Abstract
Background
Previous studies have reported additional cancers associated with BRCA mutations; however, type, magnitude of risk, and gender differences remain to be clarified. The purpose of this study was to evaluate the incidence of cancers other than breast and ovarian cancer in known mutation carriers.
Methods
An institutional review board approved study identified 1072 patients who had genetic counseling at our institution and tested positive for a deleterious BRCA mutation. The expected number of cancer cases was calculated from the number of individuals in the study sample multiplied by the general population cancer incidence rates. The expected and observed number of cases were calculated in 5 year intervals to accommodate different age-related incidence rates. Standardized incidence ratios (SIRs) for each cancer type were calculated.
Results
We identified 1177 cancers in the 1072 mutation carriers comprising 30 different cancer types. Individuals with a BRCA1 mutation did not have a significant increase in cancers other than breast and ovarian; however, a trend in melanoma was observed. Individuals with a BRCA2 mutation had a significantly higher number of observed cases compared to expected cases for pancreatic cancer (SIR = 21.7, 95%CI = 13.1–34.0, p value <0.001) in both men and women and prostate cancer in men (SIR = 4.9, 95%CI = 2.0–10.1, p value =0.002).
Conclusions
The results of this study uphold the current recommendations for HBOC screening of cancers other than breast and ovarian by the National Comprehensive Cancer Network. Larger cohorts and collaborations are needed to further verify these findings.
Keywords: Hereditary Breast and Ovarian Cancer Syndrome, Genetics, BRCA Mutation, Pancreatic Cancer, Prostate Cancer
Introduction
BRCA1 and BRCA2 tumor suppressor genes repair DNA damage to prevent tumor development. Mutations in these genes predispose an individual to malignancy. The cancers associated with mutations in BRCA1 and BRCA2 have been studied continuously since their discovery in 1994 and 1995 respectively.1,2 BRCA1 and BRCA2 mutation carriers have a significantly increased lifetime risk for developing breast and ovarian cancer, as high as 84% and 39% respectively.3–6
While the association of BRCA1 and BRCA2 mutations with breast and ovarian cancer risks is well-defined, the potential association of these mutations with other cancers is inconsistent. Prior studies have included families either at high risk for a BRCA mutation or combined BRCA1 and BRCA2 mutations carriers for analysis due to small numbers of individuals with BRCA mutations.7,8 These studies reported an increased incidence of cancers, other than breast and ovarian, in mutation carriers; however, many reports did not differentiate between BRCA1 and BRCA2 mutation carriers.
In studies that have been able to focus on BRCA1 or BRCA2 mutation carriers separately; the number of participants varied, with few studies containing more than 1000 mutation carriers. Ford et al found an increased risk for both sexes,9 while Thompson and Easton found an increased risk only in women10 and Moran et al reported BRCA1 mutations are not associated with an increased risk for other cancers.11 Other studies have described a significantly increased risk of pancreatic, prostate, and colorectal cancers in mutation carriers.9,12–15 BRCA1 mutations have also been linked to increases in cervical, esophagus, liver, stomach, and uterine cancers; however, the increased risks were inconsistent and ranged from one to four fold. 9,11,12,15 Known environmental risk factors associated with these cancers were not typically reported in these studies.
The Breast Cancer Linkage Consortium (BCLC) reported BRCA2 mutations were associated with an increased cancer risk in both sexes,16 while van Aperen found a significantly increased risk for men only.17 The most commonly reported cancers with BRCA2 mutations include pancreas, prostate, and melanoma.11,16–19 Additional cancers reported in the BRCA2 spectrum include bone, buccal cavity and pharynx, esophagus, gallbladder and bile duct, laryngeal, ocular, male breast cancer, and stomach, although inconsistently across multiple studies.11,16–18 Environmental risk factors for these cancers were not regularly reported in these studies.
The purpose of this study was to determine if cancers, other than breast and ovarian, were detected more often in BRCA mutation carriers than in the general population. The limited number of studies and variable results indicate a need for further research on the occurrence of non-breast or ovarian cancers that are associated with BRCA1 and BRCA2 mutations. Ultimately, a consensus of additional cancer risk may aid in better recognition of at-risk families where genetic testing may be warranted and in more effective screening guidelines for the types of cancer these families are at risk to develop.
