Abstract
Forkhead box P3 (Foxp3)+ regulatory T (Treg) cells maintain the immune tolerance and prevent inflammatory responses in the periphery. However, the presence of Treg cells in the central nervous system under steady state has not been studied. Here, for the first time, we show a substantial TCRαβ+CD4+Foxp3+ T-cell population (cerebral Treg cells) in the normal rat cerebrum, constituting more than 15% of the cerebral CD4+ T-cell compartment. Cerebral Treg cells showed an activated/memory phenotype and expressed many Treg-cell signature genes at higher levels than peripheral Treg cells. Consistent with their activated/memory phenotype, cerebral Treg cells robustly restrained the LPS-induced inflammatory responses of brain microglia/macrophages, suggesting a role in maintaining the cerebral homeostasis by inhibiting the neuroinflammation. In addition, brain astrocytes were the helper cells that sustained Foxp3 expression in Treg cells through IL-2/STAT5 signaling, showing that the interaction between astrocytes and Treg cells contributes to the maintenance of Treg-cell identity in the brain. Taken together, our work represents the first study to characterize the phenotypic and functional features of Treg cells in the normal rat cerebrum. Our data have provided a novel insight for the contribution of Treg cells to the immunosurveillance and immunomodulation in the cerebrum under steady state.
Keywords: Regulatory T cells, cerebrum, microglia, macrophages, inflammation
INTRODUCTION
Forkhead box P3 (Foxp3)+ regulatory T (Treg) cells maintain immune tolerance and prevent inflammation to modulate immune homeostasis [1]. Although the development and activities of Treg cells are best characterized in the lymphoid tissues, emerging evidence indicates they also sustain immune homeostasis in non-lymphoid tissues such as skin [2, 3], skeletal muscle [4, 5], visceral adipose tissue [5, 6], and the central nervous system (CNS) [7]. Studies of Treg cells in the CNS mainly focus on CNS disorders such as multiple sclerosis and experimental autoimmune encephalomyelitis [8], Alzheimer's disease [9, 10], ischemia [11] and gliobastoma [12, 13]. In such disorders the compromised blood brain barrier (BBB) allows for the entry of T lymphocytes including Treg cells into the CNS parenchyma. Similar to their functions in the periphery, these infiltrating Treg cells can inhibit immune responses to CNS antigens and restrain neuroinflammation thereby maintaining immune homeostasis, so as to protect the CNS tissues [7]. However, the existence and significance of Treg cells in the normal CNS regions such as the cerebrum is completely unknown.
The cerebrum is an immune-privileged tissue due to the presence of BBB and the lack of lymphatic vessels. It was once considered that T lymphocytes cannot extravasate the intact BBB to reach the cerebral parenchyma in the steady state [14]. However, recent findings demonstrated that T lymphocytes are present in both normal human cerebrospinal fluid [15, 16] and the normal CNS parenchyma [17-19]. It has been speculated that these T lymphocytes could contribute to immune surveillance, immune tolerance and immune memory in the CNS. Therefore, Treg cells could also be present in the normal CNS with other T-cell subsets.
In this study, we found that TCRαβ+CD4+Foxp3+ Treg cells are present in the normal rat cerebrum. Compared with their peripheral counterpart, these Treg cells are phenotypically activated/memory T cells and express higher levels of Treg-cell-associated signature genes. More importantly, in vitro assay demonstrated that cerebral Treg cells can effectively suppress the activation of conventional T cells (Tconv cells). In addition, cerebral Treg cells significantly inhibit the LPS-induced inflammatory response of brain microglia/macrophages in vitro, suggesting they could be important for restraining microglia/macrophage-mediated neuroinflammation. Our study also demonstrated that astrocytes contribute to maintaining the Foxp3 level of Treg cells, suggesting the interaction between Treg cells and astrocytes is crucial for Treg-cell maintenance and function in the normal cerebrum. Our work is the first to characterize the phenotype and function of Treg cells in the normal rat brain. Investigation on the Treg cells in the normal cerebrum will be helpful for understanding the immune homeostasis of the CNS under steady state.
RESULTS
CD4+Foxp3+ T cells are present in the normal rat cerebrum
As shown in Supporting Information Fig. 1A, a TCRαβ+ cell population was present in mononuclear cells isolated from cerebral hemispheres. The ratio of CD4+ to CD8+ T-cell subsets in the cerebrum was about 0.7 (Supporting Information Fig. 1C), which is consistent with a previous report [18]. Within cerebral CD4+ T cells, Foxp3+ cells constitute about 15% of CD4+ T cells. The frequency of Foxp3+ cells in cerebral CD4+ T cells was 2~3 folds higher than that in blood or splenic CD4+ T cells (Figure 1A). However, the percentage of CD4+Foxp3+ cells in total cerebral T cells was comparable to that in total splenic T cells (Supporting Information Fig. 1D). An average of 890.1 ± 136.3 (N=6) cerebral CD4+Foxp3+ T cells were recovered using our method (Supporting Information Fig. 1E). Immunocytochemistry for Foxp3 in sorted TCRαβ+CD4+ cells confirmed that Foxp3+ cells were present in cerebral T cells. The Foxp3 staining in cerebral T cells was broader than in blood T cells, seemingly being distributed in larger nuclei (Figure 1B). We gave the name "cerebral Treg cells" to these cerebral TCRαβ+CD4+Foxp3+ cells. In addition, Foxp3 protein levels were comparable among blood, splenic and cerebral Treg cells, while cerebral Treg cells expressed higher CD25 (Fig, 1C-D).
Figure 1. CD4+Foxp3+ T cells are present in naïve adult rat cerebrums.
(A) T cells from male Sprague-Dawley rats were gated as described in Supporting Information Fig.1A. T cells in the blood, spleen and cerebrum were analyzed for Foxp3 expression by flow cytometry. Left panel, representative dot plots showing the gating strategy. Right panel, statistical analysis for the frequency of CD4+Foxp3+ T cells in CD4+ T cells. (B) Immunocytochemical staining of Foxp3 in TCRαβ+CD4+ cells sorted from blood and cerebrums (400X magnification). Arrows indicate Foxp3+ cells. Arrow heads indicate Foxp3− cells. (C and D) Expression level of (C) Foxp3 and (D) CD25 in TCRαβ+CD4+Foxp3+ T cells. Mean fluorescent intensities (MFI) of Foxp3 and CD25 were measured by flow cytometry. Left panel, representative histograms of Foxp3 and CD25, respectively. Right panel, statistical analysis for corresponding MFI. Dashed line: isotype control. Green line: blood Treg cells. Blue line: splenic Treg cells. Red line: cerebral Treg cells. (A-D) Data are shown as mean ± SEM (n=6 samples) and are from one experiment representative of three independent experiments. . *p< 0.05; * p< 0.01; ***p< 0.001; one-way ANOVA. B, blood. S, Spleen. C, cerebrum.
