstrated reduced mRNA expression for GAD67, the enzyme that catalyzes glutamate into GABA [27,28]. Such a decrease could result in less GABA or, alternatively, could reflect a downregulation of GABA precursors in response to impaired GABA metabolism. The GAD67 expression deficit is most pronounced in PV+ cells – in fact, GAD67 is not detectable in half of PV+ cells in schizophrenia patients [29]. Moreover, expression of parvalbumin itself is reduced in patients compared to controls, though the total number of these neurons may be normal [30]. Because the strength of parvalbumin expression is activity-dependent [31], this expression deficit could be a signature of hypoactivity. This hypoactivity may in turn result from an impairment of NMDA receptor signaling, as disruption of NMDA receptor signaling in PV+ cells reproduces a range of schizophrenia-related phenotypes, including decreased parvalbumin expression and behavioral impairments [32]. Finally, computational modeling work has suggested a potential link between observed GAT-1/GAD67 deficiencies in PV+ cells and prolonged IPSCs that could account not only for the impairment of 40Hz entrainment to auditory clicks but also the preferential entrainment to slower 20Hz stimuli [33].
While there exists ample evidence linking PV+ interneurons, gamma oscillations, and schizophrenia, gamma is thought to be generated in highly localized circuits [3]. And while mechanisms have been proposed to explain long-range gamma synchrony across distant brain regions [34,35], it is questionable whether disruption of fast-spiking interneuron networks can fully account for the observed impairments in long-range synchrony. An alternative candidate link between cellular deficits and observed synchrony deficits is a schizophrenia-related disruption of myelination [36]. Myelination of long-range pathways permits for rapid signaling across long distances; disruptions in myelin might thus be expected to disrupt long-range synchrony.
Diffusion tensor imaging (DTI) allows for detection and quantification of fiber tracts using the measure of fractional anisotropy (FA), which detects the degree to which the diffusion of water molecules is confined to a particular direction in any given voxel of brain tissue. While FA represents a conflation of axon number, thickness and level of myelination, it favors detection of white matter, due to the constraint of water molecules by multiple myelin layers [37]. Studies of DTI in schizophrenia patients reveal decreases in FA in both local and long-range connections. Regarding local connectivity, widespread decreases in FA have been found in frontal and occipital regions in patients despite preserved white matter volume, suggesting axon number and thickness do not account for the difference [38]. In long-range projection pathways, schizophrenia patients have been found to have lower FA in the splenium of the corpus callosum and in the cingulum [39]. Post-mortem tissue studies also support the idea that the myelin system is disrupted. Microarray analysis of DLPFC tissue from patients reveals impaired expression of genes related to myelination and oligodendrocyte function [40]. Electron micoscopy of postmortem tissue reveals a higher density of concentric lamellar bodies, which signal damage to myelinated fibers, in caudate nucleus of patients relative to healthy controls [41]. There is also evidence of increased necrosis and apoptosis and decreased overall density of oligodentrocytes in prefrontal layer 4 in post-mortem tissue from patients [42].
Understandably, deficits in myelin have been proposed to underlie long-range connectivity abnormalities. Similarly, deficits in interneuron function have been proposed to underlie the abnormalities in local synchrony seen in schizophrenia. But direct evidence for such a division is minimal, and the impact of the two cellular deficits and could overlap (Figure 2). Long-distance synchrony can involve locally generated oscillations [43] and may thus require interneurons to maintain this oscillation-based connectivity; moreover, it is becoming increasingly clear that there are inhibitory projection neurons that may be directly involved in long-range synchrony [44]. With regard to the role of myelination, findings of heterogeneous distributions of myelin within and across neocortical neurons suggests more nuanced and potentially pathway-selective contributions to cortical communication [45]. Modeling work has found that biologically relevant conduction delays on a scale consistent with changes in myelination can account for substantial attenuation of the amplitude of gamma oscillations [46]. Thus heterogeneity of myelination could have implications for even local cortical synchrony, just as interneuron dysfunction could potentially disrupt long-range synchrony; the two deficits might even synergize.
