Abstract
Research into the comorbidity between emotional psychopathology and cigarette smoking has often focused upon anxiety and depression’s manifest symptoms and syndromes, with limited theoretical and clinical advancement. This paper presents a novel framework to understanding emotion-smoking comorbidity. We propose that transdiagnostic emotional vulnerabilities—core biobehavioral traits reflecting maladaptive responses to emotional states that underpin multiple types of emotional psychopathology—link various anxiety and depressive psychopathologies to smoking. This framework is applied in a review and synthesis of the empirical literature on three trandiagnostic emotional vulnerabilities implicated in smoking: (1) anhedonia (Anh; diminished pleasure/interest in response to rewards); (2) anxiety sensitivity (AS; fear of anxiety-related sensations); and (3) distress tolerance (DT; ability to withstand distressing states). We conclude that Anh, AS, and DT collectively: (a) underpin multiple emotional psychopathologies; (b) amplify smoking’s anticipated and actual affect enhancing properties and other mechanisms underlying smoking; (c) promote progression across the smoking trajectory (i.e., initiation, escalation/progression, maintenance, cessation/relapse); and (d) are promising targets for smoking intervention. After existing gaps are identified, an integrative model of transdiagnostic processes linking emotional psychopathology to smoking is proposed. The model’s key premise is that Anh amplifies smoking’s anticipated and actual pleasure-enhancing effects, AS amplifies smoking’s anxiolytic effects, and poor DT amplifies smoking’s distress terminating effects. Collectively, these processes augment the reinforcing properties of smoking for individuals with emotional psychopathology to heighten risk of smoking initiation, progression, maintenance, cessation avoidance, and relapse. We conclude by drawing clinical and scientific implications from this framework that may generalize to other comorbidities.
Keywords: Anxiety, Depression, Smoking, Comorbidity, Nicotine Dependence
Introduction
Despite large-scale public health campaigns to warn against the dangers of smoking and encourage cigarette smokers to quit, a significant portion of the population initiate smoking each year and existing smokers struggle to quit (CDC, 2010). Therefore, a major public health problem is to limit the incidence of new smokers, encourage current smokers to make a cessation attempt, and enhance quit success in the population of recalcitrant smokers who are interested quitting, but are unable to successfully maintain abstinence.
The field of psychology is in a unique position to address the public health burden of smoking, given that depressive and anxiety symptoms and syndromes (i.e., emotional disorders) are highly prevalent in the general population and remarkably comorbid with smoking (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Hughes, 1999, 2011; Japuntich et al., 2007; Leventhal, Japuntich, et al., 2012; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008; Piper, Cook, Schlep, Journey, & Baker, 2011; Piper et al., 2010; Zvolensky, Gibson, et al., 2008; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009). The link between smoking and emotional psychopathology: (1) generalizes across several emotional conditions, including major depression (Leventhal, Japuntich, et al., 2012), dysthymic disorder (Weinberger, Pilver, Desai, Mazure, & McKee, 2012), minor depression (Weinberger et al., 2012), panic disorder (Piper et al., 2011), social anxiety disorder (Piper et al., 2011), posttraumatic stress disorder (PTSD; Zvolensky, Gibson, et al., 2008), and generalized anxiety disorder (GAD; Piper et al., 2011); (2) extends to multiple stages of the smoking trajectory, including initiation (Leventhal, Ray, Rhee, & Unger, 2011; Patton et al., 1998), progression to regular smoking (Audrain-McGovern, Rodriguez, Rodgers, & Cuevas, 2011), development and maintenance of nicotine dependence (McKenzie, Olsson, Jorm, Romaniuk, & Patton, 2010), and risk of smoking cessation failure (Hall, Munoz, & Reus, 1994; Hitsman, Papandonatos, McChargue, Demott, Herrera, Spring et al., 2013). The smoking-emotion relation is bi-directional, as increases in tobacco use heightens risk of emotional disorder symptoms (Breslau, Novak, Kessler, 2004; Khaled, Bulloch, Williams, Hill, Lavorato, & Patten, 2012; Wu & Anthony, 1999; Breslau & Klein, 1999; Johnson, Cohen, Pine, Klein, Kasen, & Brook, 2000) and sustained abstinence decreases emotional symptoms (Kahler, Spillane, Busch, & Leventhal, 2011).
Yet, strikingly little is known about the mechanisms underlying the relation between emotional disorders and smoking relative to the volume of work published on this topic. Furthermore, behavioral, pharmacologic, or combination cessation programs designed to address emotional symptoms to facilitate quitting have generally yielded modest (e.g., Cinciripini et al., 1995; McFall et al., 2005, 2010; Piper et al., 2008) or mixed (e.g., Brown et al., 2007; Hitsman et al., 2003; Hitsman et al., 2013) results on cessation outcomes and, in some cases, poor effects on emotional outcomes (Kahler et al., 2002). Hence, the current research paradigm to studying emotion-smoking comorbidity may need to be revised to propel further progress in understanding etiologic mechanisms and advancing clinical services for this population.
The traditional paradigm in the emotion-smoking literature mainly focuses on individual psychiatric syndromes and the manifest symptoms of these disorders, which poses several scientific and clinical challenges. First, a syndrome-based approach does not adequately address the substantive heterogeneity within individual depressive and anxiety disorders. For instance, major depression may have from two to four symptom factors that are empirically, conceptually, and phenomenologically distinct (Shafer, 2006). Such heterogeneity suggests that there are multiple, distinct etiologic influences that underlie a single emotional syndrome (Hasler, Drevets, Manji, & Charney, 2004). Hence, individuals who share the same emotional disorder diagnosis may have different emotional influences on their smoking, and therefore, benefit from different smoking intervention approaches. Second, this syndrome-based approach, which often focuses on each disorder in isolation from one another, does not sufficiently address the considerable co- and multi-morbidity across multiple depressive and anxiety disorders (Gorman, 1996). Certain disorders (e.g., major depression and GAD) have exceedingly high rates of co-occurrence (Kessler et al., 2005), which has prompted some to suggest that some emotional disorders may be ‘alternate manifestations’ of a common underlying etiology (Brown, Chorpita, & Barlow, 1998). Thus, individuals with different emotional disorder syndromes may actually have shared emotional influences on their smoking and potentially benefit from a common clinical strategy. Finally, focusing on syndromes assumes that the manifest symptoms are important in explaining emotion-smoking comorbidity. However, certain manifest symptoms have limited direct relations to smoking (e.g., increased appetite in major depression, intrusive nightmares in PTSD, and worry in GAD; Greenberg et al., 2012; Leventhal, Kahler, Ray, & Zimmerman, 2009; Peasley-Miklus, McLeish, Schmidt, & Zvolensky, 2012) and for some of the pathognomonic emotional symptoms (e.g., dysphoric affect), many individuals can effectively cope with symptoms without smoking (Skrove, Romundstad, & Indredavik, 2012; Tsourtos et al., 2011). Thus, underlying processes that directly reflect the propensity to act on emotional disturbance with smoking behavior, rather than the emotional symptomatology per se, may be particularly salient to smoking. Accordingly, an approach focused on underlying vulnerability processes that govern one’s reaction to emotion states and cut across multiple forms of emotional disorder is warranted to advance research and practice.
The Current Paper
Extant review, synthesis, or theory papers on emotion-smoking comorbidity have limited their focus on manifest symptoms or syndromes, factors germane to either depression or anxiety but not both, or a particular stage of the smoking trajectory (e.g., Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005; Covey, Glassman, & Stetner, 1998; Hall, Munoz, Reus, & Sees, 1993; Hitsman, Borrelli, McChargue, Spring, & Niaura, 2003; Morissette, Tull, Gulliver, Kamholz, & Zimering, 2007; Weinberger, Mazure, Morlett, & McKee, 2013; Wilhelm, Wedgwood, Niven, & Kay-Lambkin, 2006). We are not aware of any paper that introduces an integrative theoretical framework for parsimoniously identifying key psychological mechanisms that underpin the relation of multiple manifestations of anxiety and depression to progression across the smoking trajectory. To address this major gap in the literature, the present paper puts forth a novel framework to synthesize recently emerging lines of empirical evidence on the role of transdiagnostic emotional vulnerability factors in emotion-smoking comorbidity. Here, we focus on three key trandiagnostic emotional vulnerabilities implicated in the etiology of smoking: (i) anhedonia (Anh, defined as the tendency to experience reduced happiness, pleasure, and interest in response to rewards; Leventhal, Chasson, Tapia, Miller, & Pettit, 2006); (ii) anxiety sensitivity (AS, fear that anxiety symptoms are harmful; Reiss, Peterson, Gursky, & McNally, 1986); and (iii) distress tolerance (DT, perceived or actual ability to tolerate emotional and physical distress; Leyro, Zvolensky, & Bernstein, 2010).
We first define terminology, discuss key conceptual tenets of the proposed transdiagnostic framework, explain why we extrapolate the framework to Anh, AS, and DT specifically, and describe the review methodology. In the main body of the paper, we define Anh, AS, and DT and critically review the empirical literature on the role of these three vulnerability factors in smoking, with separate subsections devoted to each vulnerability factor. The structure of these subsections is organized similarly, covering the following content areas: the vulnerability factor’s definition, measurement, and theoretical applicability to smoking, empirical relations of the vulnerability factor to stages along the smoking trajectory, and implications for smoking interventions that target the vulnerability factor. We then point out key gaps in the literature and synthesize the review results by proposing an integrative model for understanding the etiologic role of transdiagnostic vulnerabilities in emotional disorder-smoking comorbidity. We conclude with clinical and scientific implications drawn from this approach.
Trandiagnostic Emotion Vulnerability Framework: Defining Key Variables, Conceptual Tenets, and Review Methodology
Key Variable Definitions
Stages in the trajectory of cigarette smoking
Smokers often follow a generally well-specified sequence that includes the initiation, progression, maintenance, cessation, and relapse. Initiation reflects the initial cigarette smoked and further experimentation (irregular smoking). A sizeable portion of initiators continue and escalate their smoking behavior, ultimately progressing to regular smoking, ranging from weekly to daily use. The period of time in which smokers continue systematic regular smoking patterns is termed maintenance; it is in this stage that smoking behavior is likely to become habitualized. For some individuals certain mechanisms that maintain smoking may be more powerful and promote more chronic, compulsive, and severe smoking patterns, indicative of tobacco dependence (Japuntich, Piper, Schlam, Bolt, & Baker, 2009). Typically, the longer that smoking is maintained, the more entrenched and severe smoking behavior becomes. Although some smokers avoid making a cessation attempt as a result of poor motivation to quit or severe tobacco dependence that overrides any quit motivation, almost all who make a quit attempt relapse back to smoking after their first attempt (CDC, 2010). Following a relapse, many individuals return to their typical pre-quit level smoking behavior or even exceed pre-quit levels (CDC, 2010), which recapitulates and extends the maintenance of smoking. From this point forward, many smoking trajectories enter into a persistent cyclic pattern of maintenance, cessation, and relapse.
Emotional symptoms and disorders
This paper focuses on diagnostic status and dimensional variation in symptom severity within emotional syndromes within the unipolar mood and anxiety disorders. We focus on major depression, dysthymic disorder, panic disorder, social anxiety disorder, specific phobia, GAD, and PTSD and other trauma-related disorders given their (a) commonalities with one another; (b) high prevalence rate; and (c) strong relation with smoking initiation, maintenance, and relapse (Leventhal, Japuntich, et al., 2012; Piper et al., 2011; Weinberger et al., 2012; Zvolensky, Gibson, et al., 2008).
Trandiagnostic (“reactive”) emotional vulnerabilities
Recent work in psychopathological science proposes that the underlying cause of many forms of emotional symptoms and disorders as well as their comorbidity may be underpinned by a smaller set of transdiagnostic vulnerability processes (Dozois, Seeds, & Collins, 2009; Sauer-Zavala et al., 2012). This approach integrates well with the National Institute on Mental Health’s Research Domain Criteria, which proposes that common cross-cutting dimensions, traits, neural circuits, and biological pathways underpin and account for the presentation of various mental disorder diagnoses (Cuthbert and Insel, 2013). Our framework focuses on “reactive” trandiagnostic vulnerabilities, which denote characteristic maladaptive responses to emotion stimuli and states. These types of vulnerabilities play a key explanatory role in emotion experience by: (1) enhancing or diminishing the normative response to emotion stimuli and states, resulting in an excess or deficit, respectively, beyond typical emotional functioning; or (2) altering the type of response to emotion stimuli and states. In either case, these reactive processes may be maladaptive because they serve to reinforce the intensity and frequency of future manifest emotional symptoms. For example, when faced with negative emotion states, individuals with an emotional vulnerability factor that limits their capacity to handle distress may be more apt to execute avoidance behaviors that preclude habituation to negative emotion states, which could ultimately increase the intensity of future negative affect and solidify beliefs and learned responses that interfere capacity to adaptively respond to distress (Leyro et al., 2010).
Transdiagnostic Emotional Vulnerabilities and Smoking: Core Conceptual Tenets of the Framework
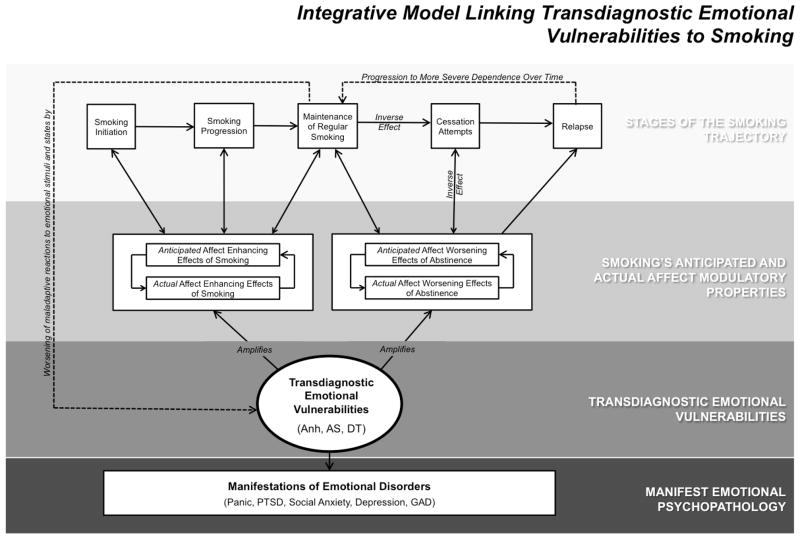
Amplification of smoking’s anticipated and actual affect modulatory effects
There is variability in smoking patterns within the subpopulation of individuals with elevated emotional pathology (e.g., Dierker & Donny, 2008), suggesting that many are able to cope with emotional disturbance without resorting to smoking whereas other may have more difficulty. We propose that underlying vulnerabilities that directly amplify the propensity to act on emotion disturbance with smoking behavior, rather than the level or quality of emotional symptomatology per se, may parsimoniously explain emotion-smoking comorbidity. The core tenet of the framework is that smoking reflects a critical manifestation of the maladaptive response to emotion states that characterize trandiagnostic emotional vulnerabilities. Specifically, people with elevated vulnerabilities may be hyper-motivated to respond to emotion states/stimuli with smoking behavior to achieve affect modulation, which they might otherwise not be able to obtain through adaptive means. As a result, people with reactive vulnerabilities may be more sensitive to the effects of smoking on affective state, place greater salience the reinforcing value of smoking-induced affect modulation, and develop of stronger expectancies for smoking-induced affect modulation, which collectively may transmit risk for movement along the smoking trajectory.
Conceptual rationale for focusing on Anh, AS, and DT
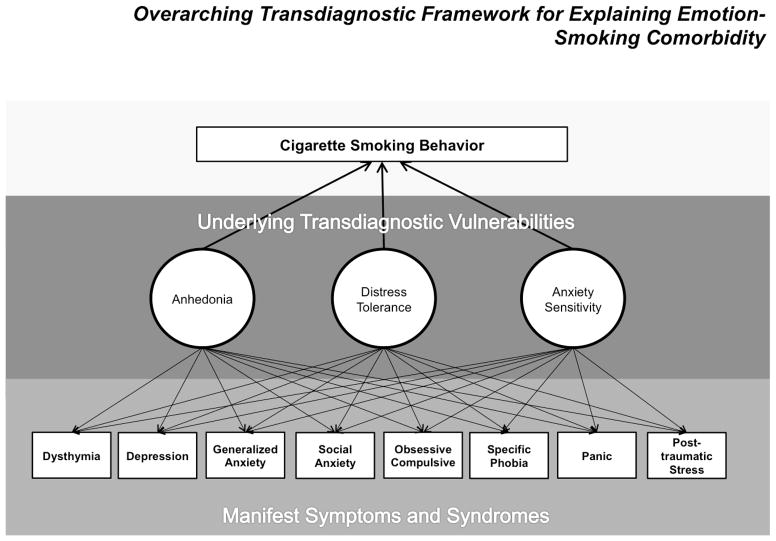
We focus on Anh, AS, and DT in this paper because these three factors are implicated in a wide variety of manifestations of emotional disorder and have theoretical relevance to smoking. Smoking posses three unique primary affect modulatory properties that make it a particularly potent reinforcer for individuals at risk for emotional psychopathology: (a) pleasure/positive affect enhancement (Strong et al., 2011); (b) anxiety reduction (Kassel & Unrod, 2000); and (c) distress termination (Kassel, Stroud, & Paronis, 2003). As reviewed below, evidence indicates that Anh amplifies smoking’s anticipated and actual pleasure enhancing properties, AS amplifies smoking’s anticipated and actual anxiolytic properties, and DT amplifies smoking’s anticipated and actual distress alleviating properties. Accordingly, Anh, AS, and DT may collectively account for a wide range of emotional psychopathology as well as multiple affective mechanisms that transmit risk along the smoking trajectory continuum. A heuristic for the transdiagnostic framework to emotion-smoking comorbidity proposed here is presented in Figure 1.
Figure 1.
General framework proposing that trandiagnostic emotional vulnerability factors explain the relation between various manifestations of emotional psychopathology and cigarette smoking. Anh = Anhedonia; AS = Anxiety Sensitivity; DT = Distress Tolerance.
Scope and Methodology for Review of Empirical Literature on the Relation of Anh, AS, and DT to Smoking
Within a portion of each succeeding section focused on Anh, AS, and DT, we critically review and integrate the available empirical literature examining the role of these three vulnerability factors in smoking. We located citations via searches of MEDLINE, PsycInfo, and Google Scholar as of May 1, 2014. Searches used the “AND” function that identified citations that crossed two types of terms: (1) a transdiagnostic vulnerability factor term; and (2) a smoking term. For the vulnerability term, searches were repeated for each of the following: “anhedonia,” “hedonia,” “hedonic,” “anhedonic,” “reduced/diminished pleasure,” “reduced/diminished interest,” “distress (in)tolerance,” “persistence,” “frustration (in)tolerance,” “anxiety sensitivity,” “bodily vigiliance,” “somatic threat,” “interoception sensitivity.” For the smoking term, searches were repeated for “smoking,” “cigarette,” “nicotine,” and “tobacco.” In addition, abstract catalogues for presentations for relevant professional meetings (e.g., Society for Nicotine and Tobacco Research) were searched. Published articles, conference abstracts, theses, and dissertations were considered for inclusion (98% of final studies included were published articles). Study selection methods resulted in approximately 500 citations. Studies that did not directly report data on the empirical relation of Anh, AS, or DT to a smoking variable or examine Anh, AS, or DT in the context of a smoking-related manipulation (e.g., tobacco deprivation, nicotine administration) were discarded. The remaining 79 studies were qualitatively reviewed and synthesized. Table 1 describes final studies included in the review.
Table 1.
