Abstract
Thrombospondin-4 (TSP4) belongs to a family of large oligomeric, extracellular matrix glycoproteins that mediate interactions between cells and interactions of cells with underlying matrix components. Recent evidence shows that TSP4 may contribute to the generation of neuropathic pain. However, there is no systematic examination of TSP4 expression in the DRGs after injury. We therefore investigated whether TSP4 protein level is changed in DRGs after injury following spinal nerve ligation (SNL) and spared nerve injury (SNI) in rats using western blot, immunohistochemistry, and immunocytochemistry. After nerve ligation, TSP4 protein level is up-regulated in the axotomized somata of the L5 DRG. There is substantial additional TSP4 in the non-neuronal compartment of the L5 DRG that does not co-stain for markers of satellite glia, microglia, or Schwann cells and appears to be in the interstitial space. Evidence of intracellular overexpression of TSP4 persists in neurons dissociated from the L5 DRG after SNL. These findings indicate that following peripheral nerve injury, TSP4 protein expression is elevated in the cytoplasm of axotomized sensory neurons and in the surrounding interstitial space.
Keywords: Neuropathic pain, Dorsal root ganglion, Thrombospondin-4
Introduction
Peripheral nerve injury often leads to chronic neuropathic pain through altered function of primary sensory neurons and central nervous system pain pathways. Improved understanding of the mechanisms causing neuropathic pain following nerve injury could lead to the development of improved treatments for this condition. Recent findings suggest that thrombospondin-4 (TSP4) may play an important role in generating neuropathic pain (Kim et al. 2012). Specifically, intrathecal administration of TSP4 protein amplifies excitatory synaptic transmission in the dorsal horn and causes mechanical and thermal sensitization that can be eliminated by specific interventions including antisense oligodeoxynucleotides, intrathecal antibodies, and TSP4 gene knockout (Kim et al. 2012). Thrombospondins are a family of large oligomeric, extracellular matrix glycoproteins that mediate interactions between cells and interactions of cells with underlying matrix components (Adams 2001; Risher and Eroglu 2012). The TSP family consists of five members (TSP 1-5) divided into two subgroups (TSP1/2 vs. TSP3/4/5) according to details of oligomerization and structure (Risher and Eroglu 2012). In the central nervous system (CNS), it is thought that TSP4 is secreted primarily by astrocytes and promotes synaptogenesis (Christopherson et al. 2005; Kim et al. 2012; Risher and Eroglu 2012).
Primary sensory neurons in the dorsal root ganglia (DRGs) are a critical element in the pain pathway that encode noxious stimuli into trains of action potentials. Also, altered sensory neuron function in neuropathic pain results in heightened excitability, making the DRG an attractive target for pharmacotherapy (Fischer et al. 2014). In a gene chip survey of genetic effects of nerve injury, we have previously observed that TSP4 gene expression is elevated in DRGs after peripheral nerve injury (Kim et al. 2009; Valder et al. 2003), but other experiments using Western immunoblotting showed decreased TSP4 protein in DRGs after injury (Kim et al. 2012). Because of the expanding recognition that TSP4 plays a role in neuropathic pain pathophysiology, we planned experiments to resolve these apparently contradictory results with more detailed analysis of TSP4 expression in DRG.
Materials and Methods
Animals and injury model preparation
Male Sprague Dawley rats weighing 125-150 g were from Taconic Farms. Transgenic mice expressing eGFP driven by the TSP4 promoter (TSP4-eGFP mice) were obtained from Mutant Mouse Regional Resource Centers (Thbs4-EGFP, MMRRC, supported by the National Center for Research Resources at the National Institutes of Health). Animals were housed in a room maintained at 22 ± 0.5 °C with a 12h/12h light/dark cycle. Food and water were available ad libitum throughout the experiments. All methods and use of animals were approved by the Institutional Animal Care and Use Committees of the Medical College of Wisconsin and the University of California Irvine.
Spinal nerve ligation
Rats and TSP4-eGFP mice were subjected to spinal nerve ligation (SNL), based on the original technique (Kim and Chung 1992) with modifications (Gemes et al. 2009; Rigaud et al. 2008). Briefly, during anesthesia by inhalation of isoflurane (1.5 −2.5% in oxygen), the right lumbar paravertebral region was exposed through a midline incision, and the sixth lumbar (L6) transverse process (L5 process in mice) was removed to expose the L5 and L6 spinal nerves (L4 and L5 in mice), which were ligated with 4-0 silk suture and severed approximately 5 mm distal to their respective DRGs. The wounds were closed in layers and the skin stapled. Control rats and mice received skin incision and closure only. To understand the role of TSP4 expression in maintaining chronic neuropathic pain, tissues for studies described below were harvested at 23.5±0.3 days after SNL or skin incision surgery (except where otherwise stated) to avoid the early influences of general surgical trauma and acute post-surgical pain.
Spared nerve injury
Other rats were subjected to SNI, based on our previous protocols (Fischer et al. 2014). Animals were anesthetized with isoflurane, the surgical field (right leg) was shaved and disinfected, an incision (~2 cm) was made on the lateral mid-thigh, and the underlying muscles separated to expose the sciatic nerve. The tibial and common peroneal were individually ligated with 6.0 sutures and cut distally to the ligature, and 2–3 mm of each nerve was removed distal of the ligation. The sural nerve was preserved and contact with it was avoided. Muscle and skin were closed using 4.0 monofilament nylon sutures.
Sensory testing
Sensory behavior was evaluated before and after surgery in a way that we have previously documented as a valid representation of pain by experiments using conditioned place avoidance (Wu et al. 2010). Briefly, right plantar skin was touched (10 stimuli/test) with a 22-G spinal needle with adequate pressure to indent but not penetrate the skin. Whereas control animals respond with only a brief reflexive withdrawal, rats following SNL or SNI may display a complex hyperalgesia response that includes licking, chewing, grooming and sustained elevation of the paw. (We hereafter term this a hyperalgesia response as this response is consistent with the definition of the International Association for the Study of Pain (http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698) of “Increased pain from a stimulus that normally provokes pain.”) Testing was performed on three different days between 10 and 17 days after surgery to avoid the influence of acute pain and general surgical tissue injury. Since pain behavior is highly variable from day to day (Hogan et al. 2004), hyperalgesia in each rat was characterized as the average frequency of hyperalgesia responses over the 3 testing days. After SNL or SNI, only rats that displayed a hyperalgesia-type response after at least 20% of stimuli were used further in this study. Behavior was not tested in the 7 transgenic mice subjected to SNL or sham surgery.
