Abstract
Background
Prostatic inflammation is reportedly associated with the development of prostatic hyperplasia. We investigated the effects of prostatic inflammation on expression levels of androgen-responsive genes and growth factors in the rat prostate.
Methods
Prostatic inflammation was induced by Escherichia coli (strain 1677) injection (0.2 mL of 1 × 108 CFU/mL) into the prostatic urethra of male Sprague-Dawley rats, and ventral lobes of the prostate were harvested on day 84. Rats were given 10 mg/kg celecoxib during the last month in the COX-2 inhibitor treated group. Histopathology and multiplex ELISA for inflammation-related proteins were performed. Glandular epithelial cells and stromal regions were separately isolated using laser-capture microdissection (LCM). Real-time RT-PCR was performed to examine mRNA levels of androgen-responsive genes in the epithelium and TGF-β1 cascade genes in the stroma.
Results
Hematoxylin and eosin staining showed that mild inflammation was distributed diffusely throughout the prostate. Polymorphonuclear cells infiltrated the slightly edematous stroma, but no morphological changes were observed in the epithelium. Immunohistochemically, expression of androgen receptor and TGF-β1 in addition to IL-6 and COX-2 were enhanced in the E. coli inoculated rats. All of these factors were suppressed in the celecoxib-treated rats. Upregulation of IL-1α, IL-1β, IL-6, and RANTES in the E. coli-inoculated rats was normalized by celecoxib treatment. Significant upregulation of androgen receptor and androgen-responsive genes such as Eaf2, ELL2, FKBP5, calreticulin and ornithine decarboxylase was observed in the LCM-dissected epithelium. Also TGF-β1 and its downstream cascade genes such as Hic-5, collagen 1, and fibronectin were upregulated significantly in the LCM-dissected stroma. The COX-2 inhibitor treatment suppressed upregulation of these genes.
Conclusions
Prostatic inflammation changed the expression of androgen-responsive genes in the epithelium and TGF-β1 cascade genes in the stroma. Activation of TGF-β1 cascade genes in the inflamed stroma, as well as altered androgen-responsive gene expression in the epithelium, might be involved in the development of BPH.
Keywords: prostate, inflammation, androgen receptor, TGF-β1, laser capture microdissection
Introduction
Benign prostatic hyperplasia (BPH) is associated with proliferation of the prostatic duct and stromal component and commonly affects older males by inducing lower urinary tract symptoms. Many investigations have been conducted; however, little is known regarding the mechanisms of BPH development. Asymptomatic chronic inflammation is often observed in BPH specimens,1–4 and recent studies have proposed a possible contribution of chronic prostatic inflammation to BPH pathogenesis.5 A correlation between histological severity of chronic prostatic inflammation and subjective symptoms was shown in the REDUCE (REduction by DUtasteride of prostate Cancer Events) trial.2 Patients with acute inflammation on baseline prostate biopsies had worse symptoms in the MTOPS (Medical Therapy Of Prostatic Symptoms) trial.6 A longitudinal observational study also reported that men with a history of prostatitis have subsequently higher rates of prostate enlargement, treatments for BPH and urinary retention.7 Increased cytokine expression in BPH tissues including IL-2, IFNγ, TNF-α, IL-4, IL-6, IL-8, IL-13, IL-15 and IL-17 has also been reported.8–15 Thus, chronic prostatic inflammation may offer new insights into the pathogenesis of BPH and strategies for its treatment and prevention.
Bacterial colonization inside the prostate, possibly through reflux of urine into the prostatic ducts, could induce chronic inflammation and oxidative stress injury. The inflammatory process causes repeated and persistent cell and genomic damage, which leads to increased cell proliferation. Chronic E. coli infections could lead to proliferation of epithelium and stroma in concert with dihydrotestosterone via the release of endotoxins.16 The proliferative signal cascade is reportedly enhanced in BPH tissue such as the androgen receptor and TGF-β1 downstream genes.8, 17
We recently reported that non-bacterial prostatic inflammation increases expression of androgen-responsive and TGF-β1 cascade genes up to 4 weeks in a chemically induced rat model of prostatic inflammation.18 In this study, we evaluated whether chronic prostatic inflammation for 12 weeks induces the elevated expression of androgen and TGF-β1 cascade genes and assessed the effects of a COX-2 inhibitor on the expression of these genes using a rat model of bacterial prostatitis.
Materials and methods
Bacterial prostatitis model
All animal experiments were performed in accordance with institutional guidelines and with approval from the University of Pittsburgh Institutional Animal Care and Use Committee.
