Abstract
OBJECTIVE
To characterize the relationship between stress and future risk of sepsis. We also evaluated the role of depression in this relationship.
METHODS
We used population-based data on 30,183 participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort, characterizing stress using the Perceived Stress Scale (PSS) and depressive symptoms using the Center for Epidemiologic Studies Depression Scale (CES-D). We identified incident sepsis events as hospitalizations for a serious infection with the presence of ≥2 SIRS criteria. We assessed associations between PSS and incidence of sepsis over one- and ten-years of follow-up, adjusting for demographics and chronic medical conditions and assessing the role of health behaviors and CES-D in these relationships.
RESULTS
During 2003–2012, 1,500 participants experienced an episode of sepsis. Mean PSS and CES-D scores were 3.2±2.9 and 1.2±2.1. PSS was associated with increased one-year adjusted incidence of sepsis (HR 1.21 per PSS standard deviation; 95% CI: 1.06–1.38); multivariable adjustment for health behaviors and CES-D did not change this association (1.20; 1.20; 1.03–1.39). PSS was also associated with increased 10-year adjusted incidence of sepsis (HR 1.07 per PSS standard deviation; 95% CI: 1.02–1.13). Multivariable adjustment showed that health behaviors did not affect this long-term association whereas addition of CES-D reduced the association between PSS and sepsis during 10-year follow-up (HR 1.04; 0.98–1.11).
CONCLUSIONS
Increased stress was associated higher one-year adjusted incidence of sepsis, even after accounting for depressive symptoms. The association between stress and ten-year adjusted incidence of sepsis was also significant, but this association was reduced when adjusting for depressive symptoms. Reduction of stress may limit short-term sepsis risk.
Keywords: sepsis, infection, stress, epidemiology, depression
INTRODUCTION
Sepsis, the clinical syndrome of microbial infection complicated by systemic inflammatory response, is a major public health problem. Severe sepsis is associated with an estimated 750,000 hospitalizations, 570,000 Emergency Department visits and over 215,000 deaths annually in the United States (US), and the national cost of sepsis care exceeds $16.7 billion.(1) Despite a thorough understanding of the pathophysiology of sepsis, relatively little is known of its associated clinical or demographic risk factors.
Psychological or social (psychosocial) stress is believed to greatly affect baseline health and has been associated with the onset and progression of diseases such as cardiovascular disease, acquired immune deficiency syndrome (AIDS), autoimmune diseases, and respiratory tract infections.(2) The interplay between stress and the immune system is complex, with different types of stressors elucidating varied natural and specific responses.(3) This is pertinent to sepsis, as down-regulation of cellular or humoral immunity could potentially lead to increased infection susceptibility, but up-regulation of pro-inflammatory cells and cytokines could lead to a state of heightened inflammation.(3, 4) Current evidence indicates a relationship between psychosocial stress and chronic, low-grade inflammation, which may be responsible for observed stress-disease associations that are not fully explained by hypothalamic-pituitary-adrenal axis and sympathetic nervous system alteration.(5) The stress-sepsis relationship could represent such an association, with prior work demonstrating a strong link between chronic inflammation and increased risk of sepsis.(6)
Depression frequently coexists with stress with much debate ongoing regarding the relationship between the two conditions.(7) Depression has plausible connections with sepsis risk. For example, depression has been linked with altered immune function and a pro-inflammatory state.(8–10) These mechanisms have been implicated in the impaired wound healing and increased risk of infection reported among individuals suffering from depression and other affective mood disorders.(8, 11)
While numerous studies have characterized the course of acute sepsis episodes, few studies have assessed the association of baseline perceived stress with future sepsis episodes. Stress has plausible links with short term health effects, but there is also evidence of its longer-term health effects. For example, in a cohort of >21,000 adults >60 years old, Draper, et al. found that childhood physical and sexual abuse were associated with poor current physical health.(19) In this study we sought to determine the association of perceived stress with short- and long-term incidence of sepsis events in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, one of the nation’s largest population-based cohorts of community-dwelling individuals in the US. In addition, because stress could lead to depression, thereby resulting in increased incidence of sepsis, we also sought to determine if the presence of depressive symptoms explained the association between stress and sepsis.(5, 8, 9)
METHODS
Study Design
This study used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a national, population-based, longitudinal cohort. The study was granted approval by the Institutional Review Board of the University of Alabama at Birmingham.
Selection of Participants
One of the largest ongoing national cohorts of community-dwelling individuals in the US, REGARDS was designed to identify the reasons for stroke geographic and racial disparities in the United States (US).(10) REGARDS includes 30,239 community-dwelling adults ≥45 years old from all regions of the continental US. The study oversampled the participants from the Southeastern US, with 21% of the cohort originating from the coastal plains of North Carolina, South Carolina and Georgia (the “stroke buckle”), and 35% originating from the remainder of North Carolina, South Carolina and Georgia plus Tennessee, Mississippi, Alabama, Louisiana and Arkansas (the “stroke belt”). The cohort is 42% African American and 45% men, and 69% of individuals are >60 years old. The cohort did not include Hispanics where stroke mortality disparities are small to non-existent. The REGARDS cohort encompasses healthy community-dwelling adults – not just individuals with a history of stroke.