Western blotting
After either 2 or 24 days following surgery, levels of TSP4 protein were determined by Western blotting with a selective TSP4 protein antibody (R&D Systems, Catalog #: AF2390) (Benner et al. 2013) or a non-commercial one (guinea pig) (Kim et al. 2012). To minimize proteolysis and avoid neuronal activation during DRG collection, rats were cardio-perfused with ice-cold oxygenated artificial CSF (aCSF) containing the following (in mM): 125 sucrose, 3 KCl, 2.5 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, saturated with 95% O2 and 5% CO2. DRGs were quickly frozen in liquid nitrogen and then broken into a fine powder with a biopulverizer (BioSpec Products Inc., Bartlesville, OK, USA). Each DRG was homogenized in 0.1 ml of lysis buffer, pH 7.6, containing 50 mM Tris-acetate, 50 mM NaF, 10 mM EDTA, 10 mM EGTA, 0.01% Triton X-100, protease inhibitors, and protein phosphatase inhibitors I and II (Sigma-Aldrich, St. Louis, MO). Samples were sonicated on ice for 1 min and then centrifuged at 14,000 × g for 20 min and the supernatant was isolated for analysis. Total protein concentrations were assayed using BCA protein assay kit (Thermo Scientific, Waltham, MA). The concentration of protein was calibrated to 1 μg/μl with 2× loading buffer containing 0.1 m Tris-HCl, pH 6.8, 4% (w/v) SDS, 20% (v/v) glycerol, 3% (v/v) dithiothreitol, and 0.04% (w/v) bromophenol blue. Samples (30 μg of protein per sample) were separated in 7.5% or 4-15% SDS-PAGE. Following the transfer, blots were blocked in solution containing 5% (w/v) milk and 0.1% (v/v) Tween 20 in Tris-buffered saline (TBS-T) for 1 h at room temperature, and incubated with antibody for TSP4 (1:1000 dilution) overnight at 4°C. Blots were then washed several times with TBS-T and probed with horseradish peroxidase-conjugated secondary antibody (1:1000, R&D Technology, Minneapolis, MN) for 2 h at room temperature before being developed using enhanced chemiluminescence immunoblotting detection system (GE Healthcare, Buckinghamshire, UK). The immunoblots were then stripped at 60°C for 30 min with stripping buffer containing 62.5 mm Tris-HCl, pH 6.8, 2% (w/v) SDS, 100 mm 2-mercaptoethanol, and reprobed with an antibody recognizing β-tubulin (1:5000; Sigma-Aldrich) used for loading control.
Some samples were deglycosylated by using an Enzymatic DeGlycoMx Kit (QA-Bio Inc, Palm Desert, CA) as directed by the instruction. Enzymes in the kit can remove N-linked oligosaccharides and most O-linked sugars.
Immunohistochemistry and imaging
Immunohistochemical procedures were based on published studies with minor modifications (Fischer et al. 2014; Yu et al. 2011). Rats were deeply anesthetized with inhalation of isoflurane and perfused through the aorta first with aCSF and then with 4% paraformaldehyde in 0.1 m sodium PBS with 4% sucrose, pH 7.4. After perfusion, DRGs were removed and fixed in the same solution overnight at 4°C. The DRGs were embedded with paraffin and prepared in a series of 5 μm sections. Sections were de-waxed, and antigen retrieval was achieved by microwave heating in citrate buffer. Sections were blocked for 1 h at room temperature with blocking solution (1% bovine serum albumin, 5% normal goat serum, and 1% Triton X-100 in 0.1 m PBS, pH 7.4). Sections were then incubated with antibodies detecting TSP4 (1:200, R&D system, Minneapolis, MN), monoclonal β3-tubulin (Tubb3, 1:400, Santa Cruz Biotechnology, SCB, Santa Cruz, CA), rabbit polyclonal GFAP (1:4000, Dako, Carpinteria, CA), and rabbit polyclonal Iba1(1:1000, Dako), at 4°C for 48 h. After rinsing three times, 5 min each, in PBS, sections were incubated in the secondary antibodies for 2 h at room temperature. The appropriate fluorophore-conjugated (Alexa 488 or Alexa 594) secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) were used to reveal the primary antibodies. After rinsing twice with PBS, sections were coverslipped. Negative control sections were processed with a nonimmune serum in place of the primary antibodies. DRGs from 2-week SNL TSP4-eGFP animals were collected both ipsilateral and contralateral, immersion fixed in 4% PFA overnight, followed by sedimentation in 30% sucrose PBS solution. DRGs were then frozen in optimal cutting tissue medium and sectioned (10 μm). Sections on slides were incubated with primary antibody eGFP (1:500; Abcam) diluted in Antibody Diluent Solution (DAKO) for 2 d, followed by incubation with secondary antibody for 2 h at room temperature. After washing, slides were mounted with Vectashield DAPI Hardmount (Vector Laboratories). The sections were examined and images captured using a Nikon TE2000-S fluorescence microscope (NIKON Instruments, Melville, NY, USA) with filters suitable for selectively detecting the green and red fluorescence, and a QuantiFire (Optronics, Goleta, CA) digital camera. To generate semi-quantitative measures of protein expression, established standardization approaches were used (Taylor and Levenson 2006; Walker 2006). Tissues from injured and control rats were prepared on the same day, and all sections were stained as a group to include controls in a single session. Sections were analyzed in a blinded fashion.