E. coli strain 1677, which was isolated from a patient with a urinary tract infection, was grown overnight in Luria broth base in a shaker at 37°C. The cells were centrifuged at 10,000 rpm for 5 min, washed three times and re-suspended in saline to give 108 colony-forming units/mL. Male Sprague-Dawley rats weighing 250–320 g were anesthetized with isoflurane and the genital area was cleaned with 70% alcohol and subsequently catheterized with PE-10 tubing. An insulin syringe was attached to the needle, and 0.2 mL of bacterial suspension or vehicle (saline; control group) was instilled into the prostatic urethra. Anesthesia was maintained for 1 h to prevent urinary leakage from movement of the rat and to allow sufficient time for the bacteria to colonize in the prostate. The ventral lobes of the prostate were excised at 84 days after inoculation. In some animals, celecoxib (10 mg/kg/day) was given by oral gavage on days 57–83 after intraprostatic E-coli inoculation to examine the effects of COX-2 inhibition.
Histological analyses
A half of the prostate was embedded in OCT Tissue-Tek compound, frozen on dry ice, and kept at −80°C until use. Samples were serially sectioned at 8-μm thickness and stained with hematoxylin and eosin.
The prostate sections (8 μm) were mounted directly on coated slides and fixed with 4% paraformaldehyde for 10 min, rinsed with phosphate-buffered saline (PBS), transferred to 3% hydrogen peroxide for 10 min to block peroxidase activity, and rinsed with distilled water and PBS for immunohistochemical analyses. Next, the sections were blocked with 10% normal goat serum (Invitrogen, Grand Island, NY) for 30 min. Without rinsing, the tissues were reacted with antibody for androgen receptor (1:1000), TGF-β1 (1:250), IL-6 (1:2000), and COX-2 (1:250) (Abcam, Cambridge, MA) for 1 h at room temperature and washed with PBS. The tissues were then incubated for 30 min with HRP labeled polymer anti-rabbit antibody (Dako, Carpentaria, CA) at room temperature and rinsed with PBS. For streptavidin–horseradish peroxidase detection, the tissues were stained with ABC reagent (Dako) for 10 min at room temperature. Hematoxylin was used as the counterstain.
Measurement of inflammatory markers
The prostate tissues of rats injected with E. coli and saline (n = 6 per group) were homogenized in RIPA lysis buffer. The homogenate was centrifuged at 10,000 rpm for 10 min, and the supernatants were stored at −80°C until assay. IL-1α, IL-1β, IL-6, TNF-α, monocyte chemotactic protein-1 (MCP-1) and regulated upon activation normal T cell expressed and presumably secreted (RANTES) levels were determined on a Luminex 200 (Bio-Rad, Hercules, CA) using a MILLIPLEX MAP Rat Cytokine/Chemokine Panel (Millipore, Billerica, MA). Protein concentration was determined using a Bio-Rad® kit with bovine serum albumin as the standard. The expression levels of the cytokines/chemokines were standardized relative to tissue protein levels and expressed as pg/mg total protein.
Laser-capture microdissection (LCM)
A part of the prostate specimen was embedded in OCT Tissue Tek (Sakura Finetek USA, Torrance, CA) and stored at −80°C until the sections were cut to 8-μm thickness. The sections were mounted on PEN membrane slides (Leica Microsystems, Wetzlar, Germany). Tissue sections were fixed in 70% EtOH for 30 s and rinsed with double-distilled H2O. The sections were stained with Mayer’s hematoxylin (Sigma-Aldrich, St. Louis, MO) for 30 s, rinsed with double-distilled H2O and placed in 0.01% eosin (Acros Organics, Geel, Belgium) for 5 s. The prostate specimens were dehydrated for 30 s twice in 95% ethanol, 30 s in 100% ethanol, and 2 min in xylene. The tissue was air-dried, and LCM was performed using a Leica LMD6000 (Leica Microsystems, Wetzlar, Germany). The stromal area and adjacent epithelium were excised and individually captured in the caps of 0.5-ml Eppendorf tubes.
