Abstract
With approximately 20 % of the world’s population living in its downstream watersheds, the Qinghai-Tibetan Plateau (QTP) is considered “Asia’s Water Tower.” However, grasslands of the QTP, where most of Asia’s great rivers originate, are becoming increasingly degraded, which leads to elevated population densities of a native small mammal, the plateau pika (Ochotona curzoniae). As a result pikas have been characterized as a pest leading to wide-spread poisoning campaigns in an attempt to restore grassland quality. A contrary view is that pikas are a keystone species for biodiversity and that their burrowing activity provides a critical ecosystem service by increasing the infiltration rate of water, hence reducing overland flow. We demonstrate that poisoning plateau pikas significantly reduces infiltration rate of water across the QTP creating the potential for watershed-level impacts. Our results demonstrate the importance of burrowing mammals as ecosystem engineers, particularly with regard to their influence on hydrological functioning.
Keywords: Burrowing mammals, Ecohydrology, Ecosystem services, Plateau pika, Qinghai-Tibetan plateau
Introduction
Approximately 20 % of the world’s human population lives in watersheds that originate on the Qinghai-Tibetan Plateau (QTP), thus this region is considered “Asia’s Water Tower” (Xu et al. 2009; Immerzeel et al. 2010). However, the grasslands of the QTP, which serve as the headwaters for many of Asia’s great rivers, are becoming increasingly degraded (Holzner and Kreichbaum 2001; Zhou et al. 2005; Harris 2008, 2010; Dong et al. 2013; Li et al. 2013). One agent of change is overgrazing by domestic livestock (yak, sheep, goats), which has resulted in elevated population densities of a native, small, burrowing mammal, the plateau pika (Ochotona curzoniae) (Shi 1983; Fan et al. 1999; Holzner and Kreichbaum 2001; Zhou et al. 2005; Harris 2010; Dong et al. 2013; Li et al. 2013). The presence of high density pika populations on degraded grassland has led local authorities to classify them as pests and initiate poisoning campaigns in an attempt to restore grassland quality. Poisoning began in 1958, and the first wide-spread attempts to control pika populations were initiated in 1962 with applications of the rodenticide zinc phosphate (Smith et al. 1990; Fan et al. 1999). By 2006, an area of 357 060 km2 had been poisoned in Qinghai province alone (An 2008). In 2006 poisoning operations utilizing type C botulinum toxin were a central feature in the allocation of a special 7.5 billion yuan ($925 million; 2006 exchange rate) fund for ecosystem management in the recently gazetted Sanjiangyuan National Nature Reserve in Qinghai province (Ma 2006). By 2013 the first phase of this extermination work directed at pikas had been carried out on 78 500 km2 of land at a cost of 157 million yuan ($25.5 million; 2014 exchange rate); over 31 000 km2 were targeted for extermination in 2014 (Gan 2014). Thus, this poisoning has gone on for over five decades, is massive in scale, yet has not improved rangeland health (Smith and Foggin 1999; Smith et al. 2006; Harris 2008; Delibes-Mateos et al. 2011).
An alternative view is that many native burrowing mammals represent keystone species for biodiversity and function as ecosystem engineers (Delibes-Mateos et al. 2011; Davidson et al. 2012), roles that have also been attributed to plateau pikas (Smith and Foggin 1999; Bagchi et al. 2006; Badingqiuying 2008; Hogan 2010; Delibes-Mateos et al. 2011). Plateau pikas occupy open alpine meadow habitat and live in adjacent social family groups, each of which occupies a large warren of burrows (Smith and Wang 1991; Dobson et al. 1998, 2000). Burrow densities may range from 120 to 500 ha−1 (Dong et al. 2013) to as high as 2000 ha−1 (Dobson et al. 1998; Pech et al. 2007). These high plateau meadows support few trees, thus most endemic plateau birds (e.g., snow finches Montifringilla spp.; Tibetan ground-tit Pseudopodoces humilis) breed almost exclusively in pika burrows; when pikas are poisoned their burrows collapse and these bird species disappear or their populations are greatly reduced (Lai and Smith 2003). Plant species richness is also higher in pika colonies compared with poisoned sites (Smith and Foggin 1999; Bagchi et al. 2006; Hogan 2010). Additionally, pikas are the main source of food of nearly every mammalian and avian carnivore on the QTP (Schaller 1998; Smith and Foggin 1999; Badingqiuying 2008). As the carnivore guild suffers in areas where pikas have been poisoned, there have been concomitant knock-on effects to human populations. For example, with pikas making up as much as 60–78 % of the diet of brown bears (Ursus arctos) on the QTP (Xu et al. 2006), bear attacks on property (primarily homes of local nomads) have increased where pikas have been eliminated (Worthy and Foggin 2008).