Methods
Study Population
This study was approved by the MD Anderson Cancer Center Institutional Review Board and by The University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects. Individuals who had received genetic counseling in the Clinical Cancer Genetics clinics at the UT MD Anderson Cancer Center (MDACC) between 1997 and 2013, and who had a confirmed BRCA1 or BRCA2 deleterious mutation, were eligible for this study. Individuals with variants suspected to be deleterious in BRCA1 or BRCA2 were included in this analysis because they were advised to follow the same high risk management guidelines as individuals with deleterious mutations in the clinical setting. Medical record number, date of birth, gene, mutation designation, number of cancers, type of cancer, and age at diagnosis were obtained from a secure Progeny database comprised of data obtained during the genetic counseling session or from the patient’s medical record. Additional information on vital status, date of last contact with the institution, ethnicity, and selected risk factors were also obtained from the individual’s medical record. Selected risk factors included tobacco use, alcohol use, radiation exposure, body mass index, and history of mastectomy and/or bilateral salpingo-oophorectomy (BSO). Information on personal cancer history was compared using information from both the medical record and the Progeny database to obtain the most current information.
Individuals with two BRCA mutations, either deleterious or suspected deleterious, in the same gene were included in the analysis. Individuals with mutations in both the BRCA1 and BRCA2 genes, or with both a BRCA mutation and another known cancer-predisposing mutation or genetic condition were excluded from the analysis.
Statistical Methods
Cancer cases were counted for the total sample as well as for BRCA1 and BRCA2 mutation carriers separately. The earliest age at diagnosis was used in the analysis for individuals that developed the same cancer more than once in their lifetime. Most cancers were analyzed independently. Similar or related cancers were grouped together for analysis. For example glioma, astrocytoma, and neuroblastoma were grouped into brain/central nervous system cancers. Ovarian cancer was also defined to include primary peritoneal and fallopian tube cancers. Within each cancer, or group of cancers, the data were stratified by sex and ethnicity.
We compared cancer incidence in our sample with the United States Cancer Statistics (USCS): 1999–2010 Incidence and Morality Web-based Report from the Centers of Disease Control and Prevention (CDC). Data from the USCS report combines the CDC’s National Program of Cancer Registries and National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program on cancer incidence in the United States population. The USCS report includes incidence data for 20 out of the 30 cancer types observed in our study population, including breast, ovarian, bladder, brain & CNS, cervical, colorectal, esophagus, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney, leukemia, lung, melanoma, myeloma, oral cavity, ovarian, pancreas, prostate, stomach, thyroid, and uterine.20 Cancers without general population incidence rates in the USCS database were excluded from analysis. The excluded cancer types were male breast cancer, eye/orbit, lower GI, lymphoma, osteosarcoma, sarcoma, skin/nonmelanoma, unknown primary site, upper GI, and vulvar. The defined reference time frame for age-specific incidence rates in USCS was 2006–2010. Standardized incidence ratios (SIR) were calculated to compare number of cases of cancer in the sample population with general population data. The expected number of cancer cases was calculated from the number of individuals in the study sample multiplied by the general population cancer incidence rates. The expected and observed number of cases was calculated in 5 year intervals to accommodate different age-related incidence rates. SIRs for each cancer type, and associated confidence intervals (CIs), were calculated for the entire sample and for BRCA1 mutation carriers and BRCA2 mutation carriers separately. Data were also stratified by sex within the three groups. To account for multiple tests, we divided the standard p value of 0.05 for statistical significance by the number of cancer types; thus with 20 tests a p value of <0.0025 was considered statistically significant.