RNA isolation and real-Time PCR analysis
The individual captured tissue specimens were lysed and RNA isolation, reverse transcription, and real-time PCR were performed using a Cells Direct™ One-Step qRT-PCR kit (Invitrogen). Gene-specific primers and TaqMan probes crossing exon/exon junctions were designed using the Primer 3 Software (Primer 3, Totowa, NJ). Probes contained the FAM fluorophore and TAMRA quencher. The primer and probe combination was optimized within suitable ranges for efficiency, and the correlation coefficient was determined using standard curve dilutions and data output from an ABI Step-One Plus thermocycler (Applied Biosystems, Foster City, CA). cDNA was amplified under the following conditions: one cycle of 50°C for 15 min, 95°C for 2 min, followed by 40 cycles of 15 s at 95°C, 45 s at 60°C. The reactions were analyzed in triplicate and normalized relative to GAPDH. Real-time PCR data were analyzed by the DCp (crossing point) method as R = 2^ (Cp sample – Cp control) to generate the relative expression ratio (R) of each target gene relative to that of GAPDH.
We measured the androgen receptor and five androgen-responsive genes including Eaf2, ELL2, FKBP5, calreticulin and ornithine decarboxylase in epithelial cells (Table 1) as wells as the androgen receptor, TGF-β1, Hic-5, collagen 1 and fibronectin in stromal cells. To determine the specificity of the primer/probe, we performed electrophoresis to verify that the primers used amplified only cDNA and not genomic DNA; the optical band was amplified without nonspecific bands. All of our primer/probe combinations showed efficiencies of 95–105%.
Table 1.
Sequences of primers and probe
| 5′ primer | 3′ primer | Taqman probe | efficacy (%) | |
|---|---|---|---|---|
| androgen receptor | TTGGCGAGAGACAGCTTGTA | ATCTGGTCATCCACATGCAA | GGTCAAGTGGGCCAAGGCCT | 95.3 |
| Eaf 2 | CACACCGTGCGCTATGACT | CCTTTGAAAACTGTGACTGGTG | GGTTGGCAAAGGTGAACAGGTGACA | 98.0 |
| ELL 2 | CGCTGGAGACTTACCAGAGC | CATTGAAGGGATCATTTTTGG | TCAGTTCCAAGGACTCCAAGGGC | 98.5 |
| FKBP 5 | GCCGTTTGTCTTTAGCCTTG | GATCTCGCCTTTCTTCATGG | GGCCAGGTTATCAAAGCCTGGGA | 99.1 |
| calreticulin | ACGAGCCAAGATTGATGACC | TCCCACTCTCCATCCATCTC | GCCTGAGGACTGGGACAAGCCA | 96.3 |
| ornithine decarboxylase | CCTGTGCTGTGAGGAGACAG | CTCGATGTGCCTCTGAAACA | TTGACCTTGTGAGAGCTGGCCA | 99.8 |
| TGF-β1 | TGAGTGGCTGTCTTTTGACG | ACTGAAGCGAAAGCCCTGTA | TCCGGCAGTGGCTGAACCAA | 98.2 |
| Hic 5 | AACCTATTGCTGGGCAAGTG | CCTCTGCAAAGGAAGTGCTC | CCGCCCTGGGTAGAGCCTGG | 102.0 |
| collagen 1 | GCCAAGAAGACATCCCTGAA | GCCATTGTGGCAGATACAGA | GGGTCCCTAATGGTGAGACGTGGA | 104.9 |
| fibronectin 1 | CAGTACGGGCACCAAGAAGT | CCAGTCACTGTGTTGCTGGT | TTCACCACCAGCGCCAGCAC | 102.3 |
| GAPDH | AGACAGCCGCATCTTCTTGT | GATACGGCCAAATCCGTTC | GCAGTGCCAGCCTCGTCTCA | 104.0 |
Statistics
The paired Student’s t-test was used to compare two values. All tests were two-sided, and p values < 0.05 were considered to indicate significant differences. All statistical analyses were performed using the SPSS software (SPSS Inc., Chicago, IL).
Results
Histopathology
Hematoxylin and eosin staining demonstrated that the ventral lobes of the prostate in the control rats consisted of simple columnar epithelium, normal prostate architecture, normal epithelial histology, and had no inflammatory cell infiltrates (Fig. 1A). Inflammatory cells in the E. coli-inoculated rats infiltrated into the stromal area with slightly edematous changes throughout the prostate whereas the epithelium had a normal appearance (Fig. 1B). No evidence of the hyperplastic epithelium was detected.
Fig. 1. Histological findings of the prostate tissue sections from control rats (A, D, G, J, and M), rats with E. coli inoculation into the prostate (B, E, H, K, and N), and E. coli-inoculated rats with celecoxib treatment (C, F, I, L, and O).
