Abstract
Background
Pulmonary arterial hypertension (PAH) has been identified as a serious complication of HIV infection.
Methods and Results
Here, we report sudden death in two pigtailed macaques (Macaca nemestrina) chronically infected (~ 1–2 years post infection) with an R5 SHIV strain. At necropsy, total occlusion of the pulmonary artery by a large fibrin thrombus was present in both animals.
Conclusion
This report describes pulmonary vascular lesions similar to PAH in R5 SHIV-infected pigtail macaques.
Keywords: Pulmonary arterial hypertension, SHIV, SIV
Introduction
Human immunodeficiency virus (HIV)-infected individuals are at increased risk of pulmonary arterial hypertension (PAH) with a prevalence of 0.5–2.0% [1, 2]. Associated with a 60% survival at two years after diagnosis, HIV-PAH has an even greater mortality in patients with more severe disease and is considered a terminal illness[2]. Non-human primates (NHPs) infected with simian immunodeficiency virus (SIV) or chimeric simian-human immunodeficiency virus (SHIV) also develop pulmonary artery lesions, characterized by intimal thickening and luminal occlusion with histologic similarity to those associated with human PAH[2–4]. Here, we describe case reports of two pigtailed macaques that were chronically infected with SHIV-B0159N4-p2; this virus had been constructed by replacing the envelope gene from the SHIV-1157ipd3N4 backbone [5, 6];unpublished data) with that of the microglia-tropic HIVBORI-15 [7]. These animals progressed to thrombocytopenia, late onset polycythemia, and sudden death. To our knowledge, this is the first report of pulmonary vascular lesions similar to PAH in R5 tropic SHIV-infected pigtailed macaques and parallels with previous reports of pulmonary arteriopathy in SIV-infected macaques.
Case Reports
Case 1 (PSp2)
This 4 year old male pigtailed macaque (PM) was born and maintained at the Yerkes National Primate Research Center (YNPRC) in accordance with their Animal Care and Use Committee. This PM was inoculated intrarectally with SHIV-Bo159N4-P2, a macrophage-microglia-tropic R5 SHIV [5, 6]; unpublished data). Peak viremia occurred at week 2 post-inoculation (Fig. 1A). The animal was persistently viremic with viral loads ranging from 104 to 1.5 × 107 copies/ml (Fig. 1A). The absolute CD4+ T cells in blood were gradually depleted and the fraction of CD4+CD29+ memory T cells declined to low levels at 8 months after post-infection (data not shown). At approximately one year post-infection (pi), the animal developed thrombocytopenia (29,000 to 124,000 µl; reference range 260–361 × 103 per µl) (Fig. 1A) that remained clinically unremarkable. Four months prior to death and about 20 months pi, the animal developed clinical polycythemia (HCT 52%; reference range 38.3–49.6%) and chronic thrombocytopenia. Despite normal appetite, the animal also failed to gain weight for the 2 months preceding death. Heart and lung auscultations during physical examinations revealed normal heart rhythm and strong peripheral pulses. Thoracic radiographs revealed moderate cardiomegaly. At twenty-one months pi, the monkey was found deceased and was submitted for post-mortem examination.