Enrollment for REGARDS occurred from 2003–7. The study obtained comprehensive baseline data for each individual from phone interviews and in-person assessments. Initial data included functional status, medical history, physical and physiological characteristics (heart rate, blood pressure, electrocardiogram), health behaviors (diet, activity, tobacco and alcohol use), and current medication use. Blood and urine samples were also taken at the initiation of the study. Self-administered questionnaires assessed psychosocial factors, family history of disease and aspects of social history (residency, education, income). During follow-up, study personnel contact participants on a semi-annual basis to collect data regarding the date, location and reason for any hospitalizations and emergency department visits during the follow-up interval. The study also collected information about participants that expired during the study period, including recent hospitalizations and the circumstances surrounding the death.
Identification of Sepsis Events
We reviewed all hospitalizations and emergency department visits for a serious infection as reported by the participant. Two trained abstractors independently reviewed relevant medical records to confirm the presence of a serious infection as the primary reason for hospitalization previously published infection classification taxonomies.(1, 11) Medical record review included clinical and laboratory findings from the first 28-hours of hospitalization. Additional physician-level review resolved discrepancies.
Using international consensus definitions, we defined sepsis as presentation to the hospital with a serious infection and two or more criteria for systemic inflammatory response syndrome (SIRS), including 1) heart rate >90 beats/minute, 2) fever (temperature >38.3°C or <36°C), 3) tachypnea (>20 breaths/min) or PCO2<32 mmHg, and 4) leukocytosis (white blood cells [WBC] >12,000 or <4,000 cells/mm3 or >10% band forms). Vital signs and laboratory findings were used for the initial 28-hours of medical care in order to account for acute changes in patient conditions during the early hospitalization period. Initial review of 1,349 hospital records indicated excellent inter-rater agreement for presence of a serious infection (kappa=0.92) and the presence of sepsis (kappa=0.90) upon hospital presentation.
Definition of Perceived Stress and Depressive Symptoms
Participants reported stress levels using a shortened four-question version of the perceived stress scale (PSS). The original PSS is a 14-item instrument designed to measure the degree to which individuals appraise life situations as stressful.(12) Cohen, et al., developed and validated a shortened four-question version of the PSS gauging feelings of control, confidence, coping and management over the prior month, with total scores ranging from 0–16.(13) (Appendix 1, Supplemental Digital Content 1) The PSS has been validated in a large probability sample in the United States and exhibits a Cronbach’s α of 0.60.(12, 14)
At the beginning of the REGARDS study, participants completed a shortened four-question version of the Center for Epidemiologic Studies Depression Scale (CES-D).(15) The original CES-D encompassing 20 questions was developed for use in epidemiologic studies of depressive symptoms in the general population. Melchior, et al. validated a shortened four-question version of the CES-D gauging feelings of depression, loneliness, crying spells and sadness over the prior week, and with total scores ranging from 0 to 12.(16) (Appendix 2, Supplemental Digital Content 2) Cronbach’s α for the four-question CES-D is 0.81.(16)
Covariates
We considered various covariates that may influence the relationship between stress and sepsis, including sociodemographic characteristics, health behaviors (tobacco and alcohol use) and chronic medical conditions. REGARDS measured all covariates at the time of participant enrollment in the study. We used categories defined by the parent REGARDS study for each of the variables.
Sociodemographic characteristics included age, sex, race, geographic region, self-reported annual household income and self-reported level of education. REGARDS recruited only participants of black and white race. Geographic region was categorized as current residence in the “stroke belt” (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina and Tennessee), “stroke buckle” (the coastal plains of Georgia, North Carolina and South Carolina) and elsewhere, included here to account for the unequal sampling used to establish the REGARDS cohort.(10) We defined alcohol use as none, moderate (1 drink per day for women or 2 drinks per day for men) and heavy (>1 drink per day for women and >2 drinks per day for men) according to the National Institute on Alcohol Abuse and Alcoholism classification.(17) Tobacco use was defined as current, past and never.
Chronic medical conditions included a history of hypertension, diabetes, obesity, dyslipidemia, coronary artery disease, chronic kidney disease and chronic lung disease. REGARDS defined hypertension as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or the self-reported use of antihypertensive medication. Diabetes was defined as a fasting glucose ≥126 mg/L, a random glucose ≥200 mg/L, or the reported use of insulin or oral hypoglycemic medication. Obesity was categorized as a body mass index ≥30 kg/m2 or a waist circumference >102 cm for men and >88 cm for women. Dyslipidemia included individuals currently using lipid lowering medication or self-reporting the presence of high cholesterol. A history of coronary artery disease consisted of individuals with a self-reported history of myocardial infarction, coronary intervention or baseline electrocardiographic evidence of myocardial infarction.
