Abstract
Objective
To better understand the high variability in response seen when treating human subjects with restorative therapies post-stroke. Preclinical studies suggest that neural function, neural injury, and clinical status each influence treatment gains, therefore the current study hypothesized that a multivariate approach incorporating these three measures would have the greatest predictive value.
Methods
Patients 3-6 months post-stroke underwent a battery of assessments before receiving 3-weeks of standardized upper extremity robotic therapy. Candidate predictors included measures of brain injury (including to gray and white matter), neural function (cortical function and cortical connectivity), and clinical status (demographics/medical history, cognitive/mood, and impairment).
Results
Among all 29 patients, predictors of treatment gains identified measures of brain injury (smaller corticospinal tract (CST) injury), cortical function (greater ipsilesional motor cortex (M1) activation), and cortical connectivity (greater inter-hemispheric M1-M1 connectivity). Multivariate modeling found that best prediction was achieved using both CST injury and M1-M1 connectivity (r2=0.44, p=0.002), a result confirmed using Lasso regression. A threshold was defined whereby no subject with >63% CST injury achieved clinically significant gains. Results differed according to stroke subtype: gains in patients with lacunar stroke were exclusively predicted by a measure of intra-hemispheric connectivity.
Interpretation
Response to a restorative therapy after stroke is best predicted by a model that includes measures of both neural injury and function. Neuroimaging measures were the best predictors and may have an ascendant role in clinical decision-making for post-stroke rehabilitation, which remains largely reliant on behavioral assessments. Results differed across stroke subtypes, suggesting utility of lesion-specific strategies.
Keywords: chronic stroke, restorative therapy, rehabilitation, neuroimaging, predictors
Introduction
Stroke is a very heterogeneous disease, with substantial variability between patients in response to treatment. This extends to clinical trials, where higher inter-subject variance means reduced study power and increased cost1. This issue has undergone considerable discussion in relation to acute stroke trials, where CT and MRI measures are under study to improve prediction of individual patient responses to reperfusion therapy2, 3. A similar challenge exists in the setting of restorative therapies, as the best approach to predicting treatment response remains undetermined4.
Efforts to identify predictors of response to a restorative treatment in humans are informed by preclinical studies5. Behavioral gains in animals given a restorative therapy after experimental stroke has been linked to growth-related events in the brain that include axonal outgrowth, increased dendritic branching, and release of growth factors6-8. Predictors of these behavioral gains fall into three categories of measurement: (1) neural injury such as infarct topography9, 10 and infarct volume9-11; (2) neural function such as motor cortex integrity12, 13; and (3) clinical status, such as baseline behavioral assessments13, age8, 14, 15 and vascular risk factors8.
Results of human studies have been largely concordant, with predictors of response to a restorative therapy post-stroke generally grouped as measures of: (1) neural injury such as extent of injury to white matter or gray matter16-20; (2) neural function such as functional activation21-24, functional connectivity25, 26, and neurophysiological status19, 22; and (3) clinical measures such as demographics27 and baseline behavioral status16, 23, 28, 29. Some data in humans suggest that genetic variation, particularly in the genes for brain-derived neurotrophic factor (BDNF) and apolipoprotein E (ApoE), might also be related to variance in response to a restorative therapy, as polymorphisms for each have been associated with poorer post-stroke outcome and reductions in some forms of cortical plasticity30-32. In addition, stroke subtype is important to many aspects of clinical decision-making for patients with cerebrovascular disease33, 34 and has been suggested as important to recovery potential and improvements with restorative therapies in the chronic phase of stroke,35 but this issue has received limited attention.
The number and diversity of these predictive measures reflects the complexity of neural repair after stroke. Optimal prediction of response to a restorative therapy may therefore likely require a multivariate approach that incorporates several types of measure36, 37. However, only a few human studies have taken this approach16, 20, 22, 23, 38, and none has taken a multivariate approach that included measures of neural injury, functional activation, functional connectivity, genetics, and clinical/demographic measures in a heterogeneous stroke population.
The current study addressed these issues, with the primary hypothesis being that pre-therapy measures of neural injury, neural function, and clinical status together would best predict response to a restorative therapy, and would surpass the performance of any single measure. An additional hypothesis was that the best predictors of treatment gains differ according to stroke subtype, focusing on lacunar vs. non-lacunar stroke given substantial differences in their pathophysiology and outcomes39-45.
Materials and methods
Patient enrollment
Forty-one patients with chronic stroke gave informed consent to be part of a longitudinal study of standardized robotic-assisted hand therapy (clinicaltrials.gov ID# NCT01244243) that was approved by the UC Irvine Institutional Review Board. Inclusion and exclusion criteria (Table 1) aimed to capture a population at a specific time point in stroke recovery (close to the time when spontaneous motor recovery is complete 46, 47), with confirmed plateau in arm motor recovery, and within a wide but prescribed range of motor deficits. In one patient, baseline MRI revealed an incidental finding that met exclusion criteria, rendering the patient ineligible for the study. The forty eligible patients were studied as below.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ≥ 18 years | Contraindication to MRI |
| Stroke with onset 11-26 weeks prior | Severe cognitive impairment |
| Residual arm motor deficit (ARAT<52 or 9-hole peg test score > 25% longer than with unaffected hand | Concurrent diagnosis affecting arm/hand function |
| Arm motor status not at stable plateau | |
| Preserved voluntary movements in distal upper extremity (≥5 degrees of active range of motion in affected wrist or index finger metacarpophalangeal joint |
Baseline assessments (Table 2) of candidate predictors were obtained, after which patients underwent a course of robotic therapy; all patients received robotic therapy, there was no comparison or placebo arm. These baseline assessments were organized into seven categories: 1) demographics/medical history; 2) cognitive/mood; 3) genetics; 4) impairment; 5) brain injury; 6) cortical function; and 7) cortical connectivity.
Table 2.
Baseline measures.