Neuron isolation, plating, and Immunocytochemistry
DRGs from rats were rapidly harvested following deep isoflurane anesthesia and decapitation. Ganglia were placed in a 35 mm dish containing Ca2+/Mg2+-free, cold HBBS (Life Technologies) and cut into four to six pieces that were incubated in in 0.01% blendzyme 2 (Roche Diagnostics, Indianapolis, IN) for 26 min followed by incubation in 0.25% trypsin (Sigma Aldrich) and 0.125% DNAse (Sigma-Aldrich) for 30 min, both dissolved in Dulbecco’s modified Eagle’s medium (DMEM)/F12 with glutaMAX (Invitrogen, Carlsbad, CA). After exposure to 0.1% trypsin inhibitor and centrifugation, the pellet was gently triturated in culture medium containing Neural Basal Media A with B27 supplement (Invitrogen), 0.5 mM glutamine, 10 ng/ml nerve growth factor 7S (Alomone Labs, Jerusalem, Israel) and 0.02 mg/ml gentamicin (Invitrogen). Dissociated neurons were plated onto poly-L-lysine coated glass cover slips (Deutsches Spiegelglas, Carolina Biological Supply, Burlington, NC) and maintained at 37°C in humidified 95% air and 5% CO2 for 2 hours, and were studied no later than 6 hours after harvest.
Immunocytochemical procedures were similar to the immunohistochemistry procedure with minor modifications. To generate semi-quantitative measures of protein expression, neurons from SNL and skin-sham rats were prepared on the same day and were randomly distributed to groups with or without Triton X-100. Negative controls were provided by replacing the primary antibody with a nonimmune serum. All sections were prepared during a single session. Specifically, neurons on the cover slips were fixed in situ for 30 min with 2% paraformaldehyde in 0.1 M PBS, pH 7.4. The slides were then washed with 0.1 M PBS for several time and ready for immunostaining. Neurons were blocked for 1 h at room temperature with blocking solution (1% bovine serum albumin, and 5% normal goat serum, with or without 1% Triton X-100 in 0.1 m PBS, pH 7.4). Sections were then incubated with 1:500 TSP4 antibody at 4°C for 24 h. After rinsing three times, 5 min each, in PBS, sections were incubated in the secondary antibodies: 1:100 goat anti-rabbit IgG-TR (Bio-Rad) for 2 h at room temperature. After rinsing twice with PBS, sections were coverslipped. Sections were analyzed in a blinded fashion using a Nikon eclipse e600 fluorescence microscope (NIKON Instruments, Melville, NY, USA) with optimized exposure parameters that were applied uniformly for all sections.
Statistical analysis
Data are presented as the mean ± SEM. Western blots and immunocytochemistry images were analyzed by densitometry using ImageJ (http://rsb.info.nih.gov/ij/index.html). Data sets were compared with either Student’s t test, paired t test, or one-way ANOVA followed by Tukey’s post hoc analysis. Results were considered to be significant at two-tailed p < 0.05.
Results
Animal behavior
Rats included as demonstrating hyperalgesia after SNL showed a frequency of hyperalgesia-type behavior upon noxious mechanical stimulation with a needle of 34.3 ± 2.4% (n=23). The 29 rats (55.8% of total SNL animals) that were subjected to SNL but developed low hyperalgesia scores (5 ± 0.9%, p<0.001 vs. hyperalgesic rats) were excluded from further evaluation. Contemporaneous sham-operated rats (skin incision only) showed 0 ± 0% hyperalgesia responses (p<0.001 vs. both other groups). Consistent with our previous report (Fischer et al. 2014), SNI produced consistent and long-lasting post-traumatic neuropathic pain behaviors (Fischer et al. 2014). Specifically, all rats subjected to SNI had high hyperalgesia scores at 2 days (71 ± 4.3%, n=10) and 24 days (74 ± 5.1%, n=5) after surgery.
The TSP4 antibody specifically detects TSP4 expression
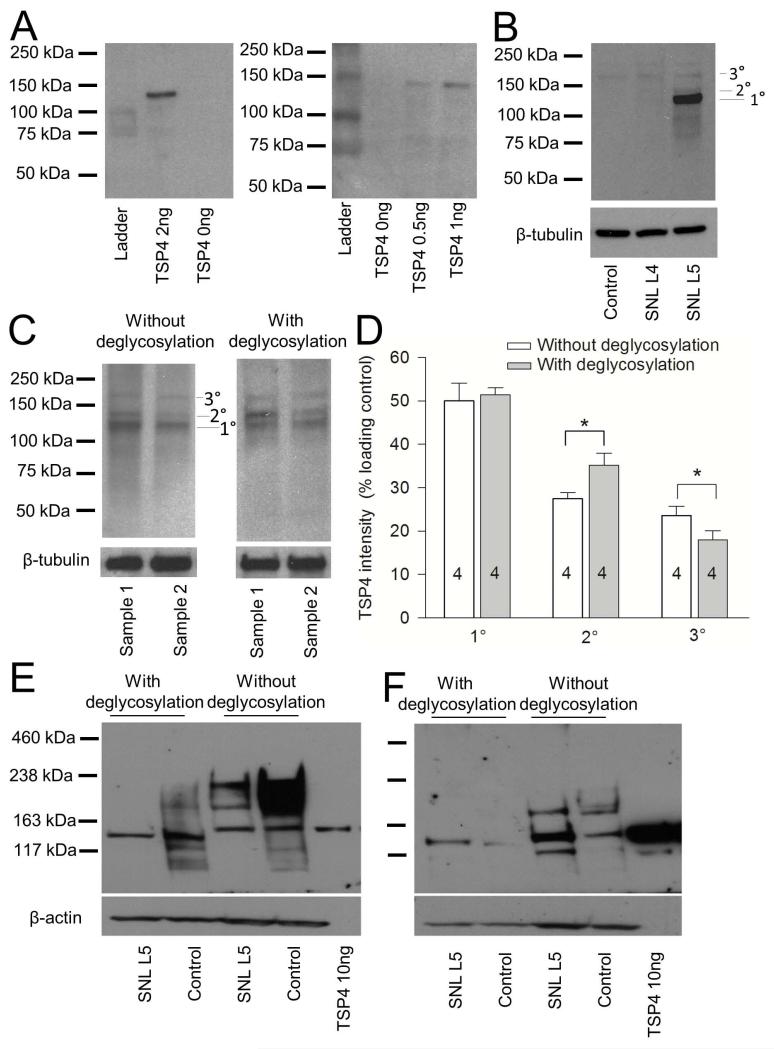
Our original work (Kim et al. 2012) used a noncommercial guinea pig TSP4 antibody that is available in amounts that are insufficient for extensive further studies, so we employed a commonly used commercial antibody to perform this study. To test this antibody’s specificity, we used it to identify recombinant TSP4 as a positive control, which has a calculated MW of 107 kDa for the unprocessed TSP4 including a 6-histadine tag. The recombinant TSP protein was detected by the antibody as a unique band that migrated with an apparent MW of 135 kDa under our experimental conditions (Figure 1A). When we used this antibody against DRG tissue homogenates prepared from control and SNL rats, we observed the main band at 135 kDa (Figure 1B), comparable to our previously used antibody (Kim et al. 2012) and to prior reports using