REGARDS defined chronic kidney disease as an estimated glomerular filtration rate <60 ml/min/1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.(18) Because REGARDS data collection did not include lung conditions (-e.g. asthma, chronic obstructive pulmonary disease), we defined as current use of pulmonary medications as a surrogate marker for chronic lung disease. Pulmonary medications included beta agonists, leukotriene inhibitors, inhaled corticosteroids, combination inhalers and other pulmonary medications such as ipratropium, cromolyn, aminophylline and theophylline.
Data Analysis
We centered and normalized PSS and CES-D scores by subtracting their mean values and dividing by their standard deviation. Using t-tests for binary variables, ANOVA for categorical variables, and Pearson’s correlation for continuous variables, we compared mean PSS and CES-D scores for each participant characteristic category.
We could not establish the exact temporal relationship between stress and each participant characteristic. However, since they likely were present before the onset of stress, we conceptualized each demographic characteristic and chronic medical condition as a confounder in the stress-sepsis relationship. Because stress may adversely affect health behaviors, leading to increased incidence of sepsis, we examined the role of tobacco and alcohol use in the stress-sepsis relationship. Finally, based upon our theoretical framework, we investigated whether the stress-sepsis relationship remained significant when adjusting for depressive symptoms (CES-D).
We used Cox proportional hazards models to determine the associations between normalized PSS and time to first sepsis event, examining the associated hazard ratios and 95% confidence intervals. We defined person-time at risk as the elapsed time in days from first interview to the first episode of sepsis or the last follow-up interview. We fit models assessing the association between normalized PSS and incidence of first sepsis events, adjusting for demographics (age, sex, race, income, education, geographic region) and chronic medical conditions (chronic kidney disease, chronic lung disease, diabetes, hypertension, myocardial infarction, obesity, stroke). To determine their effects upon the stress-sepsis association, we next added health behaviors (tobacco and alcohol use) to the multivariable model, examining the change in hazard ratio and 95% confidence interval for PSS. Finally, we added CES-D to the model, examining its effect upon the hazard ratio and 95% confidence interval for PSS.
We verified the proportional hazards assumption by examining Schoenfeld residuals and [PSS × time] interactions. We conducted all analyses using Stata v.12.1 (Stata, Inc. College Station, Texas)
RESULTS
From February 5, 2003 through December 31, 2012, a total of 1,500 REGARDS participants experienced a sepsis event (incidence 8.0 per 1000 person-years; 95% CI: 7.6–8.5). The most common infections associated with these sepsis events were pneumonia, kidney and urinary tract infections and abdominal infections. (Table 1)
TABLE 1.
Infection types associated with first hospitalizations for sepsis. Total of 1,500 first sepsis events.
| Infection Type | Number of First Sepsis Hospitalizations N (%) |
|---|---|
| Pneumonia | 592 (39.5) |
| Kidney and Urinary Tract Infections | 251 (16.7) |
| Abdominal | 230 (15.5) |
| Bronchitis, Influenza and other Lung Infections | 137 (9.1) |
| Skin and Soft Tissue | 121 (8.1) |
| Sepsis | 98 (6.5) |
| Fever of Unknown Origin | 29 (1.9) |
| Catheter (IV / Central / Dialysis) | 6 (0.4) |
| Surgical Wound | 10 (0.7) |
| Meningitis | 5 (0.3) |
| Unknown/Other | 21 (1.4) |
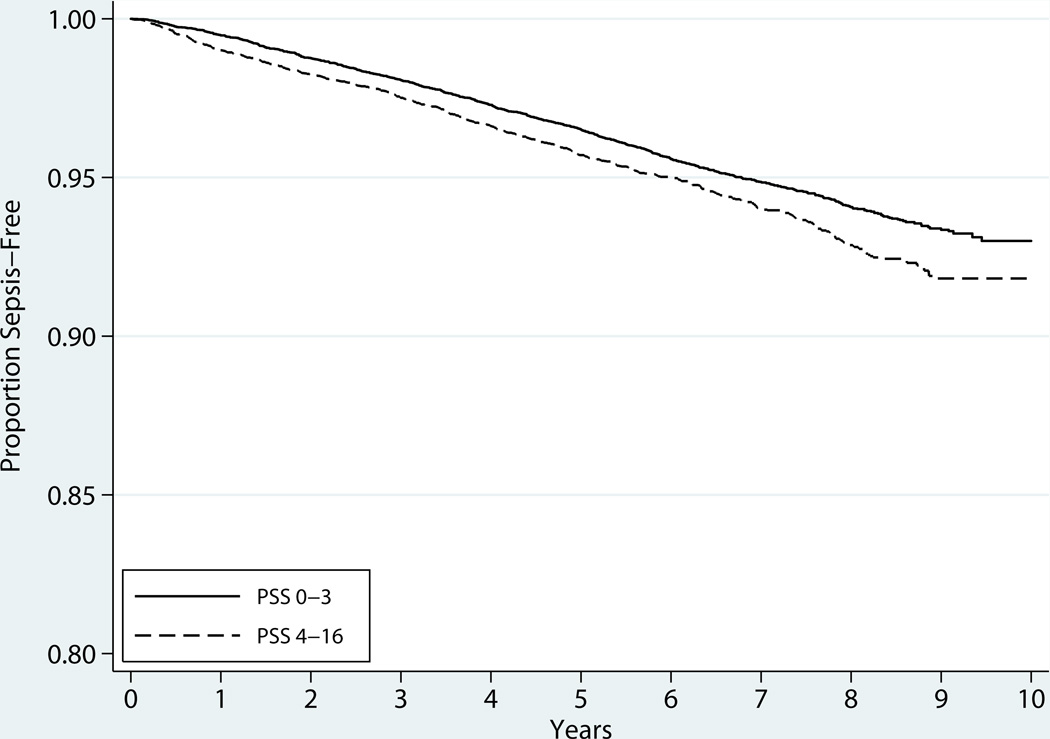
PSS and CES-D scores were available for 99.9% and 99.3% of REGARDS participants, respectively. The median and mean PSS scores were 3 (interquartile range 0–5) and 3.2 (SD 2.9). The incidence of sepsis events was higher among those with PSS 4–16 than those with PSS 0–3 (10.0 vs 5.2 per 1000 person-years). (Figure 1) The median and mean CES-D scores were 0 (interquartile range 0–2) and 1.2 (SD 2.1). Correlation between PSS and CES-D was moderate (Spearman ρ=0.43).