Cerebral Treg cells are phenotypically activated/memory T cells
Previous studies showed that T cells found in the CNS have an activated/memory phenotype [15, 20]. To characterize the phenotype of cerebral Treg cells, we determined expression of activation/memory surface markers on cerebral TCRαβ+CD4+Foxp3+ cells. Firstly, we confirmed that the cell isolation procedure did not significantly alter the surface marker staining (Supporting Information Fig. 2). We found that about 13% of splenic Treg cells were activated/memory cells (CD44H+CD62Llo/−). Over 80% cerebral Treg cells were CD44H+CD62L−, suggesting they are activated/memory T cells (Figure 2A). Consistently, compared with splenic Treg cells, very few cerebral Treg cells expressed CD45RC, which is a naïve T-cell marker (Figure 2B). Cerebral Treg cells expressed higher levels of other activation/memory markers including ICOS, CD103, KLRG1 and CTLA4 in comparison with splenic Treg cells (Figure 2C-D). Notably, cerebral Treg cells contained increased CD103+ICOS+ cells and CD103−ICOS+ cells (Supporting Information Fig. 3A). A CTLA4+KLRG1+ subpopulation, which was absent in splenic Treg cells, existed in cerebral Treg cells (Supporting Information Fig. 3B). Compared with their splenic counterparts, cerebral TCRαβ+CD4+Foxp3− Tconv cells also show an activated/memory phenotype (Figure 2).
Figure 2. Cerebral Treg cells have an activated/memory phenotype.
(A and B) Expression of (A) CD44H and CD62L and (B) CD45RC on TCRαβ+CD4+Foxp3+ and TCRαβ+CD4+Foxp3− T cells in the spleen (Sp) and cerebrum (Cere) of Sprague-Dawley rats. Cells were gated as in Figure 1. Percentages of (A) CD44H+CD62L− and (B) CD45RC+ cells were presented as mean ± SEM in the quadrants. (C) Representative histograms for the expression of ICOS, CD103, KLRG1 and CTLA4 on TCRαβ+CD4+Foxp3+ and TCRαβ+CD4+ Foxp3− T cells in the spleen and cerebrum. Dashed line: isotype control. Solid line: splenic T cells. Shaded line: cerebral T cells. (D) Quantification of the MFI of ICOS, CD103, KLRG1 and CTLA4 on TCRαβ+CD4+Foxp3+ and TCRαβ+CD4+Foxp3− T cells in the spleen and cerebrum. (D) Data are shown as mean ± SEM (n=6 samples/group). and (A-D) are from one experiment representative of three independent experiments. *p< 0.05; **p< 0.01; ***p< 0.001 in comparison with splenic counterparts; Student’s t test;.
Treg cells are distributed throughout the cerebrum
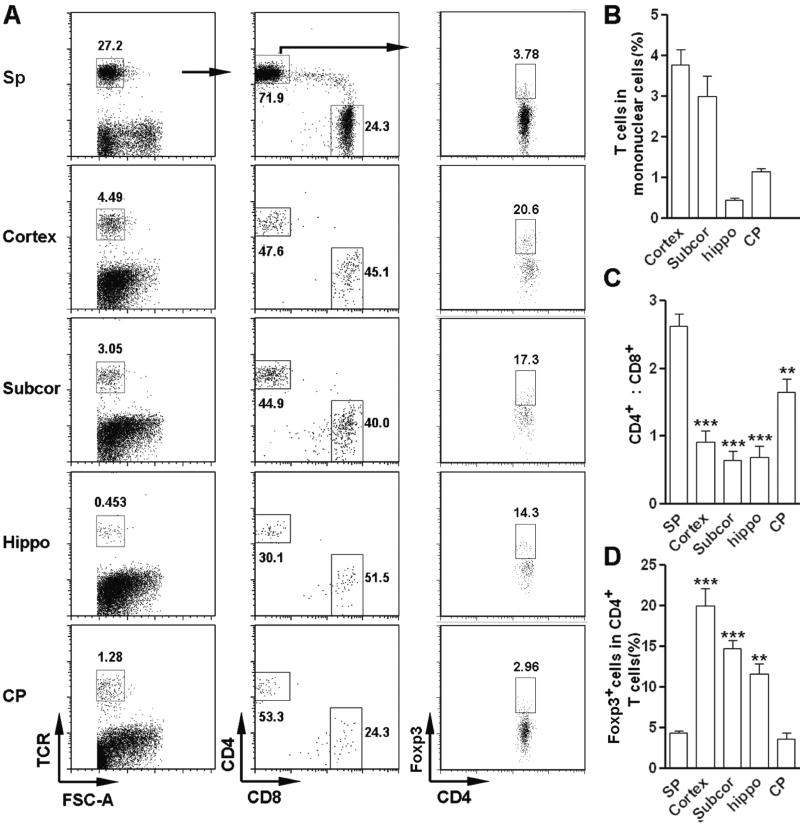
To investigate the locations of cerebral Treg cells, we determined the presence of TCRαβ+CD4+Foxp3+ cells in dissected cerebral regions. As shown in Figure 3A-B, T cells were present in all cerebral regions tested, with cortex containing the most T cells and hippocampus containing the least. The ratio of CD4+ to CD8+ T cells was much lower in the cortex, subcortex and hippocampus in comparison with that in the spleen, while in the choroid plexus this ratio was relatively close to that in spleen (Figure 3C). Cortex and subcortex contained a significantly higher Treg-cell proportion, suggesting cerebral Treg cells are mainly located in these regions (Figure 3D). The CD4+Foxp3+ T-cell fraction in the choroid plexus was comparable to that in the spleen (Figure 3D).
Figure 3. Distribution of TCRαβ+CD4+Foxp3+ cells in the normal rat cerebrum.
Mononuclear cells were isolated from each dissected region of the cerebrum of Sprague-Dawley rats. The frequency of TCRαβ+CD4+Foxp3+ Treg cells was analyzed by flow cytometry. (A) Representative dot plots. Numbers in the plots are the percentages of gated populations in their parent populations. (B-D) Statistical assay for (B) the frequency of total T cells, (C) the ratio of CD4+ versus CD8+ T subset, and (D) the frequency of Foxp3+ cells in CD4+ T cells. Data are shown as mean ± SEM (n=6 samples/group) and are from one experiment representative of three independent experiments. **p< 0.01; ***p< 0.001 in comparison with splenic counterparts; Student’s t test. Sp, spleen. Subcor, subcortical region. Hippo, hippocampus. CP, choroid plexus.