this R&D antibody on different tissues (Benner et al. 2013; Caceres et al. 2007). Additional bands with higher apparent molecular weights were found, which may represent various degrees of TSP4 glycosylation, since TSP4 is a glycoprotein that has multiple glycosylation sites. Deglycosylation treatment partially reduced the bands at higher molecular weight (Figure 1C, D), which supports the likelihood that the secondary bands are TSP4 with different levels of glycosylation. TSP4 additionally forms disulfide-linked homopentamers, and also interacts with a binding protein, polypyrimidine tract-binding protein 3, which has molecular weight of approximately 60 kDa (Sadvakassova et al. 2009). If these interactions persisted despite our denaturing conditions, it could also alter the apparent molecular weight of the protein identified by our antibody. Further experiments would be needed to resolve these details. For comparison, we tested the original guinea pig TSP4 antibody (used as an antiserum without affinity purification (Dunkle et al. 2007)) again with and without deglycosylation treatment (Figure 1E), performed in the original lab (ZDL). This antibody also detected recombinant TSP protein as a unique band with proper molecular weight of 135 kDa. In addition, it detects glycosylated TSP4 proteins that have higher apparent molecular weights than those detected by the R&D TSP4 antibody. A gel run under identical conditions using the R&D antibody (Figure 1F) again showed an injury-induced increase in TSP4 expression.
Figure 1.
TSP4 antibodies detect recombinant TSP4, as well as TSP4 in DRG samples. (A) R&D rabbit TSP4 antibody detected recombinant TSP4 with a molecular weight of approximately 140kDa. (B) When used under conditions that showed TSP4 in lumbar 5 (L5) DRG neurons injured by spinal nerve ligation (SNL), the antibody also detected TSP4 in samples prepared from DRGs. The main band (1°) has a molecular weight of approximately 135kDa. Two other bands have molecular weights of approximately 140kDa (2°) and 170kDa (3°). Identical conditions showed much TSP4 in the L4 neurons adjacent to SNL injury, or in Control animals after sham operation. (C) Deglycosylation treatment reduced the 170kDa band and expanded the 140kDa band. (D) Summary of deglycosylation data. The numbers in the bars represent the n for group size here and in subsequent figures. *p < 0.05. (E) Nonpurified guinea pig TSP4 antibody detected recombinant TSP4 with a molecular weight of approximately 140kDa and also detected glycosylated and deglycosylated TSP4 in samples prepared from DRGs. Using the R&D antibody under identical conditions (F) shows somewhat less of glycosylated TSP4 and elevated TSP4 in SNL L5 axotomized neurons.
TSP4 expression is upregulated in soma and axonal fiber DRG regions after nerve injury
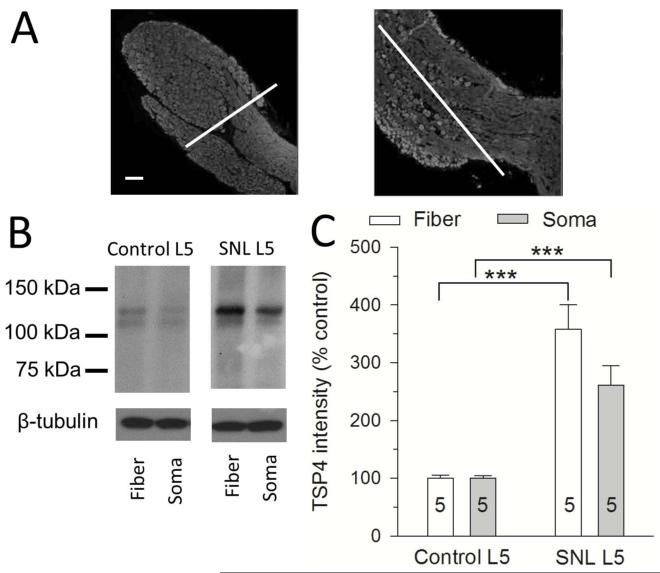
With this validated R&D antibody, our initial observations on tissue from neuropathic rats showed strongly elevated TSP4 levels in axotomized SNL L5 DRGs (Figure 1B), in contrast to our prior findings (Kim et al. 2012). In addition to different glycosylated isoforms recognized by different antibodies, one possible explanation could be different surgical preparation of the harvested tissue by different researchers. DRGs contain regions that are constituted predominantly of either neuronal somata or axonal tracts, whereas the nerve roots and spinal nerve are entirely fiber tracts (Figure 2A). Since these tissue components may have substantially different TSP4 protein levels after injury, we considered the possibility that variations in tissue preparation by different individuals might cause different Western results if TSP4 upregulation is localized in particular cell types or structures. We therefore examined TSP4 expression in tissue selectively harvested to include only predominantly DRG somata components versus purely of fiber tracts (Figure 2A). This showed that TSP4 increased in both areas (Figure 2B, C), which makes it unlikely that details of tissue preparation can alter the direction of TSP4 expression change after injury.
Figure 2.
Nerve injury elevates expression of TSP4 both in somata and axonal fiber areas of DRG. (A) Sample figures stained for β3-tubulin (neurons) demonstrate the line demarcating the separation of somatic areas and axonal fibers area used to collect these tissues separately. (B, C) Western blots comparing L5 DRG tissue 24 days after SNL to sham-operated control L5 DRGs show upregulated TSP4 protein in both the somata and axonal fiber areas. ***p < 0.001. Scale bars = 100μm.
