Abstract
Psychosis is increasingly being understood as a neurodevelopmental “dysconnection” syndrome, in which neural connectivity – at both microscopic and macroscopic levels of brain organization – becomes disrupted during late adolescence and early adulthood. Tools to quantify normative brain development and identify individuals at risk are urgently needed to tailor appropriate strategies for prevention and intervention, and could substantially improve clinical outcomes. Resting-state functional connectivity magnetic resonance imaging (rsfc-MRI) provides a rich, functional description of the brain’s macroscopic connectivity structure. Over the past several years, rsfc-MRI has evolved to be a powerful tool for studying both normal brain development and abnormalities associated with psychosis. Several recent advances highlight intriguing and potentially significant parallels between these two lines of research. For instance, rsfc-MRI work suggests that psychosis is accompanied by loss of segregation between large-scale brain association networks, a pattern that is normal in early life but typically matures into more segregated systems by young adulthood. Coupled with data sharing across large-scale neuroimaging studies, longitudinal assessments using rsfc-MRI in patients and those at risk will be essential for improving our biological understanding of psychosis and will help inform diagnosis, prognosis, and clinical decision-making.
INTRODUCTION
Schizophrenia is a major neuropsychiatric disorder that effects approximately 1% of the population worldwide, with frequently devastating consequences. Prior to the diagnosis of schizophrenia, sub-threshold psychotic-spectrum symptoms that impact functioning are common. Convergent evidence from multiple sources including animal model systems, epidemiologic data of maternal infections, and human neuroimaging has led psychosis to be increasingly understood as a disorder of neurodevelopment [1]. Such a re-conceptualization has led to the hope that a better description of the neurodevelopmental origins of psychosis will allow early interventions to “bend the curve” of abnormal brain development and lead to improved patient outcomes [2].
Resting-state functional connectivity magnetic resonance imaging (rsfc-MRI) has evolved to be a powerful tool for studying both normal brain development and abnormalities associated with psychosis. Initially described in 1995 by Biswal et al., functional connectivity is defined as time-series correlations in the blood oxygen level dependent (BOLD) signal between different brain regions, which are most predominant in low frequencies [3]. Brain regions that are functionally connected to each other are often spatially distributed, and reliably delineate large-scale functional brain networks [4,5]. Functional networks defined by rsfc-MRI accord to a remarkable degree with studies of task-based fMRI activation [6].
rsfc-MRI has certain properties that make it particularly advantageous for the study of psychosis and brain development. First, the limited behavioral demands of the acquisition procedure are an advantage: both young participants and more symptomatic patients may have difficulty performing a task paradigm appropriately in task-based fMRI studies. In contrast, the “task” of rsfc-MRI is to rest quietly. Second, the simplicity of the data acquisition procedures allow it to scale easily even to very large studies. This is a major advantage for the study of psychosis in youth, where heterogeneity is likely to be substantial. Two substantial sources of heterogeneity include normal developmental processes and biologic heterogeneity within clinical phenotypes. Effective decomposition of such heterogeneity into normative “growth charts”[7] that can be used to identify abnormalities of brain development associated with psychosis requires large-scale studies. Studies that seek to relate abnormal connectivity phenotypes to genetic data similarly require very large samples. Third and finally, rsfc-MRI provides extremely rich data regarding functional brain networks, which may be a particularly informative unit of analysis for psychotic disorders, and are amenable to many complementary analytic strategies.
Here we review the existing literature regarding how rsfc-MRI has been used to understand psychosis as a disorder of brain development. We focus on key studies of normal development, studies in adults with schizophrenia, and research from youth at-risk and those with prodromal symptoms. As described below, rsfc-MRI provides valuable evidence that a failure of functional network development may be a critical feature of psychosis, and provides a uniquely flexible tool for further research.