Overview of Articles Included in the Review
| Anhedonia | |||||
|---|---|---|---|---|---|
| Study | Sample | Emotional Vulnerability Measure | Design | Smoking Trajectory Relevance | Main Finding |
| Anh-Allen et al. (2012) |
N = 15 smokers with schizophrenia (10+ cig/day) N = 16 non-psychiatric control smokers (10+ cig/day) |
BDI-Anh | Cross-sectional correlational | M | Anh was positively associated with urge to smoke in smokers with schizophrenia (r = .60) but not non-psychiatric controls (ESU). |
| Audrain-McGovern et al. (2012) | N = 1106 adolescents (M age = 15.5) | SHAPS | Prospective correlational | I, P | Higher baseline Anh was associated with greater likelihood of past 30 day smoking at baseline (OR = 2.64) and increase in cig/day over the subsequent 18 months (d = 0.14). |
| Cook et al. (2004) | N = 35 adult non-treatment-seeking smokers (10+ cig/day) | FCPS | Lab quasi-experimental | M | Higher Anh predicted greater increases in craving (r = .47) and reductions in acute positive affect (r = − .40), but not changes in negative affect (r = .20) following 24 hours of tobacco deprivation. |
| Cook et al. (2007) | N = 50 adult non-treatment-seeking smokers (15+ cig/day) | FCPS | Lab quasi-experimental | P, M | Smokers with high (vs. low) Anh did not differ in nicotine dependence severity (d = −0.47), cig/day (d = 0.11), or years smoking (d = 0.07). Smokers with high (vs. low) Anh exhibited greater increases in positive affect during a positive mood induction when smoking a cigarette with nicotine (d = 0.72) versus placebo cigarette (d = 0.14). |
| Cook et al. (2010) | N = 45 smokers (10+ cig/day) making a quit attempt | FCPS | Prospective correlational | CR | Baseline Anh predicted faster latency to relapse (HR = 1.55). |
| Cook et al. (2012) | N = 1504 smokers (10+ cig/ay) enrolled in a cessation trial | 3-item Anh Scalea | Prospective correlational | P, M, CR | Anh was higher post (vs. pre) quit day (d = 0.13). Anh was associated with higher cig/day (r = .15) and nicotine dependence severity (r = .12) at baseline. Baseline Anh (OR = 0.93), post-quit Anh (OR = 0.91), and pre to post quit change in Anh (OR = 0.88) predicted lower 8-week point-prevalence abstinence. |
| Gregor et al. (2007) | N = 276 young adult daily smokers | MASQ-AD | Cross-sectional correlational | P, M | Anh was positively associated with cig/day (r = .17) and years as a smoker (r = .15). |
| Johnson et al. (2009) | N = 123 adult daily treatment-seeking smokers | MASQ-AD | Cross-sectional correlational | P | Anh was not associated with cig/day (r = .13). |
| Leventhal, Ramsey et al. (2008) | N = 157 heavy social drinkers (10+ cig/day) in smoking a cessation trial | CESD-Anh | Prospective correlational | P, M, CR | Anh was not associated with baseline cig/day (r = .07) or nicotine dependence severity (r = .07). Anh was associated with greater withdrawal symptoms on quit day (β = .25). Anh was positively associated with relapse at 8 (d = 0.44), 16 (d = 0.48), and 24 (d = 0.47) weeks after quit date. |
| Leventhal, Ameringer, et al. (2013) | N = 187 non-treatment seeking smokers (10+ cig/day) | MASQ-AD | Lab quasi-experimental | M | Anh predicted greater sensitivity to the dampening effects of 16-hr tobacco deprivation on emotional vigor (β = −.17) and elation (β = −.20). |
| Leventhal, Kahler, et al. (2009) | N = 1568 Psychiatric Outpatients | SCID-Anh item SADS-Anh item |
Cross-sectional correlational | I, CR | Patients with current DSM-IV nicotine dependence had higher rates of clinically significant Anh (SCID) and more severe Anh ratings (SADS) compared to those with no history of nicotine dependence (OR = 1.83; β =.33) and past (OR = 1.62; β = .25) nicotine dependence. Patients with past vs. no history of nicotine dependence did not significantly (OR = 1.13; β =.04). |
| Leventhal, Munafò, et a. (2012) | N = 75 non-treatment seeking smokers (10+ cig/day) | SHAPS TPI-R |
Lab quasi-experimental | M | Overnight tobacco deprivation moderated the relation between baseline Anh and emotional processing of happy faces (SHAPS: ηp2 = .06; TPI-R:ηp2 = .04), such that Anh predicted diminished emotional processing during deprivation (SHAPS: r = −.28; TPI-R: r = .08), but not after ad lib smoking (SHAPS: r = −.33; TPI-R: r = −.10). |
| Leventhal, Piper, et a. (2014) | N = 1469 smokers (10+ cig/day) enrolled in a cessation trial | CIDI Anticipatory Anh itemb | Prospective Correlational | P, CR | Lifetime history of Anh was associated with more severe nicotine dependence at baseline (r = .06). Anh predicted greater odds of relapse (OR = 1.42). |
| Leventhal, Waters, et al. (2009) | N = 212 non-treatment-seeking smokers (5+ cigs/day) | SHAPS | Lab quasi-experimental | P, M | Anh was not associated with baseline cig/day (r = .04), age of onset (r = .02), nicotine dependence severity (r = .09), or years as a smoker (r = .01). Anh was positively associated with a number of past quit attempts (r = .23), higher proportion of past quit attempts ending in rapid relapse (abstinence < 24hr; r = .20), and greater behavioral choice melioration smoking motives (e.g., “Very few things give me pleasure each day like cigarettes”; r = .18). Anh was positively associated with greater sensitivity to 12 hr tobacco deprivation effects on urge to smoke for pleasure (β = .19), but not urge to smoke for negative affect relief (β = .13). |
| Leventhal, Trujillo, et al. (in press) | N = 275 non-treatment-seeking smokers (10+ cigs/day) | Mulit-measure Anh Compositec | Lab quasi-experimental | P, M | Anh was associated with higher cig/day (r = .14) but not nicotine dependence severity (r = −.02) at baseline. Anh predicted shorter time to smoking initiation when delaying smoking was monetarily rewarded (β = .− 10) and more cigarettes purchased (β = .13). |
| Leventhal (2011) | N = 212 non-treatment-seeking smokers (5+ cigs/day) | CESD-Anh | Cross-sectional correlational | M | Anh was associated with higher smoking urge level (r = .27) measured after ad lib smoking. |
| Leventhal et al. (2011) | N = 1204 Chinese adolescents (M age = 12.2) | CESD-Anh | Cross-sectional correlational | I | Anh was higher in adolescents with a history of smoking experimentation compared to those without (r = .29). |
| Mickens et al. (2011) | N = 212 non-treatment-seeking smokers (5+ cigs/day) | CESD-Anh | Cross-sectional correlational | P, M | Anh was associated with more severe nicotine dependence severity (β = .19) and behavioral choice amelioration motives (β = .26), but not cig/day or other smoking motives. |
| McChargue & Cook (2007) | N = 77 adult smokers | FCPS and Anh Screenerd | Cross-sectional correlational | P | Anh level on the FCPS (r = .27), was associated with higher nicotine dependence severity. Anh status on the DSM-based screener item was not significantly associated with nicotine dependence (r = .13). |
| McLeish et al. (2006) | N = 220 non-treatment-seeking daily smokers | MASQ-AD | Cross-sectional correlational | P | Anh was positively associated cig/day (r = .18). |
| McLeish et al. (2008) | N = 222 young adults | MASQ-AD | Cross-sectional correlational | I | Anh was higher in daily (vs. never) smokers (r = .27). |
| Niaura et al. (2001) | N = 72 smokers enrolled in smoking cessation programs | Anticipatory Anh Iteme | Cross-sectional correlational | CR | Anh was positively related to more rapid relapse over a self-guided quit attempt over 30 days (ESU). |
| Pomerleau et al. (2003) | N = 931 adult women | CESD-Anh | Cross-sectional correlational | I, CR | Relative to never smokers, Anh was significantly higher in current smokers (d = 0.21) and marginally higher in ex-smokers (d = 0.18). Current and ex-smokers did not significantly differ in Anh (d = .03). |
| Powell et al. (2004) | N = 78 daily smokers | SHAPS | Lab experimental | M | Overnight deprived smokers receiving placebo (vs. nicotine) lozenge reported greater Anh (d = 0.39). |
| Stone and Leventhal (2014) | N = 504 adolescent never smokers (M age = 14.5) | SHAPS | Cross-sectional correlational | I | Anh was associated with greater expectancies that smoking would produce pleasure (β = .14), greater curiosity about trying smoking (β = .12), and lower negative smoking expectancies (β = −.16). Anh was not related to wilingness (β = .07) or intention (β = .04) to smoke. |
| Zvolensky, Kotov, et al. (2008) | N = 390 Russian adults | MASQ-AD | Cross-sectional correlational | I | Anh did not differ between smokers and non-smokers (r = .03). |
| Zvolensky, Johnson, et al. (2009) | N = 144 daily non-treatment-seeking adult smokers | MASQ-AD | Cross-sectional correlational | P, M | Anh was not associated with cig/day (r = .01), years as a smoker (r = .13), or number of past quit attempts (r = .01). |
| Anxiety Sensitivity | |||||
|---|---|---|---|---|---|
| Study | Sample | Emotional Vulnerability Measure | Design | Smoking Trajectory Relevance | Main Finding |
| Abrams, Schlosser, et al. (2011) | N = 58 heavy smokers (20+ cigs/day) and 27 nonsmokers | ASI | Lab quasi-experimental | I, M | AS was not significantly higher in smokers compared to non-smokers (ds = 0.38 to 0.53). At baseline, AS was positively correlated with reasons for smoking for tension reduction (r = .30) but not significantly correlated with reasons for smoking due to subjective addiction to smoking (r = .18) among smokers. The extent to which deprivation heightened panic response to a re-breathing challenge was diminished among smokers with high AS mental concerns (i.e., fear of the negative consequences of cognitive anxiety symptoms, “It scares me when I am unable to keep my mind on a task.”; ESU). AS social concerns (e.g., “Other people notice when I feel shaky.”) and physical concerns (e.g., “It scares me when my heart beats rapidly.”) did not moderate effects of deprivation on response to the breathing challenge (ESU). |
| Abrams, Zvolensky, et al. (2011) | N = 326 adult daily smokers (at least 5 cig/day for at least 1 year) | ASI | Cross-sectional correlational | M | AS was correlated with the Smoking Abstinence Expectancies full scale (r = .50) and subscales tapping expecting negative mood, somatic symptoms, and harmful consequences in response to abstinence (rs = .37 to .52). |
| Assayag et al. (2012) | N = 67 adult daily smokers in smoking cessation treatment | ASI | Prospective correlational | CR | Those with maintained high AS from pre-treatment to 1 month post-treatment, compared to those who experienced a significant reduction in AS levels during this time period, were at increased risk for lapse and relapse (d = 0.55). |
| Brown et al. (2001) | N = 60 smokers with past major depressed enrolled in a smoking cessation trial | ASI | Prospective correlational | M, CR | AS was associated with increased odds of lapsing during the first week after quit day (RR = 2.0). AS was correlated with the increased expectations for negative affect reduction from smoking at baseline (β = .39). |
| Evatt & Kassel (2010) | N = 32 adult smokers (average of 10 cigs/day) | ASI | Lab quasi-experimental | M | High (vs. low) AS smokers differed in the extent to which smoking reduced acute anxiety after a stressful speech (vs. control) condition (ηp2 = .11), such that smoking reduced acute anxiety in response to the speech (vs. control) condition in high-AS smokers (ηp2 = .36), but not low-AS smokers (ESU). High (vs. low) AS smokers marginally differed in the extent to which smoking reduced perceived arousal after a stressful speech (vs. control) condition (ηp2 = .07), such that smoking reduced acute anxiety in response to the speech (vs. control) condition in high-AS smokers (ηp2 = .22), but not low-AS smokers (ESU). High (vs. low) AS smokers did not differ in the extent to which smoking impacted subjective stress, heart rate, or skin conductance after a stressful speech (vs. control) condition (ESU). |
| Feldner, et al. (2008) | N = 96 high AS daily smokers in a Psychosocial intervention targeting AS and smoking or health information control group | ASI | Prospective experimental | CR | AS reduction intervention (compared to control) significantly reduced AS (η2 = .08) and marginally reduced cig/day (p = .06; η2 = .03). |
| Gregor, et al. (2008) | N = 125 daily smokers | ASI | Cross-sectional Correlational | M | AS was correlated with greater smoking expectancies for Negative Reinforcement, Negative Consequence, Positive Reinforcement, and Appetite control as well as with greater perceived barriers to cessation (rs = .33 to .55). |
| Gonzalez, et al. (2008) | N = 189 daily smokers (average of 15+ cigs/day) | ASI | Cross-sectional Correlational | M, CR | AS was significantly correlated with stimulation, habitual, addictive, and coping reasons for smoking as well as perceived barriers to quitting (βs .22 to .23). |
| Guillot et al. (in press) | N = 205 non-treatment-seeking adult smokers (≥ 10 cig/day) | ASI | Cross-sectional correlational | M | AS was associated with stronger expectancies that smoking alleviates negative affect (β = .30) and smoking abstinence exacerbates aversive withdrawal symptoms (β = .24). |
| Johnson, et al. (2012) | N = 123 adult daily smokers enrolled in a cessation study | ASI | Prospective correlational | P, M | AS was positively associated with greater baseline nicotine withdrawal symptoms (r = .55) but not nicotine dependence severity (r = .04). Greater levels of baseline AS were associated with a stronger relation between average levels of state anxiety and average levels of nicotine withdrawal symptoms experienced during the course of first two weeks of the quit attempt (ESU). AS was associated with slower decrease in withdrawal symptoms during the same time period (ESU). |
| Marshall, et al. (2009) | N = 99 daily smokers | ASI | Lab-quasi experimental | M, CR | AS was positively correlated with quit-day nicotine withdrawal (r = .53) Interaction between AS and panic responsivity to a voluntary hyperventilation challenge predicted quit-day nicotine withdrawal symptom severity above and beyond the main effects (ηp2 = 0.046, β = −.29). |
| McLeish, et al. (2008). | N = 222 young adults | ASI | Cross-sectional correlational | I | AS physical concerns subscale (e.g., “”it scares me when my heart beats rapidly) was higher in smokers vs. non-smokers (r = .24). AS physical concerns moderated the association of smoking status with body vigilance and anxiety symptoms (βs =.23 and .27), such that the positive relation of these two aspects of anxiety to smoking was higher among those with higher AS. |
| Morissette, et al. (2006) | N = 527 individuals with anxiety disorders seeking anxiety treatment | ASI | Cross-sectional correlational | I | AS was significantly higher among smokers compared to nonsmokers (d = 0.54). |
| Perkins et al. (2010) | N = 71 non-treatment-seeking adult smokers (≥10 cigarettes/day) | ASI | Lab-quasi experimental | M | Higher vs. lower AS was associated with greater cigarette liking (reward) during a stressful speech preparation procedure vs. other conditions (ηp2 = 0.046). Negative affect decreased more after smoking in those with high versus low AS, but only during stressful speech preparation vs. other conditions (ηp2 = 0.031; ηp2 = 0.044). AS did not significantly influence or interact with negative affect-related experimental conditions on smoking reinforcement (i.e., ad lib smoking) |
| Vujanovic and Zvolensky (2009) | N = 90 daily smokers (non-treatment-seeking; 15+ cig/day) | ASI | Lab-quasi experimental | M | AS by tobacco deprivation interaction was significantly predictive of acute anxiety during a CO2 challenge (anxiety during the challenge; η2 = .05), such that low-AS smokers experienced less anxiety when deprived (vs. non-deprive) and high-AS smokers experienced similar anxiety as a function of deprivation status. |
| Wong, et al. (2013) | N = 87 adult smokers (10+ cigs/day) | ASI | Lab-quasi experimental | M | AS predicted greater increases in positive affect from pre- to post-cigarette (β = .30) as well as greater smoking satisfaction and psychological reward (β = .23 to .48). AS was not significantly related to smoking-induced changes in negative affect, urge, or aversive effects of smoking (|β|s < .16) |
| Zvolensky, et al. (2007) | N = 130 “low-level smokers” (less than 10 cigs/day) from Mexico | ASI | Cross-sectional correlational | P, CR | AS physical concerns lower-order factor was related to retrospective reports of early smoking relapse (OR = 1.19). AS was correlated with higher cig/day (r = .43). |
| Zvolensky, et al. (2006) | N = 75 daily smokers (20+ cigs/day) | ASI | Cross-sectional correlational | CR | AS was significantly associated with retrospective reports of early smoking relapse (OR = 1.06), above and beyond the effects of negative affectivity. |
| Zvolensky, et al. (in press) | N= 466 adult treatment-seeking daily smokers | ASI-3 | Cross-sectional correlational | P, M, CR | AS was positively related to higher of nicotine dependence level (r = .14), perceived barriers to smoking cessation (r = .32), problematic symptoms during previous quit attempts (r = .38), and expectancies for smoking-induced negative reinforcement (r = .29). AS was not significantly associated with lifetime number of quit attempts (r = .03). AS significantly indirectly related to barriers to cessation, greater number of quit attempts, and greater negative reinforcement smoking expectancies through the tendency to avoid distressing thoughts, feelings, and internal sensations (ESU; statistical mediation of relation of AS to smoking via avoidance). |
| Zvolensky, et al. (2004) | N = 90 young adult regular smokers | ASI | Cross-sectional correlational | M | AS physical concerns and mental incapacitation concerns were signicantly related to smoking expectancies for negative reinforcement and negative health conseqeunces (rs = .30 to .32). |
| Zvolensky, et al. (2009) | N = 144 adult smokers (15+ cigs/day) | ASI | Cross-sectional correlational | CR | AS was correlated with greater number of past quit attempts (r = .28). |
| Zvolensky, Kotov, et al. (2003) | N = 95 adult smokers from Moscow | ASI | Cross-sectional correlational | P, M | AS was not significantly associated with cig/day (r = .04). AS amplified the extent to which higher cig/day predicted agoraphobic avoidance (sr2 = .10; AS x Smoking Status interaction). AS did not significantly moderate the relation of cig/day to number of panic attacks (sr2 = .02) or anxiety symptom severity experienced (sr2 = .02) during the past week. |
| Zvolensky, Lejuez, et al. (2003) | A 43-year-old Caucasian male who receiving interoceptive exposure-based program of smoking cessation and cognitive-behavioral therapy for panic disorder | ASI | Prospective case report | CR | Participant reported reduction in AS score (from 35 pre-treatment to 25 post-treatment) and maintained abstinent for 12 months following quit attempt (ipsative z-score difference .92). |
| Zvolensky, et al. (2008) | N = 3 female daily smokers with high AS and nicotine dependence receiving interoceptive exposure-based smoking cessation treatment | ASI | Prospective case series | CR | Results indicated signifcant reduction in AS compared to baseline (ipsative z-score difference .86 to1.53 for participants). All participants remained abstinent at 4-month follow-up. |
| Distress Tolerance | |||||
|---|---|---|---|---|---|
| Study | Sample | Emotional Vulnerability Measure | Design | Smoking Trajectory Relevance | Main Finding |
| Abrantes, et al. (2008) | N = 81 adult smokers completing behavioral DT tasks prior to their smoking cessation intervention | Breath holding, CO2, inhalation persistence, PASAT | Prospective correlational | CR | Compared to those high in DT on a composite index, smokers low in DT at baseline were at greater risk of lapsing on quit day (OR = 9.22). Negative affect related risk for early lapse was strongest among those with low DT vs. high DT (ESU). |
| Bernstein, et al. (2008) | N = 43 moderate smokers (11–20 cig/day) | Breath-holding duration | Lab (quasi) experimental | M | DT was lower following 12-h smoking deprivation than during a smoking-as-usual (d = 0.76). The level of psychiatric symptoms was significantly negatively correlated with DT during the smoking deprivation session (r = −.35), but not the smoking-as-usual session (r = −.18). |
| Brandon et al. (2003) | N= 144 treatment-seeking smokers | APT, MTPT | Prospective correlational | CR | DT was negatively related to, nicotine dependence at baseline (r = −.21 for APT and r = −.18 for MTPT). MTPT was a significant predictor of sustained abstinence, (HR = .998) APT was not related to laspse (ESU). |
| Brown, Lejuez, Kahler, et al. (2002) | N = 32 smokers ≥10 cig/day with history of quit attempts | Breath holding, CO2, inhalation persistence, PASAT | Cross-sectional correlational | CR | Relative to smokers with at least 1 sustained quit attempt of 3 months or longer, smokers who had failed to sustain any previous quit attempt for more than 24 hr exhibited lower DT on the PASAT (AOR= 4.6), CO2 persistence (AOR = 12.2), and breath holding duration (d = 0.93). |
| Brown et al. (2009) | N=81 smokers, motivated for cessation, prior to unaided quit attempts | PASAT; CO2 Inhalation persistence; Breath holding persistence | Prospective correlational | CR | Smokers with high (vs. low) DT on CO2 persistence and breath holding at baseline were at reduced risk of lapsing (RRs = 0.55, 0.98, respectively). DT on the PASAT did not predict lapse risk (RR = 1.0). |
| Bold et al. (2013) | N = 35 | DTS, Breath holding duration | Lab quasi-experimental | M | On a smoking choice task, smokers with lower DT on the DTS were marginally more likely to smoke smoking up to two cigarettes in a 15-minute window versus waiting and receiving four cigarettes after a delay of 45 minutes (d = 0.43, p =.08) and took more puffs (R2 = .201). DT on the DTS was not associated with other outcomes (ESU). DT on breath holding was not associated with smoking task outcomes (ESU). |
| Cameron et al. (2013) | N = 40 smokers participating in a cessation study | PASAT, MTPT | Prospective correlational | CR | Smokers with lower DT on PASAT relapsed more quickly during the quit attempt (r = .43). DT on the MTPT was not associated with time to relapse (r = −.05). |
| Dahene et al., (in press) | N = 153 adults | PASAT | Cross-sectional correlational | I | DT marginally interacted with race to predict smoking status (p = .052), such that within African Americans low DT was significantly related to smoking status (OR = 0.23), whereas within Whites there was no relation between DT and smoking status (OR = 1.07). |
| Hajek, Belcher, & Stapleton (1987) | N = 56 treatment-seeking smokers | Breath holding persistence | Prospective correlational | CR | Lower DT measured before treatment was associated with poorer smoking cessation outcome (r = .44). |
| Leyro et al. (2011) | N = 174 non-treatment seeking daily smokers | DTS | Cross-sectional correlational | P, M | Lower DT was associated with greater number of years smoking (r = −.16), more severe nicotine dependence (r = −.22), and greater expectancies of smoking-induced negative consequences (r = −.23), negative reinforcement (r = −.27), appetite reduction (r = −.30). DT was not significantly associated with cig/day (r = −.13) or expectancies of smoking-induced positive reinforcement (r = −.13). |
| MacPherson et al. 2010 | N = 230 early adolescents (aged 9–13) | BIRD | Prospective correlational | I | Lower DT was not associated with a composite risk behavior index including smoking at baseline or one-year follow-up. DT interacted with risk-taking propensity to predict high-risk behavior at one-year follow-up. |
| Perkins, et al. (2010) | N = 71 healthy adult | DTS | Lab quasi-experimental | M | Ad lib smoking behavior in the lab was greater in those with low (vs. high) DT following acute abstinence relative to other conditions (ηp2 = 0.052). DT did not directly predict or moderate the effects of negative affect manipulations on smoking reward and affect (ESU) |
| Quinn et al. (1996) | N = 52 heavy smokers (≥ 10 cig/day) and N = 57 non smokers | MTPT; APT | Lab quasi-experimental | I | Smokers compared to nonsmokers demonstrated lower DT on the APT (d = 0.68) and MTPT (d = 0.67). |
| Raglan (2013) | 38 Current smokers (≥ 10 cig/day), 21 former smokers (≥ 10 cig/day, quit for ≥ 1 yr), and 27 never smokers | MTPT, FDS | Cross-sectional correlational | I, P, M, CR | Current and former smokers exhibited lower DT on the MTPT than never smokers (ds = .76 and .71, respectively), but did not differ from each other (d = −.08) Groups did not significantly differ in DT on the FDS (ds < 0.27) Among current smokers lower DT on FDS was significantly correlated with urge to smoke (r = .37) and marginally correlated with severity of nicotine dependence (r = .30). DT on the MTPT was not associated with urge or nicotine dependence (rs < −.25) |
| Steinberg et al. (2012) | Smokers with psychotic disorder (N=71) and non-psychiatric smokers (N=78) in smoking cessation treatment | MPTP; Breath holding persistence | Prospective correlational | CR | Lower DT on the MTPT predicted great risk of relapse (ESU). DT on the breath holding persistence test was not associated with relapse likelihood (ESU) |
| Trujillo et al. (2012) | N = 212 non-treatment-seeking smokers (5+ cigs/day) | DTS | Cross-sectional correlational | P, M | DT was not significantly associated with severity of nicotine dependence on the Fagerström Test of Nicotine Dependence (ESU). Lower DT was significantly associated with higher severity of dependence on the Nicotine Dependence Syndrome Scale (β = .19) and Wisconsin Inventory of Smoking Dependence Motives total scale (β = .32) Lower DT was associated with higher smoking for negative reinforcement purposes (β = .33) and urge to smoke to alleviate negative affect (β = .34). |
| Volz et al. (in press) | 56 veterans an community members participating in a smoking cessation trial | MTPT | Prospective correlational | M, CR | Baseline DT did not have a main effect on predicting craving following the quit attempt (ESU). Baseline DT moderated the relation between daily hassles and craving during the quit attempt (explained 4% and 2% of between-person and within-person variance, respectively), such that lower DT was associated with stronger relations between hassles and craving. |
| Concomitant Relation of Multiple Emotional Vulnerabilities to Smoking | |||||
|---|---|---|---|---|---|
| Study | Sample | Emotional Vulnerability Measure | Design | Smoking Trajectory Relevance | Main Finding |
| Kramer et al. (2013) | N = 126 daily non-treatment seeking smokers | ASI-3 DTS |
Cross-sectional correlational | P, CR | AS was not significantly associated with cig/day (r = .04) or number of prior quit attempts (r = .13). AS was significantly associated with greater perceived barriers for cessation related to addiction (e.g., “quitting will make me think of cigarettes all the time”; r = .20), external (e.g., “no encouragement from work for not smoking”; r = .25), and internal (e.g., “quitting will make me feel less in control of my moods”; r = .25) sources. DT was not significantly associated with cig/day (r = .12), number of prior quit attempts (r = −.08), or addiction-related perceived barriers to cessation (r = −.12). Lower DT was significantly associated with greater perceived barriers for cessation related to external (r = −.30), and internal (r = −.42) sources. After controlling for AS, lower DT was significantly associated with greater perceived internal barriers to cessation (β = −.31), but not significantly with addiction-related (β = −.05) or external barriers to cessation (β = −.19) or prior failed quit attempts (β = .06). |
| Lagdon et al. (2013) | N = 65 smokers enrolled in a cessation study | ASI MASQ-Anh |
Prospective Correlational | CR | AS was significantly (positively) related to all of the types of individual withdrawal symptoms on quit day except craving and appetite in univariate analyses (rs = .08 to 28). Anh was significantly (positively) related to all of the individual withdrawal symptoms except craving in univariate analyses (rs = .17 to 30). After controlling for AS and other co-factors, Anh predicted quit day depression, anxiety, and insomnia but not other withdrawal symptoms (ESU). After controlling for Anh and other co-factors, AS was not significantly associated with quit day withdrawal symptoms (ESU). After controlling for AS and other co-factors, Anh significantly predicted faster declines in insomnia over time during the first 2 weeks of quit attempt but was not related to changes in other withdrawal symptoms (ESU). After controlling for Anh and other co-factors, AS significantly predicted slower declines in all withdrawal symptoms except concentration problems and craving (ESU). After controlling for co-factors, the interaction of AS and Anh predicted changes in frustration and restlessness over time but not other withdrawal symptoms (ESU), such individuals with elevated AS and Anh had disproportionately higher rates of escalation of frustration/restlessness during cessation. |
| Zvolensky, Stewart, et al. (2009) | N = 123 daily Canadian smokers enrolled in a cessation treatment study | MASQ-AD ASI |
Prospective correlational | P, M, CR | Anh was associated with higher pre-quit nicotine dependence severity (r = .21) and pre-quit nicotine withdrawal symptom severity (r = .28). After controlling for AS, baseline Anh was associated odds of lapsing on day 1 (OR = 1.04), but not day 7 (HR = 1.02) or 14 (HR = 1.02) of the quit attempt. After controlling for AS, Anh was associated with odds of relapsing on days 1 (OR = 1.04), 7 (HR = 1.02), and 14 (HR = 1.02) of the quit attempt. AS was not significantly associated with pre-quit nicotine dependence severity (r = .04) AS was significantly associated pre-quit nicotine withdrawal symptom severity (r = .25). After controlling for Anh, AS was associated with odds of lapsing on day 1 (OR = 1.04), day 7 (HR = 1.02) and 14 (HR = 1.02) of the quit attempt. After controlling for Anh, AS was not significantly associated odds of relapsing on days 1 (OR = 1.04), 7 (HR = 1.01), or 14 (HR = 1.00) of the quit attempt. |
Note. Beck Depression Inventory-II-Anh Subscale (BDI-Anh; Beck, Steer, Ball, & Ranieri, 1996); Center for Epidemiologic Studies Depression Scale-Anhedonia Scale (CESD-Anh; Radloff, 1977); Fawcett-Clark Pleasure Scale (FCPS; Fawcett, Clark, Scheftner, & Gibbons, 1983); the Mood and Anxiety Symptom Questionnaire-Anhedonic Depression Scale (MASQ-AD; Watson, Clark, Weber, Assenheimer, et al., 1995); Snaith-Hamilton Pleasure Scale (SHAPS; Snaith, et al., 1995); Schedule for Affective Disorders and Schizophrenia (SADS; Endicott & Spitzer, 1978); Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Williams, & Gibbon, 1997); Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006); Subjective Hapiness Scale (SHS; Lyubomirksy & Lepper, 1995); Tripartite Pleasure Inventory-Responsiveness Subscale (TPI-R; Leventhal et al., 2012); World Mental Health Survey Initiative Version of the Composite International Diagnostic Interview (CIDI; Kessler & Ustun, 1994; ); Anxiety Sensitivity Index –Expanded Form (ASI-X; Li & Zinbarg, 2007); Anxiety Sensitivity Index (ASI; Reiss et al., 1986); Smoking Abstinence Expectancies Questionnaire (SAEQ; Abrams et al., 2011); Anxiety Sensitivity Index-Revised (ASI-R; Taylor & Cox, 1998a); Anxiety Sensitivity Index-III (ASi-3; Taylor et al., 2007 2011); Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 2005); State-Trait Anxiety Inventory (STAI: Spielberg, Gorssuch, Lushene, Vagg, & Jacobs, 1983); Taylor Manifest Anxiety Scale (TMAS;Taylor, 1953); Child Anxiety Sensitivity Index (CASI; Silverman et al., 1991); State-Trait Anxiety Inventory for Children (STAIC;Spielberger, 1973); Child Anxiety Frequency Checklist (CAFC;Silverman et al., 1991); Anxiety Sensitivity Profile (ASP; Taylor, & Cox, 1998b); Distress Tolerance Scale (DTS; Simons and Gaher, 2005); Discomfort Intolerance Scale (DIS; Schmidt, Richey, & Fitzpatrick, 2006); Mirror Tracing Persistence Task (MTPT; Brandon et al., 2003); Anagram Persistence Task (APT; Eisenberger & Leonard, 1980); The Paced Auditory Serial Addition Task (PASAT, Lejuez, Kahler, & Brown, 2003); Behavioral Indicator of Resilancy to Distresss (BIRD; Lejuez et al., 2006); Frustration Discomfort Scale (FDS; Harrington, 2005). Relevance to stage in smoking trajectory: I = Initiation; P = Progression; M = Maintenance; CR = Cessation and Relapse. Effect size metrics: d = Cohen’s d; OR = Odds Ratio; HR = Hazards Ratio; RR = Relative Risk; r = Pearson’s correlation coefficient; β = Standardized Regression Weight; ESU = Effect Size Unavailable
Pleasure received from 1) contact with others, 2) performance (work, school, or chores), and 3) recreation;
Response to “Have you ever had a period lasting several days or longer when you lost interest in most things you usually enjoy like work, hobbies, and personal relationships?” yes/no;
Mean of TEPS-Anticipatory, TEPS-Consummatory, and the Subjective Happiness Scale;
Response to item “Have you ever lost interest or pleasure in things you typically enjoy most of the day nearly everyday for 2 weeks or more”;
Response to item “During the past week, I was a lot less interested in things”
We divided identified articles by relevance to one of the following stages: (1) smoking initiation; (2) progression to regular smoking, (3) maintenance, and (4) cessation and relapse processes. In the initiation and progression sections, we included both cross-sectional (e.g., relations of Anh, AS, DT to lifetime smoking status) and prospective studies (e.g., predicting initiation or escalation). For the maintenance sections, we focused on studies examining the relation of Anh, AS, and DT to mechanisms that maintain smoking (e.g., smoking expectancies, craving, withdrawal effects, smoking reinforcement) and indicators of smoking chronicity (e.g., years as a smoker), as well as the effects of smoking/nicotine manipulations on Anh, AS, and DT. The cessation and relapse sections incorporated prospective and retrospective studies of correlates of relapse and cessation-relevant constructs (e.g., perceived barriers to quitting).