Figure 2. Possible mechanisms underlying disruptions in synchrony.
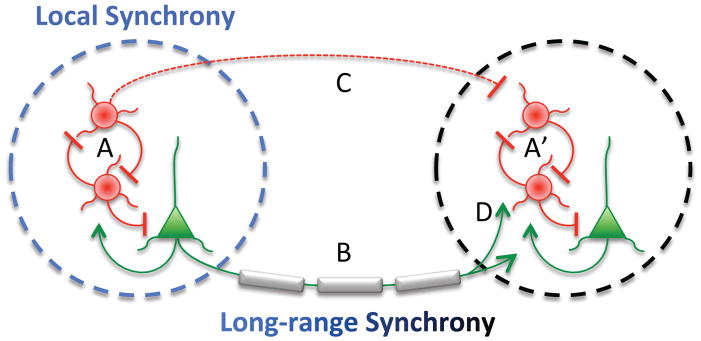
Local synchronous oscillations are thought to arise from interactions between local interneurons (red circles) and pyramidal neurons (green triangles) within a local circuit (A, A′); thus, deficits in interneuron function would lead to deficits in local synchrony. Long range synchrony is thought to rely on projections between brain regions that are predominantly glutamatergic (B); many of these projections are myelinated. Thus, deficits in myelination would lead to deficits in long-range synchrony. However, it is also possible that myelination deficits lead to local synchrony deficits if proper timing of long-range inputs also plays a role in rhythmogenesis (D). Conversely, interneuron deficits could lead to long-range synchrony deficits if local rhythms (A, A′) or inhibitory projections (B) play an important role.
Mechanisms: linking synchrony to behavior
Animal models of schizophrenia predisposition play a critical role in sorting out the complex relationships between cellular mechanisms, circuit synchrony, and behavior. Animal models allow for direct genetic, molecular and circuit manipulation, and for testing the effects of such manipulations on local and long-range synchrony as well as behavior. Indeed, disruption in synchrony is recapitulated in multiple rodent models of schizophrenia, including pharmacological, immunological and genetic models. Pharmacological models have chiefly focused on the glutamate hypothesis, employing NMDA antagonists such as ketamine and MK801 to block NMDA receptors. Systemic administration of ketamine increases baseline spontaneous gamma-range activity and decreases auditory-evoked (paired clicks, as opposed to steady-state) responses, similar to some findings in patients, as described above [47]. Stimulation of maternal immune response during pregnancy is another well-studied rodent model of schizophrenia predisposition, based on clinical evidence that early immune stress confers schizophrenia risk. Following maternal immune activation, impairments are seen in broad-spectrum hippocampal-prefrontal (HPC-PFC) synchrony [48]. Finally, several genetic mouse models of schizophrenia predisposition have replicated deficits in synchrony. In a mouse homologue to the 22q11.2 microdeletion syndrome, there is evidence of impaired long range HPC-PFC synchrony in the theta and gamma ranges; the long-range synchrony deficits correlate with cognitive impairment [49]. Moreover, a mouse heterozygous for a 22q11.2 component gene, Dgcr8, shows an inability to follow trains of 50-Hz stimulation in prefrontal layer II-V projections, a finding reminiscent of SSEP data from schizophrenia patients [50]. Mice heterozygous for the schizophrenia-associated gene Disc1 gene also show reduced HPC-PFC synchrony in the 30–50Hz gamma range [51]. Recently, a mouse homologous for the human 15q13.3 deletion, which is associated with schizophrenia and epilepsy, was found to exhibit the SSEP gamma deficits and elevated baseline gamma (prefrontal and hippocampus) often seen in schizophrenia patients [52].