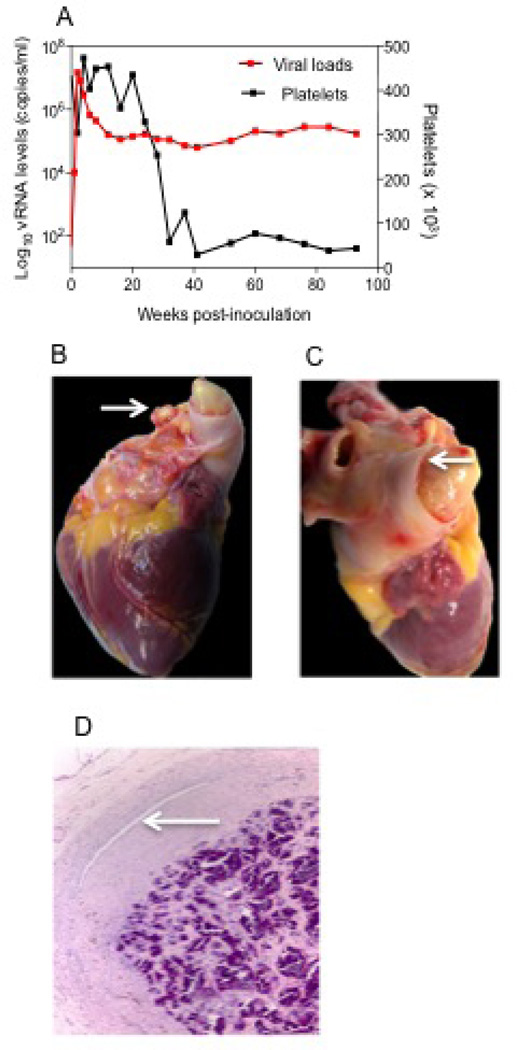
Fig. 1.
PSp2 infected with SHIV-B0159N4-p1/p2 viruses. (A) Viral loads and platelets counts kinetics (B) Occlusive fibrin thrombus protruding from both branches of pulmonary artery (arrows). (C) Marked distension of main branch of pulmonary artery and marked contraction of ventricles. (D) Fibrosis and mineralization of thrombus within pulmonary artery. There is focal recanalization lumen present (arrow). HE.
At necropsy, the heart was enlarged and both ventricles were firmly contracted. The right auricle was markedly distended. A large occlusive thrombus (3 cm in length) was present within the main pulmonary artery with extension into both branches (Fig. 1 B and C). Also present were fibrous adhesions of right lung lobes to thoracic wall, moderate serosanguineous pleural effusion, and marked pulmonary edema. Sections of all major organs were collected for routine histopathological evaluation. Tissues were collected, placed in 10% neutral buffered formalin, and processed in paraffin by routine methods for light microscopic study. Tissues were stained with hematoxylin and eosin (HE), and Masson’s Trichrome stain for collagen.
Microscopically, the pulmonary arterial thrombus was composed of dense fibrin with areas of calcification, hemosiderin and hemosiderophages and a few lymphocytes. The thrombus was attached to the vessel wall by fibroblasts and neovascularization. A smaller thrombus was almost completely calcified, with focal eccentric recanalization (Fig. 1C) and focus of multinucleated giant cells engulfing mineral. Within the heart, the left ventricle papillary muscle had marked locally extensive myocardial degeneration and necrosis with fibroplasia and minimal lymphocytic inflammation.
Histopathology of the lung showed arterial changes consisting of: arterial intimal proliferation, complex plexiform-like lesions characterized by luminal obliteration, intimal disruption, medial hypertrophy, and recanalized lumens. In a few arteries, the lumen was occluded by severe intimal thickening with a small number of inflammatory cells and multifocal recanalization. Other histopathology findings consisted of: moderate lymphoid depletion of spleen and lymph nodes.
Case 2 (PGl2)
This 4-year old male pigtailed macaque was born and maintained at the YNPRC in accordance with their Animal Care and Use Committee. He was placed on same study as case 1. The macaque was administered intravenously 1 ml of SHIV-Bo159N4-p1-infected microglial cells (MG) (2.38 × 10^7 cells) from another infected pigtailed macaque (PNi2; which had received brain tissue from PFm2 that had been inoculated with SHIV-Bo159N4-p) as shown in Fig. 2A. Monkey PGl2 was also persistently viremic with viral loads ranging from 7 × 103 to 5 × 108 copies/ml (Fig. 2B. The higher peak viral load in this animal was due to acute depletion of CD8 and B cells before it received MG cells from PNi2. The absolute CD4+ T cells in blood were gradually depleted and the fraction of CD4+ and CD29+ memory T cells declined to low levels 8 months after post-infection (data not shown). At approximately 8 months pi, the animal developed mild thrombocytopenia (137,000 µl; reference range 260–361 × 103 per µl) and prior to death, the platelet count had decreased to 52,000 per µl (Fig. 2B). Seventeen months pi, the monkey was found deceased and submitted for post-mortem examination.