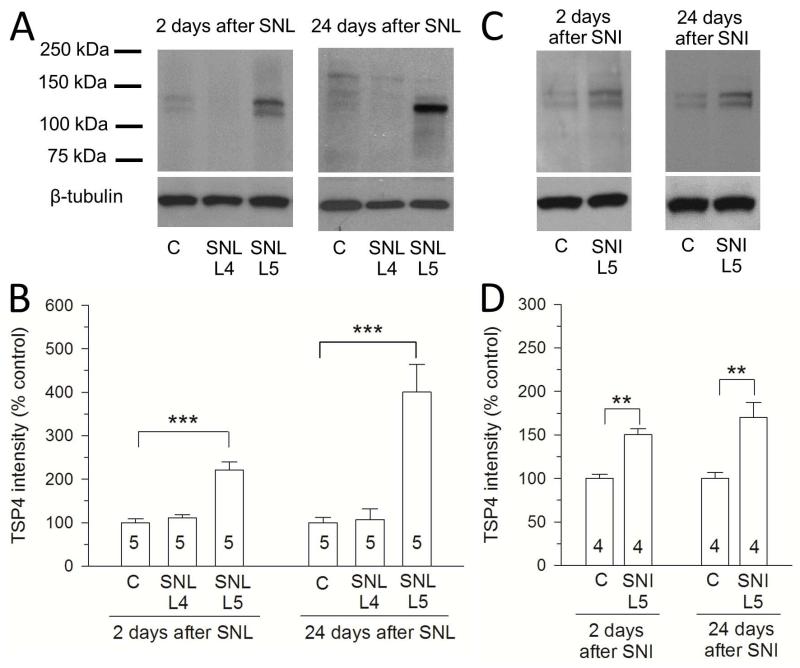
We previously showed that TSP4 mRNA was increased two days after SNL nerve injury (Kim et al. 2009). Consistent with increased gene expression, we confirmed here that the TSP4 protein also increased in the two days after SNL surgery (Figure 3A, B). Since general tissue damage could affect gene expression soon after surgery, we employed a second standard neuropathic pain model (spared nerve injury, SNI) that does not involve surgery in the vicinity of the DRG, but rather injures components of the distal sciatic nerve (Fischer et al. 2014). DRG tissue from rats after SNI surgery also showed increased TSP4 expression 2 days and 24 days after surgery (Figure 3C, D). These data suggest that increased TSP4 expression in DRGs proximal to nerve injury is unlikely to be a result of local tissue trauma and may be a consistent pathophysiological component of peripheral neuropathic pain models.
Figure 3.
Expression of TSP4 in DRG is elevated in both SNL and SNI neuropathic pain models. (A, B) Western blots show upregulated TSP4 protein after SNL compared to sham-operated control and SNL L4 DRGs. (C, B) Western blots show upregulated TSP4 protein after SNI compared to sham-operated control L5 DRGs. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars = 100μm.
TSP4 expression is elevated in neurons and in the extracellular space after nerve injury
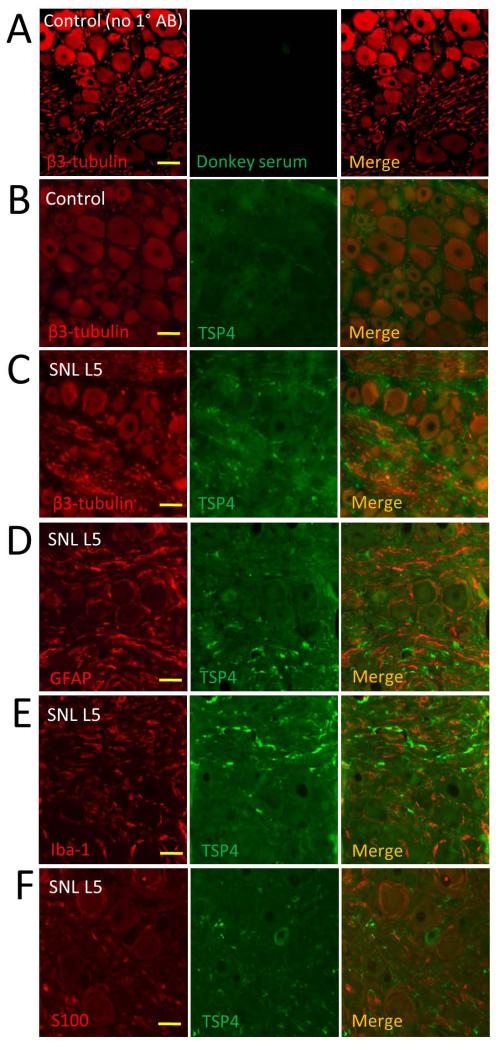
Since TSP4 is not uniformly distributed within the DRG, we next investigated where the elevated TSP4 is located at a histological level. Under basal conditions, co-staining for β3-tubulin, which exclusively stains neurons, revealed weak TSP4 expression in most neuron somata of control DRGs (Figure 4A, B). However, in SNL L5 DRGs, TSP4 expression is increased in neuronal somata and is expressed in all neuronal size groups, and is also present in an interstitial pattern (Figure 4C), with particular accumulation in fiber tract areas. This non-neuronal TSP4 was not contained in astrocytes as there was no co-staining that would indicate overlapping immunoreactivity for TSP4 and the selective astroglial/satellite glial cell protein GFAP (Figure 4D). We similarly tested whether elevated TSP4 could be occurring in microglial cells or Schwann cells, but we found only very weak co-staining of TSP4 with the selective microglial stain Iba-1 (Figure 4E) and no co-staining of TSP4 with antibody against S100 protein of Schwann cells (Figure 4F). We additionally found no immunoreactivity before or after nerve injury using stains for CD6 to identify pan-T cells and CD8 to identify cytotoxic T cells (data not shown). Taken together, our data document elevated TSP4 in both DRG neurons and extracellular spaces.
Figure 4.
TSP4 is upregulated in DRG neuronal cytoplasm and extracellular space of DRGs proximal to axotomy. (A) No autofluorescence or nonspecific staining was evident in the absence of TSP4 antibody. (B) Co-staining with β3-tubulin (red) identifies TSP4 protein in neuronal somata and the DRG extracellular space under control (uninjured) conditions. (C) SNL causes substantial intensification of TSP4 in somata and accumulation of TSP4 in interstitial areas. (D) In DRGs proximal to SNL, TSP4 is not seen to co-express with satellite glial cells, identified by staining for GFAP). Co-staining of TSP4 is also absent in microglial cells stained by Iba1 (E) or Schwann cells stained by S100 (F). Scale bars = 40μm.