Figure 1.
Kaplan-Meier survival curves for stress and long-term incidence of sepsis. Stress characterized by the short form of the Perceived Stress Scale (PSS). (12)
Higher PSS and CES-D scores were present in men, Blacks, participants with low income and education, and those with chronic medical conditions. (Table 2) Participants with higher PSS and CES-D scores were more likely to be current smokers but were less likely to regularly use alcohol.
TABLE 2.
Baseline characteristics of REGARDS participants and associations with stress and depressive symptoms.
| Characteristic | N (col %) | PSS mean (SD) |
P-value* | CES-D mean (SD) |
P-value* |
|---|---|---|---|---|---|
| Demographics | |||||
| Age(mean, SD) | 64.8 (9.4) | −0.09† | <0.001 | −0.07† | <0.001 |
| Sex | |||||
| Male | 13,551 (44.9) | 3.6 (3.0) | <0.001 | 0.9 (1.8) | <0.001 |
| Female | 16,632 (55.1) | 2.7 (2.7) | 1.4 (2.3) | ||
| Race | |||||
| White | 17,669 (58.5) | 2.9 (2.8) | <0.001 | 1.0 (1.9) | <0.001 |
| Black | 12,514 (41.5) | 3.6 (3.1) | 1.4 (2.3) | ||
| Region | |||||
| Non-Belt | 13,429 (44.5) | 3.1 (2.9) | <0.001 | 1.1 (2.0) | <0.001 |
| Belt | 10,447 (34.6) | 3.3 (3.0) | 1.2 (2.1) | ||
| Buckle | 6,307 (20.9) | 3.3 (3.0) | 1.2 (2.2) | ||
| Income | |||||
| <$20,000 | 5,478 (18.2) | 4.2 (3.4) | <0.001 | 2.1 (2.7) | <0.001 |
| $20,000–34,000 | 7,307 (24.2) | 3.3 (2.9) | 1.2 (2.1) | ||
| $35,000–74,000 | 8,914 (29.5) | 2.8 (2.7) | 0.8 (1.7) | ||
| ≥$75,000 | 4,754 (15.7) | 2.5 (2.4) | 0.6 (1.3) | ||
| Missing | 3,730 (12.4) | 3.3 (3.0) | 1.2 (2.1) | ||
| Education | |||||
| Less than High School | 3,792 (12.6) | 4.1 (3.4) | <0.001 | 2.0 (2.7) | <0.001 |
| High School Graduate | 7,804 (25.9) | 3.4 (3.0) | 1.3 (2.2) | ||
| Some College | 8,090 (26.8) | 3.1 (2.9) | 1.1 (2.0) | ||
| College Graduate | 10,472 (34.7) | 2.7 (2.6) | 0.8 (1.6) | ||
| Missing | 25 (0.1) | 3.6 (3.5) | 1.9 (3.0) | ||
| Health Behaviors | |||||
| Tobacco Use | |||||
| Never | 13,604 (45.1) | 3.2 (2.9) | <0.001 | 1.1 (1.9) | <0.001 |
| Past | 9,856 (32.7) | 3.0 (2.8) | 1.0 (1.9) | ||
| Current | 1,187 (3.9) | 3.8 (3.3) | 1.8 (2.7) | ||
| Missing | 593 (2.0) | 3.1 (2.9) | 1.2 (2.4) | ||
| Alcohol Use | |||||
| None | 18,547 (61.5) | 3.3 (3.0) | <0.001 | 1.3 (2.1) | <0.001 |
| Moderate | 9,856 (32.7) | 2.9 (2.8) | 1.0 (1.9) | ||
| Heavy | 1,187 (3.9) | 2.8 (2.9) | 1.2 (2.2) | ||
| Missing | 593 (2.0) | 3.6 (3.2) | 1.6 (2.5) | ||
| Chronic Medical Conditions | |||||
| Chronic Kidney Disease | |||||
| Yes | 3,291 (10.9) | 3.2 (3.0) | 0.32 | 1.2 (2.0) | 0.13 |
| No | 26,892 (89.1) | 3.2 (2.9) | 1.2 (2.1) | ||
| Chronic Lung Disease | |||||
| Yes | 2,765 (9.2) | 3.6 (3.1) | <0.001 | 1.5 (2.4) | <0.001 |
| No | 27,418 (90.8) | 3.2 (2.9) | 1.1 (2.0) | ||
| Diabetes | |||||
| Yes | 6,814 (22.7) | 3.6 (3.2) | <0.001 | 1.5 (2.4) | <0.001 |
| No | 23,267 (77.4) | 3.1 (2.9) | 1.1 (2.0) | ||
| Hypertension | |||||
| Yes | 17,847 (59.3) | 3.3 (3.0) | <0.001 | 1.3 (2.2) | <0.001 |
| No | 12,262 (40.7) | 3.0 (2.8) | 1.0 (1.9) | ||
| Myocardial Infarction | |||||
| Yes | 3,773 (12.8) | 3.4 (2.9) | <0.001 | 1.4 (2.3) | <0.001 |
| No | 25,823 (87.3) | 3.1 (2.9) | 1.1 (2.0) | ||
| Obesity (abnormal BMI or WC) | |||||
| Yes | 16,143 (53.6) | 3.4 (3.0) | <0.001 | 1.3 (2.2) | <0.001 |