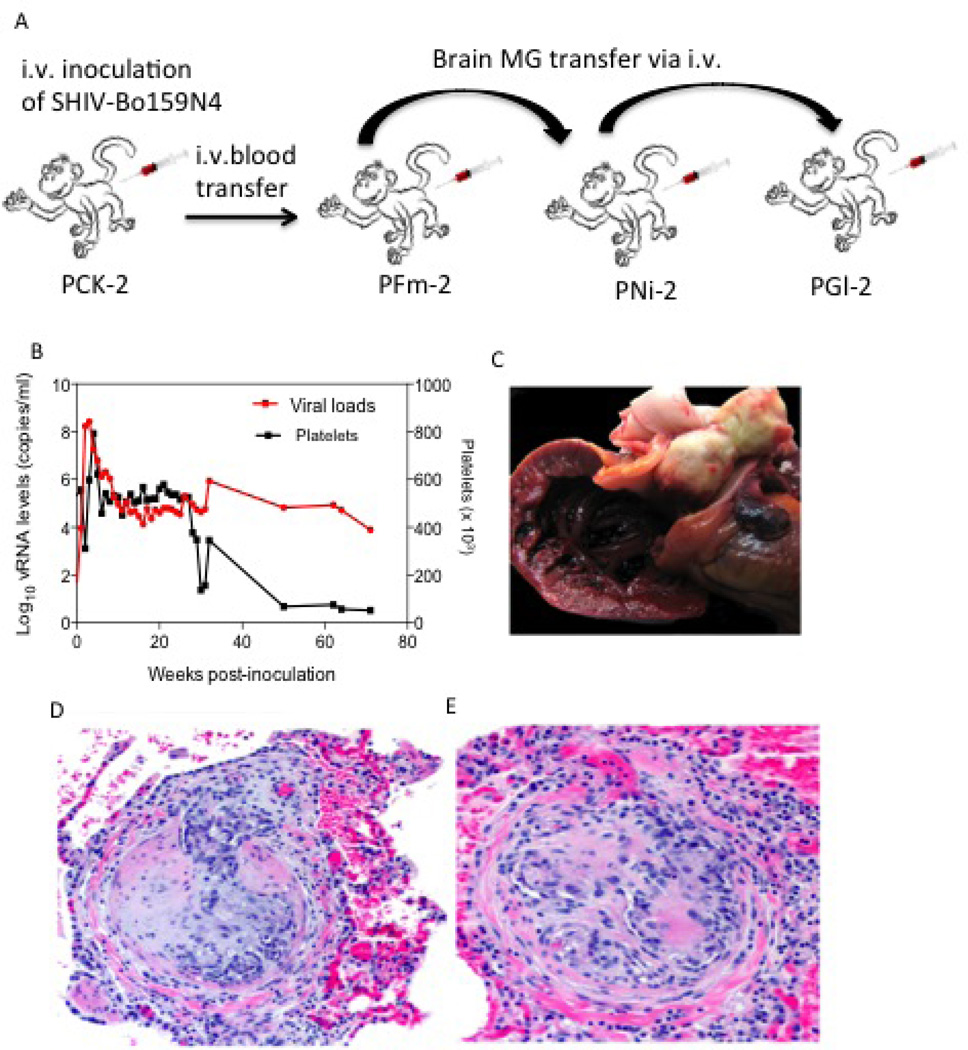
Fig. 2.