STUDIES OF NORMAL BRAIN DEVELOPMENT
While initial rsfc-MRI studies mapped functional connectivity in adults, the promise of examining how functional brain networks evolved in youth was quickly recognized. In a classic series of studies, Fair et al. initially described how the cognitive control system evolved into adult structure through segregation of fronto-parietal (FP) and cingulo-opercular (CO) elements [8]. In a follow-up study Fair et al., tracked increases in connectivity within the default mode network, and noted how default mode regions became more densely connected with each other but more segregated and less strongly connected with elements of the executive system [9]. Subsequent research has provided convergent evidence. Kelly et al. found evidence for more focal within-network connectivity from a range of anterior cingulate seed regions linked to diverse cognitive and social processes [10]. Using both a discovery and replication sample, Uddin et al. found similar evidence for increased connectivity within elements of the control network and the default mode network [11]. Notably, a recent ground-breaking study by Thomason et al. demonstrated that changes in inter-hemispheric connectivity consistent with network development can even be seen in developing fetuses [12]. Overall, these findings from studies of specific brain networks suggest that within-network connectivity increases with development while between-network connectivity diminishes (see Figure 1) [13].
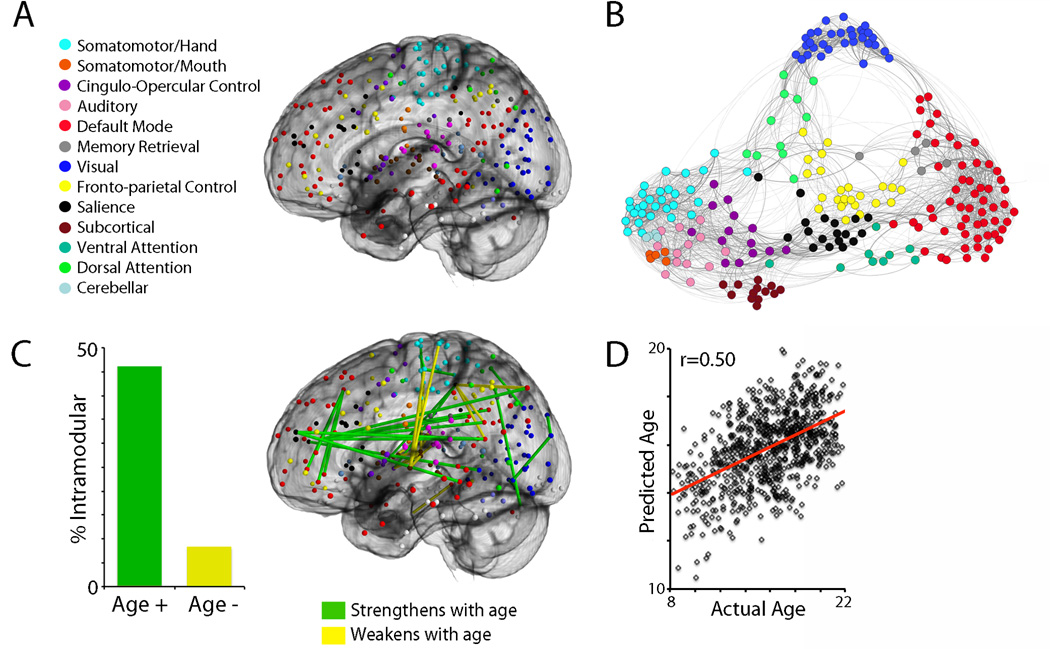
Figure 1.
(A) Functional network definition. Nodes from Power et al. (2011) define 13 large-scale functional brain networks. Nodes are colored according to module membership as indicated in the figure legend. (B) Functional network structure. Spring-embedded rendering of mean network connectivity matrix in a sample of 780 youth. Graph edge thickness is scaled according to connection strength. Nodes are colored by module assignment as in (A). As noted by Power et al. (2011), certain network modules (motor, visual, default) are more segregated, whereas networks implicated in cognitive control (frontoparietal, salience) display more inter-modular connections. (C) Large-scale functional brain networks become more segregated with development. Connections that strengthen with age are significantly more likely to be within a functional module than between functional modules. Connections that strengthen with age are displayed in green, connections that weaken with age are displayed in yellow. This effect is robust: only connections that survive that surpassed a Bonferroni-corrected statistical threshold (corrected p<0.05, uncorrected p<1.4×10−6) are displayed. Notably, this effect is enhanced when controlling for motion artifact. (D) Multivariate patterns of connectivity accurately predict an individual’s chronologic age. Using a 10-fold cross-validated support vector regression in a sample of 780 youth, the multivariate pattern of connectivity from the network defined in A can be used to accurately estimate an individual’s age. All panels adapted from Satterthwaite et al., 2013 (Ref 13).