Cerebral Treg cells suppress conventional T-cell activation
To determine the immunoregulatory activity of cerebral Treg cells, we used qRT-PCR to test the expression of regulatory activity-associated molecules in a cerebral Treg-enriched population. Firstly TCRαβ+CD4+CD25hi cells either in periphery or in the cerebrum were confirmed to be Foxp3-expressing cells (Supporting Information Fig. 4A-B). Compared with splenic Treg cells, there was an insignificant increase of TGF-β1 expression in cerebral Treg cells. Robust elevation of expression of IL-10 and IL-35 (both Ebi3 and IL-12a subunits) in cerebral Treg cells was observed (Figure 4A). Expression of CTLA4, CD39, CD73 and LAG3 were all significantly higher in cerebral Treg cells than in splenic Treg cells (Figure 4B). Intracellular staining confirmed higher IL-10 level in cerebral Treg cells compared with splenic Treg cells (Figure 4C & Supporting Information Fig. 5). The high expression of regulatory molecules suggested that cerebral Treg cells would be immunosuppressive. To test the immunosuppressive function of cerebral Treg cells, CD3+CD4+CD25hi Treg-enriched cells were sorted and were in vitro co-cultured with CFSE-labeled splenic CD4+CD25− Tconv cells in the presence of agonistic antibodies. Compared with Tconv cells cultured alone, both cerebral and splenic Treg cells effectively inhibited Tconv proliferation (Figure 4D). In addition, both cerebral and splenic Treg cells significantly restrained production of IFN-γ and TNF-α in stimulated Tconv cells, suggesting their significant immunosuppressive activity (Figure 4E). However, inconsistent with the higher regulatory molecule expression by cerebral Treg cells, the immunosuppressive effect of cerebral Treg cells was not higher than that of splenic Treg cells. This could be due to the presence of agonistic antibodies in the culture system, because they could also activate splenic Treg cells to generate a strong suppressive activity.
Figure 4. Cerebral Treg cells suppress Tconv-cell activation.
TCRαβ+CD4+CD25hi Treg-enriched cells were sorted from rats’ spleens and brains. (A and B) Expression of (A) TGF-β1, IL10 and IL-35 (IL-35 subunits: Ebi3 and IL-12a) and (B) CTLA4, CD39,,CD73 and LAG3 in Treg-enriched cells were determined by qRT-PCR. Data are presented as mean ± SEM (n= 6 samples per group) and are from three independent experiments. *p< 0.05; **p< 0.01 in comparison with splenic counterparts; Student’s t test. S, splenic Treg-enriched cells. C, cerebral Treg-enriched cells. (C) Histogram of IL-10 expression in TCRαβ+CD4+Foxp3+ Treg cells. Shaded line, splenic Treg cells; solid line, cerebral Treg cells. TCRαβ+CD4+Foxp3+ Treg cells were gated as in Figure 1. (D) Tconv cell proliferation in the presence of Treg cells. (C and D) Data are from one experiment representative of three independent experiments. (E) Expression of IFN-γ and TNF-α in un-stimulated Tconv cells and Tconv cells stimulated with agonistic antibodies. Data are shown as mean ± SEM and are from one experiment representative of three independent experiments with a total n=6 per group. *, p< 0.05; **, p< 0.01; one-way ANOVA. Ctrl: Tconv cells cultured alone; S-Treg cells: Tconv cells co-cultured with splenic Treg cells; C-Treg cells: Tconv cells co-cultured with cerebral Treg cells.
Cerebral Treg cells restrain LPS-induced inflammatory response in microglia/macrophages via IL-10
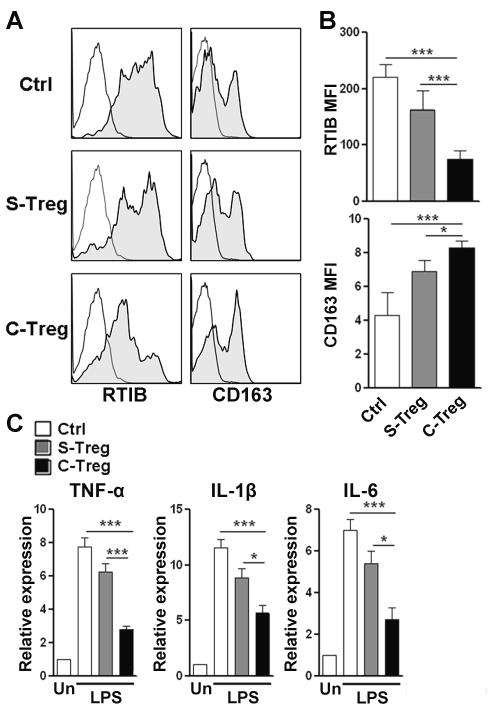
In the periphery, Treg cells are essential for controlling inflammatory response. We speculated that cerebral Treg cells also modulate neuroinflammation to maintain brain homeostasis. Microglia and perivascular macrophages are important for the initiation and progression of neuroinflammation through the production pro-inflammatory mediators. To identify the anti-inflammatory activity of cerebral Treg cells, we co-cultured splenic or cerebral Treg cells with LPS-stimulated cerebral microglia/macrophage mixture. Then Treg cells were depleted and myeloid cells were analyzed for inflammatory response (Supporting Information Fig. 6A). Microglia and macrophages can be distinguished by their differential expression of CD45 (Supporting Information Fig. 6B). We used rat MHC-II (RTIB) and CD163 as the markers of macrophage polarization, since RTIB expression is up-regulated in pro-inflammatory macrophages, and CD163 expression is up-regulated in anti-inflammatory macrophages. LPS-activated macrophages expressed high RTIB and low CD163. Naive splenic Treg cells did not significantly alter RTIB and CD163 expression (Figure 5A-B). Notably, cerebral Treg cells significantly decreased RTIB expression and increased CD163 expression in comparison with macrophages alone or co-cultured with splenic Treg cells (Figure 5A-B), suggesting cerebral Treg cells restrained macrophage polarization towards pro-inflammatory M1 type. However, RTIB and CD163 were barely expressed on microglia even after LPS stimulation, so it was difficult to distinguish the phenotypical change of microglia. Subsequently, we determined the expression of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 in LPS-stimulated microglia/macrophage mixture. Splenic Treg cells could not significantly inhibit expression of these cytokines, while cerebral Treg cells profoundly decreased each of them, suggesting the inhibitory effect of cerebral Treg cells was more robust than splenic Treg cells (Figure 5C).
Figure 5. Cerebral Treg cells inhibited inflammatory response of LPS-stimulated microglia/macrophages.
(A) Representative histograms of the expression of RTIB and CD163 on LPS-stimulated cerebral macrophages. Data are representative of four independent experiments. (B) Quantification of MFI values for RTIB and CD163 on LPS-stimulated cerebral macrophages measured by flow cytometry.. (C) Expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in LPS-stimulated microglia/macrophages was measured by q-RTPCR. Data are representative of three independent experiments. (B and C) Data are shown as mean ± SEM (n=6 per group) and are pooled from three independent experiments. *p< 0.05. **p< 0.01. ***p< 0.001; one-way ANOVA. Un: unstimulated microglia/macrophages; LPS: LPS stimulation; Ctrl: stimulated macrophages alone; S-Treg: stimulated macrophages co-cultured with splenic Treg cells; C-Treg: stimulated macrophages co-cultured with cerebral Treg cells
In the previous data we demonstrated that cerebral Treg cells express higher IL-10 than splenic Treg cells (Figure 4A). Thus, IL-10 could be involved in the anti-inflammatory function of cerebral Treg cells. To test our hypothesis, a neutralizing antibody against IL-10 was added into the co-culture and inflammatory responses in microglia and macrophages were determined as in Figure 5C. As shown in Figure 6, IL-10 antibody attenuated the inhibitory effect of cerebral Treg cells, resulting in up-regulation of RTIB and down-regulation of CD163 on macrophages, as well as partially restored production of pro-inflammatory cytokines in the microglia/macrophage mixture. However, other mediators such as IL-35, CTLA4 and CD39 might also play roles in suppressing inflammation. To exclude the possibility that IL-10 antibody directly alters inflammatory response, we incubated LPS-stimulated microglia/macrophages with IL-10 antibody. IL-10 antibody itself did not alter expression of pro-inflammatory cytokines in LPS-stimulated microglia/macrophages (Supporting Information Fig. 6C).