| No | 13,992 (46.4) | 3.0 (2.8) | 1.0 (1.9) | ||
| Stroke | |||||
| Yes | 1,930 (6.4) | 4.1 (3.3) | <0.001 | 1.8 (2.5) | <0.001 |
| No | 28,151 (93.6) | 3.1 (2.9) | 1.1 (2.0) |
Stress characterized by the short form of the Perceived Stress Scale (PSS).(20) Depressive symptoms characterized by the short form of the Center for Epidemiologic Studies DepressionScale (CES-D).(18)
P-values reflect t-tests for binary variables, ANOVA for categorical variables, and Pearson’s correlation for continuous variables.
Pearson’s correlation.
On unadjusted analysis higher PSS was associated with increased one-year incidence of sepsis (HR 1.21 per one standard deviation PSS increase; 95% CI: 1.06–1.38). (Table 3) The association between PSS and one-year sepsis incidence persisted after adjustment for demographics and chronic medical conditions. Addition of health behaviors (tobacco and alcohol use) and CES-D did not change the one-year association between PSS and sepsis (1.20 per one standard deviation PSS increase; 95% CI: 1.03–1.39).
TABLE 3.
Multivariable associations (hazard ratios and 95% confidence intervals) between stress and one- and ten-yearincidence of sepsis.
| Model | Unadjusted Model |
Add Demographics† |
Add Chronic Medical Conditions‡ |
Add Tobacco & Alcohol |
Add CES-D |
|---|---|---|---|---|---|
| Sepsis Events within 0–1 Years (n=210 sepsis events) | |||||
| Stress (PSS) | 1.30 (1.15–1.47) | 1.30 (1.15–1.48) | 1.21 (1.06–1.38) | 1.21 (1.06–1.38) | 1.20 (1.03–1.39) |
| Depressive Symptoms (CES-D) | -- | -- | -- | -- | 1.02 (0.89–1.17) |
| Sepsis Events within 0–10 Years (n=1,498 sepsis events) | |||||
| Stress (PSS) | 1.10 (1.05–1.16) | 1.11 (1.06–1.17) | 1.07 (1.02–1.13) | 1.07 (1.01–1.13) | 1.04 (0.98–1.11) |
| Depressive Symptoms (CES-D) | -- | -- | -- | -- | 1.05 (0.996–1.12) |
Hazard ratios and 95% confidence intervals reflect increased incidence of sepsis for every one standard deviation increase in PSS. Total of 30,239 participants. Stress characterized by the short form of the Perceived Stress Scale (PSS).(20) Depressive symptoms characterized by the short form of the Center for Epidemiologic Studies Depression Scale (CES-D).(18) CES-D and PSS scores centered and normalized for the analysis. Hazard ratios reflect relative increase in sepsis incidence for each standard deviation increase in CES-D or PSS.
Age decile, sex, race, geographic region, income, education.
Chronic kidney disease, chronic lung disease, diabetes, hypertension, myocardial infarction, obesity, stroke.
Higher PSS was associated with increased 10-year incidence of sepsis after adjustment for demographics and chronic medical conditions (1.07 per one standard deviation PSS increase; 95% CI: 1.02–1.13). (Table 3) While not affected by the addition of tobacco and alcohol, addition of CES-D did explain the association between PSS and 10-year incidence of sepsis (1.04 per one standard deviation PSS increase; 95% CI: 0.98–1.11)
Assessment of scaled Schoenfeld residuals verified that PSS and CES-D satisfied the proportional hazards assumption (global test p=0.50). The [PSS × time] and [CES-D × time] interactions were also not statistically significant (p=0.86 and 0.64), further supporting satisfaction of the proportional hazard assumption.