This hypothesis was explicitly tested on a whole-brain basis in a study by Anderson et al., who found that connectivity gradients between large-scale functional brain networks increased with development [14]. Barber et al. found similar evidence for the development of network segregation in the form of enhanced anti-correlation between the task-positive executive system and the default mode network [15]. Importantly, the strength of this relationship was related to cognitive capability (i.e., performance on a Go/No-Go task). Finally, in a high impact paper Dosenbach et al. used the complete multivariate pattern of connectivity to predict neurodevelopment on a single-subject level using a cross-validated support vector regression [16]. Notably, the strengthening of within-network connections and weakening of between-network connections were among the most highly predictive model features. This study demonstrated for the first time that evolving functional connectivity including patterns of network segregation could be used to create a normative growth chart of brain development, and raised the possibility that deviations from this trajectory could be detected within pathological conditions such as psychosis. Next, we examine evidence from studies of adults with psychosis and youth at risk within this context.
STUDIES OF ADULTS WITH PSYCHOSIS
Since well before the introduction of rs-fcMRI, changes within dorsolateral prefrontal cortex (DLPFC) have been reported in psychosis. Convergent evidence from animal models, human post-mortem tissue, and task-based functional imaging provides an elegant multi-level explanation for how cellular and synaptic changes (e.g., in parvalbumin-containing GABAergic interneurons) may lead to computational inefficiencies in DLPFC function that in turn lead to psychosis and cognitive dysfunction [17–19]. Resting state imaging has bolstered and extended these observations, placing them into a network framework. rs-fcMRI reveals, for instance, how variation in a large-scale system that includes DLPFC may alter relationships between systems responsible for other domains of information processing, and might help to explain disparate network-level findings in schizophrenia (see Figure 2) [20–26].
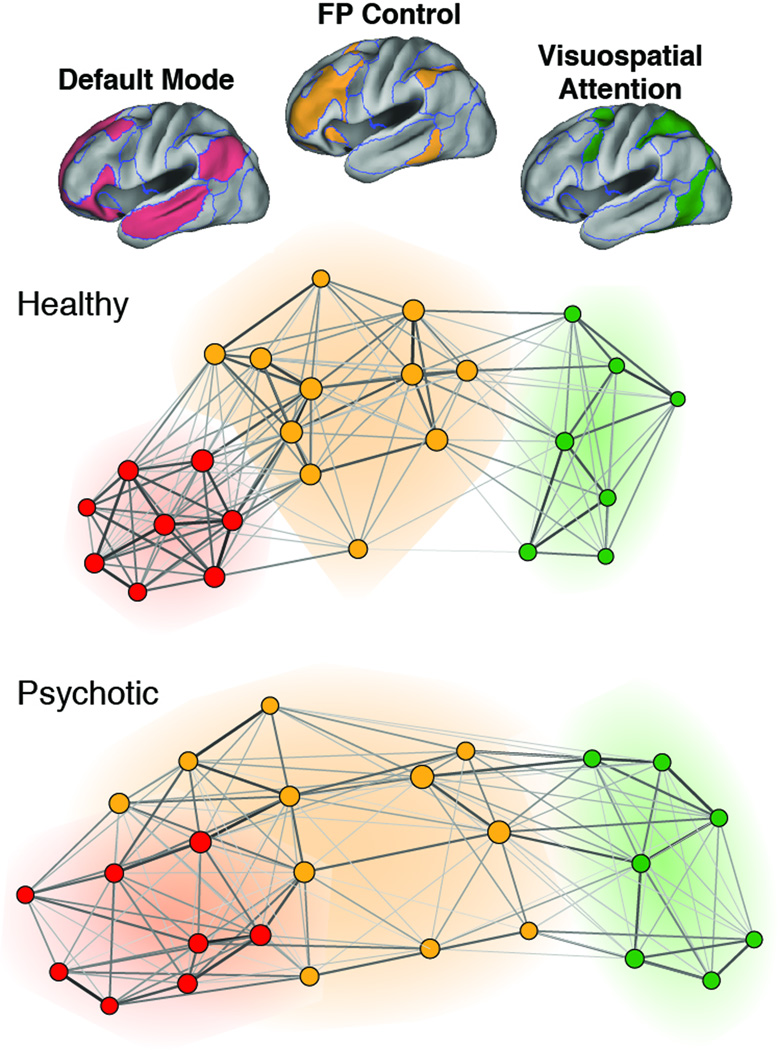
Figure 2. Variation in and among large-scale association networks in chronic psychosis.