| All patients | Non-lacunar | Lacunar | |
|---|---|---|---|
| Number | 29 | 21 | 8 |
| Demographics/medical history | |||
| Age (years) | 56.5 (13.9) | 55.3 (13.9) | 55.3 (5.4) |
| Time post-stroke (months) | 4.3 (1.0) | 4.2 (1.0) | 4.5 (0.9) |
| Gender | 8 F/ 21 M | 7 F/ 14 M | 1 F/ 7 M |
| Diabetes mellitus | 8 Y/ 21 N | 6 Y/ 15 N | 2 Y/ 6 N |
| Hypertension | 14 Y/ 15 N | 8 Y/ 13 N | 6 Y/ 2 N |
| Hypercholesterolemia | 12 Y/ 17 N | 6 Y/ 15 N | 6 Y/ 2 N |
| Cognitive/mood | |||
| Mini Mental State Exam (normal = 30) | 27.5 [25-30] | 28 [25-30] | 27 [25.25-29.75] |
| Geriatric Depression Scale (normal = 0) | 3.0 [1.5-4.5] | 2 [0.5-4] | 3 [2-6.75] |
| Impairment | |||
| NIH Stroke Scale (normal = 0) | 4.2 (1.8) | 4.4 (1.7) | 3.5 (1.9) |
| Fugl-Meyer Scale (normal = 66) | 36.3 (14.8) | 35.7 (16.1) | 37.7 (11.7) |
| Action Research Arm Test (normal = 57) | 26.2 (18.8) | 25.7 (20.2) | 27.5 (15.9) |
| Nottingham Sensory Assessment (normal = 17) | 13.5 (4.3) | 12.2 (4.4) | 16.9 (0.4) |
| Genetics | |||
| BDNF val66met polymorphism present | 8 Y/ 21 N | 5 Y/ 16 N | 3 Y/ 5 N |
| ApoE4 allele present | 4 Y/ 25 N | 2 Y/ 19N | 2 Y/ 6 N |
| Brain injury | |||
| Infarct volume (cc) | 32.6 (48.4) | 44.6 (52.3) | 1.2 (0.6) |
| Precentral gyrus injury? | 14 Y/ 15 N | 14 Y/ 7 N | 0 Y/ 8 N |
| Precentral gyrus injury (cc) | 0.61 (1.1) | 0.85 (1.2) | 0 |
| Cortical PMd injury? | 10 Y/ 19 N | 10 Y/ 11 N | 0 Y/ 8 N |
| Cortical PMd injury (cc) | 0.25 (0.52) | 0.34 (0.58) | 0 |
| Cortical injury? | 21 Y/ 8 N | 21 Y/ 0 N | 0 Y/ 8 N |
| Total cortical injury (cc) | 19.8 (33.6) | 27.3 (69.9) | 0 |
| Corticospinal tract integrity (DTI FA) | 0.39 (0.12) | 0.39 (0.13) | 0.36 (0.08) |
| Percent injury to CST | 54.5 (32.6) | 61.2 (34.2) | 35.2 (18.0) |
| Cortical function | |||
| Ipsilesional M1 activation - contrast estimate | 2.9 (1.9) | 2.6 (1.9) | 3.7 (1.7) |
| Ipsilesional PMd activation - contrast estimate | 2.0 (1.3) | 1.8 (1.5) | 2.3 (1.0) |
| Contralesional M1 activation - contrast estimate | 1.7 (1.3) | 1.6 (1.3) | 1.9 (1.2) |
| Contralesional PMd activation - contrast estimate | 2.2 (1.7) | 2.2 (1.7) | 2.3 (1.7) |
| Ipsilesional M1 activation volume (voxels) | 67 (42) | 64 (44) | 72 (38) |
| Ipsilesional PMd activation volume (voxels) | 43 (46) | 40 (46) | 49 (47) |
| Contralesional M1 activation volume (voxels) | 39 (41) | 38 (42) | 42 (42) |
| Contralesional PMd activation volume (voxels) | 49 (42) | 45 (44) | 45 (41) |
| Cortical connectivity | |||
| iM1-iPMd correlation coefficient | 0.31 (0.32) | 0.28 (0.30) | 0.38 (0.38) |
| iM1-cM1 correlation coefficient | 0.13 (0.30) | 0.14 (0.30) | 0.13 (0.31) |
| iM1-cPMd correlation coefficient | 0.10 (0.21) | 0.11 (0.19) | 0.07 (0.26) |
Robotic therapy
Patients underwent twelve treatment sessions of robotic hand therapy over three weeks: 2 hours/day, 4 days/week for a total of 24 hours. All patients completed at least eleven of twelve robotic therapy sessions. Therapy consisted of repeated grasp-release (‘close’ and ‘open’) movements of the affected hand, with grasp coupled to wrist extension and release to wrist flexion, using a pneumatically-actuated robotic device described previously48. The average number of movement repetitions at each of the 12 sessions, recorded by the robot, was 954 for the fingers, 2,579 for the thumb, and 1,298 for the wrist; the thumb is greatest because some games required movement of four fingers and thumb only while other games required movement of wrist and thumb only.
Demographics/history
Medical history was obtained and acute stroke hospitalization records reviewed.
Cognitive/mood
included the Mini Mental State Exam and Geriatric Depression Scale.
Impairment
A single rater performed all behavioral assessments. Patients were assessed before, and one-month, after a three-week course of standardized robotic hand therapy. Baseline Fugl-Meyer (FM) and Action Research Arm Test (ARAT) assessments were performed twice before beginning therapy (once at baseline screening and again 1-3 weeks later) to ensure stability of motor status (e.g., the second FM score was required to be within 3 points of the first score), and averaged for each patient.
Genetics
A blood sample was obtained, and BDNF val66met polymorphism plus ApoE4 allele status were determined as described previously 31.
Brain injury
Image acquisition
Magnetic resonance imaging was performed at the second baseline visit and acquired using a 3.0T Philips Achieva system. Anatomical imaging consisted of a high-resolution T1-weighted image, T2-FLAIR, and diffusion tensor imaging (DTI). High-resolution T1-weighted images were acquired using a 3D MPRAGE sequence (repetition time (TR)=8.5 ms, echo time (TE)=3.9 ms, slices=150, voxel size=1 × 1 × 1 mm3). T2-FLAIR (TR=11000 ms, TE=125 ms, slices=31 slices, voxel size=.58 × .58 × 5 mm3) and intracranial MR angiogram (TR=23 ms, TE=3.45 ms, slices=100, voxels size=.31 × .31 × .7 mm3) images were also acquired. One set of diffusion-weighted images was acquired (32 directions; b-value 1000 smm-2; 60 slices; voxel size=1.75 × 1.75 × 2 mm3).
Image analysis
Image analysis was performed blinded to clinical data. Three classes of brain injury metrics were extracted: (1) total brain injury (infarct volume); (2) gray matter injury (to primary motor cortex (M1), dorsal premotor cortex (PMd), plus total cortical injury); and (3) white matter injury (percent lesion overlap with corticospinal tract (CST), or CST integrity within ipsilesional cerebral peduncle using DTI fractional anisotropy (FA) values).
Infarct volume
Using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron), each patient's infarct was outlined by hand on the T1-weighted MRI image, informed by the T2-FLAIR image as described previously38.
Gray matter injury
To determine how injury to specific regions of gray matter is related to treatment-induced behavioral gains, the degree of overlap between cortical regions of interest (ROI) and each infarct was calculated. Using FSL, the whole-brain cortical ROI was generated via FAST segmentation of the 1mm3 T1 template. Precentral gyrus (M1) and PMd gray matter ROIs were manually drawn on this template. These ROI masks and the stroke masks were then multiplied to generate an overlap image in MNI space. The number of infarct voxels overlapping with each of the three ROIs was counted and expressed as cc. Injury was recorded as both a continuous and a dichotomous variable.
White matter injury
White matter integrity within the CST was quantified using FSL to probe FA within the CST at the level of the cerebral peduncle (CP) as described previously38.
Corticospinal tract injury was also evaluated as the amount of overlap in MNI stereotaxic space between the normal corticospinal tract descending from M118, 49, generated as described previously38, and each patient's infarct. This method has been shown to perform comparably to calculating lesion overlap using tracts generated from patients' brains50.
Cortical function
Image Acquisition
Three runs of blood oxygenation level-dependent fMRI images were acquired using a T2*-weighted gradient-echo echo planar imaging sequence, as described previously38. Subjects were visually guided to use the paretic distal upper extremity to execute the grasp-release movements that were at the center of robotic training, while wearing a splint that was a non-actuated plastic exoskeleton identical to the robotic interface. Prior to MRI data acquisition, patients were trained until each could independently perform this task. Throughout the fMRI scan, investigators visually monitored patient movements.
Image Analysis
Two measures of brain function were extracted from fMRI images: (1) activation beta (contrast) estimate and (2) activation volume, each measured in four ROIs, right and left M1 plus right and left PMd. Functional data were processed as described previously38.