Results
We identified 1081 individuals with a deleterious mutation or variant suspected deleterious in BRCA1 or BRCA2 (Fig. 1). We excluded 3 who had both BRCA1 and BRCA2 mutations, and 6 who had another genetic mutation or genetic condition in addition to a BRCA mutation, including neurofibromatosis (two individuals), Lynch syndrome, Turner syndrome, hereditary retinoblastoma, and 18p minus syndrome. Clinical characteristics of eligible individuals are reported in Figure 1. Demographic characteristics including sex and ethnicity are reported in Table 1. The mean age at date of last contact with MDACC was 49.3 years (± 12.76, range 17–90). Of the 1072 individuals included in our sample, most were alive at the date of last contact (912, 85%).
Figure 1.
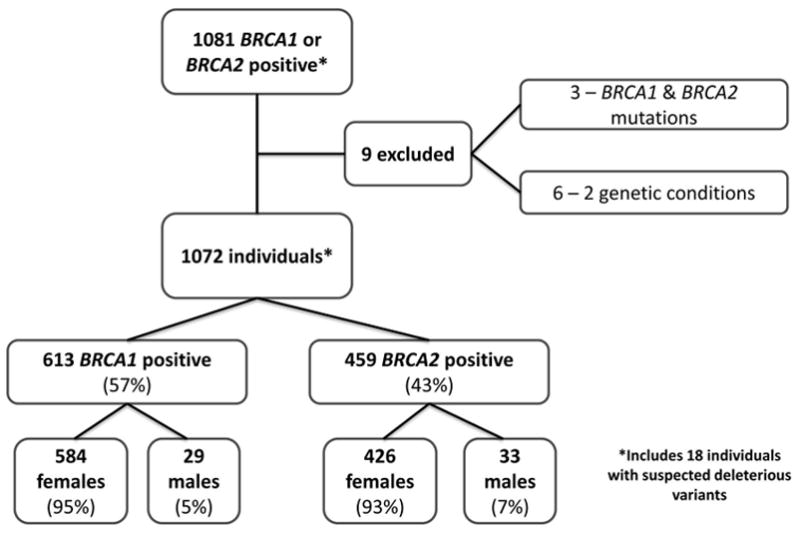
Distribution of mutations in BRCA genes in study population.
Table 1.
Frequency of BRCA1 and BRCA2 mutations by sex and ethnicity in study population.
| All Subjects |
BRCA1 n (%) |
BRCA2 n (%) |
Total n |
|
|---|---|---|---|---|
| Sex (p value=0.088*) | Male | 29 (46.77%) | 33 (53.23%) | 62 |
| Female | 584 (57.82%) | 426 (42.18%) | 1010 | |
| Total | 613 (57.18%) | 459 (42.82%) | 1072 | |
| Ethnicity (p value=0.002†) | Am Indian/Native Amer | 1 (33.33%) | 2 (66.67%) | 3 |
| Asian/Pacific Islander | 22 (53.66%) | 19 (46.34%) | 41 | |
| Black | 43 (56.58%) | 33 (43.42%) | 76 | |
| Hispanic | 103 (72.03%) | 40 (27.97%) | 143 | |
| White | 440 (54.79%) | 363 (45.21%) | 803 | |
| Total (missing=6) | 609 (57.13%) | 457 (42.87) | 1066 |
p value from Chi-Square test;
p value from Fisher’s exact test
We identified 1177 cancers in the 1072 mutation carriers comprising 30 different cancer types. After excluding duplicate cancers in same individual, the total number of cancer cases used in the analysis was reduced to 993 (Figure 2).
Figure 2.