Weighted graphs derived from groupwise regional BOLD time course correlations between selected nodes (i.e. cortical regions) of the frontoparietal control network (orange), visuospatial attention network (green), and default network (red) in healthy (n=100, upper panel) and psychotic individuals (n=100, lower panel). Healthy adults showed clear segregation between FP control and default networks (represented with non-overlapping orange and red halos), while patients with chronic psychotic illness showed less clustering within default and frontoparietal control networks and evidence of extension of FP control nodes into the default cluster (red-orange halo). Adapted from Baker et al., 2104 (Ref 21).
Several efforts that have used rsfc-MRI to survey large-scale brain systems for evidence of dysconnectivity in psychotic disorders have come to broadly similar conclusions, albeit by distinctive analytic approaches [21,23–26]. Among such studies, reduction in BOLD time course correlation among components of the FP control network appears to be the most reproducible finding, while changes in other association networks are also frequently observed, albeit to a lesser or less reliable extent, including within the “Salience” or ventral attention network [21,24,25,27] and the default network [21,27]. Network-wide analyses using data-driven selection of a priori regions and networks of interest will likely be critical to reaching consensus across these kinds of studies.[21] Primary sensory and motor networks appear to show largely intact functional connectivity patterns, according to most reports. These studies confirm the central nature of large-scale association network disruption in psychosis, and together with the task-based fMRI literature (see Barch and Caeser; [17]) support the idea that dysfunction within control networks may be a final common pathway, or shared biological substrate, for the core construct of psychosis that subsequently leads to a heterogeneous clinical presentation via idiosyncratic disruption of other large-scale brain systems in each individual.
In addition to such studies of control networks, connectivity within the default mode network has been studied extensively in psychosis. At least initially, the default network garnered considerable attention due to (a) its purported role in processing internal stimuli and representations of the self (e.g. psychological face validity), (b) its position as an information processing “hub” for the cerebral cortical network (i.e., computational face validity, (c) its amenability to rsfc-MRI analysis and relative stability across participants, and also (d) its newcomer status as a focus of study (it was first described only in the late 1990s). Whitfield-Gabrieli and colleagues found that patients with schizophrenia had increased within-network default network connectivity, as compared with healthy controls, in the context of a working memory task [28]. Remarkably, they also reported default network hyperconnectivity in unaffected first-degree relatives of the patients, as well as correlation among affected individuals between psychotic symptoms and default network hyperactivity during task performance, suggesting that “a failure to suppress default mode” function might represent an endophenotype or core, heritable feature of psychotic illness. Other studies in at risk populations show similar results (see “Studies of Children and Adolescents at risk” below), raising additional interest in this phenotype,[29,30] despite the lack of consistent default network findings in chronic patients.