Cortical connectivity
Functional connectivity was assessed as the temporal correlation using an ROI-ROI approach. After the fMRI data were preprocessed in SPM8, intra- and inter-hemispheric functional connectivity metrics were calculated using the Conn toolbox51. Time courses were filtered between 0.008 and 0.13 to minimize low-frequency drift and high-frequency noise. Within-subject realignment parameters, outliers, and main session effects were included as first-level covariates. The three connections evaluated for functional connectivity were ipsilesional M1-ipsilesional PMd (iM1-iPMd), ipsilesional M1-contralesional M1 (iM1-cM1), and ipsilesional M1-contralesional PMd (iM1-cPMd). Fisher-transformed correlation coefficients were extracted for each connection in each patient.
Patient characteristics
Data from twenty-nine patients were available for analysis (Table 2). Of the forty patients enrolled, four could not complete MRI due to claustrophobia and seven patients were excluded due to excessive head motion during fMRI scanning. These twenty-nine patients did not differ significantly from the excluded eleven patients in age or baseline FM (p>0.1). The stroke was ischemic in 27 and hemorrhagic in two.
Demographics/medical history
Mean time post-stroke was 4.3 months and mean age was 56.5 years. All but two were right-handed.
Cognitive/mood
Overall, patients were neither cognitively impaired nor clinically depressed.
Genetics
The genotypic frequencies for the BDNF val66met polymorphism and the ApoE4 allele were in Hardy-Weinberg equilibrium, and these polymorphisms were present in 28% and 14% of patients, respectively.
Impairment
Overall, patients had mild global impairment and moderate-severe upper extremity motor impairment. Preserved voluntary movement (≥5 degrees of active range of motion) was present in the affected index finger metacarpophalangeal joint in 28/29, and in affected wrist in 25/29 patients.
Brain injury
Infarct volumes were moderate overall (average=33cc, range=0.6cc-178cc). The stroke affected left hemisphere in 55% of patients; dominant hemisphere, in 48%. Approximately one-third of patients had injury to PMd cortex and half had injury to M1 cortex; among these patients, extent of infarct overlap was greater with M1 than PMd. Extent of CST injury determined by lesion overlap showed substantial variability across patients (55±33%, range 0-100%). Similar results were obtained when determining extent of CST injury using DTI, with mean FA in the affected cerebral peduncle being 0.38±0.12, significantly (p<0.0001) lower than unaffected cerebral peduncle values (0.55±0.1), indicating reduced integrity within the ipsilesional CST.
Cortical function
Patients varied in the quality of performance of the requested unilateral paretic hand grasp/release movement during the active blocks of fMRI scan acquisition, with full range of motion present in 38%, partial movement in 51%, and no visible movement (due to weakness) in 11%. In addition, 24% of patients had at least one associated movement52 in the non-affected hand, the presence of which was associated with greater contralesional M1 activation (contrast estimate, p=0.009; activation volume, p=0.03) but not with differences in any other fMRI activation/connectivity measure, nor with a difference in treatment gains (p=0.99).
This task was associated with prominent bilateral activation within M1 and PMd. Activation contrast estimates within ipsilesional M1 were larger than within contralesional M1 (p=0.0002). Activation volumes were larger within ipsilesional M1 as compared to contralesional M1 (p=0.005), ipsilesional PMd (p=0.002), and contralesional PMd (p=0.03).
Magnetic resonance angiogram, available in twenty-three patients, disclosed significant ipsilesional internal carotid artery or middle cerebral artery disease in 26% of patients; none of the fMRI activation parameters differed significantly according to presence vs. absence of these cerebrovascular observations.
Cortical connectivity
During unilateral grasp/release of the paretic hand, functional connectivity was present in all three ipsilesional M1 connections (iM1-iPMd, iM1-cM1, iM1-cPMd). Functional connectivity was significantly greater in iM1-iPMd as compared to iM1-cM1 (p=0.04) and iM1-cPMd (p=0.003). There was no significant difference between iM1-cM1 and iM1-cPMd connectivity (p=0.60). Note that correlation coefficients were not significantly related to any measure of cortical function, with the sole exception being iM1-cPMd functional connectivity and ipsilesional M1 activation volume (p=0.005), and correlation coefficients were not significantly different in relation to stroke subtype.
Statistical analyses
Predictors of treatment-induced behavioral gains - bivariate screening
Bivariate screening was performed to identify the strongest predictors of treatment-induced behavioral gains. The primary outcome measure was change in arm motor status from baseline to one month post-therapy, defined by combining change measures in impairment (change in upper extremity FM) and in functional activity (change in ARAT) via principal component analysis (PCA)53; only the first component was used to define treatment-induced motor gains, as this accounted for 84% of variance in these two behavioral outcomes.
Bivariate screening determined the significance of the relationship between each baseline measure and the dependent measure (treatment-induced motor gains). Parametric statistical methods were used for measures for which the normality assumption was valid, using raw or transformed values; otherwise non-parametric methods were used. Continuous variables were evaluated using Pearson's correlation coefficient or Spearman's rank order, while categorical variables were evaluated using Student's t-test or the Wilcoxon signed-rank test. All analyses were two-tailed with alpha=0.05 and used JMP-8 software (SAS Institute, Inc., Cary, NC). For each of the seven categories, results of this bivariate screening determined whether any baseline measure survived as a predictor of treatment gains and would be advanced to multivariate modeling.
Predictors of treatment-induced behavioral gains - multivariate modeling
The primary predictive model used a forward stepwise multivariate linear regression approach (0.1 to enter, 0.15 to leave the model), advancing the most significant predictors identified in bivariate screening. The strongest bivariate predictor within each of the seven categories was advanced as long as bivariate screening showed p<0.1. If a category had more than one variable with p<0.1 during bivariate screening, only the variable with the strongest correlation coefficient was advanced into the stepwise forward model. In order to understand whether results vary according to stroke subtype, the above analyses were repeated separately for the 8 patients with a lacunar infarct (defined as an infarct that is subcortical and has infarct volume <4cc54) and for the 21 patients with a non-lacunar infarct.
Predictors of treatment-induced behavioral gains - group Lasso regression method
To confirm findings from multivariate modeling, a secondary approach to predictive modeling used a penalized regression approach. Numerous variables have been reported to influence stroke outcomes55-59. The statistical question is therefore high-dimensional, suggesting the utility of a secondary approach that used a penalized regression, or regularization,60 method for predictive modeling, which (a) makes conclusions based on joint consideration of all variables simultaneously, and (b) reduces overfitting, which can be associated with models that perform well on one dataset but do not generalize well to new datasets). Lasso (least absolute shrinkage and selection operator61) was chosen as the penalized regression model because it selects a subset of useful predictors from a total pool of candidate predictors and, unlike linear regression, minimizes the influence of outliers. Specifically, a group Lasso model was used, which is similar to the standard Lasso regression but takes the grouping of variables (i.e., categories) into account62. The Lasso procedure requires a tuning parameter, lambda, which was chosen in a standard way through (five-fold) cross-validation. Numerical variables were standardized before running the Lasso model.
Results
Treatment-induced behavioral gains
Patients showed significant gains across the 3 weeks of robotic therapy, with improvements from baseline to 1 month post-therapy in both primary endpoints (FM: 3.7 points, p<0.0001; ARAT: 4.1 points, p=0.002).