Dissociated DRG neurons from injured rats also show increased TSP4 expression
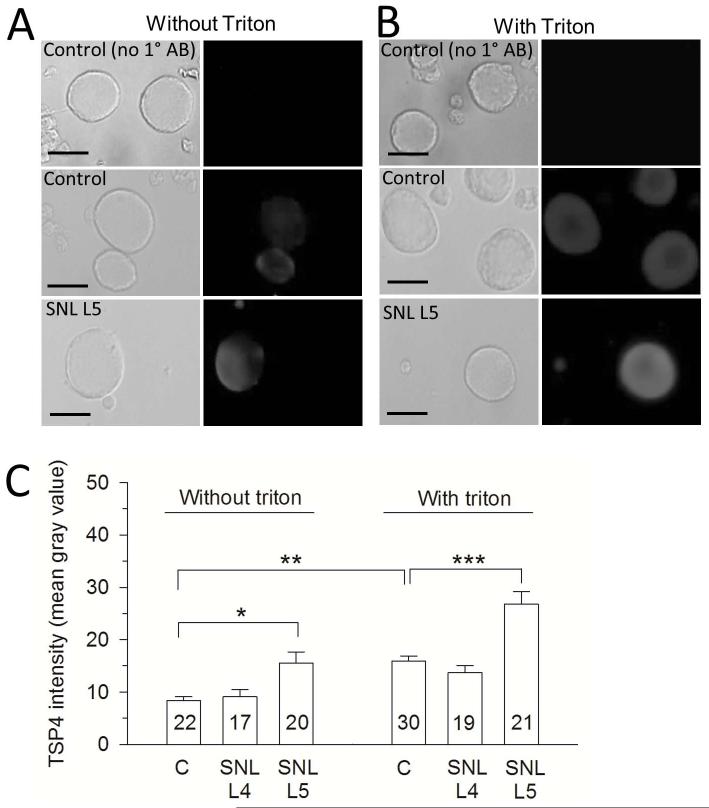
Dissociated DRG neurons are a conventional model for examination of sensory neuron physiology in our lab and others. We therefore tested whether DRG neurons held in culture for 2 hours following dissociation exhibit the same pattern of TSP4 expression as in our immunohistochemistry data after injury. We confirmed that TSP4 expression is also evident in dissociated DRG neurons. Presence of staining in the absence of permeabilization of neurons by Triton application (Figure 5A) suggests the existence of an adherent extracellular component of TSP4 expression that persists after dissociation. Using semi-quantitative methods to identify TSP4 levels, injured SNL L5 neurons have much higher TSP4 expression inside and outside of cell membrane compared with control and L4 DRG neurons, (Figure 5A-C). These observations confirm that dissociated DRG neurons retain the elevated TSP4 expression noted in intact DRGs, and that dissociated neurons provide a valid model for examining TSP4 effects.
Figure 5.
Increased expression of TSP4 in cultured neurons dissociated from DRGs harvested from nerve-injured rats. (A) Without Triton X-100 treatment, which facilitates antibody penetration into cells, neurons from injured DRGs show elevated surface-bound TSP4. Left-side panels show bright field images. (B) With treatment of Triton, neurons from injured DRGs show increased intracellular TSP4 compared with controls. (C) Summary data. Scale bars = 25μm.*p < 0.05, **p < 0.01, ***p < 0.01.
DRGs from injured TSP4-eGFP mice reveal TSP4 activation
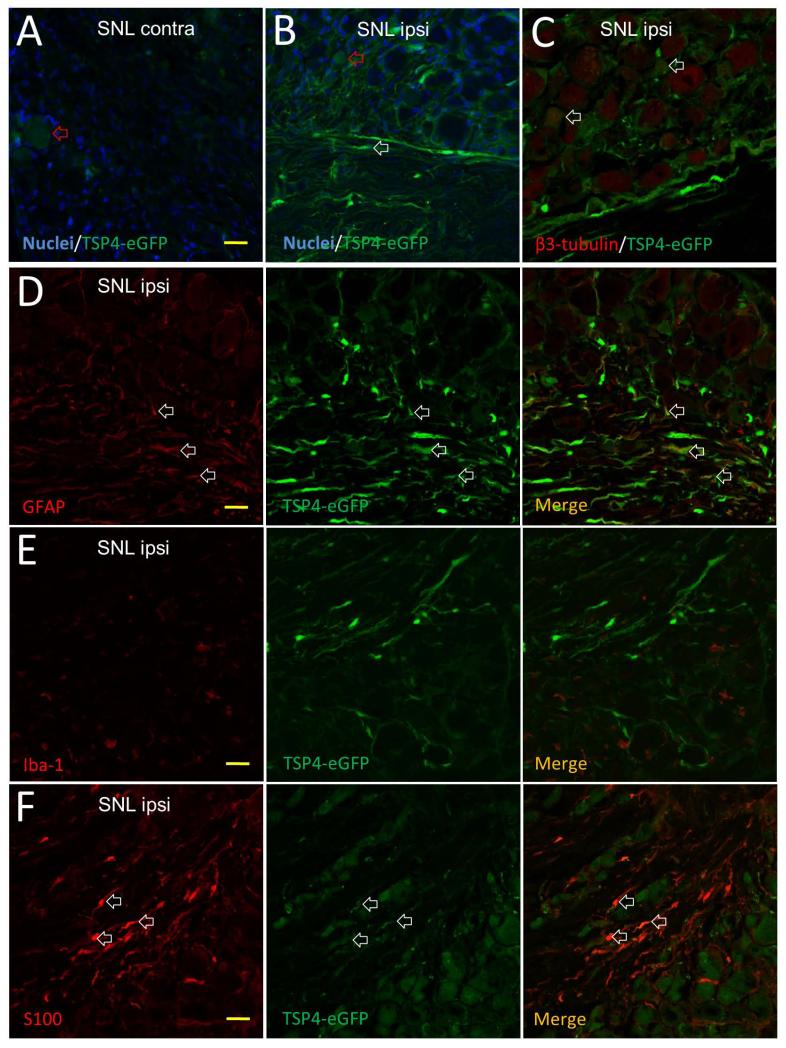
To test the effect of injury on TSP4 gene activation without depending on immunological identification, we used a transgenic mouse line in which eGFP is expressed under the control of a TSP4 promoter (Kim et al. 2012), thereby identifying cells in which the TSP4 gene is activated. DRGs from the injury side had higher eGFP expression in neurons and fiber tracts than the DRGs from contralateral side (Figure 6A, B), suggesting TSP4 upregulation after injury. Consistent with our data with the R&D TSP4 antibody, TSP4 gene expression is activated in neuronal somata of all size groups in SNL L5 DRGs (Figure 6C), However, we also saw eGFP signal around neurons and in fiber tract areas (Figure 6B, C). Co-staining showed that this non-neuronal eGFP was co-stained with GFAP (Figure 6D) and S100 (Figure 6F). Those data suggest that neurons, astrocytes and Schwann cells are activated to express TSP4 after nerve injury. In this transgenic mouse, eGFP expression identifies cells in which the TSP4 gene has been activated, but the TSP4 protein itself is not labeled by eGFP. Therefore, these findings indicate only potential sources of TSP4 after injury but do not provide insight on the natural distribution of TSP4. The eGFP marker cannot be released from neurons or astrocytes, so there is no eGFP expression in the extracellular space, as expected (Figure 6B-F). TSP4-eGFP transgenic mice show more eGFP signal in astrocytes compared with findings from wild-type rat DRGs (Figure 4), which may be attributed to intracellular accumulation of eGFP in these cells.