DISCUSSION
Prior studies suggest connections between stress, depression and a range of health risks.(2, 20–27) In this study, using the large REGARDS cohort, we observed an association between baseline stress and increased adjusted one-year sepsis incidence, a relationship that was not influenced by baseline depressive symptoms. Stress was associated with ten-year adjusted incidence of sepsis; however, this association was explained by baseline depressive symptoms.
Chronic stress, via various effects upon host immunity, may heighten the risk of infection. For instance, stress has been shown to down-regulate innate immunity, antibody production, and T-cell responses.(28) This immunomodulation is further evident in studies highlighting increased risk of viral reactivation and increased risk of infectious respiratory illness among those with increased stress.(29, 30) Alternatively, psychosocial stress also has plausible connections with increased low-grade, chronic inflammation that could be related to sepsis in a similar fashion as has been observed for CRP.(5, 6) Specifically, prior history of childhood abuse as well as self-reported social isolation, anger, hostility, and depression have been shown to be positively correlated with increased plasma concentrations of inflammatory markers that exhibit strong associations with sepsis.(5)
There are biologically plausible links between depressive symptoms and stress-sepsis relationship. Depressed states can result in elevated pro-inflammatory cytokines such as C-reactive protein (CRP) and interleukin-6, which are prominent in sepsis pathophysiology.(31) At the cellular level, clinical depression has been associated with decreased lymphocyte function manifested by reduced proliferative responses and immune cell activity.(32) These mechanisms have been implicated in the impaired wound healing and increased risk of infection reported among individuals suffering from depression and other affective mood disorders.(30, 33)
Furthermore, several studies describe the coexistence of stress and depression. Individuals with depression often also exhibit increased stress, with several studies suggesting additional infection risk among this population.(25, 31, 34) Up to 80% of depression cases have been associated with preceding stressful events.(4, 35–37) For example, in a study of 100 adults, Muscatell, et al. found that life stressors were associated with greater depression severity and symptomatology.(38) In a study of 1,898 female twins, Kendler, et al. identified a potential causal relationship between stressful life events and the onset of depression.(39) An alternative model additionally suggests that cytokine-mediated and inflammatory stress responses may have a role in the etiology of depression, as well as disease processes involving chronic low-grade inflammation.(9) While our results provide support for this contention, the study was not designed to elucidate the exact mechanisms linking depression, stress and sepsis risk. Additional investigation must identify the biologic pathways linking these conditions.
Our findings clarify that the association between stress and sepsis risk is limited to the short term. The relationship between stress and long-term sepsis hazard appears to be explained by depression, participant demographics, chronic medical conditions, and tobacco and alcohol use. This observation is sensible since chronic medical conditions are often associated with concurrent the presence of affective disorders. For example, Wells et al. found that patients with at least one chronic medical condition have a 41% increased relative risk of recent psychiatric disorder.(40) Conditions such as diabetes, obesity, chronic renal disease and cardiovascular disease have all been associated with increased incidence of depression.(41–44) In addition, depression is associated with negative health behaviors such as smoking and elevated alcohol use.(45–48) In a prior study we observed associations between chronic medical conditions, health behaviors and future sepsis events.(49)
While we examined stress and depression as precursor to sepsis events, a body of literature suggests the reverse - that infections may lead to increased depression or stress.(50–52) For example, pro-inflammatory cytokines released in response to peripheral infection can trigger major depression in previously well individuals.(53) Additionally, associations between atherosclerotic disease and depression have been noted, supporting the potential role of chronic inflammation in the etiology of psychological disorders.(5, 9) Since depressive symptoms and stress were measured at baseline, prior to the observed sepsis events, we were unable to evaluate this hypothesis. Re-assessment of all REGARDS participants is currently underway, and thus it may be possible to evaluate if sepsis events are associated with changes in measures of stress and depression.
The observations of this study support our larger hypothesis that an individual’s future sepsis risk may be potentially predicted by baseline participant characteristics. This knowledge has clinical relevance. For example, for individuals with predicted elevated sepsis risk, clinicians may exercise lower thresholds for antibiotic treatment of minor infections or for hospital admission in response to more serious infections. If validated, our findings would suggest that treatment of stress, independent of other comorbidities, may effect a reduction in future sepsis risk. While some might consider treatment of depressive symptoms as a strategy for sepsis prevention, our analysis suggests that this association is largely explained by the presence of elevated stress. Additional study must validate if changes in depressive symptoms or stress may result in transient or more sustained sepsis risk reduction.