Prediction of treatment-induced behavioral gains -- all patients
Bivariate screening
When each of the variables from the seven categories was examined in bivariate screening, four categories (demographics, impairment, genetics, and cognitive/mood) had no variables significantly predicting gains, while three categories (brain injury, cortical function, and cortical connectivity; Table 3) had at least one variable that significantly predicted treatment-induced behavioral gains. The most significant bivariate predictor of gains from each of these three, respectively, was percent CST injury determined by lesion overlap (Figure 1A: r=-0.49, p=0.007), ipsilesional M1 activation contrast estimate (r=0.37, p=0.045), and iM1-cM1 functional connectivity (Figure 1B: r=0.45, p=0.01). Excluding the two subjects with hemorrhagic stroke had no effect on results. Excluding patients with ≥50% damage to cortical ROIs63 had no effect on connectivity findings.
Table 3.
Bivariate correlations between baseline measures and arm motor gains with therapy--all patients.
| Baseline variable | Correlation with treatment-induced motor gains | |
|---|---|---|
| r | p | |
| Demographics/medical history | ||
| Age | 0.07 | 0.72 |
| Time post-stroke | 0.06 | 0.76 |
| Gender (M/F) | 0.03 | 0.80 |
| Diabetes mellitus (y/n) | -0.04 | 0.71 |
| Hypertension (y/n) | 0.04 | 0.86 |
| Hypercholesterolemia (y/n) | 0.09 | 0.60 |
| Cognitive/mood | ||
| Mini Mental State Exam | -0.13 | 0.50 |
| Geriatric Depression Scale | 0.11 | 0.58 |
| Impairment | ||
| NIH Stroke Scale | -0.26 | 0.17 |
| Mean baseline Fugl-Meyer | -0.05 | 0.79 |
| Mean baseline Action Research Arm Test | 0.04 | 0.82 |
| Nottingham Sensory Assessment | 0.25 | 0.20 |
| Genetics | ||
| BDNF val66met polymorphism present (y/n) | 0.19 | 0.35 |
| ApoE4 allele present (y/n) | 0.19 | 0.43 |
| Brain injury | ||
| Infarct volume (cc) | -0.42 | 0.02* |
| Precentral gyrus injury (y/n) | -0.15 | 0.54 |
| Precentral gyrus injury (cc) | -0.18 | 0.36 |
| Cortical PMd injury (y/n) | -0.28 | 0.07 |
| Cortical PMd injury (cc) | -0.36 | 0.06 |
| Cortical injury (y/n) | -0.46 | 0.008* |
| Total cortical injury (cc) | -0.41 | 0.03* |
| Corticospinal tract integrity (DTI FA) | 0.18 | 0.34 |
| Percent injury to CST | -0.49 | 0.007** |
| Cortical function | ||
| Ipsilesional M1 activation - contrast estimate | 0.37 | 0.045* |
| Ipsilesional PMd activation - contrast estimate | 0.36 | 0.05 |
| Contralesional M1 activation - contrast estimate | 0.28 | 0.14 |
| Contralesional PMd activation - contrast estimate | -0.01 | 0.95 |
| Ipsilesional M1 activation volume | 0.16 | 0.40 |
| Ipsilesional PMd activation volume | 0.02 | 0.92 |
| Contralesional M1 activation volume | -0.01 | 0.94 |
| Contralesional PMd activation volume | -0.10 | 0.60 |
| Cortical connectivity | ||
| Ipsilesional M1-Ipsilesional PMd connectivity | 0.11 | 0.58 |
| Ipsilesional M1-Contralesional M1 connectivity | 0.45 | 0.01* |
| Ipsilesional M1-Contralesional PMd connectivity | 0.13 | 0.50 |
For normally distributed variables, r is the Pearson correlation coefficient. For variables that are not normally distributed, r is Spearman's rho. For dichotomous variables, X2 is presented. The change in motor behavior was assessed as the principal component of change in FM and ARAT scores from baseline to one-month post-therapy.
Figure 1.
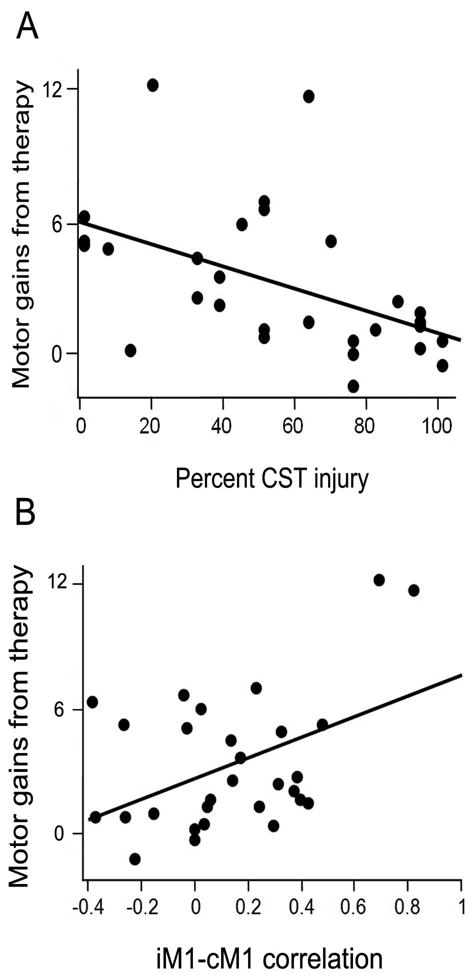
Across all subjects, (A) Smaller percent injury to M1 corticospinal tract (r=-0.49, p=0.007) and (B) greater ipsilesional M1-contralesional M1 functional connectivity (r=0.45, p=0.01) each significantly predicted larger treatment-induced behavioral gains. Behavioral gains were assessed as the principal component of the change in FM and ARAT scores from baseline to one-month post-therapy. To aid interpretation, the change in FM score is included on the Y-axis.
For one of these three measures (brain injury: percent CST injury), a threshold was identified for achieving the minimal clinically important difference (MCID) for treatment-induced motor gains in the setting of chronic stroke (4.25 points on the FM scale64): 63% injury to the CST. None of the 12 patients with ≥63% CST injury achieved the MCID. Note that most (65%) subjects with <63% CST injury did achieve the MCID, and that no MCID threshold was found for cortical function or cortical connectivity.
Multivariate modeling
To generate a predictive model, the three variables that best correlated with change in behavior based on bivariate screening were entered into multivariate analyses. The resultant model predicted 44% of variance in treatment-induced behavioral gains (p=0.002; Table 4); a measure of brain injury (percent CST injury) and a measure of cortical connectivity (iM1-cM1 functional connectivity) remained significant in this model.
Table 4.
Multivariate predictor model--all patients.
| Variable | Estimate | Standard Error | 95% CI | P |
|---|---|---|---|---|
| Intercept | 0.269 | 0.890 | -- | 0.765 |
| Ipsilesional M1 activation - contrast estimate | 0.276 | 0.161 | -0.05 to 0.61 | 0.10 |
| iM1-cM1 connectivity | 2.57 | 1.005 | 0.49 to 4.64 | 0.017* |
| Percent injury to CST | -0.020 | 0.010 | -0.40 to -0.0001 | 0.049* |
Lasso regression method
To independently verify the results from bivariate screening, Lasso regression was applied to the same 29 patients' data. This analysis identified the same two categories--brain injury and cortical connectivity--as having variables significantly predictive of treatment-induced behavioral gains. In the brain injury category, the selected variables were percent injury to the CST and cortical injury (yes/no). In the cortical connectivity category, the selected variable was the iM1-cM1 functional connectivity correlation coefficient. Estimated Lasso coefficients appear in Figure 2.