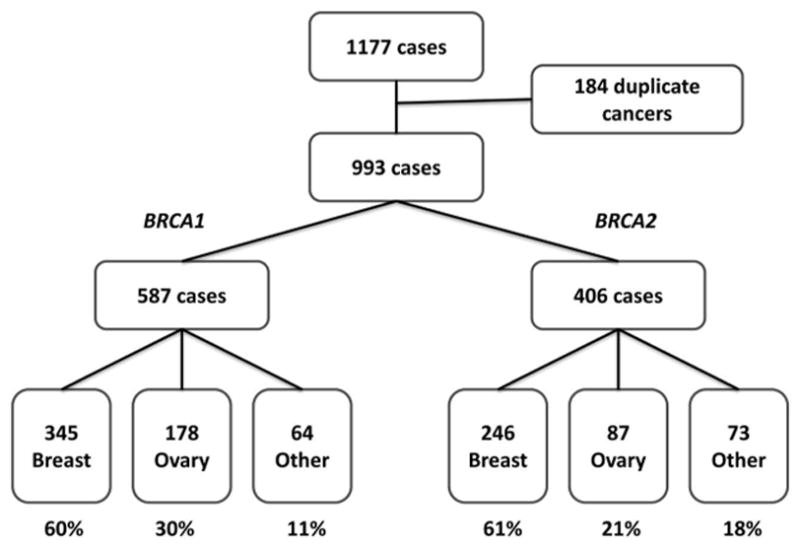
Demographics of cancers identified in study population.
Comparison of the observed and expected cases identified four types of cancer with an increased SIR (Table 2). As expected, breast and ovarian cancers were observed at significantly increased rates in BRCA1 and BRCA2 mutation carriers. Individuals with a BRCA2 mutation had a higher incidence of pancreatic cancer than expected in the general population (SIR 21.745, 95% CI 13.086–33.96, p<0.001). When males and females with BRCA2 mutations were analyzed separately the number of pancreatic cancers was significantly higher than expected in both sexes (males: SIR 82.559, 95% CI 39.524–151.84, p<0.001; females: SIR 13.809, 95% CI 6.301–26.216, p<0.001). Prostate cancer was identified in significantly more men with a BRCA2 mutation than expected in the general population (SIR 4.890, 95% CI 1.959–10.075, p=0.002).
Table 2.
Observed and expected cancers for 1072 individuals (males and females) with BRCA mutations.
| Cancer | Gene | Obs | Exp | SIR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Bladder | BRCA1 | 0 | 1.282 | 0 | 0–2.862 | 0.554 |
| BRCA2 | 1 | 1.373 | 0.728 | 0.010–4.053 | 0.791 | |
|
| ||||||
| Brain & CNS | BRCA1 | 3 | 1.268 | 2.367 | 0.849–8.078 | 0.269 |
| BRCA2 | 1 | 1.077 | 0.929 | 0.012–5.168 | 0.578 | |
|
| ||||||
| Breast – female | BRCA1 | 345 | 9.349 | 36.902 | 33.110–41.009 | <0.001* |
| BRCA2 | 246 | 8.885 | 27.688 | 24.336–31.373 | <0.001* | |
|
| ||||||
| Cervical | BRCA1 | 2 | 1.701 | 1.176 | 0.132–4.245 | 0.990 |
| BRCA2 | 6 | 1.361 | 4.410 | 1.61–9.599 | 0.006 | |
|
| ||||||
| Colorectal | BRCA1 | 6 | 3.800 | 1.579 | 0.577–3.437 | 0.367 |
| BRCA2 | 2 | 3.783 | 0.529 | 0.059–1.909 | 0.542 | |
|
| ||||||
| Esophagus | BRCA1 | 1 | 0.405 | 2.471 | 0.032–13.75 | 0.654 |
| BRCA2 | 0 | 0.422 | 0 | 0–8.694 | 0.677 | |
|
| ||||||
| Hodgkin Lymphoma | BRCA1 | 3 | 0.792 | 3.788 | 0.761–11.067 | 0.095 |
| BRCA2 | 0 | 0.634 | 0 | 0–5.787 | 0.929 | |
|
| ||||||
| Non-Hodgkin Lymphoma | BRCA1 | 0 | 2.114 | 0 | 0–1.735 | 0.237 |
| BRCA2 | 1 | 1.980 | 0.505 | 0.007–2.81 | 0.825 | |
|
| ||||||
| Kidney | BRCA1 | 2 | 1.806 | 1.107 | 0.124–3.998 | 0.925 |
| BRCA2 | 3 | 1.735 | 1.729 | 0.348–5.052 | 0.500 | |
|
| ||||||
| Leukemia | BRCA1 | 5 | 1.694 | 2.951 | 0.951–6.887 | 0.060 |
| BRCA2 | 3 | 1.493 | 2.010 | 0.404–5.872 | 0.376 | |
|
| ||||||
| Lung | BRCA1 | 2 | 4.547 | 0.440 | 0.049–1.588 | 0.335 |
| BRCA2 | 5 | 4.867 | 1.027 | 0.331–2.398 | 0.929 | |
|
| ||||||