Trandiagnostic Emotional Vulnerabilities and Smoking
Anhedonia
Anhedonia: construct and correlates
Definition
Anh reflects diminished appetitive functioning and manifests as deficient happiness and enjoyment as well as decreased pleasure from and interest in stimuli that are commonly rewarding (Hatzigiakoumis, Martinotti, Giannantonio, & Janiri, 2011). In some conceptualizations, Anh is considered a categorical symptom and acute state that onsets in conjunction with the onset of a depressive episode and offsets during remission (APA, 2013). Though often present in major depression, Anh symptom status is only modestly associated with other depressive symptoms (φs .09 – .58) and regularly occurs outside of depression among psychiatric patients (Zimmerman, McGlinchey, Young, & Chelminski, 2006b; Zimmerman et al., 2006a). The personality and psychopathology literature has conceptualized Anh as a trait-like continuous dimension normally distributed in the population (Fawcett, Clark, Scheftner, & Gibbons, 1983). Individuals at the lower end of the Anh spectrum experience higher levels of enjoyment and respond strongly to rewards, whereas those at the upper end of this spectrum exhibit prominent deficits in appetitive experience (Fawcett et al., 1983; Meehl, 1975). Anh is somewhat stable over time (Lyons et al., 1995; Meehl, 2001), but can increase following stress (Berenbaum & Connelly, 1993) and can decrease following clinical intervention (Stein, 2008). Thus, such perspectives posit that Anh is a “trait-like” dimension that is stable yet malleable (Loas, 1996), which we apply in the current paper.
We conceptualize Anh as a multi-level construct—a shared higher-order dimension indicative of diminished appetitive functioning that is composed of related, but distinct lower-order dimensions of: (a) global Anh—reduced happiness and enjoyment derived in one’s life (Carleton et al., 2013); (b) consummatory Anh—incapacity to experience pleasure in response to rewarding stimuli (Gard et al., 2006); and (c) anticipatory Anh—diminished subjective desire and anticipation of pleasant events (Gard et al., 2006). Anh is conceptually and empirically distinct from other emotional constructs, such as extraversion, positive emotionality, alexithymia, affective flattening (i.e., dampened experience of both positive and negative emotions), overall level of depressive symptoms, and negative affect (Fiorito & Simons, 1994; Franken & Muris, 2006; Leventhal et al., 2006).
Although those with higher levels of Anh may respond less strongly to typical rewards, they are not entirely incapable of feeling pleasure and do not necessarily lack a desire to experience pleasure (Gard et al., 2006). Rather, anhedonic individuals require a higher threshold of reward stimulation and more potent reinforcers to experience pleasure (Schlaepfer et al., 2008; Wise, 2008). Low to moderate potency rewarding stimuli that may be pleasant or interesting to most individuals (e.g., viewing a picturesque scene from a high vantage point) may have limited emotional and motivational effects in anhedonic individuals, whereas high potency rewards may still elicit emotional effects (Franken, Zijlstra, & Muris, 2006).
Anh is considered to be a key risk factor for depression onset and chronicity by causing a cyclic cascade of diminished levels of positive reinforcement from and engagement in rewarding anti-depressant behaviors (Lewinsohn, 1974; Loas, 1996). Specifically, repeated experience of diminished pleasure in response to activities that are enjoyable for most other individuals is likely to promote cognitive expectations that many activities are unenjoyable, which in turn, can contribute to anticipatory Anh (i.e., lack of interest/desire in pleasurable activities). Resulting elevations of anticipatory Anh may diminish reward seeking behavior and subsequent exposure to pleasure-eliciting stimuli. Reduced exposure to and pleasure from reward may promote broad deficits in happiness (i.e., global Anh) and potentially feed back into further anhedonic cognitions and experiences. These processes, either alone or in conjunction with other vulnerability factors, may escalate into a pattern of behavioral withdrawal, diminished motivation, fatigue and other depression features (Loas, 1996), which tend to present in melancholic subtypes of depression (Leventhal & Rehm, 2005). Although it is most frequently linked to depression, Anh is elevated in many psychopathologies involving dysregulated appetitive functioning, including psychosis (Cohen, Najolia, Brown, & Minor, 2011), borderline personality disorder (Bandelow, Schmahl, Falkai, & Wedekind, 2010; Marissen, Arnold, & Franken, 2012), social anxiety disoder (Watson & Naragon-Gainey, 2010), attention deficit hyperactivity disorder (Meinzer, Pettit, Leventhal, & Hill, 2012), PTSD (Kashdan, Elhai, & Frueh, 2006), and OCD (Abromovich, Pizzigalli, Reuman, & Wilhem, 2014). Hence, Anh reflects a trandiagnostic process.
Measurement
Distinct facets of the Anh construct have been measured using different methodologies. Global Anh has ben measured using questionnaires assessing reduced happiness and life enjoyment (e.g., “I enjoyed life,” Center for Epidemiologic Studies Depression Scale [CESD] Anh subscale; Radloff, 1991; Shafer, 2006). The consummatory Anh construct is often assessed in questionnaires whereby individuals rate imagined hedonic responses to various experiences that are commonly pleasurable that span hobbies, interests, food, sensory, and social activities (e.g., “Would you find pleasure in a bright sunny day?” Snaith Hamilton Pleasure Scale, [SHAPS]; Snaith, Hamilton, Morley, & Humayan, 1995). Similarly, the anticipatory Anh construct has been measured by questionnaires asking participants to rate interest, desire, and anticipation of such activities (e.g., “When I hear about a new movie starring my favorite actor, I can’t wait to see it,” Temporal Experience of Pleasure Scale [TEPS]; Gard et al. 2006). Measures of these distinct facets of Anh evidence moderate correlations with one another, suggesting that they are non-redundant, but related constructs; yet, these measures also load onto a common higher order latent dimension (Leventhal, Trujillo, et al., 2014). Each of these types of measures exhibit strong internal consistency, convergent validity, and discriminant validity from measure of global depression constructs (Franken, Rassin, & Muris, 2007; Leventhal et al., 2006; Leventhal et al., 2008; Gard et al. 2006).
Anh and Smoking
Theoretical applicability of Anh to smoking
Because anhedonic individuals may recognize that they experience significant pleasure only in response to high-potency rewards (Franken et al., 2006), they may expect particularly strong positive effects from pharmacological rewards like smoking (Stone & Leventhal, 2014). Indeed, data suggests a correlation between Anh and sensation seeking (i.e., trait indicative of needing novel situations or stimulation; Carton, Houezec, Lagrue, & Jouvent, 2000) and the tendency to seek out high-intensity reinforcers (e.g., skydiving; Franken et al., 2006). Hence, anhedonic individuals may be more prone to seek out pharmacological and other high-potency reinforcers in order to experience a pleasure response that may otherwise be deficient. Anhedonic individuals may be more likely to progress from experimentation to regular smoking because of potential psychopharmacological diatheses between Anh and nicotine (Leventhal et al., 2012). Nicotine stimulates mesolimbic dopaminergic release, which amplifies the reinforcing and pleasure-inducing properties of other rewards (Dawkins, Acaster, & Powell, 2007; Paterson, 2009). At the same time, research implicates deficient activity within brain’s mesocorticolimbic dopamine system as potential underpinning of Anh (Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002; Tremblay et al., 2005; Wise, 1982). We suspect that nicotine may temporarily counteract deficient mesolimbic activity and hedonic response to rewards in anhedonic individuals, which could sensitize anhedonic experimenters to the reward-enhancing effects of smoking (Cook et al., 2007), enhance the reinforcing properties of smoking and accelerate smoking progression
Chronic nicotine exposure produces neuroadaptations to the mesolimbic dopamine system, such that nicotine needs to be maintained in order to preserve a homeostatic level of mesolimbic (and hedonic) tone (Watkins, Koob, & Markou, 2000). When chronic nicotine use is discontinued, neuroadaptations to the mesolimbic dopamine system are expressed and the system is in a hypoactive state (Watkins et al., 2000), which may underlie abstinence-induced manifestations of deficient acute positive affect (Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010), diminished incentive salience of reward-associated stimuli (Powell, Pickering, Dawkins, West, & Powell, 2004), and acute elevations of state Anh (Dawkins et al., 2007) that have been illustrated in general samples of smokers. Anh may amplify the psychobiological effects of smoking abstinence via promoting the expression and exacerbation of pre-existing appetitive deficits due to interactions between Anh-related neuropathology and the neurobiological sequelae of nicotine withdrawal (Watkins et al., 2000). The expression of such deficits could theoretically produce a strong motivation to return to smoking in order to counteract these deficits. Overall, these processes could underlie heightened propensity to resume smoking either following brief periods of abstinence (e.g., overnight) or during an intentional cessation attempt in anhedonic individuals, which could ultimately explain Anh’s relation with maintenance of regular smoking.
Empirical data on the relation of Anh to smoking initiation
Several cross-sectional studies have examined the association between Anh and smoking status (i.e., smoker vs. nonsmoker) in adults. Pomerleau and colleagues (2003) showed that global Anh was higher in current versus never smokers in a sample of women. Similarly, global Anh was higher among daily smokers than never smokers in a sample of U.S. young adults (McLeish, Zvolensky, Yartz, & Leyro, 2008). By contrast, Zvolensky, Kotov, Bonn-Miller, Schmidt, and Antipova (2008) did not find a relation between global Anh and smoking status in a representative population-based sample of Moscow, Russia residents, which could suggest that country of origin or age may alter the strength of the Anh-smoking status relation.
Anh has also been studied as a correlate of early smoking experimentation in adolescents. In a cross-sectional study of 14-year-olds who had never had a single puff of a cigarette, those with higher Anh reported greater curiosity about trying smoking but did not differ in willingness or intention to smoke after controlling for overall depression symptoms and demographics (Stone & Leventhal, 2014). The disparate findings across susceptibility indices could reflect differential sensitivity of measures of curiosity (Pierce, Distefan, Kaplan, & Gilpin, 2005). This study also found that teens with higher Anh reported greater expectancies that smoking caused pleasure, despite never smoking a cigarette, suggesting that anticipated effects of smoking in smoking-naive anhedonic youths may confer initiation risk. It is possible that observation of others or extrapolation from direct experience of other high-potency reinforcers (e.g., drugs of abuse, high sugar foods, extreme sports) may cause anhedonic teens to develop expectancies for smoking-induced pleasure, even prior to their first smoking experience.
Research on teen initiation has found that Anh was higher among Chinese adolescents who reported ever “trying a cigarette, even a few puffs?” (Leventhal, Ray, Rhee, & Unger, 2011). A separate study of U.S. adolescents found that consummatory Anh was cross-sectionally associated with smoking status and frequency at age 15 after controlling for depressive symptoms and other co-factors (Audrain-McGovern et al., 2012). However, Anh did not predict likelihood of initiating smoking over the 1.5-year follow up period in that study, indicating that Anh’s relation with smoking experimentation may have occurred prior to age 15.
Empirical data on the relation of Anh to smoking progression and regular smoking
In the only prospective study of smoking progression, Audrain-McGovern et al. (2012) examined escalation patterns and found that age 15 Anh prospectively predicted escalation of smoking frequency over the subsequent 1.5 years. This study controlled for depressive symptoms and other co-factors, suggesting that Anh is unlikely to be an epiphenomenon of depression-related processes that confer smoking progression. Rather these finding suggest that a unique source of affective risk that perhaps may emanate from Anh.
Smoking severity level within the population of individuals who have already established a pattern of regular smoking, which may be an indirect indicator of progression, has also been studied as a cross-sectional correlate of Anh. Several studies have shown that Anh is associated with greater number of cigarettes smoked per day (cig/day) among daily smokers across a range of populations (e.g., treatment-seekers, smokers not interested in quitting, young adults), suggesting that Anh may confer risk for more severe patterns of smoking (Cook et al., 2010; Gregor, Zvolensky, Bernstein, Marshal, & Yartz, 2007; McLeish, Zvolensky, Bonn-Miller, & Bernstein, 2006; Leventhal, Trujillo, Ameringer, Tidey, Sussman, & Kahler, in press; Leventhal, Waters, et al., 2009). Furthermore, Anh is associated with measures of nicotine dependence in some investigations (Leventhal, Kahler, et al., 2009; Leventhal, Piper, et al., 2014; McChargue & Werth Cook, 2007; Mickens et al., 2011; Zvolensky, Stewart, et al., 2009). Other studies have not found evidence that Anh is related to cig/day (Cook, Spring, & McChargue, 2007; Johnson, Stewart, Zvolensky, & Steeves, 2009; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008; Leventhal, Waters, Kahler, Ray, & Sussman, 2009; Mickens et al., 2011; Zvolensky, Johnson, Leyro, Hogan, & Tursi, 2009) and nicotine dependence (Cook et al., 2007; Cook et al., 2012; Leventhal et al., 2008; Leventhal, Waters, Kahler, Ray, & Sussman, 2009; Leventhal, Trujillo, et al., 2014) in daily smokers. Hence, evidence is mixed on this topic.
Results in studies of daily smokers are apt to be at least partially conditional on study entry criteria (e.g., some studies have 10+ cig/day or regular smoking for at least 2 years as inclusion criteria, which could restrict the range at the lower end of the severity spectrum). Further, while cross-sectional analyses in samples of daily smokers are relevant to understanding progression from regular daily smoking to heavier patterns of daily smoking, they do not shed light on progression earlier in the smoking trajectory nor can they rule out alternative causal relations (e.g., smoking influences Anh). Other methodological factors (e.g., variation in sample size and statistical power across studies) may also influence the pattern of results, given data suggesting small relations between Anh and smoking severity that are statistically significant in larger samples (e.g., r = .06 for nicotine dependence, N = 1469; Leventhal, Piper, et al., 2014) but not smaller samples (e.g., r = .09; N = 212; Leventhal, Waters, et al., 2009). Given such methodological considerations, the overall pattern of data, and the prospective evidence illustrating Anh as a risk factor for smoking escalation in youths (Audrain-McGovern et al., 2012), it is likely that Anh plays some role in smoking progression.
Empirical data on the relation of and mechanisms linking Anh to the maintenance of smoking
Literature on whether Anh is cross-sectionally associated with a longer history of smoking is mixed (Cook et al., 2007; Gregor et al., 2007; Leventhal, Waters, et al., 2009; Zvolensky, Johnson, et al., 2009), although such studies are difficult to interpret because of potential confounding between age of participant and years smoking. Regarding the mechanisms maintaining smoking in anhedonic individuals, the motivation to smoke for positive affect and reward enhancement appears to be an important factor for anhedonic individuals, who may otherwise derive little pleasure or reinforcement from rewards. Cook et al. (2007) found that smokers who scored high on a measure of consummatory Anh (i.e., anhedonic smokers) showed a positive affect boost during a positive mood induction when they concurrently smoked a cigarette that contained nicotine. However, when anhedonic smokers smoked a placebo cigarette during the mood induction, their affect did not increase. By contrast, low-Anh smokers showed similar positive affect boosts regardless of the nicotine content of the cigarette smoked during the mood induction. Hence, nicotine may help anhedonic smokers affectively respond to rewards, which otherwise may have little affective impact.
On the other hand, acute smoking abstinence may induce the expression of deficits in reward and positive affect among individuals with high Anh, which may, in turn, motivate the resumption of smoking to offset such deficits. Smokers with higher consummatory and global Anh are more sensitive to the effects of overnight tobacco abstinence (vs. sated states) on declines in state positive affect and reductions in automatic cognitive processing of reward-related stimuli (Cook, Spring, McChargue, & Hedeker, 2004; Leventhal, Ameringer, Osborn, Zvolensky, & Langdon, 2013; Leventhal, Munafo, et al., 2012), even after controlling for depression, negative affect, and/or nicotine dependence. Additional results indicate that measures of consummatory Anh predict greater urge to smoke, in some cases over and above negative affect and nicotine dependence (Cook et al., 2004; Leventhal, Waters, et al., 2009); although, measures of global Anh appear to be less robustly related to urge (Ahnallen et al., 2012; Leventhal, Ameringer, et al., in 2013). Importantly, evidence suggests that these results may be specific to an appetitive (but not an aversive) drive to smoke. Cook et al. (2004) showed that the relation between Anh and abstinence-induced increases in smoking urge were mediated greater abstinence-induced reductions in state positive affect. The same mediational pathway was not found for acute negative affect. Similarly, Leventhal, Waters, et al. (2009) found that Anh predicted greater sensitivity to the amplifying effect of abstinence on the appetitive aspect of smoking urge (e.g., “a cigarette would taste good”), but did not moderate abstinence effects on aversive urge (e.g., “a cigarette would make me less depressed”).