Figure 2.
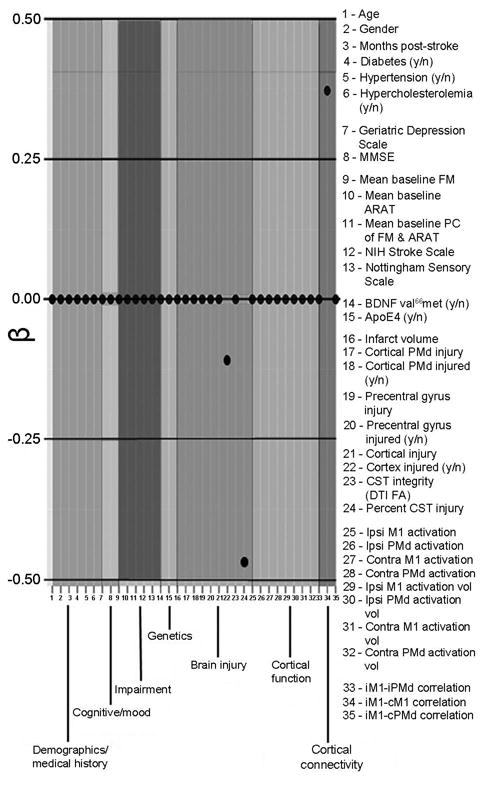
Regression coefficients determined using the group Lasso regression method. Variables identified as important for treatment-induced behavioral gains came from the cortical connectivity, cortical function, and brain injury categories.
Prediction of treatment-induced behavioral gains -- stroke subtype
In order to understand how differences in stroke pathophysiology influence prediction of treatment-induced behavioral gains, the above analyses were repeated examining only the subgroup of patients with a lacunar infarct (n=8). Baseline measures in this subgroup were overall similar to those found in the subgroup of 21 patients with a non-lacunar infarct (Table 2), except that patients with lacunar infarct had higher prevalence of hypercholesterolemia (p=0.03), less severe sensory deficits (p=0.001), and less severe injury by several measures such as percent CST injury by lesion overlap (p=0.04). Patients with a lacunar infarct had a greater treatment response compared to patients with a non-lacunar infarct (5.8±3.2 vs. 2.8±3.4 for FM, p=0.02; 8.8±7.0 vs. 2.3±5.1 for ARAT, p=0.02).
Bivariate screening in patients with a lacunar infarct found significant predictors of treatment-induced behavioral gains in only two categories: cortical function (ipsilesional M1 activation contrast estimate (r=0.79, p=0.02, Figure 3A) and ipsilesional M1 activation volume (r=0.76, p=0.03)) and cortical connectivity (iM1-iPMd correlation coefficient (r=0.81, p=0.02, Figure 3B)). Because of significant collinearity between the ipsilesional M1 contrast estimate and the iM1-iPMd correlation coefficient, a multivariate model was not pursued for the lacunar subgroup. These findings contrast with results of bivariate screening in the subgroup of 21 patients with a non-lacunar infarct, among whom the significant predictors of motor gains were the same as in the full cohort of 29 subjects: percent CST injury (r=-0.51, p=0.02) and iM1-cM1 functional connectivity (r=0.56, p=0.009).
Figure 3.
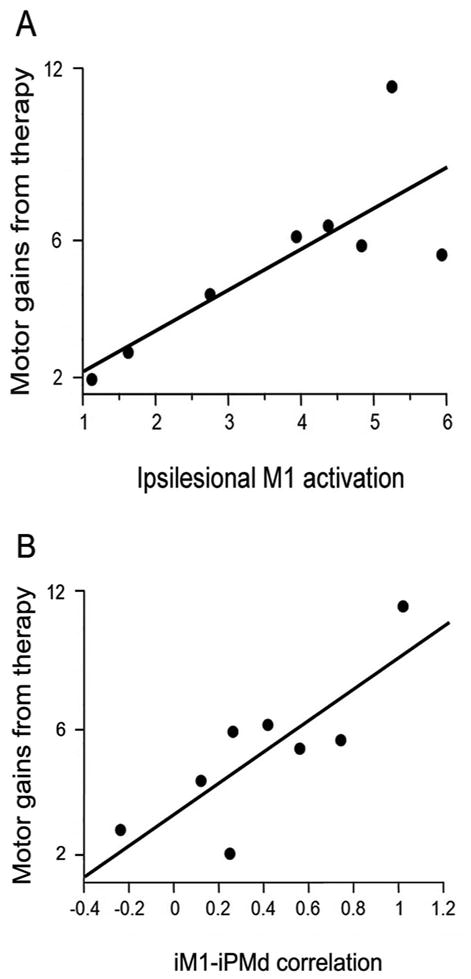
In the subgroup of patients with a lacunar infarct, greater (A) ipsilesional M1 activation (r=0.79, p=0.02) and (B) ipsilesional M1-ipsilesional PMd functional connectivity (r=0.81, p=0.02) each significantly predicted larger treatment-induced behavioral gains. Behavioral gains were assessed as the principal component of the change in FM and ARAT scores from baseline to one-month post-therapy. To aid interpretation, the change in FM score is included on the Y-axis.
Discussion
Stroke is a very heterogeneous disease, with patients showing wide differences in measures of injury, neural function, and response to therapy. These sources of variability complicate prescription of restorative therapies, as the best predictor(s) of response to a post-stroke restorative therapy remains uncertain. Concordant with preclinical studies, the results of the current study suggest that neural injury and neural function remain important in human subjects: multivariate modeling found that a measure of brain injury (smaller extent of CST injury) combined with a measure of cortical connectivity (greater iM1-cM1 functional connectivity) best predicted greater treatment-induced behavioral gains, a result independently confirmed by the group Lasso regression model. These variables outperformed traditional predictors of stroke outcome such as age or infarct volume. Different results emerged when examining only patients with a lacunar infarct. Overall, these findings emphasize the importance of a multivariate approach to patient selection and stratification for restorative therapies after stroke.
Prediction of treatment-induced behavioral gains -- all patients
The current study found that a combination of brain injury and cortical connectivity measures was best for predicting treatment gains. These findings broadly agree with a prior study that also found combining measures of neural injury (CST FA) and neural function (motor evoked potential) to be optimal for predicting upper extremity treatment gains in patients with chronic stroke16, and agree with preclinical studies that emphasize neural injury and neural function as key determinants of training-induced behavioral gains after stroke13, 15. This combined approach explained far more variance in outcome (44%, Table 4) than that provided by any single measure (up to 24%, Table 3). Clinical measures were also hypothesized to be significant predictors, but none reached significance (Tables 3, Figure 2). That neuroimaging measures were the best predictors takes on increased significance in light of the fact that triage and distribution of post-stroke rehabilitative care is still primarily based on behavioral assessments65.
Among the measures of neural injury, extent of CST injury measured with the lesion overlap method best predicted treatment-induced motor gains, consistent with prior studies using the current method18, 19 or using related methods49, 66 for estimating CST injury. The current study also confirmed a prior report18 that whenever a therapy targets a specific neural system (e.g., the motor system), an injury measure focused on that specific neural system (CST injury) is more precise than a global injury measure (e.g., infarct volume) for predicting treatment gains (Table 3). The presence of cortical injury was also an important predictor of treatment gains (Table 3) and furthermore was identified as significant by the Lasso regression method. This is consistent with previous studies that found significant differences in treatment response67 and neurophysiological integrity19 according to whether or not the stroke involved the cerebral cortex. Together, the results highlight the importance of measuring both white and gray matter injury, and of considering system-specific injury measures.