| Myeloma | BRCA1 | 1 | 0.462 | 2.164 | 0.037–12.04 | 0.728 |
| BRCA2 | 0 | 0.477 | 0 | 0–7.683 | 0.747 | |
|
| ||||||
| Oral Cavity | BRCA1 | 2 | 1.362 | 1.468 | 0.165–5.30 | 0.784 |
| BRCA2 | 1 | 1.298 | 0.770 | 0.01–4.286 | 0.739 | |
|
| ||||||
| Ovarian | BRCA1 | 178 | 1.280 | 139.115 | 119.427–161.122 | <0.001* |
| BRCA2 | 87 | 1.1614 | 74.926 | 60.011–92.422 | <0.001* | |
|
| ||||||
| Pancreas | BRCA1 | 4 | 0.846 | 4.730 | 1.273–12.11 | 0.024 |
| BRCA2 | 19 | 0.874 | 21.745 | 13.086–33.96 | <0.001* | |
|
| ||||||
| Prostate | BRCA1 | 3 | 1.788 | 3.809 | 0.766–11.13 | 0.094 |
| BRCA2 | 7 | 1.432 | 4.890 | 1.959–10.075 | 0.002* | |
|
| ||||||
| Skin – Melanoma | BRCA1 | 9 | 2.717 | 3.312 | 1.511–6.288 | 0.004 |
| BRCA2 | 2 | 2.456 | 0.814 | 0.091–2.94 | 0.887 | |
|
| ||||||
| Stomach | BRCA1 | 1 | 0.576 | 1.736 | 0.023–9.661 | 0.864 |
| BRCA2 | 1 | 0.570 | 1.755 | 0.023–9.763 | 0.858 | |
|
| ||||||
| Thyroid | BRCA1 | 5 | 2.736 | 1.828 | 0.589–4.265 | 0.283 |
| BRCA2 | 2 | 2.319 | 0.862 | 0.097–3.114 | 0.814 | |
|
| ||||||
| Uterus | BRCA1 | 4 | 2.872 | 1.393 | 0.375–3.566 | 0.645 |
| BRCA2 | 3 | 2.636 | 1.138 | 0.229–3.326 | 0.978 | |
statistically significant difference between study population and general population (p<0.0025)
Obs – observed cases; Exp – expected cases; SIR – standardized incidence ratio; CI – confidence interval
We observed a trend of increasing incidence of melanoma in BRCA1 mutation carriers (SIR 3.312, 95% CI 1.511–6.288, p=0.004) and of cervical cancer in BRCA2 mutation carriers (SIR 4.410, 95% CI 1.61–9.599, p = 0.006), compared to general population data. The p values for melanoma and cervical cancer are approaching significance although they did not reach the conservative cutoff. The 95% confidence interval does not include 1.0 indicating that the general population and study sample are likely different populations. The increased incidence for these cancers was unlikely to occur by chance.
Ten additional cancer types representing 64 total cases were identified in the study population but were not available in the CDC USCS database for statistical analysis (Table 3). Individuals with BRCA1 mutations made up 45.3% (29 cases) in this subset of cancers. Individuals with BRCA2 mutations comprised 54.7% (35 cases) in this subset of cancer types. Of note, all seven cases of male breast cancer occurred in men with BRCA2 mutations. Non-melanoma skin cancer was the most common of these 10 types of cancer in BRCA1 and BRCA2 mutation carriers (18 and 19 cases, respectively).
Table 3.