The plateau pika may be considered the most characteristic mammal of the QTP (Wei et al. 2007). Its current distributional range coincides with the geographical limits of the QTP, including the headwaters of all aforementioned rivers (2.5 million km2) (Smith et al. 1990; Smith and Xie 2008). Additionally, the phylogeographic history of the plateau pika tracks the changing uplifting and periods of glaciation across the QTP from the late Pleistocene to the present (Ci et al. 2009; Yu et al. 2012).
Within the QTP watershed, plateau pikas ubiquitously occupy the open alpine grassland/desert steppe niche, extending from flat bottomland upslope to the edge of the shrub (Potentilla fruiticosa, Caragana jubata) zone, where they tend to be replaced by the smaller Gansu (O. cansus) or Thomas’s pika (O. thomasi). This available area of natural grassland on the QTP covers about 1.4 million km2 (Fan et al. 1999), or over half of the extent of the QTP. One of us (ATS) has investigated plateau pikas on the QTP since 1984 at a variety of localities and has driven thousands of km across the QTP in Qinghai province (Smith et al. 1986; Smith and Wang 1991; Dobson et al. 1998, 2000; Smith et al. 2006; Qu et al. 2007, 2008; ongoing investigations). In drainages where pikas had not been poisoned, active pika families have been observed in all open landscapes: in wetlands, dry xeric regions, alpine meadows in flat bottomlands, on steep slopes, and in areas dominated by sedge vegetation (Kobresia spp.) and by grasses (such as Stipa spp. or Leymus). Plateau pikas even extend into the shrub zone where Gansu pikas are absent.
Historically plateau pikas were considered abundant by early explorers as reported by Prejevalsky (1876, p. 146): “Hundreds and thousands may be seen on a fine day disporting themselves in the open…” and Ekvall (1968, p. 6): “…countless mouse like pikas…” Contemporary measures of density of plateau pikas vary considerably depending on time of year, severity of overwinter conditions, and most important, rangeland condition—but generally range from about 50–200 ha−1 (Smith and Wang 1991; Dobson et al. 1998; Qu et al. 2013). With other factors controlled, plateau pika density (thus burrow density) is highest on heavily overgrazed rangeland, and may approach or exceed 300 pikas ha−1 (Shi 1983; Fan et al. 1999; Holzner and Kreichbaum 2001; Zhou et al. 2005; Harris 2010; Dong et al. 2013; Li et al. 2013).
Despite being the most abundant native mammal in the region, our understanding of the potential role plateau pikas may play in ecosystem processes, including their ecohydrological impact on this ecosystem, is limited. In the QTP hydrologic system where the plateau pika occurs, precipitation can account for as much as 40 % of annual flow and 100 % of dry season flow of downstream rivers (Immerzeel et al. 2010). Thus, infiltration, runoff, and groundwater storage in this headwaters ecosystem can potentially impact downstream ecosystems and communities, including those of the 1.4 billion people living in the QTP’s watersheds (Xu et al. 2009; Immerzeel et al. 2010). Here we hypothesized that the burrowing activity of pikas might act to increase the infiltration rate of water, particularly during summer monsoonal storms, thus providing a critical ecosystem service in this headwaters ecosystem. We show that poisoning plateau pikas significantly reduces the infiltration rate of water across the QTP with potential watershed-level impacts. These findings suggest that to help ensure the long-term sustainability of the watershed on the QTP, the indiscriminate and wide-spread poisoning of plateau pikas should be curtailed. Further, our results demonstrate the broader importance of burrowing mammals as ecosystem engineers worldwide, particularly with regard to their influence on hydrological functioning.