A novel finding was an injury threshold for achieving the minimal clinically important difference (MCID) in arm gains from robotic therapy. In 0 of the 12 patients with >63% injury did treatment gains reach the MCID (>4.25 points on the arm motor FM scale)64. This suggests that a certain amount of the CST must be intact for a patient to derive clinically significant benefit from upper extremity therapy, similar to what has been proposed for walking after CST injury68 or for control of hand movements after cortical stroke69. This threshold echoes similar findings by Stinear et al70, who found that meaningful gains from a motor intervention were not possible with CST FA asymmetry >0.25. Identification of such threshold values may be useful for stratifying individuals to an intervention in a clinical trial setting71.
Among the measures of neural function, iM1-cM1 functional connectivity best predicted therapy-induced gains, a result also confirmed by the Lasso regression method. The current finding that greater baseline iM1-cM1 functional connectivity predicted larger treatment gains agrees with one26 but not a second25 prior study, perhaps due to differences in the population studied or the connectivity technique used. Overall this finding is concordant with cross-sectional studies that have found greater inter-hemispheric connectivity to be correlated with smaller motor deficits after stroke in humans72, 73 and an animal stroke model74. The exact manner by which iM1-cM1 functional connectivity is related to treatment gains in the current cohort is uncertain and could reflect changes in motor cortex activity53, 75, 76, contralesional hemisphere excitability77, ipsilesional hemisphere excitability78-80, or inter-hemispheric inhibition81-83. The results underscore the potential value of connectivity measures for understanding behavioral state after stroke84, and support prior reports85-87 that connectivity may have advantages over regional assessments of brain function for some questions.
Prediction of treatment-induced behavioral gains -- stroke subtype
Results supported the secondary hypothesis that the best measures for predicting treatment-induced behavioral gains vary according to stroke subtype (lacunar vs. non-lacunar). Patients with a lacunar stroke tend to differ in multiple ways from patients with a non-lacunar stroke, including risk factor profile39, 88, lesion volume54, and clinical prognosis89, 90 39-45. The current results extend this list, suggesting that patients with lacunar stroke might also differ in terms of measures predicting response to a restorative therapy. The strongest predictors of treatment-induced behavioral gains among patients in the lacunar subgroup were measures of cortical function and intra-hemispheric cortical connectivity, specifically greater M1 activation and greater iM1-iPMd functional connectivity. This contrasts with findings among patients in the non-lacunar subgroup, where measures of brain injury and inter-hemispheric cortical connectivity best predicted treatment gains. The importance of cortical function to treatment gains after lacunar infarct is consistent with prior results91, 92. The basis for the observed difference between stroke subtypes is uncertain but may be related to cortical excitability, as affected arm movements increase motor cortex excitability after subcortical but not cortical stroke 93, or to lesion-specific differences in patterns of post-stroke brain plasticity91, 93.
Study considerations and limitations
Strengths of the current study include examination of multiple classes of candidate predictor variables in parallel, attention to stroke subtype, and confirmation of results using independent statistical methods. The heterogeneity of the current cohort is both a strength and a limitation. There is a balance between studying a narrowly defined population of stroke survivors, where statistical power is high due to low variance but results do not generalize, versus a broadly defined population, among whom heterogeneity limits statistical power but results are more likely to generalize. Enrollment in the current study (Table 1) prioritized time post-stroke (had to be early after the time when spontaneous arm motor recovery reaches a plateau after stroke46, 47) and severity of stroke (a wide range of motor deficits was allowed albeit minor nor devastating). Studies that enroll in relation to different priorities or that focus on different therapies might reach different findings regarding predictors of treatment gains.
Weaknesses of the study include the possibility that results of genetic analyses may suffer from Type II error. Although studies with comparable sample sizes have found significant predictive value for these genotypes with respect to motor learning94-97, the current study may have been insufficiently powered to detect an association between genotype and treatment-induced behavioral gains in the setting of stroke. The multivariate predictive model explained only 44% of the variance in treatment-induced behavioral gains; the predictive value of this model in other study populations would likely vary depending on the degree of homogeneity of such cohorts. What might account for the remaining variance? A number of candidate measures might be considered, across a range of biopsychosocial factors important to stroke outcomes, including socioeconomic factors98, stress level99, other genes100, caregiver status101, neurophysiological status37, and other cognitive/psychological tests102.
The current findings indicate that across all patients a multivariate approach combining measures of neural injury and neural function best predicts behavioral gains from a standardized course of therapy in patients who have recently reached a plateau in spontaneous recovery. The approach to predicting treatment-induced behavioral gains varies in relation to stroke subtype. The findings also emphasize that neuroimaging methods may have a role in clinical decision-making for rehabilitation therapy after stroke is currently driven by clinical assessments65.
Acknowledgments
This work was supported by the National Institutes of Health [R01 NS059909, NIH/NCRR UL1 TR000153, 1 K24 HD074722 01].
Footnotes
Potential conflicts of interest: Dr. Cramer has served as a consultant for GlaxoSmithKline, MicroTransponder, and Dart Neuroscience, and is a cofounder of personalRN.
References
- 1.Bath PM, Lees KR, Schellinger PD, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012 Apr;43(4):1171–8. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M, Albers GW. Advanced imaging to extend the therapeutic time window of acute ischemic stroke. Ann Neurol. 2013 Jan;73(1):4–9. doi: 10.1002/ana.23744. [DOI] [PubMed] [Google Scholar]
- 3.Farr TD, Wegener S. Use of magnetic resonance imaging to predict outcome after stroke: a review of experimental and clinical evidence. J Cerebr Blood F Met. 2010 Apr;30(4):703–17. doi: 10.1038/jcbfm.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer SC. Issues in clinical trial methodology for brain repair after stroke. In: Cramer SC, Nudo RJ, editors. Brain Repair After Stroke. Cambridge, UK: Cambridge University Press; 2010. pp. 173–82. [Google Scholar]
- 5.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain : a journal of neurology. 2011 Jun;134(Pt 6):1591–609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiology of disease. 2010 Feb;37(2):259–66. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupinski J, Slevin M. Emerging molecular targets for brain repair after stroke. Stroke Res Treat. 2013;2013:473416. doi: 10.1155/2013/473416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet neurology. 2012 Apr;11(4):369–80. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friel K, Nudo R. Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosensory & motor research. 1998;15(3):173–89. doi: 10.1080/08990229870745. [DOI] [PubMed] [Google Scholar]
- 10.Irle E. An analysis of the correlation of lesion size, localization and behavioral effects in 283 published studies of cortical and subcortical lesions in old-world monkeys. Brain research Brain research reviews. 1990 Sep-Dec;15(3):181–213. doi: 10.1016/0165-0173(90)90001-5. [DOI] [PubMed] [Google Scholar]