Several rsfc-MRI studies in psychotic individuals have also highlighted changes in the interactions between cortical and subcortical systems, including the thalamus and basal ganglia, both long believed critical in the pathophysiology of psychotic illness. Abnormal functional connectivity between frontal cortex and the thalamus were observed in two recent studies [26,31]. Significantly, both studies found evidence of a mixed pattern of dysconnectivity, with reduced connectivity between thalamus and prefrontal cortex and increased connectivity between thalamus and motor cortex.
Finally, several studies have examined global changes in brain organization and function in psychosis. Using graph theoretic measures, several reports describe a broad breakdown in cortical functional organization, evidenced by reduced local network connectivity, reduced modular structure, but greater global network robustness [22,32–35]. Potentially relevant to these widespread observations, Yang et al. found that BOLD signal in the low frequency range (< 0.1 hz) may also be globally elevated in schizophrenia [36]. Importantly, head motion can also lead to the appearance of reduced global modularity and increased low frequency power [37,38], making careful interpretation of global changes critical for understanding and generalization of these findings (see Limitations, below). However, recent work including that by Yang et al. has rigorously controlled for such confounds [36].
Taken together, these rs-fcMRI studies of adults with psychosis have revealed intriguing, but as yet untested, parallels between the biology of normal brain development and the emergence of psychosis. The patterns seen in patients – e.g., reduced modularity and incompletely segregated FP-CO control network – have a notable correspondence to stages of normal adolescent development, raising the intriguing possibility that psychosis may delay key developmental stages, particularly in the refinement of cortical association systems. Alternatively, psychosis may represent a developmental stage, or sensitive period, that was never properly closed, perhaps due to oxidative stress [39], leaving brain systems in a state of extended plasticity longer than appropriate and thus vulnerable to environmental insults outside the usual developmental window. Whether these speculations have etiologic relevance will depend on detailed longitudinal assessments in individuals at risk for psychosis and in the earliest stages of illness.
STUDIES OF CHILDREN AND ADOLESCENTS AT RISK
Only a relatively small number of studies have measured functional connectivity in young individuals at risk for psychosis or with early onset forms of the illness. Two recent studies recently extended upon the Whitfield-Gabrieli findings [28], focusing on changes in DMN hyperconnectivity in early forms of psychosis. Fryer et al. reported DMN hyperconnectivity in youth at clinical high risk for psychosis and early schizophrenia in the context of a working memory task, consistent with the idea that deficient suppression of DMN activity during task performance may be present early in the disease course [29], Wotruba and colleagues also reported similar findings, describing a loss of anticorrelation between a task-positive (i.e., FP control like) network and the DMN in individuals experiencing prodromal symptoms [30], Fornito and colleagues recently found that individuals with early psychosis and their unaffected relatives showed corticostriatal dysregulation, with dorsal striatal hypoconnectivity and ventral striatal hyperconnectivity with cortical targets [40], Other connectivity abnormalities have not been found to extend into the prodromal period: for example, amygdala connectivity with orbitofrontal cortex is reduced in chronic and early psychosis patients, but was preserved in high risk patients [20].
Additional research that is nearing publication also suggests intriguing dynamics of cortical network architecture around the time of psychosis onset. In a study of 129 unmedicated patients in the early stages of a psychotic illness, Anticevic et al. found evidence of hyperconnectivity between the same prefrontal-thalamic regions that show reduced connectivity in the chronic state (Anticevic et al., unpublished). Thus, early or prodromal psychosis may be qualitatively distinct biological entities compared with chronic illness. If correct, this view suggests that cortical brain systems may compensate for an early insult, which yields a state of hyperconnectivity, by systematically reducing connectivity. These tentative hypotheses require significant additional investigation in cohorts of patients who can be followed longitudinally over the course of illness, as they progress from an at-risk to disease state. Thus, although the available data do not yet paint a clear picture of how developmental connectivity changes relate to those seen in chronic psychotic illness, they highlight the utility with which rsfc-MRI can provide key information constraining the possible biological explanations for psychosis and its emergence in young adulthood.