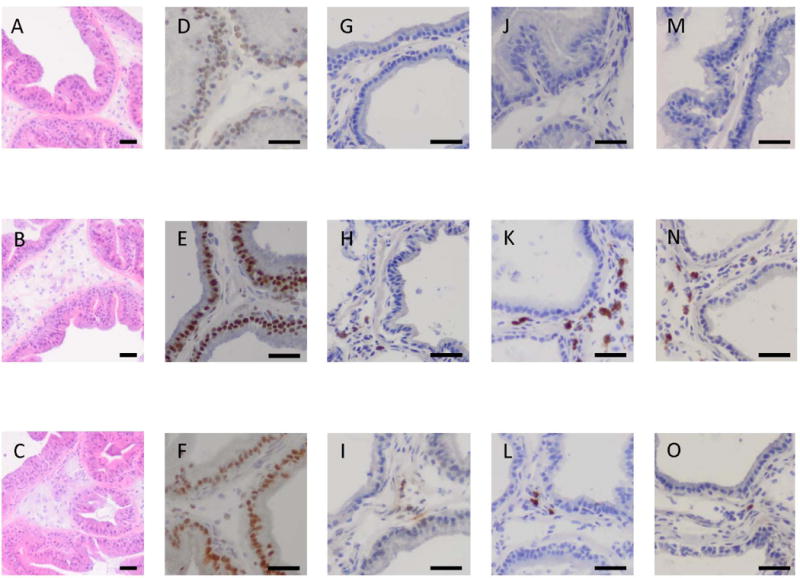
Hematoxylin and eosin staining (A–C) demonstrated that inoculation of E. coli into the prostate induced mild inflammation accompanied by inflammatory cell infiltration in the slightly expanded stromal area throughout the prostate (B) compared to those in the control (A). Immunohistochemical staining for androgen receptor (D, E and F), TGF-β1 (G, H and I), IL-6 (J, K and L), and COX-2 (M, N and O) demonstrated the increased expression of androgen receptor, TGF-β1, IL-6, and COX-2 expression was markedly enhanced in rats with E. coli-induced prostatic inflammation (E, H, K and N, respectively) compared to the control (D, G, J and M, respectively). These changes were normalized by celecoxib treatment (F, I, L and O, respectively). Images were taken at a magnification of ×100 (A–C) and ×200 (D–O). Scale bar; 100 μm.
Immunohistochemical staining for the androgen receptor, TGF-β1, IL-6, and COX-2 expression was performed on prostate tissue sections (Fig. 1). Positive staining for the androgen receptor was observed in the epithelial nuclei of the vehicle-injected control group (Fig. 1D). In the E. coli-inoculated prostate, epithelial cells were stained strongly and some stromal cells were also stained for the androgen receptor (Fig. 1E). Staining for TGF-β1 was positive in the stromal area in controls (Fig. 1G) and was strongly positive in the E.coli-inoculated prostate (Fig. 1H). Staining for IL-6 was negative in the control group (Fig. 1J), but positive around the inflammatory cells infiltrating in the stroma of E. coli-inoculated prostate (Fig. 1K). COX-2 staining showed negligible immunoreactivity in the control (Fig. 1M), but its expression level was increased in the stroma of E. coli-inoculated rats (Fig. 1N). The upregulation of androgen receptors, TGF-β1, IL-6, COX-2 proteins were suppressed in E. coli inoculated rats treated with a COX-2 inhibitor. (Figs. 1F, 1I, 1L, 1O, respectively).
Expression level of inflammation-related proteins
We measured the tissue expression levels of several inflammation-related cytokines/chemokines in the prostate including IL-1α, IL-1β, IL-6, TNF-α, MCP-1, and RANTES. Among them, IL-1α, IL-1β, IL-6, and RANTES expression was significantly increased in the E. coli-inoculated prostate compared to those in the control. Treatment with a COX-2 inhibitor prevented the upregulation of these cytokines/chemokines (Fig. 2).
Fig. 2. Inflammation-related cytokines/chemokines in the prostate.