Figure 6.
Elevated expression of eGFP immunoreactivity in DRGs of TSP4-eGFP transgenic mice 2 weeks after SNL. (A) Slight TSP4 gene activation (red arrowhead) in a DRG contralateral from SNL produces minimal eGFP in a sensory neuron soma. (B) Injured DRG ipsilateral to SNL shows elevated TSP4 gene activation, with eGFP found in neuronal somata (red arrowhead), and glial or Schwann cell elements of fiber tracts (white arrowhead). (C) Co-staining with β3-tubulin (red) identifies eGFP protein in neuronal somata (white arrowheads). (D) Co-staining with GFAP (red) identifies eGFP protein in astrocytes (white arrowheads). (E) Co-staining with Iba-1 (red) shows no eGFP in microglial cells. (F) Co-staining with S100 identifies eGFP protein in Schwann cells (white arrowheads). Images are representative from 3 sections from each of 5 mice. Scale bars, 20μm.
Discussion
In this study, we have detected TSP4 expression in primary sensory neurons under baseline conditions, and have observed that expression is heightened after injury. Additionally we have found that substantial TSP4 is secreted into the extracellular space within the DRG. Unlike in the dorsal horn, we did not observe TSP4 accumulation in glial cells in the DRG before or after neuronal trauma. Using a transgenic mouse, we identified TSP4 gene activation in astroglia/satellite cells and Schwann cells as well as in neurons. Together, these observations are compatible with an interpretation that TSP4 is synthesized in neurons and glia after injury and secreted into the extra-cellular space, where it may act on DRG neurons locally in an autocrine fashion. Our prior observations show that painful peripheral nerve injury is associated with increased TSP4 gene expression in the DRG (Kim et al. 2009; Valder et al. 2003). Our present experiments now fill the gap and show elevated TSP4 protein in DRG neurons as well. Although our previous findings showed decreased TSP4 protein in DRG homogenates after injury (SNL L5), this discrepancy is likely attributable to the fact that the former antibody has much higher sensitivity to a glycosylated form of TSP4 but the currently used affinity purified antibody has a much higher sensitivity to the core and recombinant TSP4 proteins. Our current Western blot data clearly show nerve injury induced elevation of TSP4 core proteins that is confirmed by data from immunohistology and immunocytology, as well as an injured transgenic model.
There are findings from previous reports that support our view that TSP4 may have an important role in intracellular signaling of sensory neurons. Nerve injury disrupts Ca2+ signaling in DRG neurons, such that resting voltage-gate Ca2+ channel function and resting [Ca2+]c are reduced, and sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) function is diminished, which produce depleted endoplasmic reticulum (ER) Ca2+ stores (Duncan et al. 2013; Fuchs et al. 2005; Gemes et al. 2011). The resulting ER stress is a recognized activator of JNK and p38 MAPK, which are known to become activated after peripheral nerve injury (Jin et al. 2003; Zhuang et al. 2006). Examination of cardiac myocytes has shown that ER stress results in generation of TSP4 that may provide an ER-resident adaptive mechanism in response to by regulating protein production and resolution of misfolded proteins through binding to the ER luminal domain of activating transcription factor 6α (Atf6α) (Lynch et al. 2012). Thus, elevated intracellular TSP4 following neuronal injury may provide protective effects that enhance ER function. Our data now show that injured sensory neurons with ER stress also have associated enhanced expression of TSP4. Further studies identifying TSP4’s intracellular functions in detail will be needed to determine if elevated TSP4 in DRG neurons serves the same protective role.
Painful injury also increases expression of TSP4 protein in the dorsal horn (Kim et al. 2012). At that site, the TSP4 gene is activated mainly within astrocytes in the spinal cord white matter. This contrasts with our current observations of both neuronal and glial expression of TSP4 in the DRG after injury. This may reflect the difference between the direct injury suffered by the DRG neurons as opposed to the effects at the more distant dorsal horn site, where glial responses are likely due to the arrival of injury signals such as abnormal neuronal activity levels and the release of glial activators including glutamate, brain-derived neurotrophic factor, ATP, and substance P from the central terminals of sensory neurons (Calvo and Bennett 2012; Ren and Dubner 2008), thereby producing a distinct dorsal horn response.