LIMITATIONS
Despite its numerous advantages and the results described above, rsfc-MRI is not without limitations, including limits of interpretation and susceptibility to artifact. While the low-level task in rsfc-MRI is often an advantage, this lack of constraint also limits interpretation. Effects are usually interpreted within the context of functional networks, but without supporting data from clinical, cognitive, or behavioral data such results may be easily over-interpreted.
Over-interpretation of unconstrained rsfc-MRI results is a particular risk in cases where unmeasured individual or group differences in subject motion are present. Three studies published in close synchrony recently demonstrated that even small amounts of in-scanner subject motion systematically biases measured functional connectivity [37,41,42]. As motion is often strongly related to age and clinical status, motion artifact is a major confound for developmental and psychiatric neuroimaging.
Notably, the effects of motion are the reverse of one of the most commonly reported developmental findings, which is that with development long distance connections strengthen and short-range connections weaken. Conversely, when global signal regression is used, motion is associated with a decrease in long-range connectivity and increase in short-range connectivity. Two studies systematically reassessed developmental changes in light of this artifact, and found that motion markedly inflated previous reports of distance-dependent changes in connectivity [13,43]. However, the effect of motion artifact on patterns of brain development are heterogeneous: while motion inflates estimates of distance-dependent change in connectivity, motion in fact obscures evidence of functional network segregation [13]. When motion artifact is adequately controlled, network segregation with development is more easily observable and individual age prediction remains robust (Figures 1C and 1D). More recently, advances in MRI acquisition [44] and post-processing techniques have been shown to mitigate motion artifact substantially [38,41].
ONGOING EFFORTS AND FUTURE DIRECTIONS
Taken together, the studies reviewed above emphasize that rsfc-MRI is a valuable phenotype for which to study how psychosis may be related to abnormalities of brain development. Indeed, because of its advantages, rsfc-MRI is being applied at increasingly large scales to study the developmental and genetic origins of psychosis. In the coming months, the North American Prodromal Longitudinal Study (NAPLS) is set to release its findings from its multi-site rsfc-MRI study of youth at risk for psychosis [45]. Similarly, the Philadelphia Neurodevelopmental Cohort [46] is tracking psychosis-spectrum symptoms within a large cohort of youth (9,500 phenotyped, 1,601 imaged). In combination with other large-scale imaging initiatives including PING, the NKI-Rockland Sample, IMAGEN, PSYSCAN, PRONIA, the Genome Superstruct Project [5], B-SNIP [47], and the UK BioBank Imaging Extension, this data will provide unprecedented ability to study the evolution of functional brain networks in health and disease. Because of the likely heterogeneity in brain phenotypes associated with mental illnesses such as psychosis, combining data across large samples will be critical and facilitated by efforts to harmonize imaging acquisition parameters and clinical phenotyping strategies (i.e., the NIH Common Data Elements initiative). Data from these studies and also large-scale meta-analytic efforts such as the ENIGMA consortium [48] will provide an opportunity for integrating genomic data in order to understand how genetic vulnerability may be associated with abnormalities in functional brain networks that lead to symptoms of psychosis. Finally, new multi-band fMRI acquisition techniques [49] increasingly allow higher-resolution examination of dynamic reconfiguration of resting-state networks over time [50], which may provide novel brain phenotypes related to the development of psychosis.
HIGHLIGHTS.
Psychosis is increasingly understood as a disorder of neurodevelopment.
Resting state fMRI powerfully describes brain development and psychosis.
Here we review rsfc-MRI studies of development and psychosis.
ACKNOWLEDGEMENTS
TDS was supported by K23MH098130 and the Marc Rapport Family Investigator grant through the Brain and Behavior Foundation. JTB was supported by KL2 TR001100 (Harvard Catalyst), the Maria Lorenz Pope Fellowship, and NIMH K23 MH104515-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT: Nothing declared.
REFERENCES
- 1.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 3. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. The original study demonstrating functional connectivity with resting-state fMRI.
- 4.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gur RC, Calkins ME, Satterthwaite TD, et al. Neurocognitive Growth Charting in Psychosis Spectrum Youths. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 8. Fair DA, Dosenbach NUF, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. The first in a classic series of papers demonstrating development of functional networks.