Figure 6. IL-10 is important for inhibitory effect of cerebral Treg cells on LPS-stimulated microglia/macrophages.
(A) Representative histograms of expression of RTIB and CD163 expression on LPS-stimulated cerebral macrophages. Data are representative of four independent experiments. (B) Statistical analysis for MFI of RTIB and CD163 on LPS-stimulated cerebral macrophages, measured by flow cytometry. (C) Expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in LPS-stimulated microglia/macrophages. Ctrl: stimulated macrophages alone; C-Treg: stimulated macrophages co-cultured with cerebral Treg cells; C-Treg+Ab: stimulated macrophages co-cultured with cerebral Treg cells and IL-10 neutralizing antibody. (B and C) Data are shown as mean ± SEM (n=7 per group) and are pooled from three independent experiments.*p< 0.05. **p< 0.01. ***p< 0.001 compared with control group; # p< 0.05. ##p< 0.01. ###p< 0.001 compared with C-Treg group; one-way ANOVA.
IL-2/STAT5 signaling is involved in astrocyte-induced maintenance of Treg cells.
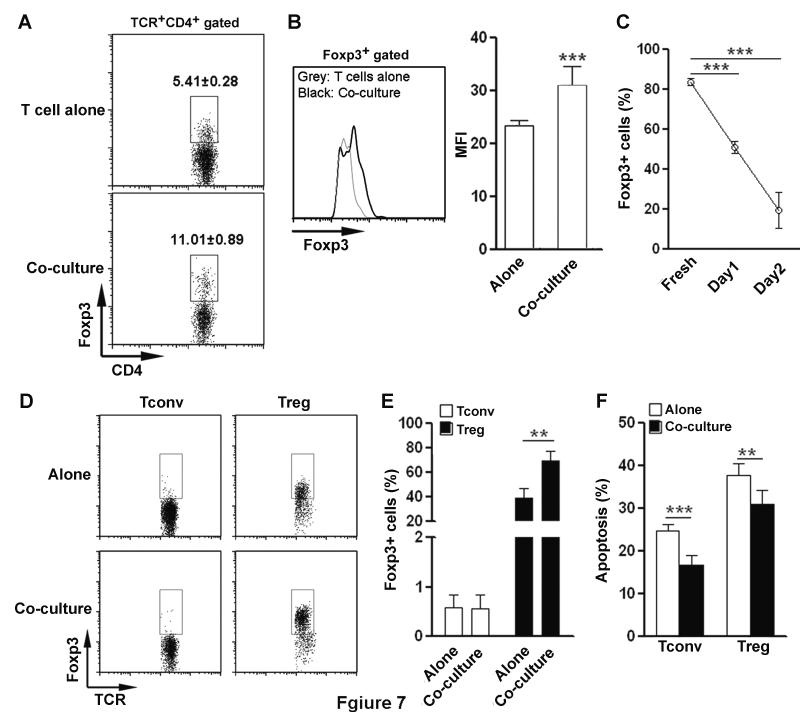
The above results led us to investigate the mechanisms by which the brain influences Treg function. Astrocytes are the most abundant cell type in the brain and are part of the BBB. Therefore astrocytes could be the first brain parenchymal cell type encountered by Treg cells. We co-cultured splenic T cells with primary astrocytes and the frequency of Foxp3+ T cells was then determined. Co-cultured T cells contained a significantly higher proportion of Foxp3+ cells in CD4+ T cells, suggesting astrocytes maintain and/or increase Treg frequency (Figure 7A). Moreover, Foxp3 expression was higher in co-cultured T cells than in those cultured alone (Figure 7B). The proportional change of Treg cells in the co-culture could be due to maintenance or induction of Foxp3 expression, or less Treg apoptosis. To test these possibilities, splenic CD3+CD4+CD25hi Treg-enriched cells or CD3+CD4+CD25− Tconv cells were cultured in the presence or absence of astrocytes, respectively. We observed a progressive decrease of Foxp3+ T cells when Treg cells were cultured alone (Figure 7C). Astrocytes maintained the proportion of Foxp3+ T cells, suggesting Foxp3 expression in Treg cells was sustained by astrocytes (Figure 7D-E). In addition, astrocytes could not induce Foxp3 expression in Tconv cells (Figure 7D-E). Astrocytes significantly reduced apoptosis of both Tconv and Treg cells after a 24 h co-culture, suggesting support of cell survival could be a mechanism underlying the Treg-cell maintenance (Figure 7F).
Figure 7. Primary astrocytes maintain Treg cells in vitro.
(A) The frequency of Foxp3+ cells in CD4+ T cells after culture. Splenic T cells were cultured in the presence or absence of primary astrocytes for 24 hours. T cells were gated as in Figure 1, except that CD3 was used to label T cells instead of TCRαβ. (B) Expression level of Foxp3 in Foxp3+ cells after in vitro culture of splenic T cells. Left panel, representative histogram of Foxp3. Right panel, statistical analysis for MFI of Foxp3. Grey line: T cells cultured alone. Black line: T cells cultured with astrocytes. (A-B) Data are presented as mean percentage ± SEM (n=10 per group) and are pooled from three independent experiments.(C) Percentage of Foxp3+ cells in cultured splenic CD3+CD4+CD25hi Treg-enriched cells. Treg-enriched cells were cultured in vitro for 1 and 2 days. The proportion of Foxp3+ cells on day 1 and day 2 was compared with freshly Treg-enriched cells. Data are shown as mean ± SEM (n=3 per group) and are from one experiment representative of three independent experiments. (D and E) Foxp3 expression in splenic CD3+CD4+CD25hi Treg-enriched cells or CD3+CD4+CD25− Tconv cells cultured for 24 h in the presence or absence of primary astrocytes. Representative dot plots were shown in (D). Statistical analysis for the frequency of Foxp3+ T cells was shown in (E). (D and E) Data are shown as mean ± SEM (n=8 per group) and are pooled from three independent experiments. (F) Apoptosis of CD3+CD4+CD25hi Treg-enriched cells or CD3+CD4+CD25− Tconv cells were quantified with Annexin-V staining after culture in the presence or absence of primary astrocytes. Data are shown as mean ± SEM (n=6 per group) and are pooled from three independent experiments. . *p< 0.05. **p< 0.01. ***p< 0.001; Student’s t test..