PGl2 infected with SHIV-B0159N4-p1/p2 viruses. (A) Serial passage of SHIV-Bo159N4-p in pigtail macaques via microglial cells (MG) (B) Viral loads and platelets counts kinetics (C) Right ventricle opened to show presence of large fibrin thrombus within main pulmonary artery and occluding the outflow tract. (D & E) Lung: Pulmonary arterial changes of plexiform endothelial proliferation and fibrosis. HE
At necropsy, the heart was firmly contracted, the right atrium was markedly distended, and a large occlusive thrombus was found within the pulmonary artery (Fig. 2C). The left middle lung lobe was adhered to the pericardium and right middle and caudal lobes were adhered to thoracic wall and diaphragm. The lungs failed to deflate upon opening of the thorax. Other findings at necropsy included: mildly enlarged spleen and inguinal, axillary and mesenteric lymph node; and edematous colonic mucosa with watery contents. Microscopically, the pulmonary arterial thrombus was composed of dense fibrin with areas of calcification and the large pulmonary artery contained marked intramural fibrosis (Fig. 2D and E). Medium-sized pulmonary arteries had mild medial hypertrophy. Histopathology of the lungs showed mild perivascular lymphocytic infiltrates, and focal subpleural bullae. Other histopathology findings included: mild lymphoid hyperplasia of lymph nodes and spleen, focal gastric mucosal erosion, moderate chronic lymphoplasmacytic colitis, and moderate lymphocytic interstitial nephritis (primate immunodeficiency virus-related findings).
Discussion
A total of 13 pigtailed macaques were infected with SHIV-Bo159N4-p1/p2 related viruses, and here, we describe occlusive pulmonary arterial thrombosis resulting in sudden death in two (2/13) pigtailed macaques with the prevalence rate of 15.3%. Pulmonary arteriopathy, consisting of medial hyperplasia, intimal hypertrophy, vascular remodeling, and plexiform-like lesions similar to HIV-related pulmonary hypertension observed in human [2–4, 8] were also present in these macaques. Histological evidence of PAH, which includes arterial medial hyperplasia and the presence of plexiform lesions, has been demonstrated in macaques infected with SIV as well as with chimeric SHIV [2–4, 8]. HIV has proven to be a risk factor for the development of PAH, and an increased prevalence of the disease in HIV-infected patients compared with the general population has been reported [1, 2, 9].
The mechanism by which infection leads to severe PAH is not known as the localization of the virus in the vascular lesions or endothelial cells in HIV-infected people has not been demonstrated [1]. This observation further suggest that a direct role of the virus is unlikely and suggests that PAH associated with HIV is related to an indirect action of virus infection, possibly through the action of pleiotropic viral proteins and/or chronic low grade inflammation. Recently, it has been demonstrated that more serum levels of immunoreactive TGF-beta1 was found in patients with Schistosomiasis-PAH compared to patients with Schistosomiasis without PAH[10]. Furthermore, they described that TGF-beta 1 is one of the isoform of TGF-beta that causes augmented cell proliferation in smooth muscle cells in the pulmonary arteries of patients with PAH and has a growth-inhibitory effect on normal cells and contribute to vascular remodeling[11]. Previously, it has been demonstrated that HIV Nef was first detected after HIV invasion of the host cell, and a possible link between HIV-1 Nef and the development of PAH has been suggested [4]. This includes the uptake of Nef into vascular endothelial cells, possibly via the CXCR4 receptor, and in conjunction with immune insufficiency, may then lead to endothelial cell dysfunction and apoptosis [4]. The HIV proteins Nef, Tat, and Env have been reported to be associated with endothelial cell dysfunction and smooth muscle cell hyperplasia and may have contributed to the development of pulmonary changes reported here [4]. Furthermore, it has also been noted that engulfment of apoptotic cells by surrounding endothelial cells may lead to an apoptosis-resistant population with increased secretion of cytokines and growth factors [12]. The uncontrolled endothelial cell proliferation leads to complex vascular remodeling, plexiform lesions, and HIV-related pulmonary hypertension [9]. Next, It has been documented that several neurotropic SIVs such as SIV/17E-Fr and SIVΔB670 viruses infect brain capillary endothelial cells by entering blood-brain barrier or by releasing toxins into the central nervous system which cause AIDS dementia[13]. Since, SHIV-Bo159N4-p1/p2 is highly macrophage-tropic virus, and causes milder forms of neurological disorders in macaques, there may be possibility that it could have been infected with brain capillary endothelial cells and caused endothelial dysfunction, which in turn could have been contributed to the massive occlusive thrombosis.