Description of additional cancers in remaining 64 cases that were not compared to the general population.
| Cancer |
BRCA1 n (%) |
BRCA2 n (%) |
Total n (%) |
|---|---|---|---|
| Total | 29 (100) | 35 (100) | 64 (100) |
| Breast – Males | 0 (0) | 7 (20) | 7 (10.9) |
| Eye and Orbit | 1 (3.4) Uveal Melanoma |
1 (2.9) Ocular Melanoma |
2 (3.1) |
| Lower GI | 2 (6.9) Anal Canal & Appendix |
0 | 2 (3.1) |
| Lymphoma | 1 (3.4) | 2 (5.7) | 3 (4.7) |
| Osteosarcoma | 0 (0) | 1 (2.9) | 1 (1.7) |
| Sarcoma | 2 (6.9) | 1 (2.9) | 3 (4.7) |
| Skin – Nonmelanoma | 18 (62.1) | 19 (54.3) | 37 (57.8) |
| Unknown Primary Site | 2 (6.9) | 2 (5.7) | 4 (6.3) |
| Upper GI | 1 (3.4) Small Intestine |
1 (2.9) Cholangiocarcinoma |
2 (3.1) |
| Vulvar | 2 (6.9) | 1 (2.9) | 1 (1.7) |
Discussion
This is one of the largest single institution studies of the cancer spectrum associated with BRCA1 and BRCA2 mutations. This study found an increased incidence in two cancers, other than breast and ovarian, in individuals with a BRCA mutation when stratified by gene and sex. The number of observed cases of pancreatic and prostate cancer was higher than expected in the general population for individuals with BRCA2 mutations. Our findings support the rationale for pancreatic and prostate cancer screening in individuals with a BRCA2 mutation. Furthermore, recent associations with additional cancers, including uterine and colorectal, were not evident in our study population.
In our analysis, the occurrence of pancreatic cancer in males and females with a BRCA2 mutation was nearly 22 times greater than expected in the study population. Separately, males had an 82.5 times higher occurrence and females had approximately 14 times higher occurrence. Other studies have reported increased risks of a lesser magnitude for pancreatic cancer in men and women with BRCA2 mutations, including relative risk estimates ranging from 3.51–5.9.11,16,17 The increased number of observed cases in this study above previous relative risks could be attributed to personal factors or a referral bias. Nearly half (8 of 19) individuals with pancreatic cancer had a history of smoking, which is a well-documented risk factor for pancreatic cancer.21 Other cancers also evaluated showed increases or trends of increases in risks. Prostate cancer occurred approximately 5 times more frequently in males with BRCA2 mutations than expected in the general population. The increased risk for prostate cancer in our study population is consistent with previous studies that have reported relative risk estimates ranging from 2.5–6.3.11,16–18 Our data confirms prior evidence that men with BRCA2 mutations are at an increased risk of prostate cancer.
The incidence of melanoma in BRCA1 mutation carriers approached significance in this study (p = 0.004). We established a conservative level of statistical significance for this study because the study sample included individuals in multiple cancer groups rather than being mutually exclusive group comparisons. The 95% confidence interval suggests the increased incidence of melanoma in BRCA1 mutation carriers differentiates it from the general population. Melanoma has been associated with BRCA2 mutations in previous studies, although the risk with BRCA1 mutations is unclear16. Therefore this study suggests screening for melanoma in BRCA1 mutation carriers may be prudent.
The incidence of cervical cancer in BRCA2 mutation carriers also approached statistical significance in this study (p=0.006). The most common risk factor for cervical cancer is human papillomavirus infection.22 We were unable to determine whether the cause of cervical cancer was viral or possibly associated with BRCA2 mutations. HPV status was available for three out of the six observed cervical cancer cases in women with a BRCA2 mutation. All three tested negative for HPV; however, the test was performed 3 to 7 years after cancer diagnosis. Thus the tests may not have accurately identified HPV because the majority of HPV infections clear or become undetectable within two years of infection.23 HPV status was not reported in the medical record for the remaining three individuals. It will be important to monitor the cancers with a trend of increasing incidence over time to determine if an association exists and what the magnitude of risk is for mutation carriers.