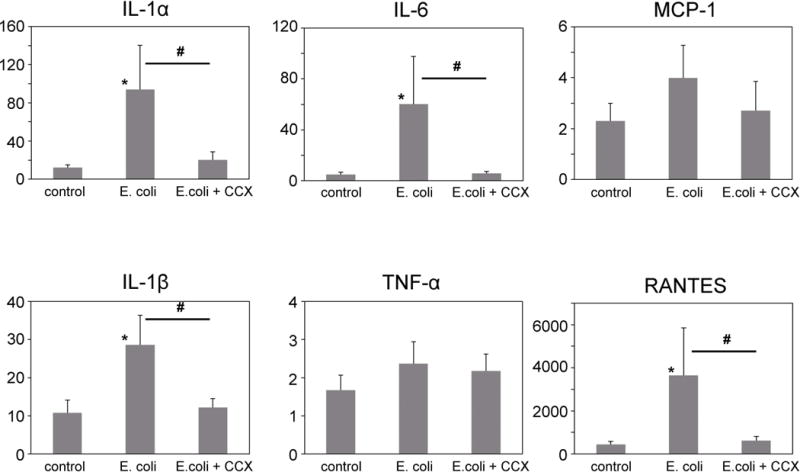
The expression levels of IL-1α, IL-1β, IL-6, TNF-α, MCP-1, and RANTES in the prostate were determined using a multiplex ELISA system. Among them, IL-1α, IL-1β, IL-6, and RANTES was significantly increased in the E. coli group compared to the control group, but were suppressed by celecoxib administration (E.coli+CCX). CCX, celecoxib. * p < 0.05, ** p < 0.01 compared to the control.
Androgen-responsive genes in the epithelium and TGF-β1 cascade genes in the stroma
In epithelial cells excised from E.coli-treated prostates by LCM, mRNA expression of the androgen receptor and five androgen-responsive genes, Eaf2, ELL2, FKBP5, calreticulin and ornithine decarboxylase increased 1.5–5 fold, compared to that in control epithelium (Fig. 3). In cells isolated from the stromal of E. coli-treated prostates, androgen receptor and TGF-β1 and its downstream genes, such as Hic-5, collagen 1, and fibronectin, were upregulated 3–15-fold (Fig. 4). The changes induced by E. coli induced inflammation in androgen receptor and TGF-ß1 signaling molecule mRNA expression in prostate epithelium and stroma were prevented upon treatment with a COX-2 inhibitor (Figs. 3 and 4).
Fig. 3. Relative expression of androgen-responsive genes in the LCM-dissected epithelium.
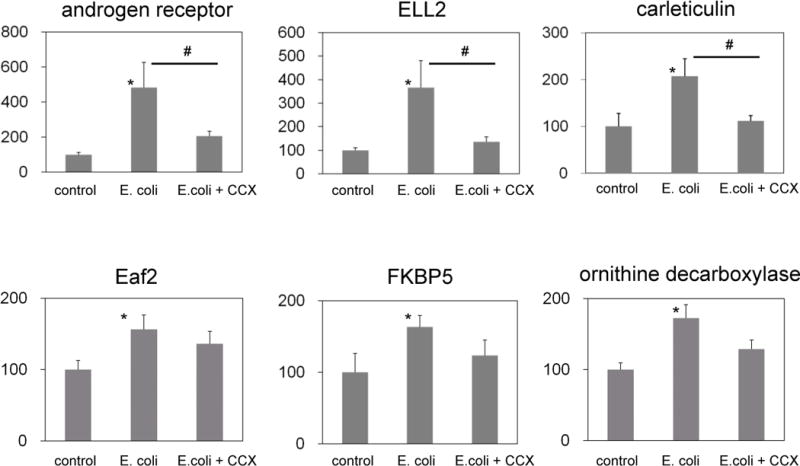
Androgen-responsive genes in the LCM-dissected epithelium were significantly upregulated in rats with E. coli-induced prostatitis compared to the control group. The COX-2 inhibitor treatment suppressed upregulation of these genes. CCX, celecoxib. * p < 0.05 compared to the control, # p < 0.05 between E.coli and E.coli+ CCX groups.
Fig. 4. Relative expression of TGF-β1 cascade genes in the LCM-dissected stroma.
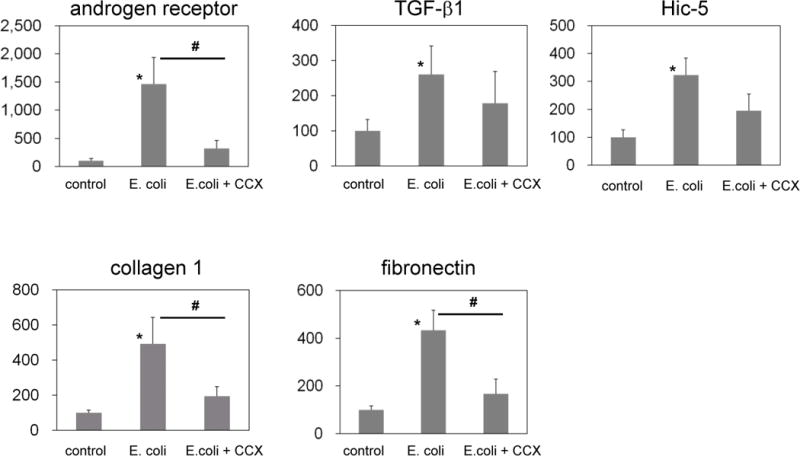
TGF-β1 cascade genes were significantly upregulated in the LCM-dissected stroma in rats with prostatitis compared to the control group. COX-2 inhibitor treatment suppressed these changes. CCX, celecoxib. * p < 0.05 compared to the control, # p < 0.05 between E. coli and E. coli+ CCX groups.