- 9.Fair DA, Cohen AL, Dosenbach NUF, et al. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences. 2008;105:4028. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly AMC, Di Martino A, Uddin LQ, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 11.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomason ME, Dassanayake MT, Shen S, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013;5:173ra24. doi: 10.1126/scitranslmed.3004978. Ground-breaking demonstration of developing brain networks in fetal MRI.
- 13.Satterthwaite TD, Wolf DH, Ruparel K, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 2011;1:147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dosenbach NUF, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. High-impact study which demonstrated that rsfc-MRI can be used to predict an individual’s age in part through measurement of network segregation.
- 17.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- 20. Anticevic A, Tang Y, Cho YT, et al. Amygdala Connectivity Differs Among Chronic, Early Course, and Individuals at Risk for Developing Schizophrenia. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt165. A rare example of a study that compared functional connectivity metrics across phases of illness, finding distinctive patterns of amygdalar connectivity in high risk and early psychosis cohorts.
- 21. Baker JT, Holmes AJ, Masters GA, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. This well-powered study (N=200) compared connectivity among functionally-defined cortical regions and also contrasted the cortical functional parcellation of healthy individuals and patients with psychosis.
- 22.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khadka S, Meda SA, Stevens MC, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. Another example of an endophenotype study, which found abnormalities in large-scale network interactions, some shared across patients and relatives and some uniquely in patients.
- 24.Mamah D, Barch DM, Repovs G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 2013;150:601–609. doi: 10.1016/j.jad.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. This landmark paper established abnormal default network functional connectivity as a potential endophenotype in schizophrenia by showing changes in both patients and unaffected relatives.
- 29.Fryer SL, Woods SW, Kiehl KA, et al. Deficient Suppression of Default Mode Regions during Working Memory in Individuals with Early Psychosis and at Clinical High-Risk for Psychosis. Front Psychiatry. 2013;4:92. doi: 10.3389/fpsyt.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wotruba D, Michels L, Buechler R, et al. Aberrant Coupling Within and Across the Default Mode, Task-Positive, and Salience Network in Subjects at Risk for Psychosis. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anticevic A, Cole MW, Repovs G, et al. Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cereb Cortex. 2013 doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander-Bloch AF, Gogtay N, Meunier D, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynall M-E, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. A small study (12 patients, 15 controls) that examined cortical network topology in schizophrenia and found both deficits and enhanced robustness to "random attack".
- 34.Tomasi D, Volkow ND. Mapping Small-World Properties through Development in the Human Brain: Disruption in Schizophrenia. PLoS One. 2014;9:e96176. doi: 10.1371/journal.pone.0096176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van den Heuvel MP, Sporns O, Collin G, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. This study reported increased correlation between structural and functional connectivity matrices in patients, as compared with controls, finding evidence from structural (diffusion) imaging of abnormalities in"rich-club” connectivity.
- 36. Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A. 2014;111:7438–7443. doi: 10.1073/pnas.1405289111. A well-powered study in chronic patients finding that low frequency power is globally increased in schizophrenia.
- 37. Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. Demonstrates relevance of resting-state motion artifact for studies of neurodevelopmental connectivity.
- 38.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fournier M, Ferrari C, Baumann PS, et al. Impaired Metabolic Reactivity to Oxidative Stress in Early Psychosis Patients. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 41.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2011;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2011;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fair DA, Nigg JT, Iyer S, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kundu P, Brenowitz ND, Voon V, et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Addington J, Cadenhead KS, Cannon TD, et al. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satterthwaite TD, Elliott MA, Ruparel K, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meda SA, Ruano G, Windemuth A, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111:E2066–E2075. doi: 10.1073/pnas.1313093111. A large multi-site study (N=1,305) that used ICA to identify genes associated with connectivity in sub-components of the default network.
- 48.Thompson PM, Stein JL, Medland SE, et al. The ENIGMA Consortium: largescale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014 doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feinberg DA, Moeller S, Smith SM, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]