BRCA mutations have been associated with uterine cancer risk, specifically more aggressive types.24 In this recent analysis by Shu and colleagues, 4 cases of high-risk uterine cancer were diagnosed out of 525 BRCA mutation carriers, which was significantly increased over the general population (SIR 14.48, p<0.001). In our overall analysis, 7 cases of uterine cancer were observed compared to 5.507 expected. Three of our 7 observed cases were classified as high risk (serous, clear cell, or sarcoma), three cases were low risk, and one case did not have pathology available for review. Thus, uterine cancer was not more prevalent in our study population than expected, although the specific occurrence of high risk uterine cancer was not statistically analyzed.
Our study has multiple limitations. Although our overall sample size of individuals with BRCA mutations is large in comparison to other published studies, the sample size remains a limitation for discovering small differences. MDACC is also a tertiary care center and individuals with complex cancer histories, poor prognosis, or multiple cancer diagnoses are often referred for treatment. Another limitation is the use of general population incidence rates. The largest date range (2006–2010) in the USCS dataset was used; however, individuals in our sample developed cancer outside of this date range which required us to infer statistical associations. The SIR used in this study is not relative risk. The SIR is an approximation of the relative risk, however, discrepancies can arise because the general population is composed of individuals with and without BRCA mutations. Also, cancers diagnosed at centers other than ours did not require pathological confirmation; thus there may be inaccurate reporting for some cancers. Because our study population was predominantly white (75%), the information learned from this study may not be generalizable across all ethnicities.
Our study observed more than the expected number of cases of pancreatic and prostate cancer in BRCA2 mutation carriers. A trend toward statistical significance in the incidence of melanoma with BRCA1 mutations was observed. The presence of male breast cancer which was exclusively with seen in the BRCA2 mutation carriers in our cohort is consistent with previous studies. Our findings do not rule out an increase of these cancers in both BRCA genes given the limitations of our cohort. Additionally why some cancers may be more prevalent in one BRCA gene vs. another is not yet well understood. These findings do, however, support the current National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Hereditary Breast and Ovarian Cancer syndrome management.25 Recommendations or considerations for prostate cancer, male breast cancer, and melanoma screening have been included for individuals with BRCA1 or BRCA2 mutations. These suggestions currently include digital rectal exam (DRE) and prostate specific antigen (PSA) serum test beginning at age 40, clinical male breast exams beginning at age 35 followed by baseline mammogram at age 40, and full-body skin exams for men and women. While the risk for pancreatic cancer has been acknowledged by NCCN, specific screening guidelines do not exist. Lack of effective procedures for early pancreatic cancer detection prevents the development of screening guidelines. Investigational protocols into pancreatic screening include endoscopic ultrasound (EUS) and/or magnetic resonance imaging (MRI) cholangiopancreatography rather than computed tomography (CT) scans, however, inconsistency in follow up intervals and when fine needle aspirations are needed continues to be debated.26,27,28
The high rate of pancreatic cancer in men and women with BRCA2 mutations in this study further emphasizes the need for effective screening and recommendations in this high-risk population.
Acknowledgments
Financial Support: Litton funding from the Woolf-Toomim Fund and Institutional database funding from NCI Cancer Center Support Grant P30CA016672
Footnotes
Financial Disclosures: Dr. Litton has research funding from: Novartis, BMS, Biomarin and Steering Committee Membership for trials supported by Novartis and Biomarin; all uncompensated. None of these relationships are related to this manuscript/analysis.
Contributor Information
Jacqueline Mersch, Genetic Counseling Program at The University of Texas Graduate School of Biomedical Science at Houston, Houston, TX 77030.
Michelle Jackson, Breast Medical Oncology at The University of Texas MD Anderson Cancer Center, Houston, TX 77030.
Minjeong Park, Division of Quantitative Sciences at The University of Texas MD Anderson Cancer Center, Houston TX 77030.
Denise Nebgen, Gynecologic Oncology and Reproductive Medicine at The University of Texas MD Anderson Cancer Center, Houston, TX 77030.
Susan K. Peterson, Department of Behavioral Science at The University of Texas MD Anderson Cancer Center, Houston, TX 77030.