Additional lines of evidence implicate the importance of a disparity between the lack of positive reinforcement from non-smoking rewards the reinforcement derived from smoking as a maintaining mechanism in anhedonic smokers. Two cross-sectional studies of daily smokers have explored relations between Anh and 13 qualitatively unique types of self-reported smoking dependence motives (Leventhal, Waters, et al., 2009; Mickens et al., 2011). These studies found that global and consummatory forms of Anh were positively associated with the behavioral choice melioration subscale of the Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004). This scale taps the tendency to place higher priority on smoking as a reinforcer in comparison to other reinforcers (e.g., “ Very few things give me pleasure each day like cigarettes,” “smoking is the fastest way to reward myself.”). Leventhal, Trujillo, Ameringer, et al. (2014) explored this notion further in a laboratory study of daily smokers who completed an objective behavioral economics choice procedure. They showed that Anh predicted choices indicative of a biased relative reward value of smoking versus an alternative reinforcer (i.e., money), such that anhedonic participants were less willing to delay smoking for money and were more likely to pay for cigarettes when given the opportunity to smoke. These relations were mediated by high negative affect and low positive affect prior to completing the task and persisted after controlling for depressive symptoms, nicotine dependence, and gender.
In addition to studies examining Anh as a trait-like construct, changes in acute Anh as a result of nicotine exposure have been reported. Laboratory studies of regular smokers show that experimentally-manipulated acute tobacco abstinence increases states of consummatory Anh, diminishes ability to modulate behavior as a function of reward (i.e., reward learning), and attenuates the attentional salience of reward-associated stimuli (Dawkins et al., 2007; Powell, Dawkins, & Davis, 2002; Powell, Tait, & Lessiter, 2002; Powell et al., 2004). Additional data indicate that acute nicotine administration alleviates Anh on some of these outcomes (Barr, Pizzagalli, Culhane, Goff, & Evins, 2008; Dawkins, Powell, West, Powell, & Pickering, 2006). Effects of nicotine administration and deprivation on state Anh are thought to be mediated by enhancement of and neuroadaptations to mesolimbic pathway, respectively (Caggiula et al., 2009; D’Souza & Markou, 2010). Thus, Anh appears to be a consequence of regular smoking.
It is plausible that there may be a bi-directional etiological positive feedback loop whereby Anh increases vulnerability to regular smoking, and chronic smoking increases Anh, which, in turn, increases smoking and so on. A clinical study showed that consummatory Anh increased from pre to post-quit and the degree of increase predicted relapse following cessation treatment (Cook, Piper, Kim, Schlam, & Baker, 2012). Hence, those with higher trait Anh prior to smoking may be at risk for smoking initiation and maintenance, as well as the exacerbation of their Anh as a result of nicotine-induced neuroadaptations, which may further motivate smoking.
Empirical data on the relation of Anh to smoking cessation and relapse
Studies have found that Anh increases risk of smoking cessation failure. Leventhal, Ramsey, et al. (2008) assessed the predictive influence of depression symptom constructs on cessation outcomes in smokers enrolled in a clinical trial involving smoking cessation counseling and nicotine replacement therapy (NRT). Four dimensions were measured prior to quit date: global Anh, negative affect (i.e., sadness, crying), somatic features (i.e., sleep, appetite, psychomotor, and concentration problems), and interpersonal problems (i.e., social difficulties). When each dimension was examined in isolation, Anh, negative affect, and somatic features all predicted lower cessation success, with Anh having the strongest effect. When the dimensions were considered concomitantly, only Anh significantly predicted poorer outcomes incrementally to the other dimensions. Both negative affect and somatic features no longer significantly predicted outcomes when controlling for the influence of Anh. These relations remained after controlling for gender, nicotine dependence, cig/day, and history of major depression.
Evidence that Anh incrementally increases risk of cessation failure over and above other factors has been replicated in three separate studies. Among smokers receiving NRT and counseling, Zvolensky, Stewart, et al. (2009) showed that pre-quit levels of global Anh significantly predicted increased risk of lapse (i.e., any smoking) within 24-hours of quitting and increased likelihood of relapse at three successive post-quit assessments over and above nicotine dependence and anxiety symptoms. Among smokers with a history of major depression who attended a one-day smoking cessation counseling workshop, Cook, Spring, McChargue, and Doran (2010) found that greater pre-cessation consummatory Anh was associated with shorter time to relapse after covarying for depressive symptom level and cig/day across a 25 day follow up. More recently, Leventhal, Piper, Japuntich, Cook, and Baker (2014) examined smokers taking part in a clinical trial involving multi-session cessation counseling and randomization to one of several medication or placebo treatment conditions. They found that participants with a lifetime history of anticipatory Anh were more likely to relapse at 8-weeks and 6-months post quit over and above gender, depressed mood, depressive disorder, anxiety or substance use disorder, and nicotine dependence. History of major depression or recurrent depression did not significantly predict cessation after controlling for Anh.
Anh also appears to predict rapid relapses shortly after quitting. Global Anh predicted lapse within the first day following cessation in smokers receiving counseling and NRT (Zvolensky, Stewart, et al., 2009). In a study of smokers motivated to quit who obtained minimal self-help cessation reading materials, Niaura and colleagues (2001) showed that smokers who endorsed anticipatory Anh lapsed quicker (median time to lapse = 0.9 days) than those with no anticipatory Anh (median time to lapse = 1.75 days). Similarly, a cross-sectional study of non-treatment seeking smokers found that consummatory Anh was positively associated with a greater number of prior failed quit attempts as well as a greater proportion of quit attempts that ended in a lapse within the first 24-hours of the attempt (Leventhal, Waters, et al., 2009). These results were incremental to variance in negative affect and suggest that while anhedonic smokers are indeed interested in quitting, their quit attempts tend to end in rapid failure.
Implications for targeting Anh in smoking prevention and cessation
Given evidence implicating Anh in smoking initiation and progression, smoking prevention that targets Anh may be fruitful. Though a formal smoking prevention program that specifically targets Anh has yet to be investigated, Sussman and Leventhal (2014) suggest that educational strategies to promote normalization or acceptance of one’s anhedonic status and increase recognition of subtle increases in pleasure in anhedonic youths might prevent them from experimenting with smoking to obtain pleasure. Methods to counteract Anh directly, such as facilitating exposure to novel, high-threshold rewards that are healthy (e.g., roller coasters, vigorous exercise), might naturally engender pleasure and offset motivation to initiate smoking to obtain pharmacological reward in Anh individuals. School-based activities could be developed that can be completed by individuals, small workgroups, or the whole classroom for universal prevention of smoking that targets Anh. For instance, in the self-esteem enhancement session of the Towards No Drug Abuse prevention program utilized in high school settings (Sussman, Dent, & Stacy, 2002), students note their personal assets and pass compliment to other students, which may perhaps be potent enough social rewards to enhance pleasure in anhedonic teens.
When considering the role of Anh in cessation treatment it is important to note that Anh predicts poor quit outcomes across a number of studies that apply different efficacious medications, including NRT and bupropion (Leventhal, Piper, et al., 2014; Leventhal et al., 2008; Zvolensky, Stewart, et al., 2009), as well as studies that apply different behavioral intervention approaches, including self-help materials (Niaura et al., 2001), one-day workshops (Cook et al., 2010), and standard multi-session cessation counseling (Leventhal, Piper, et al., 2014; Leventhal et al., 2008; Zvolensky, Stewart, et al., 2009). Furthermore, one of these studies found that medication condition (NRT/bupropion vs. placebo) did not significantly moderate the relation of pre-quit lifetime Anh to smoking cessation outcome (Leventhal, Piper, et al., 2014). Thus, existing standard interventions may do little to offset Anh-related risk of cessation failure and identifying tailored treatments that may offset the particular mechanisms underlying the Anh’s effects on smoking is warranted.
Diminished reward and positive affective response to non-smoking alternative rewards as well as heightened reward and affective response to smoking may be important factors that maintain smoking in anhedonic individuals. Hence, candidate medications for offsetting Anh’s effects might successfully mitigate nicotine’s subjective effects without having detrimental effects on the hedonic properties of non-smoking rewards. A recent laboratory study showed that, in comparison to placebo, varenicline—a partial agonist of the ∂4β2 nicotinic acetylcholine receptor that has shown strong efficacy for smoking cessation (Jorenby et al., 2006)—reduced the subjective rewarding effects of smoking following abstinence and diminished the relative reward value of smoking versus an alternative reinforcer (i.e., money) on a behavioral economics measure (McClure et al., 2014). Hence, future exploration of whether varenicline may be particularly efficacious for anhedonic smokers may be fruitful.
Other pharmacotherapy targets for the nicotine receptor system may be warranted. Anh predicts poorer outcomes and higher post-quit withdrawal symptoms among studies involving standard doses of NRT (Langdon et al., 2013; Leventhal, Piper, et al., 2014; Leventhal et al., 2008; Zvolensky, Stewart, et al., 2009). A standard dose of NRT may not provide enough nicotine to entirely offset nicotine withdrawal in anhedonic smokers. Future research is needed to determine whether a higher dose of NRT may be required to more fully ameliorate reward and positive affect deficits that are prominent when anhedonic individuals make a quit attempt.
Candidate behavioral interventions that may heighten the reward and hedonic effects of alternative non-drug reinforcers are worthy of consideration. Behavioral activation (BA), which is based on behavioral approaches to addressing diminished appetitive functioning in depression, aims to enhance one’s ability to access healthy reinforcers and recognize their mood-enhancing effects (Lejuez, Hopko, & Hopko, 2001). This treatment has recently been adapted for smoking cessation (Behavioral Activation Treatment for Smoking; BATS) by integrating BA-specific strategies (e.g., activity monitoring, mood tracking, identifying and planning valued activities) to standard cessation counseling, with the overall aim of structuring reinforcing activities to promote a more rewarding nonsmoking lifestyle (MacPherson et al., 2010). In a pilot trial of smokers with elevated depressive symptoms, an 8-session BATS (vs. standard cessation counseling alone) produced significantly higher rates of smoking abstinence (MacPherson et al., 2010). Pending replication and extension, these results highlight the promise of BA as a strategy to prevent relapse in anhedonic smokers.
Positive psychotherapy (PPT; Seligman, Rashid, & Parks, 2006) is another candidate intervention for offsetting Anh’s impact on smoking. PPT aims to cultivate positive emotions and traits and has recently been adapted into an integrated format for smoking cessation (i.e., Positive Psychotherapy for Smoking Cessation; PPT-S; Kahler et al., 2014). PPT-S includes several PPT exercises that are designed to teach smokers means of obtaining pleasure, satisfaction, and meaning without relying on smoking. For instance, in the savoring exercise, individuals are asked to savor at least two experiences (e.g. their morning coffee; the sun on their face) each day for one week, for at least 2– 3 min per experience. To effectively savor, participants were encouraged to be mindful, ‘in the moment,’ and ‘take in’ all that a given experience had to offer. In a preliminary open-label trial for PPT-S in high-Anh smokers, point prevalence abstinence rates were 47.4% at 8-weeks, 36.9% at 16 weeks, and 31.6% at 26 weeks, which exceeds abstinence benchmarks reported in meta-analyses in U.S. Public Health Service Guidelines clinical practice guidelines for smoking cessation (USDHHS, 2008). Hence, PPT may be a promising strategy for addressing the role of Anh in smoking cessation.
Summary
Empirical data suggestively implicates Anh as a risk factor for smoking initiation and progression across early stages of the smoking trajectory. There consistent empirical evidence that Anh increases risk of smoking cessation failure both early and late in quit attempts. Anticipated and actual amplification of smoking effects on reward and positive affect appears to be a key mechanism that maintains smoking in anhedonic individuals and underlies Anh’s possible effects on progression across several stages of the smoking trajectory. Efforts to cultivate pleasure and prevent smoking behavior as an unhealthy means of obtaining pleasure in anhedonic individuals are candidate smoking interventions that require future evaluation. In many cases, the relation of Anh to smoking processes are incremental to other factors, such as depressive, anxiety symptoms, and nicotine dependence. Thus, evidence is broadly consistent with the transdiagnostic formulation proposed here that Anh may be one common pathway that channels distal risk of smoking carried by emotional disorders.
Anxiety Sensitivity
Anxiety sensitivity: construct and correlates
Definition
The AS construct, defined as the extent to which individuals believe anxiety and anxiety-related sensations have harmful personal consequences (Reiss & McNally, 1985), is a relatively stable, but malleable, factor. The global AS construct encompasses lower-order fears of physical, mental, and publicly observable experiences (Zinbarg, Barlow, & Brown, 1997). High-AS individuals are afraid to experience interoceptive sensations indicative of arousal or other anxiety symptoms because they believe these experiences signal or will lead to cardiac arrest or other feared outcomes; low-AS individuals believe such sensations to be benign. If a person perceives anxiety-related experiences are a sign of imminent harm, they will likely experience anxiety and arousal in response to such cognitions, which could trigger physiological arousal reactions and more anxiety sensations, and in turn increase risk for panic.
Empirically, AS is distinguishable from the tendency to experience more frequent anxiety symptoms (trait anxiety) and other negative affect propensity variables (e.g., neuroticism; Rapee & Medoro, 1994; Zvolensky, Kotov, Antipova, & Schmidt, 2003). In addition to a robust influence of AS on panic psychopathology (McNally, 2002), research also documents AS’s role in the etiology of PTSD (Fedoroff, Taylor, Asmundson, & Koch, 2000), major depressive disorder (Taylor, et al., 1996), social anxiety disorder (Scott, Heimberg, & Jack, 2000), hypochondrias (Eifert & Zvolensky, 2004), chronic pain (Asmundson, Wright, & Hadjistavropoulos, 2000), OCD (Naragan-Gainy, 2010), and other clinical conditions (e.g., asthma; McLeish, Zvolensky, & Luberto, 2011). The data implicating AS in mood and anxiety pathology is strong, consistent across cultures and distinct national groups, and incremental to other risk factors (Hayward, Killen, Kraemer, & Taylor, 2000; Li & Zinbarg, 2007; Maller & Reiss, 1992; Marshall, Miles, & Stewart, 2010; Schmidt, Keough, Mitchell, Reynolds, MacPherson, Zvolensky, & Lejuez, 2010; Schmidt, Lerew, & Jackson, 1997, 1999; Schmidt, Zvolensky, & Maner, 2006). AS may play a role in multiple disorders involving negative mood dysregulation as AS-related tendencies may promote avoidance behavior and prevent the development of adaptive coping responses to a wide variety of emotionally aversive circumstances, and hence negatively reinforce more frequent negative emotional states. Further, AS reduction to be a chief explanatory element (mechanism) that promotes the reduction and remission in emotional symptomatology (Smits, Berry, Tart, and Powers; 2008).
Measurement
AS is most commonly assessed via self-report means on the 16-item Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986). Items are rated on a 5-point Likert scale and cover physical (e.g., “When I notice that my heart is beating rapidly, I worry that I might have a heart attack”), mental (e.g., “When I cannot keep my mind on a task, I worry that I might be going crazy”), and publicly observable experiences (e.g., “It is important not to appear nervous”), which may reflect empirically distinct manifestations of AS (Zinbarg, Barlow, & Brown, 1997). Efforts to improve the psychometric properties the ASI have yielded additional variants of the measures, including Anxiety Sensitivity Index–Revised (ASI-R; Taylor & Cox, 1998a), Anxiety Sensitivity Profile (ASP; Taylor & Cox, 1998b), and the 18-item Anxiety Sensitivity Index–3 (ASI-3; Taylor et al., 2007). The ASI-R and ASP perform relatively poorly in terms of replicability of factor structure and discriminant validity (Armstrong, Khawaja, & Oei, 2006; Deacon, Abramowitz, Woods, & Tolin, 2003), whereas the ASI-3 has shown generally strong psychometric properties (Taylor et al., 2007). Among youth, AS has been measured via the Childhood Anxiety Sensitivity Index, which has shown good psychometric properties (CASI; Silverman, Fleisig, Rabian, & Peterson, 1991). The 18-item CASI asks youth to rate their fear to similar experiences on the adult ASI, but its language is simplified and context is tailored (e.g., “When I cannot keep my mind on my schoolwork I worry that I might be going crazy”).
Anxiety sensitivity and smoking
Theoretical applicability of AS to smoking
A major theoretical premise of work linking AS and smoking is that smoking serves critically important and immediate acute affect regulatory functions, which may override fears about the long-term health consequences of smoking. Perhaps for those high in AS, smoking may be a potential means for offsetting anxiety symptoms or limiting the negative consequences of anxiety in those yet to initiate, which could in turn enhance motivation to experiment with smoking. These expectations may be based in a pharmacological reality, as the administration of tobacco and nicotine acutely diminishes anxiety symptoms and tobacco abstinence increases anxiety in regular smokers (Evans, Blank, Sams, Weaver, & Eissenberg, 2006; Evatt & Kassel, 2010; Leventhal, Waters, et al., 2010). As a result, high-AS individuals may find the anxiolytic effects of smoking highly negatively reinforcing, which could accelerate the progression from experimentation to regular smoking. In the absence of other more adaptive coping strategies, smokers high in AS may learn to rely on smoking to manage anxiety states and fears of bodily sensations in the relatively short-term and overlook long-term health consequences of smoking (Zvolensky & Bernstein, 2005).
Over longer periods of time, smoking itself will lead to increased anxiety-related sensations via a number of routes, including nicotine-based withdrawal symptoms, perceived and objective health impairment, and physical illness (CDC, 2010). For those with pre-existing elevated AS, the development of smoking-induced aversive sensations may heighten one’s fear of such symptoms. Hence, smoking may increase AS. Furthermore, bi-directional learning mechanisms may serve to create a positive feedback loop in which smoking and AS both increase one another. Indeed, because high-AS individuals may experience short-term anxiolysis from smoking, particularly in the presence of anxiety-inducing situations (Evatt & Kassel, 2010), high-AS smokers’ cognitions that internal cues can be personally harmful, dangerous, and anxiety-evoking, may be maintained because of limited opportunity for exposure to anxiety symptoms and resulting extinction and learning processes to ensue. Once regular smoking is established, high-AS smokers may be afraid to make quit attempts, because these persons are apt to be particularly fearful of, and emotionally reactive to, distressing nicotine withdrawal-related sensations (e.g., anxiety, heart rate slowing, concentration difficulty) that occur during smoking abstinence. Thus, a forward feed cycle may develop, whereby smoking is used as a coping strategy for managing aversive states in the short term yet paradoxically confers longer-term risk for the maintenance of smoking in high-AS indivdiausl (Zvolensky et al., 2003).
Empirical data on the relation of AS to smoking initiation
Some cross-sectional studies have found that smokers report higher levels of AS than non-smokers (McLeish et al., 2008; Morissette, Brown, Kamholz, & Gulliver, 2006; c.f., Abrams, Schlosser, et al., 2011), which provide indirect evidence that AS may be related to smoking initiation. Given AS-smoking status relations extend to a sample of individuals with anxiety disorders (Morissette et al., 2006), it is possible that AS is not solely a proxy for manifest anxiety psychopathology and rather a transdiagnostic factor explains variance in smoking status among the population of anxiety-disordered individuals. McLeish et al. (2008) found that the extent to which panic-relevant anxiety symptoms was cross-sectionally associated with greater likelihood of smoking was amplified for individuals who scored high on the ASI-Physical Concerns subscale (e.g., “It scares me when my heart beats rapidly”). These findings raise the possibility that high-AS individuals may initiate smoking as a protective response to their own physiologic anxiety symptoms, as they may expect smoking to help them cope with anxiety and have a strong drive to limit anxiety due to their fear of anxiety-related consequences. Perhaps high-AS individuals might be prone to developing expectancies for smoking-induced anxiety reduction even prior to initiation based on observation of other smokers and extrapolation from other behaviors that have acute anxiolytic properties (e.g., alcohol use; O’Connor, Farrow, & Colder, 2008).
Empirical data on the relation of AS to smoking progression and regular smoking
Although no study has examined AS in the progression from initiation to regular smoking, cross-sectional studies have examined if AS is associated with more severe smoking profiles in those who have already established daily smoking patterns. Results are mixed on this issue, with a wide range of effect sizes for relations of AS to cig/day and nicotine dependence severity in daily smokers (rs = .03 to .44; Johnson et al., 2012; Zvolensky, Farris, Schmidt, & Smits, in press; Zvolensky, Kotov, et al., 2003). Given the methodological caveats with these studies (e.g., cross-sectional, do not assess progression from non-daily to daily smoking), prospective work is warranted to clarify if AS accelerates progression from irregular to regular smoking.
Empirical data on the relation of and mechanisms linking AS to the maintenance of smoking
AS has frequently been linked to stronger smoking outcome expectancies for negative reinforcement (e.g., beliefs smoking will reduce negative affect) across adult treatment-seeking heavy smokers (> 20 cig/day; Brown, Kahler et al., 2001), college student daily smokers (Zvolensky, Feldner, et al., 2004), and adult daily smokers from the community (Abrams, Schlosser, Leger, Donisch, Widmer, & Minkina, 2011; Gonzalez, Zvolensky, Vujanovic, Leyro, & Marshall, 2008; Gregor, Zvolensky, McLeish, Bernstein, & Morrisette, 2008; Guillot, Pang, & Leventhal, 2014). Similarly, AS is correlated with greater expectations that abstinence will exacerbate negative affect and other undesired outcomes in regular smokers (Abrams, Zvolensky, Dorman, Gonzalez, & Mayer, 2011; Guillot et al., 2014). In many of these studies, relations involving AS were evident above and beyond the variance accounted for demographic, manifest psychopathology, and nicotine dependence level. AS also relates to stronger beliefs of smoking leads to health consequences (e.g., Zvolensky et al., 2004), which is in line with theoretical models of AS (i.e., expectancy of negative consequences). The overall weight of scientific evidence is consistent with the perspective that expectations of (and motivation to obtain) smoking’s acute affect-modulatory effects are paramount and outweigh heightened perceptions of smoking’s potential negative consequences in high-AS individuals
Consistent with evidence linking AS to expectancies that abstinence provokes negative affect, AS also is related to the actual experience of more severe nicotine withdrawal symptoms among those initiating an abstinence attempt in the early phases of abstinence (i.e., one week post quit; Johnson, Stewart, Rosenfield, Steeves, & Zvolensky, 2012; Marshall, Johnson, Bergman, Gibson, & Zvolensky, 2009), but less so in later phases of abstinence (Mullane, Stewart, Rhyno, Steeves, Watt, & Eisner, 2008). Importantly, AS-related amplification of withdrawal during abstinence is more robust for withdrawal symptoms that are affective and anxiety-related in nature (i.e., frustration, restlessness, depression, anxiety, irritability) than non-affective withdrawal symptoms (i.e., cigarette craving, concentration problems).
Experimental psychopathology paradigms to study AS, which often utilizes some type of emotion elicitation paradigm and monitors ‘real-time’ responsivity (Forsyth & Zvolensky, 2002), has been leveraged to understand AS-smoking relations. For example, two studies have examined the effect of 12-hour tobacco deprivation or ad lib smoking on subjective anxiety during a carbon dioxide (CO2) procedure provocation challenge. Both studies showed that, although AS amplifies response to CO2 provocation, high-AS (vs. low-AS) smokers do not appear to show an enhanced sensitivity to the subjective effects of CO2 provocation during abstinence (Abrams, Schlosser, Leger, Donisch, Widmer, & Minkina, 2011; Vujanovic and Zvolensky, 2009). Taken together with results above, high (vs. low) AS smokers may be more susceptible to the anticipated and actual effects of abstinence on sustained (“tonic”) levels of subjective anxiety, yet may not experience an amplification of subjective reaction to anxiogenic stimuli (“phasic” anxiety responses) during abstinent states.