There are important limitations that must be considered when interpreting these results. The REGARDS study measured depressive symptoms using the shortened version of the CES-D scale.(15) More comprehensive batteries may be able to discern and identify other dimensions of depression including the time course, severity and functional limitations produced by the syndrome. However, a prior effort to validate the 4-item CES-D found the 4-item scale to be less specific than the full 20-item scale for depression screening, and thus we would not expect the observations of the current study to change significantly with the use of the full CES-D scale.(16) Also, REGARDS measured depressive symptoms and stress at only at the beginning of the observation period. Studies have verified that depressive symptoms are cyclical in nature and may change over time.(54) However, while the symptom windows for CES-D and PSS are relatively short, gauging symptoms within the prior week or month, numerous studies have linked these measures with long-term health outcomes.(55–58) Repetition of the study with more comprehensive or robust assessment tools and periodic reassessments may alter the observations. The REGARDS study is currently re-examining all members of the cohort, and in a future effort it may be possible to determine if interval changes in reported depressive symptoms may be associated with sepsis incidence.
An additional limitation is that individuals with depressive symptoms accounted for a relatively small number of sepsis events, potentially affecting our ability to detect more subtle associations. We did not examine severity variants of sepsis, such as severe sepsis or septic shock, and did not examine longer term outcomes such as long-term death. By design, the REGARDS cohort includes only African Americans and whites. While we were able to detect the presence of chronic medical conditions, we did not characterize their level of severity. We were also unable to differentiate the specific types of stress most strongly associated with sepsis, and could not examine the specific components of the immune system involved in this process. This is an important area of future study, as greater understanding of the processes mediating the stress-sepsis relationship could improve risk stratification, or identification of those most vulnerable to sepsis.
In conclusion, individuals reporting elevated stress exhibited higher one-year adjusted incidence of sepsis, even after accounting for the influence of depressive symptoms. Elevated stress was associated with higher ten-year adjusted incidence of sepsis; this association was explained by increased depressive symptoms. Reduction or management of stress may provide a strategy for reducing short-term sepsis risk.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by award R01-NR012726 from the National Institute for Nursing Research, UL1-RR025777 from the National Center for Research Resources, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
FINANCIAL SUPPORT
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org and http://www.regardssepsis.org.
Mr. Donnelly is currently supported by grant 2 T32 HS013852 from the Agency for Healthcare Research and Quality, Rockville, MD, USA.
ABBREVIATIONS
- CES-D
Center for Epidemiologic Studies Depression Scale
- CI
Confidence Interval
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- HR
Hazard Ratio
- PSS
Perceived Stress Scale
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- SIRS
Systemic Inflammatory Response Syndrome
Footnotes
CONFLICTS OF INTEREST
Dr. Safford reports the following potential conflicts of interest: Amgen - salary support to study patterns of statin use in Medicare and other large databases; diaDexus - salary support for a research grant on lipids and CHD outcomes; diaDexus - consulting to help with FDA application; NIH, AHRQ - salary support for research grants.
Mr. Ojard, Mr. Donnelly and Dr. Wang do not report any related conflicts of interest.
HEW, CO, JD and MMS conceived the study. HEW and MMS organized and oversaw data collection. HEW and MMS obtained funding for the study. CO, JD and HEW conducted the analysis, and all authors contributed to review of results. CO drafted the manuscript, and all authors contributed to its editorial review and revision. HEW assumes responsibility for the work as a whole.
Contributor Information
Connor Ojard, University of Alabama School of Medicine.
John P. Donnelly, Department of Emergency Medicine, University of Alabama School of Medicine.
Monika M. Safford, Division of Preventive Medicine, Department of Medicine, University of Alabama School of Medicine.
Russell Griffin, Department of Epidemiology, University of Alabama at Birmingham.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA : the journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? Journal of neuroendocrinology. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 5.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic medicine. 2014;76:181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 6.Wang HE, Shapiro NI, Safford MM, Griffin R, Judd S, Rodgers JB, Warnock DG, Cushman M, Howard G. High-sensitivity C-reactive protein and risk of sepsis. PloS one. 2013;8:e69232. doi: 10.1371/journal.pone.0069232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tennant C. Life events, stress and depression: a review of recent findings. The Australian and New Zealand journal of psychiatry. 2002;36:173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- 8.Slavich GM, Irwin MR. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychological bulletin. 2014 doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosovich SA, Boone RT, Reichenberg A, Bansilal S, Shaffer J, Dahlman K, Harvey PD, Farkouh ME. New insights into the link between cardiovascular disease and depression. International journal of clinical practice. 2008;62:423–432. doi: 10.1111/j.1742-1241.2007.01640.x. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 11.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Critical care medicine. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 13.Cohen S, Williamson GM. Perceived Stess in a Probability Sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 14.Burt CE, Cohen LH, Bjorck JP. Perceived family environment as a moderator of young adolescents' life stress adjustment. American journal of community psychology. 1988;16:101–122. doi: 10.1007/BF00906074. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 16.Melchior LA, Huba GJ, Brown VB, Reback CJ. A Short Depression Index for Women. Educational and Psychological Measurement. 1993;53:1117–1125. [Google Scholar]