But while numerous studies have found impaired synchrony in rodent models of schizophrenia risk, recapitulation of the disruption in circuit dynamics does not complete the picture. A model that links risk with circuit pathophysiology requires elucidation of the cellular and molecular signaling components of the disruption; for the models listed above, these mechanisms are unclear to date, though work continues to define them. One promising model where the cellular mechanisms have been more clearly defined involves neuregulin, a candidate schizophrenia-related gene that facilitates glutamate receptor subunit expression and is involved in synapse formation and stabilization. Neuregulin enhances cortical gamma oscillations in vivo and in vitro, acting on GABAergic interneurons and mediated by synapses (as opposed to gap-junctions) [53]. Moreover, deletion of Erbb4, a receptor for neuregulin, from PV+ interneurons results in decreased HPC-PFC theta coherence under anesthesia and increased HPC gamma power during running (Figure 1c) [54]. Thus investigating the interactions between neuregulin and Erbb4 and their downstream consequences on synapse and circuit function is one potential avenue by which we might begin to mechanistically link genetic risk with neural synchrony deficits. Similar links upward from synchrony to behavior might be forged with any of the models mentioned here.
Conclusions
One could reasonably argue that the observation of abnormal circuit-level brain dynamics are a trivial sequitur of the cognitive impairments and disorganized thinking associated with schizophrenia; if cognitive processes are disrupted, so must be their neurophysiological substrates. But a mounting body of research increasingly points to a more selective disruption. Consistent findings of an impaired capacity to sustain rhythmic (specifically gamma-frequency) activity, a decreased response in this frequency range to task-related stimuli, and diminished global functional connectivity, lead to a common conclusion: the idea of schizophrenia as a “disconnection syndrome”, originally based on evidence from clinical symptomology, has a neurophysiological basis in impaired temporal coordination of neural activity. Deficits in synchrony can therefore be seen as a mid-level phenotype that can be related to causal mechanisms as well as behavioral consequences, and thus serve as a potentially useful starting point for understanding the pathophysiology of schizophrenia.
Much work remains to be done in elucidating the mechanisms of these disruptions at the level of microcircuits. Pharmacological models must be increasingly targeted to receptor and neuronal subtypes, in relevant brain regions, perhaps making use of pharmaco- and optogenetics to elucidate fine-grained circuit dysfunction. Genetic models have done much to validate the phenotypic homology resulting from targeted mutations by recapitulating synchrony deficits seen in human patients, but genetic and pharmacological rescue experiments are needed to demonstrate which cellular mechanisms downstream of these genes of interest are critical to the impairment. Animal studies of myelin disruption typically model severe, global myelin impairments involving peripheral neuropathy and motor impairments not typically seen in schizophrenia. The development of more subtle, neuroanatomically restricted models of myelin disruption (such as inducible oligodendrocyte hypofunction), paired with in vivo recordings, could prove valuable in linking clinically observed myelin impairments with disruption of rhythmic synchrony and functional connectivity. Finally, careful elucidation of the effects of particular manipulations on both local and long-range synchrony have to potential to determine whether these are two separable phenomena that happen to occur in the same syndrome, or whether they share overlapping pathophysiological causes and consequences.
Highlights.