In the tobacco administration literature, evidence suggests that AS enhances sensitivity to the anxiolytic effects of acute smoking during stressful anxiogenic conditions (i.e., smoking-induced “phasic” anxiety reductions). For example, using a stressful speech paradigm, Evatt and Kassel (2010) found that laboratory in vivo smoking reduced anxiety in high AS smokers who smoked during a stressful situation, but not a no stress situation; low AS smokers endorsed anxiolysis in both conditions. These results are in line with those of Perkins et al. (2010) who reported that AS predicted greater smoking-induced changes in some measures of state positive and negative affect under certain conditions of stress or smoking abstinence. More recently, Wong and colleagues (2013) indicated that AS predicted greater increases in positive affect pre- to post-cigarette as well as greater smoking satisfaction and psychological reward during a cigarette administration following ad lib smoking; effects remained significant after adjusting for anxiety symptom severity. AS did not predict degree of negative affect and craving suppression or post-cigarette aversive effects. Thus, positive reinforcement mechanisms may be salient etiological processes that maintain smoking in high-AS individuals, at least when not acutely abstinent or stressed. Yet, negative reinforcement (including smoking-induced anxiolysis) mechanisms may be enhanced in high AS-smokers in a stressed state.
Empirical data on the relation of AS to smoking cessation and relapse
Studies of cessation outcomes have shown that AS is associated with greater risk of lapse and relapse. One study found AS was associated with an increased likelihood of smoking lapse (any smoking behavior) during the first week of a quit attempt among depressed smokers receiving combination psychosocial and nicotine replacement therapy (Brown, Kahler et al., 2001). A separate prospective investigation found AS was related to increased relapse likelihood among adult daily smokers by one-month following cessation (Mullane et al., 2008). In a more recent of smokers receiving cessation counseling and NRT, Zvolensky et al. (2009) found that pre-quit AS was significantly associated with an increased risk of early smoking lapse (i.e., any smoking behavior) at days 1, 7, and 14 following the quit day, but not full relapse (i.e., seven consecutive days of smoking). In separate work, adult daily smokers with higher levels of AS reported their longest (lifetime) quit attempts as consisting of relapse within one week post-cessation in smokers residing in Mexico (Zvolensky, Bernstein, Jurado, Colotla, Marshall, & Feldner, 2007) and U.S. (Zvolensky, Bonn-Miller, Bernstein, & Marshall, 2006). Finally, a recent study examined lapse and relapse during a four-week group NRT-aided cognitive-behavioral tobacco intervention program (Assayag, Bernstein, Zvolensky, Steeves, & Stewart, 2012). Results indicated that, participants whom maintained high levels of AS from pre-treatment to 1-month post-treatment, compared to those who demonstrated a significant reduction in AS levels during this time period, showed a significantly increased risk for lapse and relapse. Importantly, the observed AS-smoking quit effects across the reviewed studies are not explained by smoking rate, nicotine dependence, gender, other concurrent substance use (e.g., alcohol, cannabis), manifest emotional symptomatology, withdrawal symptoms, or trait-like negative mood propensity. Hence, high-AS individuals who do not show a reduction in AS during cessation treatment may be at risk of cessation failure, suggesting that treatments that attenuate AS during the smoking cessation process may perhaps improve quit outcomes.
Evidence also highlights the importance of barriers and other factors that thwart quit attempts in high-AS smokeres. AS is related to greater perceived barriers for quitting among daily adult smokers and such an effect is not attributable to negative affectivity, Axis I psychopathology, history of nonclinical panic attacks, smoking rate, and alcohol consumption (Zvolensky et al., 2009). Thus, AS may prevent regular smokers from making a cessation attempt. Consistent with this perspective, Zvolensky, Farris, Schmidt, and Smits (in press) recently found in treatment-seeking daily smokers that AS was indirectly related (i.e., statistical mediation) to greater perceived barriers to cessation, greater number of prior quit attempts, and greater mood-management smoking expectancies through the the tendency to escape and avoid aversive smoking-related thoughts, feelings and internal sensations. Because high-AS individuals may be more apt to excessively worry about the stress of quitting because they inflexibly rely on smoking to cope with anxiety, they may be at risk for treatment drop out. Indeed, in a study of smokers recruited to participate in a self-guided (unaided) quit attempt, Langdon and colleagues (2013) found that that after controlling for the effects of a number of co-factors including pre-quit levels of motivation to quit, AS predicted increased odds of study dropout prior to scheduled quit day. Despite these barriers, AS has been related to increased motivation to quit (Zvolensky et al., 2004), perhaps due to concerns about the health effects of smoking (Zvolensky et al., 2007). Thus, if practitioners could harness such motivation prior to and early in cessation attempts, quit success might be enhanced for high-AS smokers.
Implications for targeting AS in smoking prevention and cessation
If AS in fact confers risk for smoking initiation and expectancies that smoking offsets anxiety plays a role in smoking experimentation, smoking prevention targeting AS-related beliefs may be useful. For example, psychoeducation which acknowledges that although smoking may have some acute anxiolytic properties, the long-term harmful cardiovascular effects of nicotine smoking may actually exacerbate anxiety symptoms and other negative health outcomes, may have preventive effects for high-AS individuals through harnessing healthy fears of smoking-related negative consequences. In addition, extensive mental illness prevention programs that challenge maladaptive fears of anxiety-related sensations and prevent avoidance behaviors might have useful indirect effects on smoking prevention as speculated by some authors (Dudas, Hans, & Barabas, 2005). Existing prevention programs that aim to reduce AS by incorporating behavioral exposure exercises to anxiety-related sensations without executing escape behaviors in which individuals learn corrective information that such sensations are not harmful could be expanded (Schmidt, Eggleston, et al., 2007). We speculate that encouraging youths to avoid smoking (and other substances) as escape behaviors in anxiety prevention programs may perhaps enhance any preventive effect that AS-reduction interventions have on smoking. Yet, prospective evidence linking AS to risk of smoking initiation and progression among youth will be needed prior to full-fledged application of AS-based smoking prevention.
Based on the potential negative effects of AS on smoking cessation outcomes, efforts have been made to target reductions in AS to improve cessation success. Non-tobacco oriented intervention programs for anxiety-mood psychopathology, which target AS via psychoeducation, cognitive restructuring, and interoceptive exposure (Broman-Fulks & Storey, 2008; Gardenswartz & Craske, 2001; Schmidt et al., 2007; Vujanovic, Bernstein, Berenz, & Zvolensky, 2012), have been integrated with smoking cessation programs. In an initial case study, a 16-session integrated AS-smoking cessation treatment successfully reduced AS and improved quit success (Zvolensky, Lejuez, Kahler, & Brown, 2003). Subsequent controlled work has shown that a single session program can reduce AS and facilitate reductions in smoking rate at one-month follow-up (Feldner, Zvolensky, Babson, Leen-Feldner, & Schmidt, 2008). Based upon such work, an 8-session program for smokers high in AS entitled the Anxiety Sensitivity Reduction Program for Smoking Cessation was developed (Zvolensky, Yartz, Gregor, Gonzalez, & Bernstein, 2008). The treatment applied cognitive restructuring and acceptance-oriented behavioral strategies as well as interoceptive exposure to anxiety-related sensations with a specific focus on reducing AS-related beliefs, combined with evidenced-based behavioral counseling for smoking cessation (see Zvolensky, Yartz, et al., 2008; Zvolensky & Farris, 2012, for comprehensive session by session descriptions of the treatment). In a case series evaluation (n = 3), this program reduced AS and facilitated smoking cessation success at one-month follow-up (Zvolensky, Yartz, et al., 2008). These findings have now been replicated and extended to 3-month follow-up (Zvolensky, Bogiaizian, et al., 2014), yileding positive results in terms of acceptability and adherence, positive smoking cessation outcome (5 out of 6 participants were abstinent at 12-week follow-up), and statistically significant reductions in AS. While additional work testing AS-reduction strategies in larger controlled trials is necessary, initial results suggest possible clinical value of such approaches.
Summary
While AS may theoretically play a role in smoking initiation and potentially progression, the empirical evidence base is too limited to draw firm conclusions regarding AS’s role early in the smoking trajectory. Yet, AS is consistently associated with factors that likely drive smoking behavior across the later stages of the smoking trajectory, including impeding the initiation of cessation attempts and derailing their success very early upon abstinence. A key mechanism underlying and maintaining smoking in high-AS individuals involves amplification of anticipated and actual anxiolytic and negative reinforcing effects of smoking. Applying psychoeducation and exposure based strategies to alleviate AS may perhaps be useful in smoking intervention and should be further evaluated. In many studies, AS is associated with smoking-related processes over and above variance in emotional symptomatology and other factors, which is consistent with the notion that AS is a transdiagnostic processes that is a key underlying factor linking anxiety and depressive pathology to smoking.
Distress Tolerance
Distress tolerance: construct and correlates
Definition
The literature general characterizes have two broad, conceptually distinct forms of DT (Leyro et al., 2010): (a) the perceived capacity to withstand negative emotional or other aversive states (e.g., physical discomfort), and (b) the objective behavioral act of withstanding distressing internal states elicited by some type of stressor. We conceptualize DT as a higher-order construct that reflects one’s ability to tolerate and withstand any type of experience that is aversive in nature, which spans a variety of negative emotional states (e.g., stress, frustration, anxiety, non-specific perceptions of feeling upset) or physical sensations that often provoke negative affect, such as pain or other forms of physical discomfort. DT is theorized to be related to, though conceptually distinct from, other variables (e.g., avoidant coping, emotion regulation, experiential avoidance; Leyro et al., 2010). Individuals with lower DT are prone to maladaptively respond to distress, a common manifestation of which involves avoidance and escape of distress-eliciting contexts. In contrast, high-DT individuals may be more able to adaptively respond to distress or distress-eliciting contexts. Theoretically, DT may affect, and be affected by, a variety of processes involved in self-regulation, including attention, cognitive appraisals of distressing emotional and physical states, and emotional as well as behavioral responses to distress. Individuals with a more extensive or qualitatively unique history of emotional experiences may have a greater opportunity to develop a more entrenched or qualitatively distinct type of perceived or behavioral response to distress, which may manifest in low or high DT. The literature shows that DT can be context-dependent (e.g., exacerbated by triggers such as stress, ameliorated by intervention), yet tends to be somewhat stable over time (Leyro & Zvolensky, 2010; Cummings et al., 2013). Hence, it is plausible that, similar to Anh and AS, DT reflects a stable, yet malleable, construct that can be targeted by intervention.
DT is believed to be an explanatory construct implicated in a wide variety of psychopathological symptoms and disorders (Leyro et al., 2010). There is evidence of a consistent empirical relation of low DT to depressive symptoms in a variety of samples (Buckner, Keough, & Schmidt, 2007; Dennhardt & Murphy, 2011; Gorka, Ali, & Daughters, 2012) and poor depression treatment outcome (Williams, Thompson, & Andrews, 2013). There is also a consistent relation between poor DT and anxiety symptoms (Keough et al., 2010), including in samples of children (O’Neil, Rodriguez & Kendall, 2013). DT’s relations extend across several manifestations of anxiety including, PTSD, panic, obsessive compulsive, general worry, and social anxiety symptoms (Marshall-Berenz, Vujanovic, Bonn-Miller, Bernstein, & Zvolensky, 2010; Norr et al., 2013). In addition to its role in emotional pathology, DT is inversely associated with substance-dependence status, substance abstinence duration, and substance use treatment retention (Quinn, Brandon, & Copeland, 1996), antisocial (Daughters, Sargeant, Bornovalova, Gratz, & Lejuez, 2008), and borderline (Bornovalova et al., 2008) personality disorder, eating psychopathology (Anestis, Selby, Fink, & Joiner, 2007), and several other maladaptive processes (e.g., risk taking propensity and risk taking behavior; MacPherson et al., 2010). Hence, DT has trandiagnostic relevance to various emotional disorders and other psychopathological conditions involving emotional disturbance.
Poorer DT is likely to confer risk for emotional pathology via a number of mechanisms. Individuals with low DT are likely to avoid and engage in escape behavior in response to stimuli and situations that produce distress. As a result, negative affective reactions to aversive stimuli are unlikely to habituate due to limited extinction learning and a proneness towards avoidance, which may escalate emotional pathology in various contexts (e.g., social events, physiological arousal). Furthermore, certain manifestations of DT (e.g., tendency to become cognitively absorbed when experiencing distress, feeling ashamed in response to distress; Simons & Gaher, 2005) may prolong negative affect, contribute to maladaptive depressogenic and anxiogenic cognitions, and interfere with one’s ability to effectively function. In addition, avoidance behavior associated with poor DT may prevent the development of positive coping skills, which may in turn worsen emotional psychopathology trajectories.
Measurement
The perceived capacity to tolerate distress has been operationalized in several ways, with two conceptualizations most often applied in the smoking literature (Zvolensky et al., 2010). Tolerance of negative emotional states reflects individual differences in the perceived capacity to withstand negative emotional states (Simons & Gaher, 2005). This construct can be measured via self-report indices, such as the Distress Tolerance Scale (Simons & Gaher, 2005), which instruct participants to agree or disagree with self-statements regarding responses to distressing states (e.g., “I’ll do anything to avoid feeling distressed or upset.”). Tolerance of physical sensations reflects perceived capacity to withstand uncomfortable physical sensations (Schmidt, Richey, & Fitzpatrick, 2006). This construct has been assessed via self-report questionnaires, such as the Discomfort Intolerance Scale (Schmidt, Ritchey, & Fitzpatrick, 2006), which instruct respondents to indicate the extent to which self-statements of discomfort tolerance are characteristic of their typical behavior (e.g., “When I begin to feel physically uncomfortable, I quickly take steps to relieve the discomfort.”).
Behavioral indicators of DT typically measure the duration of time that an individual can withstand exposure to a specific type of experimentally-presented aversive task or stimulus. Tolerance to the experiential distress elicited by such stimuli/tasks is inferred by longer persistence on such tasks. In one area of study, procedures that acutely change levels of oxygen and CO2 in order to induce physiological sensations associated with anxious arousal (e.g., shortness of breath, dizziness) are applied (e.g., voluntary hyperventilation or breath holding, carbon-dioxide-enriched air challenge [CO2 challenge]). In another line of study, DT has been evaluated by means of persistence in completing stressful or frustrating cognitive tasks, such as the Paced Auditory Serial Addition Test (PASAT), Mirror-tracing persistence test (MTPT), and Anagram Persistence Task (APT). DT is operationalized as how long one can continue engagement in the distressing task prior to termination.
DT and Smoking
Theoretical applicability of DT to smoking
Application of DT to smoking has grown, in part, out of Eisenberger’s (1992) learned industriousness theory, which proposed that receiving reinforcement for high-effort behaviors would lead to an increased likelihood of a person putting forth greater effort in later endeavors. As applied to smoking, low-DT individuals may be more apt to smoke because they have learned to utilize ‘low-effort coping skills’ for distress throughout their lives (Quinn, Brandon, & Copeland, 1996). Thus, low-DT individuals are likely to be attuned to identifying behaviors are low-effort and maximum efficacy for reducing or avoiding distress. Hence, low-DT individuals may be susceptible to the development of affect modulation expectancies for such behaviors that circumvent distress and are likely to extrapolate that smoking is one such distress-terminating behavior via observation of other individuals who smoke or translation of expectancies from other acute affect-modulatory behaviors (e.g., emotional eating). Hence, those with poor DT should presumably be more likely to initiate smoking in order to manage experiential discomfort. Upon smoking initiation, those with low DT may be more susceptible to any negative reinforcement from smoking because of the high priority they place on distress escape, which ultimately may accelerate smoking progression for low-DT individuals. Continued negative reinforcement via smoking-induced alleviation of a variety of aversive states, including physical pain (e.g., Ditre, Brandon, Zale, & Meagher, 2011), may be salient factors maintaining smoking for low-DT smokers.
DT theoretically impacts smoking cessation, as individuals who have lower DT may be at the greatest risk of terminating their long-term goal (e.g., abstinence, reductions in use) when in discomfort in favor of the short-term goal of distress termination. During a quit attempt, low-DT smokers may have a low threshold for tolerating aversive states that routinely occur during cessation (e.g., withdrawal symptoms, bodily sensations associated with declining nicotine levels, coping with the stress and frustration of not being able to smoke). Thus, low-DT smokers are likely to respond to such states experienced during cessation with the resumption of smoking behavior aimed at temporarily ameliorating experiential distress.
Empirical data on the relation of DT to smoking initiation
In an early study, Quinn and colleagues (1996) found that nonsmokers persisted significantly longer than smokers on frustrating cognitive tasks. Later, MacPherson et al. (2010) found that levels of self-reported risky behavior index that included cigarette smoking were highest among adolescents lower DT and higher levels of impulsivity; this interactive effect was evident above and beyond the variance accounted for by a number of sociodemographic factors. There was no main effect of DT on risky behaviors in that study. In a more recent study, Raglan (2013) explored DT across current smokers, former smokers, and never smokers. Participants completed the self-report of tolerance for frustration scale (e.g., “I can’t stand doing tasks that seem too difficult.”) and a behaviorally-based DT task that involves mirror tracing (i.e., MTPT). There were no significant differences between groups on perceived tolerance for frustration. Yet, never smokers persisted longer on the MTPT than former or current smokers; there was not a significant difference between current smokers and former smokers. Although these results are notion that low DT may precede smoking, the cross-sectional design precludes conclusions regarding temporality.
Empirical data on the relation of DT to smoking progression and regular smoking
We know of no study of DT in relation to progression across the early end of the smoking trajectory (e.g., initiation to irregular to regular smoking). Studies on the association of DT to nicotine dependence and smoking heaviness among daily smokers tend to show modest or nonsignificant associations. For example, across numerous behavioral tasks measuring DT among daily smokers, no significant associations of DT to cig/day or nicotine dependence were found (Brown et al., 2009). Similar findings have been reported in treatment-seeking smoking samples (Brandt, Johnson, Schmidt, 2012). By contrast, two studies reported modest significant associations of perceived DT to nicotine dependence severity among daily smokers not seeking treatment (Leyro et al., 2010; Trujillo et al., 2012), but DT was not associated with cig/day. Hence, perceived incapacity to handle distress may be more strongly linked with more severe dependence than actual behavioral persistence in the face of distress.
Empirical data on the relation of and mechanisms linking DT to the maintenance of smoking
Cross-sectional evidence indicates that daily smokers with lower DT may have a longer history of smoking (Leyro et al., 2010), suggesting that DT may be a maintaining factor that prolongs regular smoking. Work on the mechanisms linking DT and smoking maintenance indicates that DT may prolong smoking behavior by amplifying the reinforcing properties of smoking. Perkins and colleagues (2010) found lower self-reported DT enhanced acute smoking reinforcement (defined as ad lib smoking) due to abstinence in a laboratory study; DT did not moderate smoking’s effect on mood state. Similarly, in a small exploratory study, Bold et al. (2013) found that while completing a smoking choice task, smokers with lower perceived DT: (1) were marginally more likely to choose to smoke now versus delay smoking for a greater reward; and (2) took more puffs after smoking. Other work indicates that perceived DT is significantly and uniquely related to smoking motives aimed at negative affect management and addiction as well as expectancies for smoking-induced negative affect reduction (Leyro, Zvolensky, Vujanovic, & Bernstein, 2008; Trujillo et al., 2012). Indeed, one study found that, even after controlling for level of anxiety and depressive symptoms, lower perceived DT was associated with greater reported urge to smoke to alleviate negative affect and greater motivation to smoke for negative reinforcement purposes, but not motives for smoking or desire to smoke for positive affect enhancement (Trujillo et al., 2012). Hence, DT may be a transdiagnostic factor incremental to emotional disorder symptoms that maintains smoking via anticipation that smoking aids in negative affect reduction motivation.
Some work has explored the possible exacerbating influence of smoking on acute DT. In a laboratory tobacco deprivation study, Bernstein and colleagues (2008) found that smokers’ breath-holding duration was significantly shorter following 12-hour smoking deprivation period than during a smoking-as-usual session. They also found that psychiatric symptoms were negatively correlated with breath-holding duration during smoking deprivation, but not after smoking-as-usual. These findings suggest that: (1) DT may be context-sensitive and perhaps acutely diminished by smoking abstinence; and (2) the expression of poor DT during abstinence may couple with the expression psychiatric symptoms.
Empirical data on the relation of DT to smoking cessation and relapse
There are fairly consistent associations between poor DT and lower ability to sustain abstinence using retrospective reports of quit history (Brown, Lejuez, Kahler, & Strong, 2002), prospective analyses of pre-quit DT as a predictor of cessation outcomes (Brandon et al., 2003; Brown et al., 2009; Cameron et al., 2013; Hajek, 1991; Hajek, Belcher, & Stapleton, 1987; Steinberg et al., 2012), and laboratory experimental analogues of relapse behavior (Kahler et al., 2013). Much of this work documents that low DT increases risk of very early lapse behavior, including within the first several hours or days of abstinence (e.g., Abrantes et al., 2008; Brown et al., 2002; Kahler et al., 2013). Associations of low DT to faster relapse latency generally extend across various measures, including breath holding duration (Brown et al., 2002; Brown et al., 2009; Hajek, 1991; Hajek, Belcher, & Stapleton, 1987; c.f., Steinberg et al., 2012), persistence on a CO2 challenge (Brown et al., 2002; Brown et al., 2009), and persistence on psychologically stressful and frustrating tasks (Brandon et al., 2003; Brown et al., 2009; Brown et al., 2002; Cameron et al., 2013). They have also been documented in several populations, including a mixed sample of smokers with and without schizophrenia who were provided cessation counseling and pharmacotherapy (Steinberg et al., 2012). In one of these studies, precessation DT reflected as persistence on a stressful cognitive task increased monotonically across: (1) cessation treatment dropouts, (2) lapsers, and (3) abstinence maintainers (Brandon et al., 2003). Hence, low DT is prognosticative of poor cessation outcome in several contexts.
In a mechanistic analysis of the role of DT in cessation outcome, Abrantes and colleagues (2008) divided smokers who completed laboratory-based, behavioral DT tasks (PASAT, breath holding duration, and CO2 persistence) prior to an unaided quit attempt into low, average, and high DT on the tasks. Low DT smokers were significantly more likely to lapse on the assigned quit day than high DT smokers. Furthermore, the extent to which negative affect on quit day predicted lapse was stronger in smokers with low versus high DT. These results were not explained by overall level of depressive symptoms, which did not meaningfully predict cessation outcome in this study. Addressing a similar mechanism, Volz et al. (in press) showed that low baseline DT amplified the relation between daily self-reported hassles and cigarette craving that took place during a cessation attempt. Hence, low-DT smokers appear to be more likely to respond to the distress occurring in abstinence with strong motivations to resume smoking, perhaps to terminate such distress, and this risk pathway is potentially more proximal than any distal influence on relapse caused by depressive symptomatology.
Implications for targeting DT in smoking prevention and cessation
Although prospective studies examining relations of DT with smoking initiation in youth samples is needed prior to developing smoking prevention interventions that specifically target DT, a potential contextual role of DT in smoking prevention might be considered. Many empirically-supported smoking prevention programs teach youths refusal skills to promote assertive resistance to social pressures by their peers to smoke (Botvin & Griffin, 2007). In theory, individuals with lower DT might find it a particularly difficult to tolerate stress associated with going against social influences to smoke and ultimately give in and smoke to terminate uncomfortable feelings that may accompany resisting offers to smoke. While targeting the DT per se in prevention may not yet be indicated based on the dearth of DT and initiation research, considering the contextual backdrop of DT in relation to the psychosocial processes that confer smoking initiation risk may be a worthwhile pursuit in smoking prevention efforts.