The effect of astrocytes on maintenance of Treg cells indicates they could be a substitute for Tconv cells in support of Treg cells. Tconv cell-derived interleukin-2 (IL-2) maintains Treg cells in the periphery [21]. Previous studies indicated that astrocytes can also produce IL-2 both in vitro and in vivo [22-25]. Moreover, previous research showed that astrocytes can produce transforming growth factor-β (TGF-β) [26, 27], a potent Foxp3-inducing cytokine. Since STAT5 is critical for the IL-2 signal pathway, we firstly used a STAT5 inhibitor and/or TGF-β RI kinase inhibitor VI (SB431542) in the co-culture to block the STAT5 and/or TGF-β signaling. Only the STAT5 inhibitor effectively decreased the frequency of Foxp3+ T cells (Figure 8A). To further confirm the role of STAT5, we determined STAT5 phosphorylation in Treg cells after co-culture with astrocytes. The STAT5 phosphorylation level was significantly higher in co-cultured Treg cells than in Treg cells alone, suggesting STAT5 is indeed activated in co-cultured Treg cells (Figure 8B). ELISA demonstrated low but substantial IL-2 in 24 h astrocyte supernatant, suggesting at least in vitro cultured astrocytes can produce IL-2 (Figure 8C). Subsequently, we used an anti-IL-2 neutralizing antibody to block the potential effect of IL-2. Consistent with our expectation, the neutralizing antibody abolished astrocyte-induced maintenance of Foxp3+ cells during a 24-hour culture (Figure 8D). However, the above data were all generated from splenic Treg cells. To further confirm the effect of astrocyte-derived IL-2 on cerebral Treg cells, we co-cultured a small quantity of cerebral Treg cells with astrocytes for 24 hours. Due to the difficulty in staining Foxp3 in such few cells, we sorted T cells from the co-culture instead and used qRT-PCR to determine the expression of Foxp3. Consistent with splenic Treg cells, cerebral Treg cells maintained Foxp3 expression at a relatively high level, in comparison with Treg cells alone (Figure 8E). Therefore, IL-2/STAT5 signaling is involved in astrocyte-mediated maintenance of Treg cells. It is likely that astrocytes support the expression of Foxp3 in existing Treg cells, rather than inducing Foxp3 expression in Tconv cells.
Figure 8. IL-2/STAT5 signaling is involved in astrocyte-induced maintenance of Treg cells.
(A) Treg-enriched cells were co-cultured with astrocytes in the presence or absence of STAT5 inhibitor and/or TGF-β RI kinase inhibitor VI for 24 h. Subsequently the proportion of Foxp3+ cells was determined by flow cytometry. U: untreated; Si: STAT5 inhibitor; Ti:, TGF-β RI kinase inhibitor VI. Data are shown as mean ± SEM (n=8 per group) and are pooled from three independent experiments. (B) STAT5 phosphorylation in Treg cells was analyzed by flow cytometry. Black line: Treg cells cultured alone; grey line: Treg cells cultured with astrocytes. Plots are from one experiment representative of three independent experiments with n=3 per group. (C) IL-2 concentration in the supernatant of astrocyte culture was measured by ELISA. (D) Foxp3 expression in splenic Treg cells after IL-2 neutralizing antibody treatment. Splenic Treg-enriched cells were cultured alone or co-cultured with astrocytes in the presence or absence of an IL-2 neutralizing antibody. Foxp3 expression was measured by flow cytometry. (E) Foxp3 expression in cerebral Treg cells after IL-2 neutralizing antibody treatment. Cerebral Treg-enriched cells were cultured alone or co-cultured with astrocytes in the presence or absence of an IL-2 neutralizing antibody. Foxp3 expression was measured by q-RTPCR. Fresh: freshly sorted Treg-enriched cells; Alone: Treg-enriched cells cultured alone; Co-culture: Treg-enriched cells cultured with astrocytes; V: vehicle; Ab: IL-2 neutralizing antibody. (C-E) Data are shown as mean ± SEM (n=6 per group) and are pooled from three independent experiments. .*p< 0.05; **p< 0.01; ***p< 0.001; one-way ANOVA.
It is possible that astrocytes maintain Treg-cell identity through signaling triggered by MHC-II and co-stimulatory molecules on astrocytes. To test this possibility, we tested expression of MHC-II and CD80 on primary astrocytes by flow cytometry. We found that both MHC-II and CD80 were expressed at very low levels on astrocytes, and less than 3% of astrocytes express both molecules (Supporting Information Fig. 8A). Hence, it is unlikely that astrocytes induce significant signaling in Treg cells through MHC-II and CD80, at least in our experimental settings.
We further tested whether Treg-derived IL-10 could induce IL-2 expression in astrocytes. Astrocytes were treated with 10 μg/ml rat IL-10 for 24 hs and IL-2 concentration in the supernatant was analyzed by ELISA. We did not observe significant changes in IL-2 levels, suggesting IL-10 is not important for inducing IL-2 expression in astrocytes (Supporting Information Fig. 8B).
DISCUSSION
The potent immunosuppressive and anti-inflammatory activities of Treg cells make them crucial for maintenance of immune homeostasis. However, it remains elusive whether Treg cells have different subtypes which play distinct roles in different organs and tissues. Under steady state, Treg cells are present in both lymphoid and non-lymphoid tissues. The Treg-containing normal non-lymphoid tissues include skin, lung, liver, and intestinal lamina propria [5]. Altering the distribution of Treg cells leads to the development of tissue-specific inflammatory disease [28]. Until recently the presence of Treg cells in the normal cerebrum has not been carefully studied, probably because the cerebrum has long been considered an immune-privileged tissue. Some studies have shown the existence of T cells in the normal cerebrum [18, 19]. As an important T-cell subset, Treg cells might also be present in the cerebrum. Our study showed a substantial TCRαβ+CD4+Foxp3+ Treg population in the rat brain. The fraction of Treg cells in cerebral CD4+ T cells was higher than that in splenic CD4+ T cells, similar to the case in skin [5]. In cerebral Treg cells, Foxp3 was more broadly stained in the nuclei in comparison to the blood Treg cells. We speculated it might be related to the activation status of these cells [29].
Blood Treg cells are likely the source of cerebral Treg cells, due to lack of lymphatic vessels and abundance of blood vasculature in the brain [14]. There are two possible sites for leukocyte entry into the brain. One is the choroid plexus, which lacks tight junction between vascular endothelial cells and the glia limitans [14]. Leukocytes enter the CSF through this site, but whether they can go further into the brain parenchyma has not been determined. The second site is the vascular network in the subventricular zone (SVZ). SVZ contains a rich plexus of blood vessels that has a leaky BBB, with areas along the blood vessel that lack endothelial tight junctions, pericytes and astrocytic end feet [30, 31]. If Treg cells can cross the SVZ microvessels, they would easily enter the parenchyma proximal to the vasculature. However, we cannot rule out the possibility that large brain blood vessels could be the site of Treg recruitment. Furthermore, recruitment of Treg cells into non-lymphoid tissues require different sets of adhesion molecules and chemokine receptors, which bind to corresponding ligands expressed in specific tissues [5]. Future studies on the expression of adhesion molecules and chemokine receptors will provide more details about the homing of Treg cells into the brain.