Claire Singletary, Department of Pediatrics and Department of Obstetrics, Gynecology, and Reproductive Sciences at The University of Texas Medical School at Houston, Houston, TX 77030.
Banu K. Arun, Breast Medical Oncology at the University of Texas MD Anderson Cancer Center.
Jennifer K. Litton, Breast Medical Oncology at The University of Texas MD Anderson Cancer Center, Houston, TX 77030.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PDP, Narod S, et al. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. The American Journal of Human Genetics. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24(6):863–871. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56(1):265–271. [PMC free article] [PubMed] [Google Scholar]
- 6.Ford D, Easton DF, Stratton M, et al. Genetic Heterogeneity and Penetrance Analysis of the BRCA1 and BRCA2 Genes in Breast Cancer Families. The American Journal of Human Genetics. 1998;62(3):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermejo JL, Hemminki K. Risk of cancer at sites other than the breast in Swedish families eligible for BRCA1 or BRCA2 mutation testing. Ann Oncol. 2004;15(12):1834–1841. doi: 10.1093/annonc/mdh474. [DOI] [PubMed] [Google Scholar]
- 8.Noh JM, Choi DH, Baek H, et al. Associations between BRCA Mutations in High-Risk Breast Cancer Patients and Familial Cancers Other than Breast or Ovary. Journal of Breast Cancer. 2012;15(3):283. doi: 10.4048/jbc.2012.15.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford D, Easton DF. Risks of cancer in BRCA1-mutation carriers. Lancet. 1994;343(8899):692. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 10.Thompson D, Easton DF. Cancer Incidence in BRCA1 Mutation Carriers. JNCI J Natl Cancer Inst. 2002;94(18):1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 11.Moran A, O’Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Familial Cancer. 2012;11(2):235–242. doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 12.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer Risk Estimates for BRCA1 Mutation Carriers Identified in a Risk Evaluation Program. JNCI J Natl Cancer Inst. 2002;94(18):1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107(12):2005–2009. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelan CM, Iqbal J, Lynch HT, et al. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: results from a follow-up study. Br J Cancer. 2014;110(2):530–534. doi: 10.1038/bjc.2013.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson D, Easton DF. Cancer Incidence in BRCA1 Mutation Carriers. JNCI J Natl Cancer Inst. 2002;94(18):1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 16.The Breast Cancer Linkage Consortium. Cancer Risks in BRCA2 Mutation Carriers. JNCI J Natl Cancer Inst. 1999;91(15):1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 17.Van Asperen CJ. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. Journal of Medical Genetics. 2005;42(9):711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Easton DF, Steele L, Fields P, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. American Journal of Human Genetics. 1997;61(1):120. doi: 10.1086/513891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai YC, Domchek S, Parmigiani G, Chen S. Breast Cancer Risk Among Male BRCA1 and BRCA2 Mutation Carriers. J Natl Cancer Inst. 2007;99(23):1811–1814. doi: 10.1093/jnci/djm203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. [Google Scholar]
- 21.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Practice & Research Clinical Gastroenterology. 2006;20(2):197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 23.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132(2):277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 24.Shu CA, Pike M, Jotwani AR, et al. Risk of developing uterine corpus cancer (Ut Ca) following risk-reducing salpingo-oophorectomy (RRSO) in women with BRCA mutations. SGO Annual Meeting; March 24, 2014. [Google Scholar]
- 25.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. V.1.2014. Fort Washington (PA): NCCN; 2014. Genetic/familial high-risk assessment: breast and ovarian. [DOI] [PubMed] [Google Scholar]
- 26.Canto MI, Hruban RH, Fishman EK, et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology. 2012;142(4):796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canto MI, Harnick F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larghi A, Verna EC, Lecca PG, et al. Screening for Pancreatic Cancer in High-Risk Individuals: A Call for Endoscopic Ultrasound. Clin Cancer Res. 2009;15:1907–1914. doi: 10.1158/1078-0432.CCR-08-1966. [DOI] [PubMed] [Google Scholar]