- 17.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much, a Clinician's Guide. [cited 2012 February 13];2005 Available from: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draper B, Pfaff JJ, Pirkis J, Snowdon J, Lautenschlager NT, Wilson I, Almeida OP for the D, Early Prevention of Suicide in General Practice Study G. Long-Term Effects of Childhood Abuse on the Quality of Life and Health of Older People: Results from the Depression and Early Prevention of Suicide in General Practice Project. Journal of the American Geriatrics Society. 2008;56:262–271. doi: 10.1111/j.1532-5415.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 20.Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovascular psychiatry and neurology. 2013;2013:695925. doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatric disease and treatment. 2011;7:3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penninx BW, Leveille S, Ferrucci L, van Eijk JT, Guralnik JM. Exploring the effect of depression on physical disability: longitudinal evidence from the established populations for epidemiologic studies of the elderly. American journal of public health. 1999;89:1346–1352. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baune BT, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, Berger K. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Translational psychiatry. 2012;2:e92. doi: 10.1038/tp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 27.Burg MM, Edmondson D, Shimbo D, Shaffer J, Kronish IM, Whang W, Alcantara C, Schwartz JE, Muntner P, Davidson KW. The 'perfect storm' and acute coronary syndrome onset: do psychosocial factors play a role? Progress in cardiovascular diseases. 2013;55:601–610. doi: 10.1016/j.pcad.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser R, Rabin B, Chesney M, Cohen S, Natelson B. Stress-induced immunomodulation: implications for infectious diseases? JAMA. 1999;281:2268–2270. doi: 10.1001/jama.281.24.2268. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. The New England Journal of Mmedicine. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 30.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. Journal of consulting and clinical psychology. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 31.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. The American journal of cardiology. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 32.Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychological bulletin. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 33.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. Journal of psychosomatic research. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 34.Aneshensel CS, Stone JD. Stress and depression: a test of the buffering model of social support. Arch Gen Psychiatry. 1982;39:1392–1396. doi: 10.1001/archpsyc.1982.04290120028005. [DOI] [PubMed] [Google Scholar]
- 35.Muscatell KA, Slavich GM, Monroe SM, Gotlib IH. Stressful life events, chronic difficulties, and the symptoms of clinical depression. The Journal of nervous and mental disease. 2009;197:154–160. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiology of disease. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. The American journal of psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 38.Muscatell KA, Slavich GM, Monroe SM, Gotlib IH. Stressful life events, chronic difficulties, and the symptoms of clinical depression. J Nerv Ment Dis. 2009;197:154–160. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 40.Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. The American journal of psychiatry. 1988;145:976–981. doi: 10.1176/ajp.145.8.976. [DOI] [PubMed] [Google Scholar]
- 41.Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. The American journal of psychiatry. 1998;155:4–11. doi: 10.1176/ajp.155.1.4. [DOI] [PubMed] [Google Scholar]
- 42.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Archives of general psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 43.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes care. 1997;20:585–590. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
- 44.Tossani E, Cassano P, Fava M. Depression and renal disease. Seminars in dialysis. 2005;18:73–81. doi: 10.1111/j.1525-139X.2005.18217.x. [DOI] [PubMed] [Google Scholar]
- 45.Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking. A longitudinal investigation. Archives of general psychiatry. 1998;55:161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- 46.Dierker LC, Avenevoli S, Stolar M, Merikangas KR. Smoking and depression: an examination of mechanisms of comorbidity. The American journal of psychiatry. 2002;159:947–953. doi: 10.1176/appi.ajp.159.6.947. [DOI] [PubMed] [Google Scholar]
- 47.Dixit AR, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. The American journal of psychiatry. 2000;157:751–758. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- 48.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 49.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Chronic medical conditions and risk of sepsis. PLoS One. 2012;7:e48307. doi: 10.1371/journal.pone.0048307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel A. Review: the role of inflammation in depression. Psychiatria Danubina. 2013;25(Suppl 2):S216–S223. [PubMed] [Google Scholar]
- 51.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. Journal of affective disorders. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrews G. Should depression be managed as a chronic disease? BMJ. 2001;322:419–421. doi: 10.1136/bmj.322.7283.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richard E, Reitz C, Honig LH, Schupf N, Tang MX, Manly JJ, Mayeux R, Devanand D, Luchsinger JA. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurology. 2013;70:374–382. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye S, Muntner P, Shimbo D, Judd SE, Richman J, Davidson KW, Safford MM. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (Reason for Geographic and Racial Differences in Stroke) study. Journal of the American College of Cardiology. 2013;61:622–630. doi: 10.1016/j.jacc.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teng PR, Yeh CJ, Lee MC, Lin HS, Lai TJ. Depressive symptoms as an independent risk factor for mortality in elderly persons: results of a national longitudinal study. Aging & mental health. 2013;17:470–478. doi: 10.1080/13607863.2012.747081. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal A, Freund K, Sato A, Adams-Campbell LL, Lopez AM, Lessin LS, Ockene J, Wallace RB, Williams CD, Bonds DE. Are depressive symptoms associated with cancer screening and cancer stage at diagnosis among postmenopausal women? The Women's Health Initiative observational cohort. Journal of women's health. 2008;17:1353–1361. doi: 10.1089/jwh.2007.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.