It should be noted that cerebral Treg cells have an activated/memory phenotype. This phenomenon is consistent with previous publications, stating that most T cells found in the normal CNS have an activated/memory phenotype [15, 16, 20]. Consistent with activated/memory phenotype, cerebral Treg cells express higher levels of regulatory activity-associated molecules. In vitro suppression assays confirmed the immunosuppression induced by cerebral Treg cells. However, it is still not clear whether the activated/memory state is acquired before or after Treg cells enter the brain. It has long been considered that activated T cells can relatively easily extravasate the blood brain barrier, probably because of their altered expression profile of adhesion molecules and chemokine receptors [32-35]. Hence, activated/memory Treg cells in the blood vasculature might efficiently penetrate the BBB to enter the brain. However we cannot exclude the possibility that naïve Treg cells become activated after they contact the cellular or humoral components of the brain.
Although Treg cells are widely spread in the normal brain, the significance of their existence is still not understood. We hypothesized that cerebral Treg cells might contribute to the anti-inflammatory feature of the brain. Cerebral Treg-derived IL-10, TGF-β and IL-35 might work together to maintain the homeostasis of the brain. In addition, previous data have shown that Treg cells can maintain or promote polarization of macrophages towards anti-inflammatory M2 type [36, 37], and tissue-resident macrophages generate Foxp3+ regulatory T cells and promote immune tolerance [38]. Thus, cerebral Treg cells might interact with microglia/macrophages to restrain neuroinflammation. Our data suggest that cerebral Treg cells are able to inhibit LPS-induced inflammatory response of microglia/macrophages. It is possible that cerebral Treg cells could react with and fight against some stress-or-injury-induced neuroinflammatory responses such as in ischemic stroke and Alzheimer's disease. On the other hand, cerebral Treg cells could also play a critical role in suppressing the autoimmune response against the brain tissue in the steady state. Note that cerebral Tconv cells are also activated/memory cells. These Tconv cells could be responsive to cerebral antigens, and cerebral Treg cells might suppress the responsiveness of cerebral Tconv cells. Interestingly, previous studies have indicated that mononuclear cells within the CNS suppress the response of myelin basic protein-specific T cells [18]. The cerebral Treg cells are likely involved in this suppression. The exact role of cerebral Treg cells in maintaining brain homeostasis needs further investigation.
In normal brains, Treg cells might lose the support for survival and function from blood-derived cellular and humoral components. Hence, they would acquire the supporting signals from the brain components. Previous research indicates that in vitro cultured neurons can generate Foxp3+ Treg cells from encephalitogenic T cells and suppress experimental autoimmune encephalomyelitis [39]. Another major cerebral cell type — astrocytes — may interact with Treg cells as well. Astrocytes are the most abundanT-cell population in the CNS [40, 41]. Previous studies have suggested some interactions between astrocytes and T lymphocytes under pathological conditions [42-45]. Our data demonstrates that primary astrocytes maintain the Foxp3 expression in Treg cells via IL-2/STAT5 signaling. Hence, astrocytes could be a partial substitute for Tconv cells in the maintenance of Treg cells. To our knowledge, this is the first study showing evidence for the effect of astrocytes on Foxp3+ Treg cells.
In summary, our study has provided novel insight for the homeostatic maintenance of immunomodulation in the normal brain. We postulate that Treg cells are also present in the normal brains of other species such as mice and humans. Further studies are needed to elucidate the significance of cerebral Treg cells under pathological conditions.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (8~12 week old, weighing 250~300g) were purchased from the Jackson Laboratory. All animal procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee. All experiments were conducted in compliance with institutional guidelines and NIH Guidelines for the Use of Animals in Neuroscience and Behavioral Research.
Isolation of mononuclear cells from rat brains
Isolation of brain mononuclear cells was performed following the well-established method with a few modifications [46, 47]. Briefly, anesthetized rats were cardially perfused with 250 ml of ice-cold phosphate buffered saline (PBS). Each cerebral hemisphere was digested with RPMI1640 containing 1 mg/ml collagenase type IV (Sigma-Aldrich), 100 U/ml DNase I (Roche), 5 mM CaCl2 and 10% fetal calf serum (FCS) for 45 min at 37°C. Released cells were then subjected to Percoll (GE Healthcare) gradient isolation by centrifugation at 500 g for 20 min. The cells in the interlayer between 37% Percoll and 70% Percoll were collected.
Flow cytometry analysis and cell sorting
For cell surface marker staining, the following anti-rat antibodies purchased from BioLegend were used: Alexa Fluor® 647 anti-TCRαβ monoclonal Ab (R73), Biotin anti-rat CD3 Ab (OX-19), Allophycocyanin-Cy7 anti-CD4 monoclonal Ab (W3/25), PE-Cy7 anti-CD8 monoclonal Ab (G28), PE anti-CD25 monoclonal Ab (OX-39), FITC anti-CD25 monoclonal Ab (OX-39), PE anti-CD44H Ab (OX-49), PerCP anti-CD62L Ab (OX-85), PE anti-CD45RC Ab (OX-22), Biotin anti-CD103 Ab (OX-62), PE/Cy7 anti-CD278 (ICOS) Ab (C398.4A), PE anti-CTLA4 (WKH203), PE/Cy7 anti-CD45 Ab (OX-1), PE anti-CD11b/c Ab (ox-42), FITC anti-RTIB (OX-6), PE anti-CD80. Biotin anti-mouse/rat KLRG1 Ab (2F1) was from BD Biosciences. Alexa Fluor® 647 anti-CD163 (ED2) was from AbD serotec. Cells were incubated with antibodies on ice for 15 min before analysis. For Treg-cell detection, Alexa fluor 488 anti-Foxp3 antibody (150D, BioLegend) was used. Cells were stained using Foxp3 Fix/Perm Buffer Set (BioLegend) following manufactures’ instructions and analyzed on a BD LSR-II flow cytometer. For intracellular staining for pSTAT5 and IL-10, purified anti-Phospho-STAT5 (Tyr694) antibody (C11C5, Cell Signaling Technology) and PE-anti-IL10 (JES3-19F1, BD Biosciences) were used, respectively. For Fluorescence-activated cell sorting (FACS), cells were sorted on a BD InFlux Cell Sorter. For Magnetic-activated cell sorting (MACS), cells were sorted with Dynabeads® Biotin Binder kit (Invitrogen) according to the manufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNAs were extracted using PicoPure® RNA Isolation Kit (Invitrogen) following the manufacture’s manual. cDNAs synthesis was performed using SuperScript® III First-Strand Synthesis System (Invitrogen) according to the manufactures’ instructions. Quantitative PCR was performed using Fast SYBR® Green Master Mix (Invitrogen) on a 7300 Real-Time PCR System (Invitrogen). 2−ΔΔCt method was used to calculate relative expression of each gene. Primer sequences are shown in Table 1.