The dominant recognized function of thrombospondins is as extracellular matrix molecules, where they may play an important role in regeneration after peripheral nerve injury (Hoffman and O’Shea 1999). It is therefore particularly interesting that we found greatly increased TSP4 in the extracellular space. In the central nervous system, TSPs secreted from astrocytes can induce excitatory synaptogenesis through interactions with the Ca2+ channel α2δ1 subunit (Christopherson et al. 2005; Eroglu et al. 2009). At the same time, the α2δ1 subunit plays important roles in the neuropathic pain. Specifically, peripheral nerve injury and inflammation result in a marked upregulation of α2δ1 in sensory neurons of the DRG (Li et al. 2006; Lu et al. 2010; Luo et al. 2001). Furthermore, transgenic α2δ1 overexpression enhances ICa, accelerates channel opening, and hyperpolarizes channel activation in sensory neurons (Li et al. 2006), while chronic lack of α2δ1 or application of its ligand gabapentin decreases high-voltage-activated Ca2+ channel trafficking to the neuronal membrane (Hendrich et al. 2008). We and others have previously observed that peripheral nerve injury decreases ICa in the somata of injured sensory neurons (Abdulla and Smith 2001; Baccei and Kocsis 2000; Gemes et al. 2009; Hogan et al. 2000; McCallum et al. 2006; Tang et al. 2012). Taken together, our findings suggest that elevated TSP4 in the DRGs might contribute to neuropathic pain via dysregulation of voltage-gated Ca2+ channels in the DRG by activating the α2δ1 subunit. Additionally, exposure of sensory neuron somata in the DRG to high levels of TSP4 may affect presynaptic factors in dorsal horn synaptogenesis (Kim et al. 2012) that may underlie post-injury synaptogenesis by which acute pain may transition into chronic pain.
Acknowledgements
We would like to thank Dr. Frank Zaucke for the guinea pig anti-TSP4 antibody for some experiments.
Grant information: The study was supported by National Institutes of Health Grant R01DE021847 (to Z.D.L. & Q.H. H.).
References
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. Journal of neurophysiology. 2001;85(2):644–658. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- Baccei ML, Kocsis JD. Voltage-gated calcium currents in axotomized adult rat cutaneous afferent neurons. Journal of neurophysiology. 2000;83(4):2227–2238. doi: 10.1152/jn.2000.83.4.2227. [DOI] [PubMed] [Google Scholar]
- Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497(7449):369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres M, Suwyn C, Maddox M, Thomas JW, Preuss TM. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17(10):2312–2321. doi: 10.1093/cercor/bhl140. [DOI] [PubMed] [Google Scholar]
- Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234(2):271–282. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Duncan C, Mueller S, Simon E, Renger JJ, Uebele VN, Hogan QH, Wu HE. Painful nerve injury decreases sarco-endoplasmic reticulum Ca(2)(+)-ATPase activity in axotomized sensory neurons. Neuroscience. 2013;231:247–257. doi: 10.1016/j.neuroscience.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle ET, Zaucke F, Clegg DO. Thrombospondin-4 and matrix three-dimensionality in axon outgrowth and adhesion in the developing retina. Experimental eye research. 2007;84(4):707–717. doi: 10.1016/j.exer.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Pan B, Vilceanu D, Hogan QH, Yu H. Sustained relief of neuropathic pain by AAV-targeted expression of CBD3 peptide in rat dorsal root ganglion. Gene Ther. 2014;21(1):44–51. doi: 10.1038/gt.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102(6):1217–1225. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- Gemes G, Bangaru ML, Wu HE, Tang Q, Weihrauch D, Koopmeiners AS, Cruikshank JM, Kwok WM, Hogan QH. Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci. 2011;31(10):3536–3549. doi: 10.1523/JNEUROSCI.5053-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemes G, Rigaud M, Weyker PD, Abram SE, Weihrauch D, Poroli M, Zoga V, Hogan QH. Depletion of calcium stores in injured sensory neurons: anatomic and functional correlates. Anesthesiology. 2009;111(2):393–405. doi: 10.1097/ALN.0b013e3181ae63b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JR, O’Shea KS. Thrombospondin expression in nerve regeneration I. Comparison of sciatic nerve crush, transection, and long-term denervation. Brain Res Bull. 1999;48(4):413–420. doi: 10.1016/s0361-9230(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101(2):476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86(1-2):43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23(10):4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, Luo ZD. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors [corrected] Pain. 2009;143(1-2):114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Li KW, Boroujerdi A, Peter Yu Y, Zhou CY, Deng P, Park J, Zhang X, Lee J, Corpe M, Sharp K, Steward O, Eroglu C, Barres B, Zaucke F, Xu ZC, Luo ZD. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J Neurosci. 2012;32(26):8977–8987. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125(1-2):20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SG, Zhang XL, Luo ZD, Gold MS. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151(3):633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21(6):1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149(6):1257–1268. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum JB, Kwok WM, Sapunar D, Fuchs A, Hogan QH. Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons. Anesthesiology. 2006;105(1):160–168. doi: 10.1097/00000542-200607000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21(5):570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136(1-2):188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31(3):170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadvakassova G, Dobocan MC, Difalco MR, Congote LF. Regulator of differentiation 1 (ROD1) binds to the amphipathic C-terminal peptide of thrombospondin-4 and is involved in its mitogenic activity. J Cell Physiol. 2009;220(3):672–679. doi: 10.1002/jcp.21817. [DOI] [PubMed] [Google Scholar]
- Tang Q, Bangaru ML, Kostic S, Pan B, Wu HE, Koopmeiners AS, Yu H, Fischer GJ, McCallum JB, Kwok WM, Hudmon A, Hogan QH. Ca(2)(+)-dependent regulation of Ca(2)(+) currents in rat primary afferent neurons: role of CaMKII and the effect of injury. J Neurosci. 2012;32(34):11737–11749. doi: 10.1523/JNEUROSCI.0983-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CR, Levenson RM. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4):411–424. doi: 10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. Journal of neurochemistry. 2003;87(3):560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Walker RA. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49(4):406–410. doi: 10.1111/j.1365-2559.2006.02514.x. [DOI] [PubMed] [Google Scholar]
- Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain. 2010;11(3):280–286. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Fischer G, Jia G, Reiser J, Park F, Hogan QH. Lentiviral gene transfer into the dorsal root ganglion of adult rats. Mol Pain. 2011;7:63. doi: 10.1186/1744-8069-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26(13):3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]