To the extent low DT impairs sustained smoking abstinence, interventions that cultivate a greater willingness to tolerate or accept experiential distress should promote greater success in quitting or maintaining abstinence (e.g., Brown et al., 2005). In fact, there have been efforts to apply acceptance-oriented treatment strategies to smokers more generally (Bricker, Mann, Marek, Liu, & Peterson, 2010; Gifford et al., 2004; Hernandez-Lopez, Luciano, Bricker, Roales-Nieto, & Montesinos, 2009), although this work has not focused on DT per se. In the first smoking study that specifically targeted DT, Brown and colleagues (2008) developed an intervention for smokers with a history of early lapses (i.e., individuals with no quit attempts lasting longer than 72 hours in the last 10 years). The treatment included six 50-minute individual sessions, nine 2-hour group sessions, and 8 weeks of nicotine replacement therapy over the course of ten weeks. Specific treatment components included psychoeducation about smoking triggers, self-management strategies for dealing with external triggers, withdrawal-based exposure exercises aimed at enhancing tolerance of withdrawal-related states and sensations via increasing exposure to tobacco abstinence, NRT, and a collection of acceptance strategies. In a small single-group pilot study, the end of treatment (4 weeks post-quit) 7-day point prevalence of abstinence was 31%. By 8-, 13-, and 26-weeks post-quit, abstinence rates were 25%, 18%, and 0% respectively. Despite the low abstinence rates, these participants, who reported a history of no quit attempts lasting longer than 3 days in the last 10 years, achieved a median of 24 days of continuous abstinence, and 40.5 days of non-continuous abstinence. Further, although most participants lapsed quickly, they did not evidence full-blown relapse (7 consecutive days of smoking) until much later into their attempt (median = 45.5 days), and many continued to make quit attempts after lapsing (median number of attempts = 2.5).
In a follow-up preliminary randomized trial of smokers with early lapse history, Brown and colleagues (in press) compared this DT-based cessation treatment (n = 27) to standard smoking cessation counseling (n = 22), with all receiving transdermal NRT. Results indicated that DT treatment participants were more likely to be abstinent at the end of behavioral treatment and were also more likely to recover from lapses that occurred during treatment. Relative to standard cessation treatment, DT treatment participants also reported a larger decrease in emotional avoidance, a hypothesized DT treatment mediator, prior to quit day. The trajectory of negative mood and withdrawal symptoms in DT treatment differed from standard treatmetn and was largely consistent with hypotheses. However, there were no group differences in abstinence rates at long-term follow-ups (8-, 13-, and 26-weeks after quit day).
Summary
Although direct empirical evidence of the role of DT in the early stages of the smoking trajectory is sparse, DT is theoretically relevant to smoking initiation and progression and is worthy of consideration at least as a contextual factor in smoking prevention. Evidence consistently implicates DT in the maintenance of regular smoking behavior over time and the precipitation of relapse early in a quit attempt. A key putative mechanism is that individuals with lower DT may be more likely to anticipate or act on sources of distress (elicited by a variety of mechanisms) with smoking in order to terminate such distress. While efforts to cultivate DT may improve cessation outcomes, therapeutic tactics to target DT applied up to this point show promise but require further refinement to meaningfully enhance cessation outcomes. Some results suggest that DT independently relates to smoking processes over and above depressive and anxiety symptoms, which is consistent with a transdiagnostic formulation of DT as a linking mechanism between emotional symptomatology and smoking.
Concomitant Relations of Multiple Trandiagnostic Emotional Vulnerabilities to Smoking
We have thus far focused on how each of the three trandiagnostic emotional vulnerabilities operate independently without considering their collective relation to smoking. Understanding the extent to which Anh, AS, and DT have unique, overlapping, or interactive relations to smoking would provide a comprehensive, yet nuanced, view of how transdiagnostic vulnerabilities may underlie the comorbidity between smoking and a variety manifestations of emotional disorders that may involve multiple emotional influences on smoking. We are aware of only three studies of these concomitant relations below.
In a cross-sectional test, Kraemer et al. (2013) showed that after controlling for the covariance between AS and DT as well as other factors, lower perceived DT significantly predicted self-reported internal barriers to cessation (e.g., “quitting will make me feel less in control of my moods”) but not external or addiction-related barriers to cessation or number of prior quit attempts. The effects of AS over and above DT were not reported. Univariate analyses illustrated that higher AS and lower DT were correlated with higher levels of perceived cessation barriers across a number of domains in that study.
In a cessation study, Zvolensky et al. (2009) found that after controlling for the covariance between AS and Anh and other factors, pre-quit levels of AS incrementally predicted risk of early smoking lapse (i.e., any smoking behavior) at each assessment point during the first 14 days post-quit but did not predict not relapse (i.e., seven consecutive days of smoking). In addition, Anh incrementally predicted lapse only on quit day, but also predicted relapse throughout the 14 days of follow up. In a follow up report, pre-quit levels of Anh but not AS predicted quit day levels of mood-based nicotine withdrawal symptoms when the covariance of Anh and AS was adjusted for (Langdon et al., 2013). Alternatively, AS, but not Anh predicted change in most nicotine withdrawal symptoms across the 14 days following cessation with high-AS individuals showing slower declines in withdrawal symptoms over time. Furthermore, there were interactive effects between the two vulnerability factors, such that individuals with elevated AS and Anh showed continued escalation of frustration and restlessness during the first two weeks of cessation, whereas the majority of the sample showed a decline in these symptoms. Collectively, these findings suggest that AS and Anh may be play unique roles in smoking cessation failure, with regards to both risk prediction, mechanisms of relapse, and which stage of the cessation process they exert their influence (e.g., lapse vs. relapse and quit day vs. later).
Remaining Gaps in the Literature
Little Integrative Work Across Emotional Vulnerabilities and their Role in Diverse Manifestations of Emotional Disorders
Importance of Anh, AS, and DT relative to one another
There is very little study of possible unique, overlapping, and interactive relations of Anh, AS, and DT to smoking. Hence, it is unclear the extent to which these vulnerabilities relate to smoking via unique etiological mechanisms. Although theory suggests some unique mechanisms (e.g., anxiety specific avoidance through smoking vs. general distress avoidance and termination through smoking), it is plausible that AS and DT may have some common linkages to smoking via shared negative reinforcement mechanisms whereby both vulnerability factors affect and result from efforts to avoid or terminate any type of aversive state (e.g., Kraemer et al., 2013). If relations of the vulnerability factors to smoking are indeed non-overlapping, as has been shown with regard to AS and Anh in the prediction of lapse and relapse risk and withdrawal (Zvolensky et al., 2009; Langdon et al., 2013), smoking interventions may benefit from targeting multiple transdiagnostic vulnerabilities. Further, if several factors have interactive effects that multiplicatively increase smoking processes (e.g., Langdon et al., 2013), individuals high on multiple transdiagnostic vulnerability factors may require especially high intensity and specialized smoking interventions.
Explanatory power of Anh, AS, and DT relative to multiple manifestations of emotional disorder
The transdiagnostic formulation proposed here purports that Anh, AS, and DT reflect common pathways that explain risk marked by manifest symptoms of emotional disorders (Figure 1). There is a significant body of work supporting this proposition, by illustrating that Anh, AS, and DT relate to smoking incrementally to emotional disorders and symptoms (e.g., Audrain-McGovern et al., 2012; Brown et al., 2009; Leventhal et al. 2008; Leventhal, Piper et al., 2014; Wong et al., 2013; Zvolensky et al., 2009). However, much of this work has studied such effects relative to a limited set of emotional syndromes (e.g., Anh relative major depression and an overarching category of anxiety disorder, Leventhal, Piper, et al, 2014). Hence, the extent of trandiagnostic relevance to various manifestations of emotional psychopathology (e.g., major depression vs. panic disorder vs. social anxiety disorder vs. PTSD) is not entirely clear. Furthermore, besides a few examples (e.g., emotion regulation; Brandt et al., 2012), there has been limited investigation of Anh, AS, and DT relative to other emotional constructs in the smoking literature. Such research would clarify the ‘catchment area’ of potential types of patients and areas of the literature that could benefit from the transdiagnostic framework put forth here. The application of statistical approaches, such as mediation, to quantify the extent of covariation between emotional disorders and smoking that is accounted for by transdiagnostic emotional vulnerability factors is warranted. Such data could clarify the relative importance of the three factors identified here and whether additional variance indirectly linking smoking and emotional disorder symptoms is unaccounted for and may perhaps reflect the influence of other (possibly transdiagnostic) processes outside of Anh, AS, and DT in emotion-smoking relations. For example, in a recent study of treatment-seeking smokers, emotional disorders were predictive of higher levels of nicotine dependence, greater perceived barriers to cessation, and greater severity of problematic symptoms while quitting in past attempts (Zvolensky, Farris, Leventhal, & Schmidt, in press). Each of these relations was accounted for by the indirect effect of AS, suggesting this construct may partially account for the link between emotional disorders and various clinically-relevant smoking processes.
Targeting multiple trandiagnostic vulnerability factors using a single treatment framework
Though some research for applying psychosocial treatment strategies that target Anh, AS, and DT to enhance smoking cessation have been used (e.g., Brown et al., 2008), the majority of this literature is preliminary and lacks definitive randomized controlled trials. Furthermore, this work has largely been unintegrated, which could reflect, in part, the common emphasis on a single manifest disorder (e.g., Anh treatment is considered more often in populations with depression than those with a primary anxiety concern). Accordingly, efforts to target multiple trandiagnostic vulnerabilities within a common treatment framework are needed, as such a framework could be applicable to a large diagnostically heterogeneous population of smokers with emotional problems.
Limited Conceptualization of Trandiagnostic Emotional Vulnerabilities as Dynamic Factors Over Time and That Reciprocally Relate with Smoking
The majority of smoking research has examined Anh, AS, and DT as static traits. However, as noted above, each of these factors are somewhat malleable in response to certain factors, including smoking. For instance, there is evidence that these factors may be temporarily altered in acute abstinence among regular smokers (Powell, Dawkins, et al., 2002; Powell et al., 2004) and some data suggesting that the shape of trajectories of transdiagnostic emotional vulnerabilities during a cessation attempt may demarcate of smoking relapse risk (AS, Assayag et al., 2012; Anh; Cook et al., 2012). Hence, additional work is needed to examine within-person variation across multiple stages of the smoking uptake or cessation process as well as in response to relevant factors, such as stress or intervention. Such work, which lends itself to ecological momentary assessment methods (Shiffman, 2009), is likely to refine the precision of theoretical models, enhance knowledge of treatment mechanisms, and identify which stages of smoking uptake and cessation processes may require particular prevention and cessation interventions tailored to Anh, AS, and DT levels.
Sparse Research on Developmental Context and Early Risk
There is limited work on how trandiagnostic factors increase risk of (and may result from) smoking initiation and progression in youths. Adolescence is a critical time for the development of neural pathways underlying emotional processing and risk taking (Dahl, 2004). Hence, it is likely that this period may be associated with significant and clinically-relevant malleability in Anh, AS, and DT, changes in smoking behavior, and the coupling of these processes. Not only can studying relations of Anh, AS, and DT to smoking in adolescents be used to identify youths at risk in need of intervention, studying such relations is critical for informing targets for preventive interventions aimed to offset the risk of smoking update due to emotional disorder; and preventing emotional disorder risk impacted by smoking. To date, we are aware of no smoking prevention intervention that specifically targets any of the three transdiagnostic factors.
Poor Understanding of Moderating Factors
Almost all smoking research on trandiagnostic emotional vulnerabilities have used a ‘main effect approach’ with the goal of isolating a bivariate relation between Anh, AS, and DT to smoking processes as they apply to general populations and contexts. However, demographic characteristics such as gender, age, ethnicity, and socioeconomic status have been shown to impact the relation of emotional disorders to smoking and other health behaviors and outcomes (Gavin, Rue, & Takeuchi, 2010; Husky, Mazure, Paliwal, & McKee, 2008). Such factors may mark important sociocultural or biological processes, such as cultural differences in the experience or expression of emotional distress and psychopathology (Hunter & Schmidt, 2010), that could influence how emotional vulnerabilities couple with smoking. Indeed, one study found suggestive evidence that low DT was associated with greater likelihood of being a smoker (vs. non smoker) in African Americans, but the DT-smoking association was attenuated in Whites (Dahene et al., in press). Other factors, such as additional medical or psychiatric comorbidity, which may heighten emotion disturbance and limit alternative coping mechanisms, could ultimately enhance the link between vulnerabilities and smoking. Identifying moderators of the relation of Anh, AS, and DT to smoking could also inform the identification of individuals and contexts in which smoking interventions focused on vulnerabilities may be most effective.
Modeling Variability in Transdiagnostic Factors in the Smoking Literature
Much research on transdiagnostic vulnerability factors in smoking have utilized observed raw score variables and have modeled linear relations of quantitative levels of Anh, AS, and DT to smoking. This traditional approach is based upon two assumptions that may not always be upheld: (1) observed scores are accurate reflections of latent traits; and (2) each successive increase in Anh, AS, or DT is associated with a proportional increase in a smoking variable. Item response theory (IRT) based scaling methods that map particular items (and response categories within items) to different points upon a latent severity dimension might result in more accurate reflections of the latent traits of Anh, AS, and DT (Muthen and Lehman, 1985). Certain items may map differently onto latent dimensions across disparate settings and populations (e.g., males vs. females) and hence require unique modeling strategies. Techniques to assess differential item functioning using IRT can address this phenomenon (Muthen and Lehman, 1985), which may be particularly relevant for modeling how variation in transdiagnostic constructs may change throughout the smoking trajectory. Perhaps in later (vs. earlier) stages of tobacco dependence (e.g., relapse vs. early progression) certain manifestations of Anh, AS, or DT may be more or less discriminatory of underlying latent constructs. IRT has yet to be applied to the emotional vulnerability and smoking literature.
Taxometric analysis, which involves identifying the existence of meaningful breaks in the distribution of continuous observations (Meehl, 2001), may also be a useful application to transdiagnostic factors in smoking. For instance, AS was shown to have some taxonic properties (with dimensional variation within the a taxon) suggesting a qualitatively unique group of individuals with high-AS (Berstein et al., 2005). Thus identifying whether taxons of individuals with high levels of Anh, AS, and DT are at greater risk of smoking is warranted, as continuous variation in these transdiagnostic factors may not proportionally relate to successive increases in smoking. Along similar lines, exploring non-linear models (e.g., quadratic) to the relation of transdiagnostic factors to smoking may also be useful.
Limited Information on Biological Mechanisms
The limited work that has been conducted on biological processes in transdiagnostic vulnerabilities in smoking has focused on whether Anh, AS, and DT moderate the effects of nicotine administration and abstinence manipulations on behavioral outcomes (e.g., Leventhal et al., 2009; Leventhal et al., 2012; Vujanovic & Zvolensky, 2009). Work incorporating pharmacological challenges other than nicotine and positron emission tomography (PET) imaging are likely to yield insights into other neuropharmacological pathways that may innervate with the nicotinic-Acetylcholine receptor circuits to underpin why transdiagnostic emotional vulnerabilities may be linked with smoking. Furthermore, other technologies, including fMRI may yield insight into the neuroanatomical substrates underlying the relation between emotional vulnerabilities and smoking. Finally, molecular genetic research studies are needed. As evidence emerges that links certain gene regions to risk of developing transdiagnostic vulnerabilities (e.g., Corticotropin-Releasing Hormone Receptor Type 1 gene region and Anh; Bogdan, Santesso, Fagerness, Perlis, & Pizzagalli, 2011), the proteins coded for by such genes might be integral into relation between such vulnerabilities and smoking.
Integrative Theoretical Model Linking Transdiagnostic Emotional Vulnerabilities to Smoking
Model Overview
Though important gaps in the literature remain, we apply the existing knowledge base to an integrative theoretical model to explain how and why transdiagnostic emotional vulnerabilities are key to the comorbidity between smoking and emotional disorders (see Figure 2). A central element of this model is that transdiagnostic emotional vulnerabilities directly amplify expected and actual experience of smoking’s three primary acute affect-modulatory effects, which transmit risk of progressing across the smoking trajectory (i.e., experimentation, progression, maintenance, inability to make a cessation attempt, relapse following cessation, and recurrence). Based on empirical findings and theoretical notions reviewed above, we propose that Anh amplifies smoking’s reward (and pleasure) enhancing effects, AS amplifies smoking’s anxiolytic effects, and poor DT amplifies smoking’s distress terminating effects. Given that these three affect modulatory effects may collectively account for a significant portion of critical pathways underlying addiction motivation (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Morissette et al., 2007; Watkins et al., 2000), we argue that it is plausible that Anh, AS, and DT are key factors linking emotional disorders to smoking.
Figure 2.
Integrative theoretical model identifying transdiagnostic emotional vulnerability factors as key elements linking emotional symptoms and syndromes to stages of the smoking trajectory. Different transdiagnostic emotional vulnerabilities are proposed to putiatiavely amplify the impact of different types of smoking’s affect-modulatory effects on smoking behavior: (1) Anh amplifies smoking-induced reward; (2) AS amplifies smoking-induced anxiolysis; and (3) Poor DT amplifies smoking-induced distress reduction. Anh = Anhedonia; AS = Anxiety Sensitivity; DT = Distress Tolerance.
We propose that the effects of Anh, AS, and DT on these three affect-modulatory effects may be manifested across two types of changes in acute tobacco use (i.e., tobacco administration and abstinence) and in two ways (e.g., actual in the moment experience and anticipated effects). That is, we hypothesize that Anh, AS, and DT not only amplify smoking-induced reward, anxiolysis, and distress termination, they also amplify the effects of smoking abstinence on reward declinations, anxiogenisis, and distress exacerbation. We further propose that Anh, AS, and DT amplifies the formation of beliefs that tobacco administration and abstinence produce affect enhancement and worsening, respectively.
Initiation and Progression of Smoking
We suspect that these processes could play out as follows. Never-smoking youths with elevated transdiagnostic emotional vulnerabilities may have stronger expectations that smoking produces affect-enhancing effects (Stone & Leventhal, 2014), based on their learning history in relation to other affect-modulatory behaviors (e.g., low-DT never smokers may learn that certain foods help to alleviate distress due to eating-induced stress reduction) or observational learning (e.g., seeing and hearing from others’ experiences with smoking, tobacco advertising). Additionally, individuals with these vulnerabilities may find information and stimuli in their environment that promote expectancies for affect modulation more personally salient (e.g., high Anh may be hyper-attuned to learning opportunities for behaviors that might produce pleasure, high AS may be hyper-attuned to learning opportunities for behaviors that might produce anxiolysis, low DT individuals may be hyper-attuned to learning opportunities for behaviors that might alleviate distress). Hence, they may be more likely to develop expectancies for smoking-induced affect modulation prior to initiation. Stronger expectations of smoking’s anticipated effects on emotional state along with additional tendencies toward pursuing high-potency and low effort affect-modulatory behaviors and agents, such as nicotine, may heighten willingness to experiment with smoking. Once smoking is initiated, those with elevated transdiagnostic emotional vulnerabilities may experience stronger smoking-induced affect modulation, which is likely to enhance the reinforcing properties of smoking, motivate further smoking behavior, and ultimately accelerate progression from infrequent to regular smoking and possible nicotine dependence. During this process, any cognitive expectations of smoking’s affect modulatory effects may be strengthened based on actual experience. Furthermore, smoking expectancies may serve to enhance actual emotion experienced from smoking (Juliano and Brandon, 2002; i.e., placebo/expectancy effects), and ultimately, promote a positive feedback loop whereby cognitive anticipatory and emotional experiential processes of smoking’s effects bi-directionally enhance one another over time. Such processes may further elevate the reinforcing value of smoking promote progression to regular smoking for individuals with elevated vulnerabilities.
Maintenance of Regular Smoking
Once habitual smoking is established, many smokers are likely to experience temporary states of deprivation prior to any interest in quitting smoking (e.g., smoking restrictions at work; waking up after not smoking overnight). Smokers with elevated transdiagnostic emotional vulnerabilities may experience amplified exacerbations in affect disturbance after these temporary abstinence states (e.g., Cook et al., 2004). These experiences may motivate the quick resumption of smoking behavior to counteract these temporary abstinence-provoked states of affect disturbance, which may be an important maintaining factor for regular smoking. Furthermore, actual experience with smoking-induced affect exacerbation may be highly personally salient for individuals with elevated vulnerabilities and therefore may promote the development of strong cognitive expectations that smoking abstinence worsens affect (e.g., Abrams et al., 2011). Simply believing that abstinence worsens affect may directly enhance any actual emotion disturbance experienced during abstinence (Hendricks and Leventhal, 2013; i.e., placebo/expectancy effects). Hence, cognitive anticipatory and experiential processes involving abstinence-provoked affect disturbance may bi-directionally enhance one another over time further maintaining smoking behavior. Overall, a strong drive to experience smoking-induced affect enhancement and avoid abstinence-induced affect exacerbation may be important factors that maintain regular smoking in those with elevated transdiagnostic emotional vulnerabilities.
Cessation, Relapse, and Recurrence
Due to heightened anticipated and actual experiences that abstinence may exacerbate affect, smokers with elevated transdiagnostic emotional vulnerabilities may perceive greater barriers to cessation and avoid quitting altogether (e.g., Kraemer et al., 2013). Emotionally vulnerable smokers who overcome such barriers and ultimately make cessation attempts may experience strong affect disturbance upon quitting, which may motivate lapses back to smoking. Such lapses are likely to produce particularly strong smoking-induced affect enhancement for those with elevated transdiagnostic emotional vulnerabilities (e.g., Perkins et al., 2010). The strong reinforcement (i.e., affect-enhancing) experience of lapses is likely to motivate additional lapses, as well as further reinforce cognitive expectancies regarding smoking’s affect modulatory effects. Ultimately, the lapse process is likely to eventually promote a full-blown relapse back to pre-cessation smoking level and recurrence and worsening of nicotine dependence. Relapse propensity is a key indicator of loss of control over smoking and abstinence-induced worsening of affect reflects withdrawal. This model and empirical review illustrates that Anh, AS, and DT amplify both of these phenomena, which are core elements of tobacco use disorder (APA, 2013). Accordingly, we can infer that transdiagnostic emotional vulnerabilities play key roles in nicotine dependence risk.
We further hypothesize that during the smoking dependence process, individuals with elevated transdiagnostic emotional vulnerabilities may experience a worsening of Anh, AS, and DT as a result of their smoking. As noted in the sections above, experiencing smoking-induced affect modulation may reinforce maladaptive responses to emotional cues and worsen transdiagnostic emotional vulnerabilities processes. For instance, poor-DT smokers may avoid experiencing a natural habituation to distressing states due to smoking-induced escape of distress and may therefore not learn to strengthen their DT skills. High-Anh smokers may exhibit a narrowing in their repertoire of non-smoking reinforcers, which may lead to less non-smoking positive reinforcement and heightened Anh. High-AS smokers may experience physiological effects from smoking (e.g., smoking-induced cardiovascular symptoms), which could reinforce AS-related fears of anxiety-related sensations. These are some examples of many types of mechanisms whereby smoking might worsen transdiagnostic vulnerability processes. As a result, a positive feedback loop in between transdiagnostic emotional vulnerabilities and smoking behavior may develop (as well as a further heightening of manifest emotional symptomatology produced by worsening Anh, AS, and DT given that these factors putatively underlie emotional pathology risk). Such a feedback loop may produce vicious cycles that may link smoking and a various manifest emotional disorders with trandiagnostic vulnerability factors playing a key intermediate role (see Figure 1). Finally, given the possibility that multiple transdiagnostic vulnerability factors may operate through independent, overlapping, and possibly interactive pathways to and from smoking (e.g., Zvolensky et al., 2009), each of the abovementioned mechanisms may have cumulative impact and account for multi-morbidity of several emotional symptoms and syndromes with smoking.