Table 1.
| Genes | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| IL-10 | TAAAAGCAAGGCAGTGGAGC | GATGCCGGGTGGTTCAATTT |
| TGF-β | GTCAACTGTGGAGCAACACG | TTCCGTCTCCTTGGTTCAGC |
| Ebi3 | TTCTAGCCTTTGTGGCGGAA | AGCGAAGTCGGTACTTGAGAG |
| IL-12a | CCAGGCCATAAATGCAGCAC | GGAGCTTTCTGGTGCAGAGT |
| CTLA4 | TGCAGTTAGTTCGGGGTTGT | TCACATTCTGGCTCTGTTGGG |
| CD39 | TGCATCGATATGGCCAGTCC | AGATGGGCACTCGACACTTG |
| CD73 | ACTCCCAGTGTGCCTTCAAC | AGACACTGTCGTTCGCCATC |
| Perforin | CACAGTGGAGTGTCGCATGTA | GTGGGGAAGGTTCTTGAGTGC |
| LAGS | CCTACACCTGCAGCATCCATC | GCCGGGACTACCTCACATAAC |
| Foxp3 | AGTGGCAGGGAAGGAGTGTC | TTCCAAGTCTCGTGTGAAGGC |
| TNF-α | TCGGTCCCAACAAGGAGGAG | GGGCTTGTCACTCGAGTTTTG |
| IL-1β | TGTCTGACCCATGTGAGCTG | GCCACAGGGATTTTGTCGTT |
| IL-6 | ACTTCACAAGTCGGAGGCTT | TTCTGACAGTGCATCATCGCT |
| GAPDH | GATGGTGAAGGTCGGTGTGA | TGAACTTGCCGTGGGTAGAG |
Immunocytochemical staining
FACS-Sorted TCRαβ+CD4+ cells were seeded on glass slides coated with 0.01% poly-L-lysine (Sigma-Aldrich) and were air-dried for 30 min. Cells were fixed and permeabilized with Foxp3 Fix/Perm Buffer Set (BioLegend). Cells were stained with Alexa Fluor® 488 anti-Foxp3 (150D). For astrocytes, cells were fixed with BD Cytofix/perm buffer, permeabilized with BD perm/wash buffer and then were incubated with anti-rat GFP monoclonal Ab (Santa Cruz Biotechnology). Astrocytes were washed and incubated with Alexa Fluor® 488 goat anti-mouse IgG (Invitrogen). Cells were observed on an Axio Observer Z1 fluorescent microscope (Zeiss).
In vitro cell culture
For T-cell activation assay, 96-well half-area microplates (Greiner Bio-One) were coated with 500 μg/ml sheep anti-mouse IgG (Jackson ImmunoResearch Laboratories) overnight at 4 °C. Microplates were then washed with PBS and coated with 5μg/ml mouse anti-rat TCRαβ Ab (R73, BioLegend) for 1 h at 37 °C. FACS-sorted splenic CD3+CD4+CD25− conventional T cells (Tconv cells) were labeled with 5 μM Carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes) following the manufacture’s instructions. 1 × 104 Tconv cells were then co-cultured with 1 × 103 CD3+CD4+CD25hi Treg-enriched cells in supplemented RPMI 1640 containing 200 ng/ml mouse anti-rat CD28 Ab (JJ319, BioLegend) and 300 U natural IL-2 (Gibco) for 4 days. At day 2 after stimulation, CFSE+ Tconv cells were sorted by flow cytometry and were subject to RNA extraction and qRT-PCR. At day 4 after stimulation, CFSE dilution was evaluated by flow cytometry.
Microglia/macrophage inflammatory response was induced based on previous study with modifications [37]. Briefly, CD11b/c+ microglia/macrophages in the rat cerebrum were sorted by FACS and were cultured in RPMI 1640 supplemented with 1% penicillin/streptomycin, 1% glutamine, and 10% heat-inactivated FCS. 1 × 105 per ml Microglia/macrophages were stimulated for 6 h with lipopolysaccharide (LPS, 50ng/ml, Sigma). The cells were then washed with PBS twice and were mixed with 2.5 × 104 per ml TCRαβ+CD4+CD25hi Treg-enriched cells freshly isolated from either spleen or cerebrum. The cell mixture was cultured in the 96-well untreated round bottom microplates (Greiner Bio-One) for 40 h. The cells were then incubated with 5 mM EDTA-PBS at 37 °C for 15 min followed by vigorous pipetting. Detached cells were incubated with 5 μg/ml biotinylated anti-rat CD3 Ab (OX-19, BioLegend) on ice for 15 min followed by depletion of CD3+ cells with Dynabeads® Biotin Binder kit (Invitrogen) according to the manufacturer’s instructions. Unbound CD3− cells were subject to flow cytometry and qRT-PCR. In some experiments, 1 μg/ml neutralizing polyclonal goat anti-mouse/rat IL-10 IgG (R&D systems) was added into culture.
Primary astrocyte culture was performed as described [48]. 1 × 105 per ml astrocytes were seeded into each well of 48-well plates and cultured overnight to reach 80%~90% confluent. Splenic T cells (CD3+ total T cells, CD4+CD25hi Treg cells or CD4+CD25− Tconv cells, respectively) were sorted by FACS. T cells were seeded onto the astrocyte monolayer and were cultured at 37°C in a humidified incubator for indicated periods of time. The ratio of T cells to astrocytes was 1:2. In some experiments, 100 μM STAT5 inhibitor, 10 μM TGF-β RI kinase inhibitor VI (Cat# 573108 and 616461, respectively. Calbiochem) or 1 μg/ml polyclonal rabbit anti-rat IL-2 neutralizing antibody (PeproTech) was added into culture.
To determine the effect of astrocytes on cerebral Treg cells, FACS-sorted 2 × 103 TCRαβ+CD4+CD25hi Treg-enriched cells from pooled cerebrums were seeded onto primary astrocyte monolayer (1× 104/well) in 96-well half-area microplates (Greiner Bio-One). Twenty four hours after co-culture, cells were lifted by TrypLE-Express (Invitrogen) treatment at room temperature for 10 min. Then cells were stained with anti-CD3 antibody and CD3+ cells were sorted by FACS before being subject to RNA extraction and cDNA synthesis. Foxp3 expression was analyzed using q-PCR.
ELISA
IL-2 concentration in the supernatant of astrocyte culture was determined using Rat IL-2 Platinum ELISA kit (eBioscience) following the manufacture instructions.
Statistical analysis
Data were analyzed and results were presented as mean ± SEM. Student’s t test or one-way ANOVA were used for comparison of mean between the groups, and p < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
1. This work was supported by US Public Health Service Grants NS57186 and AG21980 to KJ, and NS054651 and NS088596 to SY.