- 11.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. The European journal of neuroscience. 2005 Feb;21(4):989–99. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 12.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008 Feb;51(1):S225–39. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 13.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Experimental neurology. 2008 Jul;212(1):14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008 May-Jun;22(3):250–61. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr AL, Cheng SY, Jones TA. Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord. 2011 Sep-Oct;44(5):538–48. doi: 10.1016/j.jcomdis.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan;130(Pt 1):170–80. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 17.Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012 May;33(5):1040–51. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley JD, Le V, Der-Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011 Feb;42(2):421–6. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011 Aug 31; doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain : a journal of neurology. 2012 Aug;135(Pt 8):2527–35. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006 Jun;37(6):1552–5. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 22.Milot MH, Spencer SJ, Chan V, et al. Corticospinal Excitability as a Predictor of Functional Gains at the Affected Upper Limb Following Robotic Training in Chronic Stroke Survivors. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314527351. in press; online 3/20/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer SC, Parrish TB, Levy RM, et al. Predicting functional gains in a stroke trial. Stroke. 2007 Jul;38(7):2108–14. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- 24.Laible M, Grieshammer S, Seidel G, Rijntjes M, Weiller C, Hamzei F. Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabil Neural Repair. 2012 Sep;26(7):881–8. doi: 10.1177/1545968312437939. [DOI] [PubMed] [Google Scholar]
- 25.Sergi F, Krebs HI, Groissier B, et al. Predicting efficacy of robot-aided rehabilitation in chronic stroke patients using an MRI-compatible robotic device. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference; 2011. pp. 7470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varkuti B, Guan C, Pan Y, et al. Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehab Neural Repair. 2013 Jan;27(1):53–62. doi: 10.1177/1545968312445910. [DOI] [PubMed] [Google Scholar]
- 27.Graham JE, Ripsin CM, Deutsch A, et al. Relationship between diabetes codes that affect Medicare reimbursement (tier comorbidities) and outcomes in stroke rehabilitation. Archives of physical medicine and rehabilitation. 2009 Jul;90(7):1110–6. doi: 10.1016/j.apmr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dam M, Tonin P, Casson S, et al. The effects of long-term rehabilitation therapy on poststroke hemiplegic patients. Stroke. 1993 Aug;24(8):1186–91. doi: 10.1161/01.str.24.8.1186. [DOI] [PubMed] [Google Scholar]
- 29.Kononen M, Tarkka IM, Niskanen E, et al. Functional MRI and motor behavioral changes obtained with constraint-induced movement therapy in chronic stroke. Eur J Neurol. 2012 Apr;19(4):578–86. doi: 10.1111/j.1468-1331.2011.03572.x. [DOI] [PubMed] [Google Scholar]
- 30.McCarron MO, Muir KW, Nicoll JA, et al. Prospective study of apolipoprotein E genotype and functional outcome following ischemic stroke. Archives of neurology. 2000 Oct;57(10):1480–4. doi: 10.1001/archneur.57.10.1480. [DOI] [PubMed] [Google Scholar]
- 31.Cramer SC, Procaccio V. Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International Studies. Eur J Neurol. 2012 May;19(5):718–24. doi: 10.1111/j.1468-1331.2011.03615.x. [DOI] [PubMed] [Google Scholar]
- 32.Pearson-Fuhrhop KM, Burke E, Cramer SC. The influence of genetic factors on brain plasticity and recovery after neural injury. Current opinion in neurology. 2012 Dec;25(6):682–8. doi: 10.1097/WCO.0b013e32835a360a. [DOI] [PubMed] [Google Scholar]
- 33.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014 May 1; [Google Scholar]
- 34.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Mar;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 35.Seitz RJ, Donnan GA. Role of neuroimaging in promoting long-term recovery from ischemic stroke. J Magn Reson Imaging. 2010 Oct;32(4):756–72. doi: 10.1002/jmri.22315. [DOI] [PubMed] [Google Scholar]
- 36.Burke E, Cramer SC. Biomarkers and predictors of restorative therapy effects after stroke. Curr Neurol Neurosci Rep. 2013 Feb;13(2):329. doi: 10.1007/s11910-012-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinear CM, Ward NS. How useful is imaging in predicting outcomes in stroke rehabilitation? International journal of stroke. 2013 Jan;8(1):33–7. doi: 10.1111/j.1747-4949.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 38.Burke E, Dodakian L, See J, et al. A multimodal approach to understanding motor impairment and disability after stroke. J Neurol. 2014 Jun;261(6):1178–86. doi: 10.1007/s00415-014-7341-8. [DOI] [PubMed] [Google Scholar]
- 39.Pullicino P, Nelson RF, Kendall BE, Marshall J. Small deep infarcts diagnosed on computed tomography. Neurology. 1980 Oct;30(10):1090–6. doi: 10.1212/wnl.30.10.1090. [DOI] [PubMed] [Google Scholar]
- 40.Sprigg N, Gray LJ, Bath PM, et al. Early recovery and functional outcome are related with causal stroke subtype: data from the tinzaparin in acute ischemic stroke trial. J Stroke Cerebrovasc. 2007 Jul-Aug;16(4):180–4. doi: 10.1016/j.jstrokecerebrovasdis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Lavados PM, Sacks C, Prina L, et al. Incidence, case-fatality rate, and prognosis of ischaemic stroke subtypes in a predominantly Hispanic-Mestizo population in Iquique, Chile (PISCIS project): a community-based incidence study. Lancet Neurol. 2007 Feb;6(2):140–8. doi: 10.1016/S1474-4422(06)70684-6. [DOI] [PubMed] [Google Scholar]
- 42.Mustanoja S, Meretoja A, Putaala J, et al. Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke. 2011 Jan;42(1):102–6. doi: 10.1161/STROKEAHA.110.597534. [DOI] [PubMed] [Google Scholar]
- 43.Uchino K, Billheimer D, Cramer S. Entry criteria and baseline characteristics predict outcome in acute stroke trials. Stroke. 2001;32(4):909–16. doi: 10.1161/01.str.32.4.909. [DOI] [PubMed] [Google Scholar]
- 44.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001 Dec 1;32(12):2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 45.Landi G, D'Angelo A, Boccardi E, et al. Venous thromboembolism in acute stroke: prognostic importance of hypercoagulability. Archives of neurology. 1992;49(3):279–83. doi: 10.1001/archneur.1992.00530270093024. [DOI] [PubMed] [Google Scholar]
- 46.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006 Sep;37(9):2348–53. doi: 10.1161/01.STR.0000238594.91938.1e. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama H, Jorgensen H, Raaschou H, Olsen T. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehab. 1994;75(4):394–8. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008 Feb;131(Pt 2):425–37. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 49.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010 May;41(5):910–5. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park CH, Kou N, Boudrias MH, Playford ED, Ward NS. Assessing a standardised approach to measuring corticospinal integrity after stroke with DTI. Neuroimage Clin. 2013;2:521–33. doi: 10.1016/j.nicl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 52.Nelles G, Cramer S, Schaechter J, Kaplan J, Finklestein S. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–7. doi: 10.1161/01.str.29.6.1182. [DOI] [PubMed] [Google Scholar]
- 53.Ward N, Brown M, Thompson A, Frackowiak R. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003 Jun;126(Pt 6):1430–48. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982 Aug;32(8):871–6. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 55.Kotila M, Waltimo O, Niemi M, Laaksonen R, Lempinen M. The profile of recovery from stroke and factors influencing outcome. Stroke. 1984;15(6):1039–44. doi: 10.1161/01.str.15.6.1039. [DOI] [PubMed] [Google Scholar]
- 56.Macciocchi S, Diamond P, Alves W, Mertz T. Ischemic stroke: relation of age, lesion location, and initial neurologic deficit to functional outcome. Arch Phys Med Rehab. 1998 Oct;79(10):1255–7. doi: 10.1016/s0003-9993(98)90271-4. [DOI] [PubMed] [Google Scholar]