Conclusion
We are hopeful that the framework put forth here will stimulate exciting new work that meaningfully advances understanding of the comorbidity between emotional disorders and cigarette smoking. On the basis of this review, we conclude that Anh, AS, DT: (a) are distinct from other emotion constructs, manifest symptomatology, and each other; (b) are aptly represented as overarching vulnerability factors that give rise to a variety of different types of emotional symptomatology; (c) are malleable (can be changed, and therefore, targeted in treatment), (d) drive movement across multiple stages of the smoking trajectory (i.e., initiation, escalation/progression, maintenance, cessation/relapse); (e) collectively amplify the anticipated and actual affect modulatory (and reinforcing) properties of smoking in independent ways to drive smoking behavior; (f) are exacerbated by smoking in some cases; and (g) appear to be non-overlapping contributors to certain smoking processes (e.g. relapse risk).
To the extent the conceptualization proposed here is accurate, a primary clinical implication is that an integrated, transdiagnostic approach for smoking intervention (and mental health promotion) that specifically assesses and targets these processes may be warranted for the individuals with one or more emotional comorbidities. First and foremost, the current review suggests that it would behoove smoking cessation practitioners to not solely assess manifest emotional symptomatology. Rather, assessment of trandiagnostic emotional vulnerabilities, including Anh, AS, and DT, may provide clinically-important information for prognosticating which patients may be likely to experience certain treatment barriers (e.g. nicotine withdrawal, cigarette craving, avoidance of quitting, treatment drop out) and be at high risk for relapse at certain stages of the cessation process (e.g., all three factors have been linked to early lapse, Anh has been linked to long-term relapse). Such information could inform the nature and timing cessation treatments and could suggest that patients with concomitant emotional vulnerabilities would at the very least require more intensive intervention than the typical patient. Given some of evidence reviewed above indicates that certain vulnerabilities may confer incremental non-overlapping sources of smoking relapse risk, patients with elevations on multiple transdiagnostic emotional vulnerabilities may require particular clinical attention. Furthermore, this review points toward an eventual transdiagnostic treatment model that may be useful for the overall population of smokers with one or multiple emotional comorbidities. Such a transdiagnostic treatment may ultimately be more efficient and cost-effective than diagnosis-specific treatments, given the increased expense of training in different protocols for each type of comorbidity. For example, a set of distinct therapeutic tactics could be used to target specific emotional vulnerability processes within a common treatment framework (e.g., behavioral exercises will incorporate exposure to smoking-related cues following quit day to enhance tolerance of high-anxiety and distress states without smoking as well as BA to counter the Anh and behavioral and social withdrawal associated with depression that may motivate smoking). Because the treatment literature for targeting transdiagnostic factors is still emerging, future work is sorely needed to develop such integrated protocols, evaluate their efficacy, and explore their relevance to smoking prevention and cessation.
Although the current framework focuses on only three transdiagnostic vulnerability factors and emotion-smoking comorbidity, we believe that the “big picture” elements of this framework can serve as a prototype for other related research agendas. These concepts may potentially generalize to smoking research on other transdiagnostic emotional vulnerabilities (e.g., emotional acceptance). Furthermore, one could envision these transdiagnostic concepts being applied to: (1) understanding the comorbidity between emotional disorder and use, abuse, and addiction to substances other than tobacco; (2) elucidating the underlying elements linking emotional disorders to other behaviors that influence health and are emotionally determined (e.g., physical activity, eating); and (3) identifying transdiagnostic vulnerabilities that span emotional and non-emotional disorders—Anh for instance is implicated in psychotic disorders (Horan, Kring, & Blanchard, 2006)—and the extent to which they account for broad comorbidities with smoking and other behaviors and conditions. Ultimately, theoretically-guided work focusing on transdiagnostic psychopathologic vulnerabilities may be a key paradigm for advancing clinical and scientific efforts dedicated to reducing the public health burden associated with emotion-smoking co-occurrence and possibly other important comorbidities.
Acknowledgments
Dr. Leventhal was supported by National Institute on Drug Abuse Grant DA025041
References
- Abrams K, Zvolensky MJ, Dorman L, Gonzalez A, Mayer M. Development and Validation of the Smoking Abstinence Expectancies Questionnaire. Nicotine & Tobacco Research. 2011;13(12):1296–1304. doi: 10.1093/ntr/ntr184. [DOI] [PubMed] [Google Scholar]
- Abrams K, Schlosser L, Leger K, Donisch K, Widmer A, Minkina A. Panic-relevant cognitive processes among smokers. Journal of Cognitive Psychotherapy. 2011;25(1):71–81. [Google Scholar]
- Abrantes AM, Strong DR, Lejuez CW, Kahler CW, Carpenter LL, Price LH, Brown RA. The role of negative affect in risk for early lapse among low distress tolerance smokers. Addictive behaviors. 2008;33(11):1394–1401. doi: 10.1016/j.addbeh.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnallen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012;196(1):9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anestis MD, Selby EA, Fink EL, Joiner TE. The multifaceted role of distress tolerance in dysregulated eating behaviors. International Journal of Eating Disorders. 2007;40(8):718–726. doi: 10.1002/eat.20471. [DOI] [PubMed] [Google Scholar]
- Armstrong KA, Khawaja NG, Oei TP. Confirmatory factor analysis and psychometric properties of the Anxiety Sensitivity Index-Revised in clinical and normative populations. European Journal of Psychological Assessment. 2006;22(2):116–125. [Google Scholar]
- Asmundson GJG, Wright KD, Hadjistavropoulos HD. Anxiety sensitivity and disabling chronic health conditions: State of the art and future directions. Scandinavian Journal of Behaviour Therapy. 2000;29:100–117. [Google Scholar]
- Assayag Y, Bernstein A, Zvolensky MJ, Steeves D, Stewart SS. Nature and Role of Change in Anxiety Sensitivity During NRT-Aided Cognitive-Behavioral Smoking Cessation Treatment. Cognitive Behaviour Therapy. 2012;41(1):51–62. doi: 10.1080/16506073.2011.632437. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, Sass J. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine Tob Res. 2012;14(10):1187–1196. doi: 10.1093/ntr/nts017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106(1):178–187. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychol Rev. 2010;117(2):623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63(11):1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories- IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Connelly J. The effect of stress on hedonic capacity. J Abnorm Psychol. 1993;102(3):474–481. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Trafton J, Ilgen M, Zvolensky MJ. An evaluation of the role of smoking context on a biobehavioral index of distress tolerance. Addictive behaviors. 2008;33(11):1409–1415. doi: 10.1016/j.addbeh.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci. 2011;31(37):13246–13254. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Gratz KL, Daughters SB, Nick B, Delany-Brumsey A, Lynch TR, Lejuez CW. A multimodal assessment of the relationship between emotion dysregulation and borderline personality disorder among inner-city substance users in residential treatment. Journal of Psychiatric Research. 2008;42(9):717–726. doi: 10.1016/j.jpsychires.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Bold KW, Yoon HY, Chapman GB, McCarthy DE. Factors precicting smoking in a laboratory-based smoking-choice task. Experimental & Clinical Psychopharmacology. 2013;21(2):133–143. doi: 10.1037/a0031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvin GJ, Griffin KW. School-based programmes to prevent alcohol, tobacco and other drug use. International Review of Psychiatry. 2007;19(6):607–615. doi: 10.1080/0954026070179775. [DOI] [PubMed] [Google Scholar]
- Brandt CP, Johnson KA, Schmidt NB, Zvolensky MJ. Main and interactive effects of emotional dysregulation and breath-holding duration in relation to panic-relevant fear and expectancies about anxiety-related sensations among daily smokers. Journal of Anxiety Disorders. 2012;26:173–18. doi: 10.1016/j.janxdis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112(3):448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Breslau N, Klein DF. Smoking and panic attacks: an epidemiologic investigation. Archives of general psychiatry. 1999;56(12):1141. doi: 10.1001/archpsyc.56.12.1141. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychological medicine. 2004;34(2):323–333. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- Bricker JB, Mann SL, Marek PM, Liu J, Peterson AV. Telephone-delivered Acceptance and Commitment Therapy for adult smoking cessation: a feasibility study. Nicotine & Tobacco Research. 2010;12(4):454–458. doi: 10.1093/ntr/ntq002. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Storey KM. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Anxiety, Stress, & Coping. 2008;21(2):117–128. doi: 10.1080/10615800701762675. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addictive Behaviors. 2001;26:887–899. doi: 10.1016/s0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse (theory and clinical implications) Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, Miller IW. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9(7):721–730. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV. Distress Tolerance Treatment for Early-Lapse Smokers Rationale, Program Description, and Preliminary Findings. Behavior Modification. 2008;32(3):302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of abnormal psychology. 1998;107(2):179. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Keough ME, Schmidt NB. Problematic alcohol and cannabis use among young adults: The roles of depression and discomfort and distress tolerance. Addictive Behaviors. 2007;32(9):1957–1963. doi: 10.1016/j.addbeh.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A, Reed KP, Ninnemann A. Reactivity to negative affect in smokers: The role of implicit associations and distress tolerance in smoking cessation. Addictive Behaviors. 2013;38:2905–2912. doi: 10.1016/j.addbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Carton S, Houezec JL, Lagure G, Jouvent R. Relationships between sensation seeking and emotional symptomology during smoking cessation with nicotine patch therapy. Addictive Behaviors. 2000;25(5):653–662. doi: 10.1016/s0306-4603(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Thibodeau MA, Teale MJ, Welch PG, Abrams MP, Robinson T, Asmundson GJ. The center for epidemiologic studies depression scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS One. 2013;8(3):e58067. doi: 10.1371/journal.pone.0058067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2010 Surgeon General’s Report - How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease. Atlanta, Ga: 2010. [PubMed] [Google Scholar]
- Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K, Van Vunakis H. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? Journal of consulting and clinical psychology. 1995;63(3):388. doi: 10.1037//0022-006x.63.3.388. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clin Psychol Rev. 2011;31(3):440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Kim SY, Schlam TR, Baker TB. Anhedonia in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Paper presented at the Annual Meeting for the Society for Research on Nicotine and Tobacco; Houston, TX USA. 2012. [Google Scholar]
- Cook JW, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res. 2010;12(9):978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl) 2007;192(1):87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6(1):39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. Journal of addictive diseases. 1998;17(1):35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- Cummings JR, Bornovalova MA, Ojanen T, Hunt E, MacPherson L, Lejuez C. Time Doesn’t Change Everything: The Longitudinal Course of Distress Tolerance and its Relationship with Externalizing and Internalizing Symptoms During Early Adolescence. Journal of abnormal child psychology. 2013:1–14. doi: 10.1007/s10802-012-9704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Curr Top Behav Neurosci. 2010;3:119–178. doi: 10.1007/7854_2009_20. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Sargeant MN, Bornovalova MA, Gratz KL, Lejuez CW. The relationship between distress tolerance and antisocial personality disorder among male inner-city treatment seeking substance users. Journal of Personality Disorders. 2008;22(5):509–524. doi: 10.1521/pedi.2008.22.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict Behav. 2007;32(2):425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--effects on incentive motivation. Psychopharmacology (Berl) 2006;189(3):355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Deacon BJ, Abramowitz JS, Woods CM, Tolin DF. The Anxiety Sensitivity Index-Revised: psychometric properties and factor structure in two nonclinical samples. Behaviour Research and Therapy. 2003;41(12):1427–1449. doi: 10.1016/s0005-7967(03)00065-2. [DOI] [PubMed] [Google Scholar]
- Dennhardt AA, Murphy JG. Associations between depression, distress tolerance, delay discounting, and alcohol-related problems in European American and African American college students. Psychol Addict Behav. 2011;25(4):595–604. doi: 10.1037/a0025807. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin. 2011;137(6):1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois DJA, Seeds PM, Collins KA. Transdiagnostic approaches to the prevention of depression and anxiety. Journal of Cognitive Psychotherapy. 2009;23(1):44–59. [Google Scholar]
- Dudas RB, Hans K, Barabas K. Anxiey, depression, and smoking in schoolchildren - implications for smoking prevention. The Journal of the Royal Society for the Promotion of Health. 2005;125(2):87–92. doi: 10.1177/146642400512500213. [DOI] [PubMed] [Google Scholar]
- Eifert GH, Zvolensky MJ. Somatoform disorders. Psychopathology: Foundations for a Contemporary Understanding. 2004:313. [Google Scholar]
- Eisenberger R, Leonard JM. Effects of conceptual task difficulty on generalized persistence. American Journal of Psychology. 1980;93:285–298. [PubMed] [Google Scholar]
- Eisenberger R. Learned industriousness. Psychological review. 1992;99(2):248–267. doi: 10.1037/0033-295X.99.2.248. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14(2):121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evatt DP, Kassel JD. Smoking, arousal, and affect: the role of anxiety sensitivity. J Anxiety Disord. 2010;24(1):114–123. doi: 10.1016/j.janxdis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients: The Pleasure Scale. Archives of General Psychiatry. 1983;40(1):79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Babson K, Leen-Feldner EW, Schmidt NB. An integrated approach to panic prevention targeting the empirically supported risk factors of smoking and anxiety sensitivity: Theoretical basis and evidence from a pilot project evaluating feasibility and short-term efficacy. Journal of anxiety disorders. 2008;22(7):1227–1243. doi: 10.1016/j.janxdis.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito ER, Simons RF. Emotional imagery and physical anhedonia. Psychophysiology. 1994;31(5):513–521. doi: 10.1111/j.1469-8986.1994.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Muris P. Gray’s impulsivity dimension: A distinction between reward sensitivity versus rash impulsiveness. Personality and Individual Differences. 2006;40(7):1337–1347. [Google Scholar]
- Franken IHA, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) J Affect Disord. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Zijlstra C, Muris P. Are nonpharmacological induced rewards related to anhedonia? A study among skydivers. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):297–300. doi: 10.1016/j.pnpbp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory component of the experience of pleasure: A scale development study. Personality and Individual Differences. 2006;40:1086–1102. [Google Scholar]
- Gardenswartz CA, Craske MG. Prevention of panic disorder. Behavior Therapy. 2001;32(4):725–737. [Google Scholar]
- Gavin AR, Rue T, Takeuchi D. Racial/ethnic differences in the association between obesity and major depressive disorder: findings from the Comprehensive Psychiatric Epidemiology Surveys. Public Health Rep. 2010;125(5):698–708. doi: 10.1177/003335491012500512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behavior Therapy. 2004;35:689–705. [Google Scholar]
- Gonzalez A, Zvolensky MJ, Vujanovic AA, Leyro TM, Marshall EC. An evaluation of anxiety sensitivity, emotional dysregulation, and negative affectivity among daily cigarette smokers: Relation to smoking motives and barriers to quitting. Journal of psychiatric research. 2008;43(2):138–147. doi: 10.1016/j.jpsychires.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Ali B, Daughters SB. The role of distress tolerance in the relationship between depressive symptoms and problematic alcohol use. Psychol Addict Behav. 2012;26(3):621–626. doi: 10.1037/a0026386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996;4(4):160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Greenberg JB, Ameringer KJ, Trujillo MA, Sun P, Sussman S, Brightman M, Leventhal AM. Associations between posttraumatic stress disorder symptom clusters and cigarette smoking. Psychol Addict Behav. 2012;26(1):89–98. doi: 10.1037/a0024328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor K, Zvolensky MJ, Bernstein A, Marshall EC, Yartz AR. Smoking motives in the prediction of affective vulnerability among young adult daily smokers. Behaviour research and therapy. 2007;45(3):471–482. doi: 10.1016/j.brat.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Gregor KL, Zvolensky MJ, McLeish AC, Bernstein A, Morissette S. Anxiety sensitivity and perceived control over anxiety-related events: Associations with smoking outcome expectancies and perceived cessation barriers among daily smokers. Nicotine & Tobacco Research. 2008;10(4):627–635. doi: 10.1080/14622200801978706. [DOI] [PubMed] [Google Scholar]
- Guillot CR, Pang RD, Leventhal AM. Anxiety sensitivity and negative urgency: A pathway to negative reinforcement-related smoking expectancies. Journal of Addiction Medicine. doi: 10.1097/ADM.0000000000000017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P. Individual differences in difficulty quitting smoking. British Journal of Addiction. 1991;86:555–558. doi: 10.1111/j.1360-0443.1991.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Hajek P, Belcher M, Stapleton J. Breath-holding endurance as a predictor of success in smoking cessation. Addictive Behaviors. 1987;12:285–288. doi: 10.1016/0306-4603(87)90041-4. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. Journal of consulting and clinical psychology. 1994;62(1):141. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hall SM, Munoz RF, Reus VI, Sees KL. Nicotine, negative affect, and depression. Journal of consulting and clinical psychology. 1993;61(5):761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- Harrington N. The frustration discomfort scale: Development and psychometric properties. Clinical Psychology & Psychotherapy. 2005;12(5):374–387. [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Kraemer HC, Taylor C. Predictors of panic attacks in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(2):207–214. doi: 10.1097/00004583-200002000-00021. [DOI] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Leventhal AM. Abstinence related expectancies predict smoking withdrawal effects: Implications for possible causal mechanisms. Psychopharmacology. 2013;230(3):363–373. doi: 10.1007/s00213-013-3169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of Addictive Behaviors. 2009;23(4):723–730. doi: 10.1037/a0017632. 2009-24023-020 [pii] [DOI] [PubMed] [Google Scholar]
- Hitsman B, Borrelli B, McChargue DE, Spring B, Niaura R. History of depression and smoking cessation outcome: a meta-analysis. Journal of consulting and clinical psychology. 2003;71(4):657. doi: 10.1037/0022-006x.71.4.657. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Papandonatos GD, McChargue DE, Demott A, Herrera MJ, Spring B, Niaura R. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev. 2003;23(5):699–717. doi: 10.1016/s0272-7358(03)00070-9. S0272735803000709 [pii] [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Comorbidity and smoking. Nicotine & Tobacco Research. 1999;1(Suppl 2):S149–152. doi: 10.1080/14622299050011981. discussion S165–146. [DOI] [PubMed] [Google Scholar]
- Hughes JR. The hardening hypothesis: Is the ability to quit decreasing due to increasing nicotine dependence? A review and commentary. Drug Alcohol Depend. 2011;117(2–3):111–117. doi: 10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LR, Schmidt NB. Anxiety psychopathology in African American adults: literature review and development of an empirically informed sociocultural model. Psychol Bull. 2010;136(2):211–235. doi: 10.1037/a0018133. [DOI] [PubMed] [Google Scholar]
- Husky MM, Mazure CM, Paliwal P, McKee SA. Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend. 2008;93(1–2):176–179. doi: 10.1016/j.drugalcdep.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Piper ME, Schlam TR, Bolt DM, Baker TB. Do smokers know what we’re talking about? The construct validity of nicotine dependence questionnaire measures. Psychol Assess. 2009;21(4):595–607. doi: 10.1037/a0017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Smith SS, Jorenby DE, Piper ME, Fiore MC, Baker TB. Depression predicts smoking early but not late in a quit attempt. Nicotine and Tobacco Research. 2007;9(6):677–686. doi: 10.1080/14622200701365301. doi:0.1080/14622200701365301. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA: the journal of the American Medical Association. 2000;284(18):2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychology of Addictive Behaviors. 2012;26:289–297. doi: 10.1037/a0024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Stewart SH, Zvolensky MJ, Steeves D. Evaluating the mediating role of coping-based smoking motives among treatment-seeking adult smokers. Nicotine & Tobacco Research. 2009;11(11):1296–1303. doi: 10.1093/Ntr/Ntp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of Abnormal Psychology. 2002;111:88–97. doi: 10.1037/0021-843X.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, Miller IW. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J Abnorm Psychol. 2002;111(4):670–675. doi: 10.1037//0021-843x.111.4.670. [DOI] [PubMed] [Google Scholar]
- Kahler CW, McHugh RK, Metrik J, Spillane NS, Rohsenow DJ. Breath Holding Duration and Self-Reported Smoking Abstinence Intolerance as Predictors of Smoking Lapse Behavior in a Laboratory Analog Task. Nicotine & Tobacco Research. 2013;15(6):1151–1154. doi: 10.1093/ntr/nts231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Busch AM, Leventhal AM. Time-varying smoking abstinence predicts lower depressive symptoms following smoking cessation treatment. Nicotine Tob Res. 2011;13(2):146–150. doi: 10.1093/ntr/ntq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Day A, Clerkin E, Parks A, Leventhal AM, Brown RA. Positive Psychotherapy for Smoking Cessation: Treatment Development, Feasibility and Preliminary Results. Journal of Positive Psychology. 2014;9(1):19–29. doi: 10.1080/17439760.2013.826716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behav Res Ther. 2006;44(3):457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. J Abnorm Psychol. 2000;109(1):161–166. doi: 10.1037//0021-843x.109.1.161. [DOI] [PubMed] [Google Scholar]
- Keough ME, Riccardi CJ, Timpano KR, Mitchell MA, Schmidt NB. Anxiety symptomatology: The association with distress tolerance and anxiety sensitivity. Behavior Therapy. 2010;41(4):567–574. doi: 10.1016/j.beth.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):617. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Iniative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) The International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled SM, Bulloch AG, Williams JV, Hill JC, Lavorato DH, Patten SB. Persistent heavy smoking as risk factor for major depression (MD) incidence–evidence from a longitudinal Canadian cohort of the National Population Health Survey. Journal of psychiatric research. 2012;46(4):436–443. doi: 10.1016/j.jpsychires.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Kraemer KM, McLeish AC, Jeffries ER, Avallone KM, Luberto CM. Distress Tolerance and Perceived Barriers to Smoking Cessation. Substance Abuse. 2013 doi: 10.1080/08897077.2013.771597. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: prospective relationships to nicotine withdrawal symptoms during smoking cessation. J Stud Alcohol Drugs. 2013;74(3):469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Daughters SB, Danielson CW, Ruggiero K. Thebehavioral indicator of resiliency to distress (BIRD) 2006 Unpublished manual. [Google Scholar]