2. Flow Cytometry facility in UNTHSC is supported by National Institutes of Health award ISIORR018999-01A1.
Abbreviations
- IL
interleukin
- TGF-β
tumor growth factor beta
- TNF-α
tumor necrosis factor alpha
- Treg
regulatory T cells
- Tconv
conventional T cells
- BBB
blood brain barrier
- FACS
Fluorescence-activated cell sorting
- MACS
Magnetic-activated cell sorting
- CFSE
Carboxyfluorescein succinimidyl ester
- CTLA4
Cytotoxic T-Lymphocyte Antigen 4
- LAG3
Lymphocyte-activation gene 3
- ICOS
Inducible T-cell costimulator
- LPS
Lipopolysaccharides
- STAT5
Signal Transducer and Activator of Transcription 5
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
REFERENCES
- 1.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 2.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elst EF, Klein M, de Jager W, Kamphuis S, Wedderburn LR, van der Zee R, Albani S, Kuis W, Prakken BJ. Hsp60 in inflamed muscle tissue is the target of regulatory autoreactive T cells in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58:547–555. doi: 10.1002/art.23202. [DOI] [PubMed] [Google Scholar]
- 5.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunol Rev. 2012;248:156–169. doi: 10.1111/j.1600-065X.2012.01130.x. [DOI] [PubMed] [Google Scholar]
- 8.Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4:384–398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- 9.Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Calvo MG, Nemni R, Clerici M. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2010;21:927–938. doi: 10.3233/JAD-2010-091696. [DOI] [PubMed] [Google Scholar]
- 10.He F, Balling R. The role of regulatory T cells in neurodegenerative diseases. Wiley Interdiscip Rev Syst Biol Med. 2013;5:153–180. doi: 10.1002/wsbm.1187. [DOI] [PubMed] [Google Scholar]
- 11.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 12.Cantini G, Pisati F, Mastropietro A, Frattini V, Iwakura Y, Finocchiaro G, Pellegatta S. A critical role for regulatory T cells in driving cytokine profiles of Th17 cells and their modulation of glioma microenvironment. Cancer Immunol Immunother. 2011;60:1739–1750. doi: 10.1007/s00262-011-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonabend AM, Rolle CE, Lesniak MS. The role of regulatory T cells in malignant glioma. Anticancer Res. 2008;28:1143–1150. [PubMed] [Google Scholar]
- 14.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 15.de Graaf MT, Smitt PA, Luitwieler RL, van Velzen C, van den Broek PD, Kraan J, Gratama JW. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom. 2011;80:43–50. doi: 10.1002/cyto.b.20542. [DOI] [PubMed] [Google Scholar]
- 16.Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gemechu JM, Bentivoglio M. T-cell Recruitment in the Brain during Normal Aging. FronT-cell Neurosci. 2012;6:38. doi: 10.3389/fncel.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–880. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 20.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T-cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eizenberg O, Faber-Elman A, Lotan M, Schwartz M. Interleukin-2 transcripts in human and rodent brains: possible expression by astrocytes. J Neurochem. 1995;64:1928–1936. doi: 10.1046/j.1471-4159.1995.64051928.x. [DOI] [PubMed] [Google Scholar]
- 23.Gabryel B, Labuzek K, Malecki A, Herman ZS. Immunophilin ligands decrease release of pro-inflammatory cytokines (IL-1beta, TNF-alpha and IL-2 in rat astrocyte cultures exposed to simulated ischemia in vitro. Pol J Pharmacol. 2004;56:129–136. [PubMed] [Google Scholar]
- 24.Hanisch UK, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain Res Brain Res Rev. 1995;21:246–284. doi: 10.1016/0165-0173(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 25.Labuzek K, Kowalski J, Gabryel B, Herman ZS. Chlorpromazine and loxapine reduce interleukin-1beta and interleukin-2 release by rat mixed glial and microglial cell cultures. Eur Neuropsychopharmacol. 2005;15:23–30. doi: 10.1016/j.euroneuro.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Tran ND, Correale J, Schreiber SS, Fisher M. Transforming growth factor-beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors. Stroke. 1999;30:1671–1678. doi: 10.1161/01.str.30.8.1671. [DOI] [PubMed] [Google Scholar]
- 27.Wahl SM, Allen JB, McCartney-Francis N, Morganti-Kossmann MC, Kossmann T, Ellingsworth L, Mai UE, Mergenhagen SE, Orenstein JM. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991;173:981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood AJ, Bickmore WA. A condensed view of chromatin during T-cell development. EMBO J. 2011;30:235–236. doi: 10.1038/emboj.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 2009;4:879–897. doi: 10.2217/rme.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westland KW, Pollard JD, Sander S, Bonner JG, Linington C, McLeod JG. Activated non-neural specific T cells open the blood-brain barrier to circulating antibodies. Brain. 1999;122:1283–1291. doi: 10.1093/brain/122.7.1283. Pt 7. [DOI] [PubMed] [Google Scholar]
- 33.Tan KH, Purcell WM, Heales SJ, McLeod JD, Hurst RD. Activated T cells mediate direct blood-brain barrier endothelial cell death and dysfunction. Neuroreport. 2002;13:2587–2591. doi: 10.1097/00001756-200212200-00041. [DOI] [PubMed] [Google Scholar]
- 34.Lou J, Dayer JM, Grau GE, Burger D. Direct cell/cell contact with stimulated T lymphocytes induces the expression of cell adhesion molecules and cytokines by human brain microvascular endothelial cells. Eur J Immunol. 1996;26:3107–3113. doi: 10.1002/eji.1830261242. [DOI] [PubMed] [Google Scholar]
- 35.Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123:1092–1101. doi: 10.1093/brain/123.6.1092. Pt 6. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 37.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 40.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barcia C, Sr., Mitxitorena I, Carrillo-de Sauvage MA, Gallego JM, Perez-Valles A, Barcia C., Jr. Imaging the microanatomy of astrocyte-T-cell interactions in immune-mediated inflammation. FronT-cell Neurosci. 2013;7:58. doi: 10.3389/fncel.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barcia C, Sanderson NS, Barrett RJ, Wawrowsky K, Kroeger KM, Puntel M, Liu C, Castro MG, Lowenstein PR. T cells' immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS One. 2008;3:e2977. doi: 10.1371/journal.pone.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meinl E, Aloisi F, Ertl B, Weber F, de Waal Malefyt R, Wekerle H, Hohlfeld R. Multiple sclerosis. Immunomodulatory effects of human astrocytes on T cells. Brain. 1994;117:1323–1332. doi: 10.1093/brain/117.6.1323. Pt 6. [DOI] [PubMed] [Google Scholar]
- 45.Garg SK, Banerjee R, Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol. 2008;180:3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 46.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 47.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 48.Xie L, Poteet EC, Li W, Scott AE, Liu R, Wen Y, Ghorpade A, Simpkins JW, Yang SH. Modulation of polymorphonuclear neutrophil functions by astrocytes. J Neuroinflammation. 2010;7:53. doi: 10.1186/1742-2094-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.