Schizophrenia is characterized by deficits in local and long-range synchrony
These deficits provide circuit-level phenotypes
Animal models can link synchrony deficits to cellular mechanisms
Acknowledgments
J.A.G. is supported by grants from the NIMH (R01 MH096274 and P50MH096891), the International Mental Health Research Organization, and the Hope for Depression Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 2.Ulhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 3.Buzsaki G, Wang X. Mechanisms of Gamma Oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Krishnan G, Hetrick W, Brenner C, Shekhar A, Steffen A, O’Donnell B. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. Examined spatial distribution and frequency resonance of SSEPs from auditory clicks and found a distinct reponse curve, with stimuli at 45Hz inducing the strongest response, which was spatially widespread but strongest over frontal midline. Schizophrenia patiens were found to have reduced response, measured by both the mean gamma power and the inter-trial coherence, a measure of the temporal precision and reliability of the response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon J, O'Donnell B, Wallenstein G, Greene R, Hirayasu Y, Nestor P, Hasselmo M, Potts G, Shenton M, McCarley R. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner C, Sporns O, Lysaker P, O’Donnell B. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 7.Wilson T, Hernandez O, Asherin R, Teale P, Reite M, Rojas D. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18(2):371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer KM, Salisbury D, Shenton M, McCarley R. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer K, Niznikiewicz M, Nestor P, Shenton M, McCarley R. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Light G, Hsu J, Hsieh M, Meyer-Gomes K, Sprock J, Swerdlow N, Braff D. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60(11):1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 11**.Spencer K, Nestor P, Perlmutter R, Niznikiewicz M, Klump M, Frumin M, Shenton M, McCarley R. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci. 2004;101(49):17288–17293. doi: 10.1073/pnas.0406074101. Following on a finding of impaired reaction time and impaired 40Hz stimulus-evoked responses in schizophrenia patients, Spencer et al analyzed these data temporally aligned to the behavioral output, rather than the stimulus. They found that, rather than an absence of response, there was a pronounced but lower-frequency response in patients relative to controls. Moreover, the strength of this lower frequency response correlated positively with scores for visual hallucinations, global thought disorder, and conceptual disorganization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 13.Haenschel C, Bittner R, Waltz J, Haertling F, Wibral M, Singer W, Linden D, Rodriguez E. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29(30):9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho R, Konecky R, Carter C. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grützner C, Wibral M, Sun L, Rivolta D, Singer W, Maurer K, Uhlhaas P. Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front Hum Neurosci. 2013;7:88. doi: 10.3389/fnhum.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Gandal MJ, Edgar JC, Klook K, Siegel SJ. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Neuropharmacology. 2012;62(3) This study used EEG to examine the comparative strength of resting-state oscillatory activity (in eyes-open and eyes-closed states), in schizophrenia patients, relatives, and healthy controls. In both conditions, schizophrenia patients had strongly and significantly higher theta-frequency activity than relatives and controls (across all recorded sites), while both patients and relatives had higher gamma power relative to controls in the eyes-open condition, particularly in more posterior sites corresponding with visual cortex. [Google Scholar]
- 17.Rutter L, Carver F, Holroyd T, Nadar S, Mitchell-Francis J, Apud J, Weinberger D, Coppola R. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldeweg T, Spence S, Hirsch S, Gruzelier J. γ-band electroencephalographic oscillations in a patient with somatic hallucinations. The Lancet. 1998:352. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- 19.Chai X, Whitfield-Gabrieli S, Shinn A, Gabrieli J, Nieto C, McCarthy J, Cohen B, Ongür D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang X, Shen H, Wang L, Liu Z, Xin W, Hu D, Miao D. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 2014;1562:87–99. doi: 10.1016/j.brainres.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Argyelan M, Ikuta T, DeRosse P, Braga R, Burdick K, John M, Kingsley P, Malhotra A, Szeszko P. Resting-state FMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull. 2014;40(1):100–110. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkataraman A, Whitford T, Westin C, Golland P, Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res. 2012;139(1–3):7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Bassett D, Nelson B, Mueller B, Camchong J, Lim K. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59(3):2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. Here Bassett et al quantified complexity in resting-state fMRI data, using bivariate and multivariate measures. This yeilded a brain-wide measure of pairwise functional connectivity, which was found to be lower in schizophrenia patients. Moreover, this measure allowed for classification of disease state, which was 75% accurate and 85% sensitive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohal V, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardin J, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L, CIM Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis D, Curley A, Glausier J, Volk D. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti A, Auta J, Davis J, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson D, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 28.Curley A, Arion D, Volk D, Asafu-Adjei J, Sampson A, Fish K, Lewis D. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168(9):921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto T, Volk D, Eggan S, Mirnics K, Pierri J, Sun Z, Sampson A, Lewis D. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo T, Miller J, Lewis D. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154(7):1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 31.Donato F, Rompani S, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2010;504(7479):272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 32.Belforte J, Zsiros V, Sklar E, Jiang Z, Yu G, Li Y, Quinlan E, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99(5):2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vicente R, Golloc L, Mirassoc C, Fischerd I, Pipaa G. Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proc Natl Acad Sci. 2008;10544:17157–17162. doi: 10.1073/pnas.0809353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajagovindan R, Ding M. Decomposing Neural Synchrony: Toward an Explanation for Near-Zero Phase-Lag in Cortical Oscillatory Networks. PLOS One. 2008;3(11):e3649. doi: 10.1371/journal.pone.0003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis K, Stewart D, Friedman J, Buchsbaum M, Harvey P, Hof P, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 37.Kubicki M, McCarley R, Westin C, Park HJ, Maier S, Kikinis R, Jolesz F, Shenton M. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim K, Hedehus M, Moseley M, de Crespigny A, Sullivan E, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56(4):367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 39.Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, Cong Z, Zhang H, Li B, Hong N, et al. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14(14):1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- 40.Hakak Y, Walker J, Li C, Wong W, Davis K, Buxbaum J, Haroutunian V, Fienberg A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55(5):597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 42.Uranova N, Vostrikov V, Orlovskaya D, Rachmanova V. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67(2–3):269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 43.Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21(3):486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caputi A, Melzer S, Michael M, Monyer H. The long and short of GABAergic neurons. Current Opinion in Neurobiology. 2013;23:179–186. doi: 10.1016/j.conb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Tomassy G, Berger D, Chen H, Kasthuri N, Hayworth K, Vercelli A, Seung H, Lichtman J, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344(6181):319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pajevic S, Basser P, Fields R. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.007. S0306-4522(13)00947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazarewicz M, Ehrlichman R, Maxwell C, Gandal M, Finkel L, Siegel S. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22(7):1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 48.Dickerson D, Wolff A, Bilkey D. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30(37):12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigurdsson T, Stark K, Karayiorgou M, Gogos J. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464(7289):763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fénelon K, Mukai J, Xu B, Hsu P, Drew L, Karayiorgou M, Fischbach G, Macdermott A, Gogos J. Deficiency of Dgcr8, a gene disrupted by the 22q11. 2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci. 2011;108(11):4447–4452. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurihara M, Dunlop J, Bandon N, Hughes Z, Randall A, Brown J. Soc Neurosci. Altered hippocampal-medial prefrontal cortex connectivity In vitro and In vivo in a transgenic Disc1 mouse model of psychiatric disease. Edited By 2013. [Google Scholar]
- 52.Fejgin K, Nielsen J, Birknow M, Bastlund J, Nielsen V, Lauridsen J, Stefansson H, Steinberg S, Sorensen H, Mortensen T, et al. A Mouse Model that Recapitulates Cardinal Features of the 15q13.3 Microdeletion Syndrome Including Schizophrenia- and Epilepsy-Related Alterations. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.014. S0006-3223(13)00767-1. [DOI] [PubMed] [Google Scholar]
- 53.Hou X, Ni K, Yang JM, Li X. Neuregulin 1/ErbB4 enhances synchronized oscillations of prefrontal cortex neurons via inhibitory synapses. Neuroscience. 2014;261:107–117. doi: 10.1016/j.neuroscience.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 54**.Del Pino I, García-Frigola C, Dehorter N, Brotons-Mas J, Alvarez-Salvado E, Martínez de Lagrán M, Ciceri G, Gabaldón M, Moratal D, Dierssen M, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79(6):1152–1168. doi: 10.1016/j.neuron.2013.07.010. Del Pino generated conditional knock-out mice for the Erbb4 receptor in PV+ interneurons. Awake, freely moving mutants showed an increase in cortical gamma power. Under anesthesia, mutants showed impairment in synchrony between hippocampus and prefrontal cortex in the theta range. Mutants also displayed hyperlocomotion, decreased anxiety and grooming, decreased social behavior, impaired sensorimotor gating, and impaired performance on a spatial alternation task. [DOI] [PubMed] [Google Scholar]