Discussion
Castrated males before puberty do not develop BPH, and prostate volume shrinks during androgen blockade therapy; thus androgens play an important role in the pathogenesis of BPH. However, serum androgen levels generally fall in elderly males19 and are not correlated with the prostate volume.20 Therefore, prostate reactivity to androgens may increase in patients with BPH. Previous studies have shown that androgen receptor levels in epithelial nuclei are elevated by 47% in BPH specimens relative to those in normal controls.21 Our previous study showed that not only the androgen receptor but also androgen-responsive genes are upregulated in hypertrophied epithelium relative to adjacent normal epithelium of BPH patients.17
The association between chronic prostate inflammation and the development of BPH has been proposed in previous clinical studies.2, 6, 7 Cytokines secreted from inflammatory cells stimulate androgen receptor activity in prostate cancer cells.22, 23 We have previously reported molecular changes similar to those identified in human BPH specimens in an abacterial rat model of prostatitis,18 which is characterized by infiltration of inflammatory cells up to 4 weeks after intraprostatic formalin injection. In this study, we further demonstrated that E. coli-induced chronic inflammation also induces the long-lasting (12 weeks) upregulation of epithelial androgen-responsive and stromal TGF-β1 cascade genes. Taken together, these results suggest that the rat with bacterially-induced chronic prostatic inflammation is a suitable animal model to study the androgen and TGF-β1-mediated mechanisms underlying the development of prostatic proliferation in BPH.
Increased expression of not only cytokines but also some growth factors is reportedly associated with the presence of inflammatory cells in human BPH tissues.24–26 Among them, TGF-β1 is critical for prostatic smooth muscle regulation, as it induces transdifferentiation of prostatic fibroblasts into myofibroblasts that secrete extracellular matrix components such as collagen and fibronectin.27 In addition, the transcriptional co-regulator and focal adhesion protein Hic-5 modulates the response of many downstream targets of TGF-β1 that have been implicated in tissue homeostasis and crosstalk between TGF-β1 and androgen signaling.28
COX-2, a proinflammatory enzyme involved in the conversion of arachidonic acid to prostaglandin, also mediates prostatic epithelial cellular proliferation. Macrophage and epithelial levels of COX-2 are upregulated in BPH tissues.13 COX-2 metabolites also upregulate expression of the anti-apoptotic gene BCL-2.13, 29 Some studies have reported high BCL-2 expression in BPH tissue specimens accompanied with chronic inflammation.30 The daily use of non-steroidal anti-inflammatory drugs prevents an increase of prostate volume31 and the treatment with a COX-2 inhibitor combined with an α1-adrenoceptor blocker or a 5α-reductase inhibitor improves urinary flow rate.32–34 In addition, COX-2 inhibitors decrease androgen receptor activity in prostate cancer cells.35 These observations suggest that COX-2 may represent a convergent mediator of prostate growth in the inflammatory pathway. Our results demonstrate that inhibition of COX-2 normalized the upregulated expression of androgen-responsive and TGF-β1 associated genes induced by chronic prostatic inflammation.
Bacterial compounds cause a wide range of cellular responses in prostatic cells, including hypersecretion of epithelial proteins and the proliferation in the epithelial and stromal compartments. Induction of prostatitis results in proliferation of approximately 80% of epithelial cells during acute inflammation.36 Yatkin et al. also reported that rats with endocrine prostatitis show epithelial proliferation 3 weeks after castration combined with estradiol administration.37 Quintar et al. demonstrated rapid epithelial hyperplasia 48 h after inoculating E. coli into the rat prostate.38 Furthermore, Elkahwaji et al. demonstrated that Propionibacterium acnes isolated from the human prostate enhances the expression of the androgen receptor in the mouse prostate, which was more often observed in areas close to intense inflammatory infiltrates.39 These results suggest that prostatic inflammation, even at the acute stage of infection, is a strong proliferative stimulus for epithelial cells. Although we did not see apparent proliferation of prostatic epithelium in this study, it is possible that a longer follow-up may have revealed prostatic proliferation.