- Lejuez CW, Hopko DR, Hopko SD. A brief behavioral activation treatment for depression. Treatment manual. Behav Modif. 2001;25(2):255–286. doi: 10.1177/0145445501252005. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and Depressive Symptoms and Affective Patterns of Tobacco Withdrawal. Drug Alcohol Dependence. 2013;133(2):324–329. doi: 10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62(12):1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Japuntich SJ, Piper ME, Jorenby DE, Schlam TR, Baker TB. Isolating the Role of Psychological Dysfunction in Smoking Cessation: Relations of Personality and Psychopathology to Attaining Cessation Milestones. Psychol Addict Behav. 2012 doi: 10.1037/a0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Zimmerman M. Refining the depression-nicotine dependence link: patterns of depressive symptoms in psychiatric outpatients with current, past, and no history of nicotine dependence. Addict Behav. 2009;34(3):297–303. doi: 10.1016/j.addbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Munafo M, Tidey JW, Sussman S, Monterosso JR, Sun P, Kahler CW. Anhedonia predicts altered processing of happy faces in abstinent cigarette smokers. Psychopharmacology (Berl) 2012;222(2):343–351. doi: 10.1007/s00213-012-2649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology. 2014;82(1):122–129. doi: 10.1037/a0035046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine and Tobacco Research. 2008;10(3):507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ray LA, Rhee SH, Unger JB. Genetic and Environmental Influences on the Association Between Depressive Symptom Dimensions and Smoking Initiation Among Chinese Adolescent Twins. Nicotine Tob Res. 2011 doi: 10.1093/ntr/ntr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Rehm LP. The empirical status of melancholia: implications for psychology. Clin Psychol Rev. 2005;25(1):25–44. doi: 10.1016/j.cpr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KA, Tidey J, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. Journal of Abnormal Psychology. 2014;123(2):375–386. doi: 10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine and Tobacco Research. 2009;11(9):1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35(12):1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. A behavioral approach to depression. In: Friedman R, Katz M, editors. The psychology of depression: Contempory theory and research. Silver Spring, MD: Winston Sons Publishers; 1974. [Google Scholar]
- Leyro T, Bernstein A, Vujanovic A, McLeish A, Zvolensky M. Distress Tolerance Scale: A Confirmatory Factor Analysis Among Daily Cigarette Smokers. Journal of Psychopathology and Behavioral Assessment. 2010:1–11. doi: 10.1007/s10862-010-9197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ, Bernstein A. Distress tolerance and psychopathological symptoms and disorders: a review of the empirical literature among adults. Psychol Bull. 2010;136(4):576–600. doi: 10.1037/a0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ, Vujanovic AA, Bernstein A. Anxiety sensitivity and smoking motives and outcome expectancies among adult daily smokers: Replication and extension. Nicotine & Tobacco Research. 2008;10:985–994. doi: 10.1080/14622200802097555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zinbarg RE. Anxiety Sensitivity and Panic Attacks A 1-Year Longitudinal Study. Behavior Modification. 2007;31(2):145–161. doi: 10.1177/0145445506296969. [DOI] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41(1):39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Faraone SV, Kremen WS, Yeung AS, Tsuang MT. Correlates of psychosis proneness in relatives of schizophrenic patients. Journal Of Abnormal Psychology. 1995;104(2):390–394. doi: 10.1037//0021-843x.104.2.390. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Lepper H. A measure of subjective happiness: Preliminary reliability and construct validation. Social Indicators Research. 1999;(46):137–155. [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Lejuez CW. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of consulting and clinical psychology. 2010;78(1):55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Reynolds EK, Cassidy J, Daughters S, Mayes L, Wang F, Lejuez CW. Positive and negative reinforcement underlying risk behavior in early adolescents. Prevention Science. 2010;11:331–342. doi: 10.1007/s11121-010-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller RG, Reiss S. Anxiety sensitivity in 1984 and panic attacks in 1987. Journal of Anxiety Disorders. 1992;6:241–247. [Google Scholar]
- Marissen MA, Arnold N, Franken IH. Anhedonia in borderline personality disorder and its relation to symptoms of impulsivity. Psychopathology. 2012;45(3):179–184. doi: 10.1159/000330893. [DOI] [PubMed] [Google Scholar]
- Marshall-Berenz EC, Vujanovic AA, Bonn-Miller MO, Bernstein A, Zvolensky MJ. Multimethod study of distress tolerance and PTSD symptom severity in a trauma-exposed community sample. J Trauma Stress. 2010;23(5):623–630. doi: 10.1002/jts.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EC, Johnson K, Bergman J, Gibson LE, Zvolensky MJ. Anxiety sensitivity and panic reactivity to bodily sensations: Relation to quit-day (acute) nicotine withdrawal symptom severity among daily smokers making a self-guided quit attempt. Experimental and Clinical Psychopharmacology. 2009;17(5):356–364. doi: 10.1037/a0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GN, Miles JN, Stewart SH. Anxiety sensitivity and PTSD symptom severity are reciprocally related: evidence from a longitudinal study of physical trauma survivors. Journal of abnormal psychology. 2010;119(1):143. doi: 10.1037/a0018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChargue DE, Werth Cook JW. Depression vulnerability within smoking research: How accurate are one-item screening items? Addict Behav. 2007;32(2):404–409. doi: 10.1016/j.addbeh.2006.05.006. [DOI] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Malte CA, Chow B, Bailey S, Baker DG, Lavori PW. Integrating tobacco cessation into mental health care for posttraumatic stress disorder. JAMA: The Journal of the American Medical Association. 2010;304(22):2485–2493. doi: 10.1001/jama.2010.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Thompson CE, Yoshimoto D, Malte C, Straits-Troster K, Steele B. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. American Journal of Psychiatry. 2005;162(7):1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: findings from a 10-year longitudinal study. Addiction. 2010;105(9):1652–1659. doi: 10.1111/j.1360-0443.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Bonn-Miller MO, Bernstein A. Perceived health moderates the association between smoking rate and panic vulnerability variables among daily smokers. Depress Anxiety. 2006;23(5):257–265. doi: 10.1002/da.20170. [DOI] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Luberto CM. The role of anxiety sensitivity in terms of asthma control: a pilot test among young adult asthmatics. Journal of Health Psychology. 2011;16(3):439–444. doi: 10.1177/1359105310382584. [DOI] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Yartz AR, Leyro TM. Anxiety sensitivity as a moderator of the association between smoking status and anxiety symptoms and bodily vigilance: replication and extension in a young adult sample. Addict Behav. 2008;33(2):315–327. doi: 10.1016/j.addbeh.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine & Tobacco Research. 2013;15(1):139–148. doi: 10.1093/ntr/nts101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biological Psychiatry. 2002;52:938–946. doi: 10.1016/S0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bulletin Of The Menninger Clinic. 1975;39(4):295–307. [PubMed] [Google Scholar]
- Meehl PE. Primary and secondary hypohedonia. Journal of Abnormal Psychology. 2001;110(1):188–193. doi: 10.1037//0021-843x.110.1.188. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Comorbidity and Taxometrics. Clinical Psychology: Science and Practice. 2001;8:507–519. [Google Scholar]
- Meinzer MC, Pettit JW, Leventhal AM, Hill RM. Explaining the covariance between attention-deficit hyperactivity disorder symptoms and depressive symptoms: the role of hedonic responsivity. J Clin Psychol. 2012;68(10):1111–1121. doi: 10.1002/jclp.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickens L, Greenberg J, Ameringer KJ, Brightman M, Sun P, Leventhal AM. Associations between depressive symptom dimensions and smoking dependence motives. Eval Health Prof. 2011;34(1):81–102. doi: 10.1177/0163278710383562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette SB, Brown TA, Kamholz BW, Gulliver SB. Differences between smokers and nonsmokers with anxiety disorders. J Anxiety Disord. 2006;20(5):597–613. doi: 10.1016/j.janxdis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychol Bull. 2007;133(2):245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Mullane JC, Stewart SH, Rhyno E, Steeves D, Watt M, Eisner A. Anxiety sensitivity and difficulties with smoking cessation. In: Columbus F, editor. Advances in Psychology Research. Vol. 54. Nova Science Publishers; Hauppauge, NY: 2008. [Google Scholar]
- Muthen BO, Lehman J. Multiple group IRT modeling: application to item bias analysis. Journal of Education Studies. 1985;10:133–142. [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15(1):13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Norr AM, Oglesby ME, Capron DW, Raines AM, Korte KJ, Schmidt NB. Evaluating the unique contribution of intolerance of uncertainty relative to other cognitive vulnerability factors in anxiety psychopathology. J Affect Disord. 2013;151(1):136–142. doi: 10.1016/j.jad.2013.05.063. [DOI] [PubMed] [Google Scholar]
- O’Connor RM, Farrow S, Colder CR. Clarifying the anxiety sensitivity and alcohol use relation: considering alcohol expectancies as moderators. Journal of Studies on Alcohol and Drugs. 2008;69(5):765–772. doi: 10.15288/jsad.2008.69.765. [DOI] [PubMed] [Google Scholar]
- O’Neil Rodriguez KA, Kendall PC. Suicidal Ideation in Anxiety-Disordered Youth: Identifying Predictors of Risk. J Clin Child Adolesc Psychol. 2013 doi: 10.1080/15374416.2013.843463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE. The neuropharmacological substrates of nicotine reward: reinforcing versus reinforcement-enhancing effects of nicotine. Behav Pharmacol. 2009;20(3):211–225. doi: 10.1097/FBP.0b013e32832c7083. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health. 1998;88(10):1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peasley-Miklus CE, McLeish AC, Schmidt NB, Zvolensky MJ. An examination of smoking outcome expectancies, smoking motives and trait worry in a sample of treatment-seeking smokers. Addict Behav. 2012;37(4):407–413. doi: 10.1016/j.addbeh.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Concklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210(1):25–34. doi: 10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Distefan JM, Kaplan RM, Gilpin EA. The role of curiosity in smoking initation. Addictive Behaviors. 2005;30:685–696. doi: 10.1016/j.addbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106(2):418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of consulting and clinical psychology. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010;78(1):13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Zucker AN, Stewart AJ. Patterns of depressive symptomatology in women smokers, ex-smokers, and never-smokers. Addict Behav. 2003;28(3):575–582. doi: 10.1016/s0306-4603(01)00257-x. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51(2):151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Powell J, Tait S, Lessiter J. Cigarette smoking and attention to signals of reward and threat in the Stroop paradigm. Addiction. 2002;97(9):1163–1170. doi: 10.1046/j.1360-0443.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29(7):1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH, Copeland AL. Is task persistence related to smoking and substance abuse: The application of learned industriousness theory to addictive behaviors. Experimental and Clinical Psychopharmacology. 1996;4:186–190. doi: 10.1037/1064-1297.4.2.186. [DOI] [Google Scholar]
- Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of Youth and Adolescence. 1991;20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Medoro L. Fear of physical sensations and trait anxiety as mediators of the response to hyperventilation in nonclinical subjects. Journal of Abnormal Psychology. 1994;103:696–699. doi: 10.1037//0021-843x.103.4.693. [DOI] [PubMed] [Google Scholar]
- Raglan GB. Master’s thesis. 2013. Distress tolerance and smoking status: Differences between smokers, former smokers, and never smokers. Retrieved from Washington Reseach Library Contorium. [Google Scholar]
- Reiss S, McNally RJ. Expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. Orlando, FL, US: Academic Press, Inc; 1985. pp. 107–121. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. 0005-7967(86)90143-9 [pii] [DOI] [PubMed] [Google Scholar]
- Sauer-Zavala S, Boswell JF, Gallagher MW, Bentley KH, Ametaj A, Barlow DH. The role of negative affectivity and negative reactivity to emotions in predicting outcomes in the unified protocol for the transdiagnostic treatment of emotional disorders. Behav Res Ther. 2012;50(9):551–557. doi: 10.1016/j.brat.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. 1301408 [pii] [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Eggleston AM, Woolaway-Bickel K, Fitzpatrick KK, Vasey MW, Richey JA. Anxiety Sensitivity Amelioration Training (ASAT): A longitudinal primary prevention program targeting cognitive vulnerability. Journal of Anxiety Disorders. 2007;21(3):302–319. doi: 10.1016/j.janxdis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Keough ME, Mitchell MA, Reynolds EK, MacPherson L, Zvolensky MJ, Lejuez CW. Anxiety sensitivity: prospective prediction of anxiety among early adolescents. Journal of anxiety disorders. 2010;24(5):503–508. doi: 10.1016/j.janxdis.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. The role of anxiety sensitivity in the pathogenesis of panic: Prospective evaluation of spontaneous panic attacks during acute stress. Journal of Abnormal Psychology. 1997;106:355–364. doi: 10.1037//0021-843x.106.3.355. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. Prospective evaluation of anxiety sensitivity in the pathogenesis of panic: Replication and extension. Journal of Abnormal Psychology. 1999;108:532–537. doi: 10.1037//0021-843x.108.3.532. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Fitzpatrick KK. Discomfort intolerance: Development of a construct and measure relevant to panic disorder. Journal of Anxiety Disorders. 2006;20(3):263–280. doi: 10.1016/j.janxdis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: Prospective prediction of panic attacks and Axis I pathology. Journal of Psychiatric Research. 2006;40:691–699. doi: 10.1016/j.jpsychires.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Scott EL, Heimberg RG, Jack MS. Anxiety sensitivity in social phobia: comparison between social phobics with and without panic attacks. Depression and anxiety. 2000;12(4):189–192. doi: 10.1002/1520-6394(2000)12:4<189::AID-DA1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774–788. doi: 10.1037/0003-066X.61.8.774. [DOI] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21(4):486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Fleisig W, Rabian B, Peterson RA. Childhood anxiety sensitivity index. Journal of Clinical Child and Adolescent Psychology. 1991;20(2):162–168. [Google Scholar]
- Simons J, Gaher R. The Distress Tolerance Scale: Development and validation of a self-report measure. Motivation and Emotion. 2005;29:83–102. doi: 10.1007/s11031-005-7955-3. [DOI] [Google Scholar]
- Skrove M, Romundstad P, Indredavik MS. Resilience, lifestyle and symptoms of anxiety and depression in adolescence: the Young-HUNT study. Soc Psychiatry Psychiatr Epidemiol. 2012 doi: 10.1007/s00127-012-0561-2. [DOI] [PubMed] [Google Scholar]
- Smits JA, Berry AC, Tart CD, Powers MB. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: A meta-analytic review. Behaviour research and therapy. 2008;46(9):1047–1054. doi: 10.1016/j.brat.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Smits JA, Powers MB, Cho Y, Telch MJ. Mechanism of change in cognitive-behavioral treatment of panic disorder: evidence for the fear of fear mediational hypothesis. Journal of Consulting and Clinical Psychology. 2004;72(4):646. doi: 10.1037/0022-006X.72.4.646. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A. A scale for the assessment of the hedonic tone: The Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spielberger C. STAIC preliminary manual. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr. 2008;13(7):561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Gandhi KK, Foulds J, Epstein EE, Brandon TH. Task persistence predicts smoking cessation in smokers with and without schizophrenia. Psychology of Addictive Behaviors. 2012;26(4):850–858. doi: 10.1037/a0028375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MD, Leventhal AM. Associations between Anhedonia and Smoking Risk Factors in Never-Smokers. Paper presented at the Society for Nicotine and Tobacco Research Annual Scientific Meeting; Seattle, WA USA. 2014. [Google Scholar]
- Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, Niaura R. Positive reactions to tobacco predict relapse after cessation. Journal of Abnormal Psychology. 2011;120(4):999–1005. doi: 10.1037/a0023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Dent CW, Stacy AW. Project Towards No Drug Abuse: A review of the findings and future directions. American Journal of Health Behavior. 2002;26 (5):354–365. doi: 10.5993/ajhb.26.5.4. [DOI] [PubMed] [Google Scholar]
- Sussman S, Leventhal AM. Substance misuse prevention: Addressing anhedonia. New Directions in Youth Development. 2014;141:45–56. doi: 10.1002/yd.20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA. A Personality Scale of Manifest Anxiety. Journal Abnormal and Social Psychology. 1953;48(2):285–290. doi: 10.1037/h0056264. [DOI] [PubMed] [Google Scholar]
- Taylor S, Cox BJ. An expanded Anxiety Sensitivity Index: Evidence for a hierarchic structure in a clinical sample. Journal of anxiety disorders. 1998a;12(5):463–483. doi: 10.1016/s0887-6185(98)00028-0. [DOI] [PubMed] [Google Scholar]
- Taylor S, Cox BJ. Anxiety sensitivity: multiple dimensions and hierarchic structure. Behaviour research and therapy. 1998b;36(1):37–51. doi: 10.1016/s0005-7967(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Taylor S, Koch WJ, Woody S, McLean P. Anxiety sensitivity and depression: how are they related? Journal of Abnormal Psychology. 1996;105(3):474. doi: 10.1037//0021-843x.105.3.474. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Cardenas SJ. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological assessment. 2007;19(2):176. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: Altered response to dextroamphetamine. Archives of General Psychiatry. 2002;59(5):409–417. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional Neuroanatomical Substrates of Altered Reward Processing in Major Depressive Disorder Revealed by a Dopaminergic Probe. Archives of General Psychiatry. 2005;62(11):1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Trujillo MA, Greenberg JB, Pitts SR, Ameringer KJ, Palau JJ, Leventhal AM. Associations between dimensions of distress tolerance and tobacco dependence characteristics: incremental relations over and above anxiety and depressive symptoms. Poster presented at The College on Problems of Drug Dependence Annual Meeting; Palm Springs, California. 2012. Jun, [Google Scholar]
- Tsourtos G, Ward PR, Muller R, Lawn S, Winefield AH, Hersh D, Coveney J. The importance of resilience and stress to maintaining smoking abstinence and cessation: a qualitative study in Australia with people diagnosed with depression. Health Soc Care Community. 2011;19(3):299–306. doi: 10.1111/j.1365-2524.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (USDHHS) Clinical practice guideline. Rockville, MD: U.S. Public Health Service; 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- Volz AR, Dennis PA, Dennis MF, Calhoun PS, Wilson SM, Beckham JC. The role of daily hassles and distress tolerance in predicting cigarette craving during a quit attempt. Nicotine & Tobacco Research. doi: 10.1093/ntr/ntt286. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Bernstein A, Berenz EC, Zvolensky MJ. Single-session anxiety sensitivity reduction program for trauma-exposed adults: A case series documenting feasibility and initial efficacy. Behavior therapy. 2012;43(3):482–491. doi: 10.1016/j.beth.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Zvolensky MJ. Anxiety sensitivity, acute nicotine withdrawal symptoms, and anxious and fearful responding to bodily sensations: a laboratory test. Experimental and clinical psychopharmacology. 2009;17(3):181. doi: 10.1037/a0016266. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2(1):19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin Psychol Rev. 2010;30(7):839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Mazure CM, Morlett A, McKee SA. Two decades of smoking cessation treatment research on smokers with depression: 1990–2010. Nicotine & tobacco research. 2013;15(6):1014–1031. doi: 10.1093/ntr/nts213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pilver CE, Desai RA, Mazure CM, McKee SA. The relationship of dysthymia, minor depression, and gender to changes in smoking for current and former smokers: Longitudinal evaluation in the U.S. population. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm K, Wedgwood L, Niven H, Kay-Lambkin F. Smoking cessation and depression: current knowledge and future directions. Drug and alcohol review. 2006;25(1):97–107. doi: 10.1080/09595230500459560. [DOI] [PubMed] [Google Scholar]
- Williams AD, Thompson J, Andrews G. The impact of psychological distress tolerance in the treatment of depression. Behaviour research and therapy. 2013;51(8):469–475. doi: 10.1016/j.brat.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neuroleptics and operant behavior: The anhedonia hypothesis. Behavioral and Brain Sciences. 1982;5(1):39–87. [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Krajisnik A, Truong L, Lisha NE, Trujillo M, Greenberg JB, Leventhal AM. Anxiety sensitivity as a predictor of acute subjective effects of smoking. Nicotine & Tobacco Research. 2013;15(6):1084–1090. doi: 10.1093/ntr/nts208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. American Journal of Public Health. 1999;89(12):1837–1840. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Young D, Chelminski I. Diagnosing major depressive disorder I: A psychometric evaluation of the DSM-IV symptom criteria. J Nerv Ment Dis. 2006a;194(3):158–163. doi: 10.1097/01.nmd.0000202239.20315.16. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Young D, Chelminski I. Diagnosing major depressive disorder: II: is there justification for compound symptom criteria? J Nerv Ment Dis. 2006b;194(4):235–240. doi: 10.1097/01.nmd.0000207423.36765.89. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Barlow DH, Brown TA. Hierarchical structure and general factor saturation of the Anxiety Sensitivity Index: Evidence and implications. Psychological Assessment. 1997;9:277–284. [Google Scholar]
- Zvolensky MJ, Bernstein A. Cigarette smoking and panic psychopathology. Current Directions in Psychological Science. 2005;14:301–305. doi: 10.1177/0963721410388642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A, Cardenas SJ, Colotla VA, Marshall EC, Feldner MT. Anxiety sensitivity and early relapse to smoking: A test among Mexican daily, low-level smokers. Nicotine & Tobacco Research. 2007;9(4):483–491. doi: 10.1080/14622200701239621. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J. An anxiety sensitivity reduction smoking cessation program for Spanish-speaking smokers. Cognitive and Behavioral Practice. 2014;21:350–363. [Google Scholar]
- Zvolensky M, Bonn-Miller M, Bernstein A, Marshall E. Anxiety sensitivity and abstinence duration to smoking. Journal of Mental Health. 2006;15(6):659–670. [Google Scholar]
- Zvolensky MJ, Farris SG, Leventhal AM, Schmidt NB. Anxiety sensitivity mediates relations between emotional disorders and smoking. Psychology of Addictive Behaviors. doi: 10.1037/a0037450. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG. Theory and practice of an integrated approach for the treatment of comorbid anxiety psychopathology and tobacco use and dependence: The example of panic. In: Serebrisky D, Muller F, editors. Smoking and psychiatric illness: Tools for action. Buenos Aires, Argentina: Sciens; 2012. pp. 113–130. [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner E, Bonn-Miller MO, McLeish AC, Gregor K. Evaluating the role of anxiety sensitivity in smoking outcome expectancies among regular smokers. Cognitive Therapy and Research. 2004;28:473–486. [Google Scholar]
- Zvolensky MJ, Forsyth JP, Bernstein A, Leen-Feldner EW. A concurrent test of the anxiety sensitivity taxon: Its relation to bodily vigilance and perceptions of control over anxiety-related events in a sample of young adults. Journal of Cognitive Psychotherapy. 2007;21:72–90. [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Feldner MT. Impact of Posttraumatic Stress Disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine and Tobacco Research. 2008;10(8):1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Johnson KA, Leyro TM, Hogan J, Tursi L. Quit-Attempt History: Relation to Current Levels of Emotional Vulnerability Among Adult Cigarette Users. J Stud Alcohol Drugs. 2009;70(4):551–554. doi: 10.15288/jsad.2009.70.551. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Kotov R, Bonn-Miller MO, Schmidt NB, Antipova AV. Anxiety sensitivity as a moderator of association between smoking status and panic-related processes in a representative sample of adults. J Psychiatr Res. 2008;42(1):69–77. doi: 10.1016/j.jpsychires.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Kotov R, Antipova AV, Schmidt NB. Cross cultural evaluation of smokers risk for panic and anxiety pathology: A test in a Russian epidemiological sample. Behaviour Research and Therapy. 2003;41:1199–1215. doi: 10.1016/s0005-7967(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Leen-Feldner EW, Feldner MT, Bonn-Miller MO, Lejuez CW, Kahler CW, Stuart G. Emotional responding to biological challenge as a function of panic disorder and smoking. Journal of anxiety disorders. 2004;18(1):19–32. doi: 10.1016/j.janxdis.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Integrating an interoceptive exposure-based smoking cessation program into the cognitive-behavioral treatment of panic disorder: Theoretical relevance and case demonstration. Cognitive and Behavioral Practice. 2003;10(4):347–357. [Google Scholar]
- Zvolensky MJ, Schmidt NB, McCreary BT. The impact of smoking on panic disorder:: An initial investigation of a pathoplastic relationship. Journal of anxiety disorders. 2003;17(4):447–460. doi: 10.1016/s0887-6185(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Bernstein A, Leyro T. Distress Tolerance Theory, Measurement, and Relations to Psychopathology. Current Directions in Psychological Science. 2010;19(6):406–410. doi: 10.1177/0963721410388642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine and Tobacco Research. 2009;11(3):323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy. 2008;22(4):346–365. [Google Scholar]