In summary, we reported here that sudden death by thrombosis of pulmonary arteries should be added to the array of pulmonary vascular lesions that can be seen in SHIV-infected pigtailed macaques. Our findings parallel the PAH in HIV-infected humans and suggest that our new SHIV model appears to facilitate the development of PAH pathology, providing an attractive model. However, additional work would be required to confirm this conjecture.
Acknowledgments
The authors thank the animal care and pathology staff of the YNPRC for their excellent care of the animals and technical support for this study. This work was supported by NIH grants R21 NS063877 and R37 AI034266 to RMR as well as base grant P51OD11132 to the Yerkes National Primate Research Center.
References
- 1.Barbaro G, Fisher SD, Lipshultz SE. Pathogenesis of HIV-associated cardiovascular complications. The Lancet infectious diseases. 2001;1:115–124. doi: 10.1016/S1473-3099(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 2.George MP, Brower A, Kling H, Shipley T, Kristoff J, Reinhart TA, Murphey-Corb M, Gladwin MT, Champion HC, Morris A, Norris KA. Pulmonary vascular lesions are common in SIV- and SHIV-env-infected macaques. AIDS research and human retroviruses. 2011;27:103–111. doi: 10.1089/aid.2009.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Laboratory investigation; a journal of technical methods and pathology. 1992;67:338–349. [PubMed] [Google Scholar]
- 4.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, Voelkel NF, Flores SC. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. American journal of respiratory and critical care medicine. 2006;174:437–445. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrareddy SN, Thorat S, Sharma P, Hemashettar G, Matsuda K, Hirsch V, Girolami U, Novembre F, Villinger F, Ruprecht RM. Macrophage/microglia lineage-related R5-tropic simian-human immunodeficiency viruses as tools to induce and study HAND. J Neurovirol. 2013;19:S1–S101. [Google Scholar]
- 6.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD, Xu W, Whitney JB, Goins LM, Ong H, Li PL, Shai-Kobiler E, Wang T, McCann CM, Zhang H, Wood C, Kankasa C, Secor WE, McClure HM, Strobert E, Else JG, Ruprecht RM. Molecularly cloned SHIV-1157ipd3N4: a highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. Journal of virology. 2006;80:8729–8738. doi: 10.1128/JVI.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strizki JM, Albright AV, Sheng H, O'Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. Journal of virology. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. Journal of the American College of Cardiology. 2004;43:25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Voelkel NF, Cool CD, Flores S. From viral infection to pulmonary arterial hypertension: a role for viral proteins? AIDS. 2008;22(Suppl 3):S49–S53. doi: 10.1097/01.aids.0000327516.55041.01. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira Rde C, Montenegro SM, Domingues AL, Bandeira AP, Silveira CA, Leite LA, Pereira Cde A, Fernandes IM, Mertens AB, Almeida MO. TGF beta and IL13 in schistosomiasis mansoni associated pulmonary arterial hypertension; a descriptive study with comparative groups. BMC infectious diseases. 2014;14:282. doi: 10.1186/1471-2334-14-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 12.George MP, Champion HC, Simon M, Guyach S, Tarantelli R, Kling HM, Brower A, Janssen C, Murphy J, Carney JP, Morris A, Gladwin MT, Norris KA. Physiologic changes in a nonhuman primate model of HIV-associated pulmonary arterial hypertension. American journal of respiratory cell and molecular biology. 2013;48:374–381. doi: 10.1165/rcmb.2011-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. The American journal of pathology. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]