Inflammation in BPH specimens may not have been associated with bacterial infection, which is a major limitation of this study. However, some studies have suggested that prostatic bacterial infections are common. The percentage of BPH specimens positive for a bacterial infection varies around 20%40, and molecular epidemiological studies suggest that bacterial colonization of the prostate may occur more commonly than previously thought. Considering these facts and our results together, bacterial inflammation may trigger the development of prostatic enlargement in BPH tissues although further studies are needed to clarify this point.
Conclusion
Chronic prostatic inflammation up to 12 weeks causes changes in the expression of androgen-responsive genes in the epithelium and a TGF-β1 gene cascade in the stroma. Activation of androgen-responsive and TGF-β1–cascade genes might contribute to epithelial and stromal proliferation, respectively, during the development of BPH. In addition, COX-2 inhibitors, which suppress inflammation-induced changes in the expression of these genes, might be beneficial to treat prostatic hypertrophy associated with prostatic inflammation.
Acknowledgments
This project was supported by NIH P20-DK090919. We thank Dr. Wade A. Bushman at University of Wisconsin for kindly providing us with Escherichia coli (strain 1677).
Key of Definitions for Abbreviations
- BPH
Benign prostatic hyperplasia
- COX-2
cyclooxigenase-2
- TGF-β1
Tumor growth factor-β1
- ABC
avidin–biotin complex
- ELISA
enzyme-linked immunosorbent assay
- LCM
laser-capture microdissection
Footnotes
Conflicts of interest
The authors have nothing to disclose.
References
- 1.Di Silverio F, Gentile V, De Matteis A, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, Roehrborn CG, O’Leary MP, et al. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra VC, Allen DJ, Nicolaou C, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100:327. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- 4.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of Inflammation and Benign Prostatic Hyperplasia on Autopsy in Asian and Caucasian Men. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.06.026. in press. [DOI] [PubMed] [Google Scholar]
- 5.De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60:106. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 6.Roehrborn CG. Definition of at-risk patients: baseline variables. BJU Int. 2006;97(Suppl 2):7. doi: 10.1111/j.1464-410X.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 7.St Sauver JL, Jacobson DJ, McGree ME, et al. Longitudinal association between prostatitis and development of benign prostatic hyperplasia. Urology. 2008;71:475. doi: 10.1016/j.urology.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Fibbi B, Penna G, Morelli A, et al. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2009 doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 10.Penna G, Fibbi B, Amuchastegui S, et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009;182:4056. doi: 10.4049/jimmunol.0801875. [DOI] [PubMed] [Google Scholar]
- 11.Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51:524. doi: 10.1016/j.eururo.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Royuela M, Ricote M, Parsons MS, et al. Immunohistochemical analysis of the IL-6 family of cytokines and their receptors in benign, hyperplasic, and malignant human prostate. J Pathol. 2004;202:41. doi: 10.1002/path.1476. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61:60. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Li Q, Han P, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009;74:340. doi: 10.1016/j.urology.2009.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Steiner GE, Newman ME, Paikl D, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 16.Roper WG. The etiology of benign prostatic hypertrophy. Med Hypotheses. 1998;50:61. doi: 10.1016/s0306-9877(98)90179-7. [DOI] [PubMed] [Google Scholar]
- 17.O’Malley KJ, Dhir R, Nelson JB, et al. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69:1716. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funahashi Y, O’Malley KJ, Kawamorita N, et al. Upregulation of Androgen-Responsive Genes and Transforming Growth Factor-b1 Cascade Genes in a Rat Model of Non-bacterial Prostatic Inflammation. Prostate. 2014;74:337. doi: 10.1002/pros.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partin AW, Oesterling JE, Epstein JI, et al. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991;145:405. doi: 10.1016/s0022-5347(17)38353-2. [DOI] [PubMed] [Google Scholar]