- 57.Niskakangas T, Ohman J, Niemela M, Ilveskoski E, Kunnas TA, Karhunen PJ. Association of apolipoprotein E polymorphism with outcome after aneurysmal subarachnoid hemorrhage: a preliminary study. Stroke. 2001 May;32(5):1181–4. doi: 10.1161/01.str.32.5.1181. [DOI] [PubMed] [Google Scholar]
- 58.Siironen J, Juvela S, Kanarek K, Vilkki J, Hernesniemi J, Lappalainen J. The Met allele of the BDNF Val66Met polymorphism predicts poor outcome among survivors of aneurysmal subarachnoid hemorrhage. Stroke. 2007 Oct;38(10):2858–60. doi: 10.1161/STROKEAHA.107.485441. [DOI] [PubMed] [Google Scholar]
- 59.van de Weg FB, Kuik DJ, Lankhorst GJ. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil. 1999 Jun;13(3):268–72. doi: 10.1191/026921599672495022. [DOI] [PubMed] [Google Scholar]
- 60.Hastie TT, Robert, Friedman J, Jerome H. The elements of statistical learning. New York: Springer; 2001. [Google Scholar]
- 61.Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society, Series B. 1996;58:267–88. [Google Scholar]
- 62.Simon N, Tibshirani R. Standardization and the group lasso penalty. Stat Sinica. 2012;22:983–1001. doi: 10.5705/ss.2011.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabilitation and neural repair. 2012 Jan;26(1):7–19. doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Physical therapy. 2012 Jun;92(6):791–8. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 65.Hakkennes S, Hill KD, Brock K, Bernhardt J, Churilov L. Selection for inpatient rehabilitation after severe stroke: what factors influence rehabilitation assessor decision-making? J Rehabil Med. 2013 Jan;45(1):24–31. doi: 10.2340/16501977-1065. [DOI] [PubMed] [Google Scholar]
- 66.Binkofski F, Seitz R, Arnold S, Classen J, Benecke R, Freund H. Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Annals of neurology. 1996;39(4):460–70. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- 67.Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Annals of neurology. 2009 Sep;66(3):298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- 68.Young W. Spinal cord regeneration. Science. 1996 Jul 26;273(5274):451. doi: 10.1126/science.273.5274.451. [DOI] [PubMed] [Google Scholar]
- 69.Crafton K, Mark A, Cramer S. Improved understanding of cortical injury by incorporating measures of functional anatomy. Brain. 2003 Jul;126(Pt 7):1650–9. doi: 10.1093/brain/awg159. [DOI] [PubMed] [Google Scholar]
- 70.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan;130(Pt 1):170–80. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 71.Trochim WM, Cappelleri JC. Cutoff assignment strategies for enhancing randomized clinical trials. Control Clin Trials. 1992 Jun;13(3):190–212. doi: 10.1016/0197-2456(92)90003-i. [DOI] [PubMed] [Google Scholar]
- 72.Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of neurology. 2010 Mar;67(3):365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urbin MA, Hong X, Lang CE, Carter AR. Resting-State Functional Connectivity and Its Association With Multiple Domains of Upper-Extremity Function in Chronic Stroke. Neurorehabilitation and neural repair. 2014 doi: 10.1177/1545968314522349. in press, online Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Meer MP, van der Marel K, Wang K, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010 Mar 17;30(11):3964–72. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006 Mar;129(Pt 3):791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 76.Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006 May 31;26(22):6096–102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takechi U, Matsunaga K, Nakanishi R, et al. Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clin Neurophysiol. 2014 Feb 28; doi: 10.1016/j.clinph.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 78.Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003 Nov;34(11):2653–8. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- 79.Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cerebral cortex. 2008 Aug;18(8):1909–22. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hummel FC, Steven B, Hoppe J, et al. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009 May 19;72(20):1766–72. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murase N, Duque J, Mazzocchio R, Cohen L. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of neurology. 2004 Mar;55(3):400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 82.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005 Dec;36(12):2681–6. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 83.Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. NeuroImage. 2010 Mar;50(1):233–42. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rowe JB. Connectivity Analysis is Essential to Understand Neurological Disorders. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Annals of neurology. 2009 Nov;66(5):604–16. doi: 10.1002/ana.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011 Mar 16; doi: 10.1093/brain/awr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? NeuroImage. 2012 Oct 1;62(4):2271–80. doi: 10.1016/j.neuroimage.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke. 2005 Apr;36(4):891–901. doi: 10.1161/01.STR.0000157949.34986.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005 Nov;128(Pt 11):2507–17. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landi G, Cella E, Boccardi E, Musicco M. Lacunar versus non-lacunar infarcts: pathogenetic and prognostic differences. J Neurol Neurosurg Psychiatry. 1992 Jun;55(6):441–5. doi: 10.1136/jnnp.55.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luft A, Waller S, Forrester L, et al. Lesion location alters brain activation in chronically impaired stroke survivors. NeuroImage. 2004 Mar;21(3):924–35. doi: 10.1016/j.neuroimage.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 92.Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, et al. Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007 Dec;17(12):2980–7. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- 93.Renner CI, Schubert M, Jahn M, Hummelsheim H. Intracortical excitability after repetitive hand movements is differentially affected in cortical versus subcortical strokes. J Clin Neurophysiol. 2009 Oct;26(5):348–57. doi: 10.1097/WNP.0b013e3181baaa86. [DOI] [PubMed] [Google Scholar]
- 94.Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010 Apr 29;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010 May;20(5):1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore AB, Bondi MW, Salmon DP, Murphy C. Eyeblink classical conditioning to auditory and olfactory stimuli: performance among older adults with and without the apolipoprotein E epsilon 4 allele. Neuropsychology. 2005 Jul;19(4):437–45. doi: 10.1037/0894-4105.19.4.437. [DOI] [PubMed] [Google Scholar]
- 97.Bookheimer S, Strojwas M, Cohen M, et al. Patterns of brain activation in people at risk for Alzheimer's disease. The New England journal of medicine. 2000;343(7):450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ostwald SK, Swank PR, Khan MM. Predictors of functional independence and stress level of stroke survivors at discharge from inpatient rehabilitation. J Cardiovasc Nurs. 2008 Jul-Aug;23(4):371–7. doi: 10.1097/01.JCN.0000317435.29339.5d. [DOI] [PubMed] [Google Scholar]
- 99.Edmondson D, Richardson S, Fausett JK, Falzon L, Howard VJ, Kronish IM. Prevalence of PTSD in Survivors of Stroke and Transient Ischemic Attack: A Meta-Analytic Review. PLoS One. 2013;8(6):e66435. doi: 10.1371/journal.pone.0066435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pearson-Fuhrhop KM, Minton B, Acevedo D, Shahbaba B, Cramer SC. Genetic variation in the human brain dopamine system influences motor learning and its modulation by L-dopa. PloS one. 2013;8(4):e61197. doi: 10.1371/journal.pone.0061197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han B, Haley WE. Family caregiving for patients with stroke. Review and analysis. Stroke. 1999;30(7):1478–85. doi: 10.1161/01.str.30.7.1478. [DOI] [PubMed] [Google Scholar]
- 102.Cramer SC, Fitzpatrick C, Warren M, et al. The beta-hCG+erythropoietin in acute stroke (BETAS) study: a 3-center, single-dose, open-label, noncontrolled, phase IIa safety trial. Stroke. 2010 May;41(5):927–31. doi: 10.1161/STROKEAHA.109.574343. [DOI] [PMC free article] [PubMed] [Google Scholar]