- 20.Cooper CS, Perry PJ, Sparks AE, et al. Effect of exogenous testosterone on prostate volume, serum and semen prostate specific antigen levels in healthy young men. J Urol. 1998;159:441. doi: 10.1016/s0022-5347(01)63944-2. [DOI] [PubMed] [Google Scholar]
- 21.Coffey DS, Walsh PC. Clinical and experimental studies of benign prostatic hyperplasia. Urol Clin North Am. 1990;17:461. [PubMed] [Google Scholar]
- 22.Chun JY, Nadiminty N, Dutt S, et al. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res. 2009;15:4815. doi: 10.1158/1078-0432.CCR-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinowska K, Neuwirt H, Cavarretta IT, et al. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr Relat Cancer. 2009;16:155. doi: 10.1677/ERC-08-0174. [DOI] [PubMed] [Google Scholar]
- 24.Monti S, Di Silverio F, Iraci R, et al. Regional variations of insulin-like growth factor I (IGF-I), IGF-II, and receptor type I in benign prostatic hyperplasia tissue and their correlation with intraprostatic androgens. J Clin Endocrinol Metab. 2001;86:1700. doi: 10.1210/jcem.86.4.7413. [DOI] [PubMed] [Google Scholar]
- 25.Kramer G, Steiner GE, Handisurya A, et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52:43. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 26.Penna G, Fibbi B, Maggi M, et al. Prostate autoimmunity: from experimental models to clinical counterparts. Expert Rev Clin Immunol. 2009;5:577. doi: 10.1586/eci.09.37. [DOI] [PubMed] [Google Scholar]
- 27.Tuxhorn JA, Ayala GE, Smith MJ, et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912. [PubMed] [Google Scholar]
- 28.Inui S, Itami S. Androgen receptor transactivity is potentiated by TGF-beta1 through Smad3 but checked by its coactivator Hic-5/ARA55 in balding dermal papilla cells. J Dermatol Sci. 2011;64:149. doi: 10.1016/j.jdermsci.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996;27:668. doi: 10.1016/s0046-8177(96)90396-2. [DOI] [PubMed] [Google Scholar]
- 30.Gerstenbluth RE, Seftel AD, MacLennan GT, et al. Distribution of chronic prostatitis in radical prostatectomy specimens with up-regulation of bcl-2 in areas of inflammation. J Urol. 2002;167:2267. [PubMed] [Google Scholar]
- 31.St Sauver JL, Jacobson DJ, McGree ME, et al. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006;164:760. doi: 10.1093/aje/kwj258. [DOI] [PubMed] [Google Scholar]
- 32.Gorgel SN, Sefik E, Kose O, et al. The effect of combined therapy with tamsulosin hydrochloride and meloxicam in patients with benign prostatic hyperplasia symptoms and impact on nocturia and sleep quality. Int Braz J Urol. 2013;39:657. doi: 10.1590/S1677-5538.IBJU.2013.05.07. [DOI] [PubMed] [Google Scholar]
- 33.Di Silverio F, Bosman C, Salvatori M, et al. Combination therapy with rofecoxib and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) Eur Urol. 2005;47:72. doi: 10.1016/j.eururo.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Jhang JF, Jiang YH, Kuo HC. Adding Cyclooxygenase-2 inhibitor to alpha blocker for patients with benign prostate hyperplasia and elevated serum prostate specific antigen could not improve prostate biopsy detection rate but improve lower urinary tract symptoms. Int J Clin Pract. 2013;67:1327. doi: 10.1111/ijcp.12220. [DOI] [PubMed] [Google Scholar]
- 35.Narayanan BA, Narayanan NK, Davis L, et al. RNA interference-mediated cyclooxygenase-2 inhibition prevents prostate cancer cell growth and induces differentiation: modulation of neuronal protein synaptophysin, cyclin D1, and androgen receptor. Mol Cancer Ther. 2006;5:1117. doi: 10.1158/1535-7163.MCT-05-0520. [DOI] [PubMed] [Google Scholar]
- 36.Haverkamp JM, Charbonneau B, Crist SA, et al. An inducible model of abacterial prostatitis induces antigen specific inflammatory and proliferative changes in the murine prostate. Prostate. 2011;71:1139. doi: 10.1002/pros.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yatkin E, Bernoulli J, Talvitie EM, et al. Inflammation and epithelial alterations in rat prostate: impact of the androgen to oestrogen ratio. Int J Androl. 2009;32:399. doi: 10.1111/j.1365-2605.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 38.Quintar AA, Doll A, Leimgruber C, et al. Acute inflammation promotes early cellular stimulation of the epithelial and stromal compartments of the rat prostate. Prostate. 2010;70:1153. doi: 10.1002/pros.21150. [DOI] [PubMed] [Google Scholar]
- 39.Elkahwaji JE, Zhong W, Hopkins WJ, et al. Chronic bacterial infection and inflammation incite reactive hyperplasia in a mouse model of chronic prostatitis. Prostate. 2007;67:14. doi: 10.1002/pros.20445. [DOI] [PubMed] [Google Scholar]
- 40.Nickel JC, Downey J, Young I, et al. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]