Materials and methods
To test the hypothesis that plateau pikas, through their burrowing activity, increase infiltration rates we measured this parameter directly at three treatment sites. These were defined as: (1) adjacent to an active pika burrow entrance (On Burrow) (Fig. 1a); (2) between two (or more) active pika burrows, but at a distance of at least 1 m from an active burrow entrance and its surface disturbance (On Colony) (Fig. 1b); and (3) areas where pikas had been thoroughly eradicated due to poisoning campaigns and absent for more than 2 years (where burrows had collapsed; Poisoned Site) (Fig. 1c).
Fig. 1.

Portrayal of sites identified for ecohydrological measurements on the Qinghai-Tibetan Plateau. a “On Burrow.” Infiltrometer was placed centered in disturbed area outside the plateau pika burrow entrance; b “On Colony.” Infiltrometer was placed at least 1 m from an active pika burrow; c “Poisoned Site” showing the condition of pika-free grassland. Infiltrometer placement at each site was randomly determined (see text)
Measurements of infiltration rate of water were obtained using a double-ring infiltrometer (Turf-Tech International—Model IN8-W; http://www.turf-tec.com/) with an inner ring diameter of 15.24 cm and an outer ring diameter of 30.48 cm, and accompanying Mariotte tubes. Infiltrometer placement at each site was randomly determined by throwing a piece of yak dung over one’s shoulder in a randomly determined direction. The apparatus was then situated adjacent to the closest site meeting the specifications of the treatment. All placements were approximately level as the thick sod mat inhibited driving the apparatus more than 1–2 cm deep, and leakage could only be prevented on nearly flat surfaces. To assure consistency of measurement, the constant head (ponded) method was used, and testing sites were brought to, or near, saturation by allowing a minimum of 20 cm of water to infiltrate into the soil before measurements were taken (Wu et al. 1997; Bodhinayake 2004). To assure precision, infiltration rates were measured and averaged over two or three, 15 min periods, depending on local conditions (i.e., availability of water, etc.).
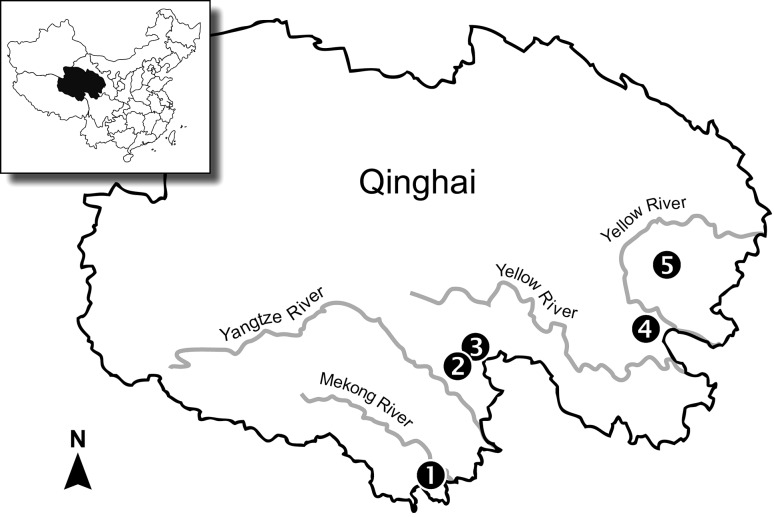
Data were collected from 16 May to 15 July 2010 and 18 May to 23 June 2011. This experiment took place at five localities broadly spread across Qinghai Province in the Sanjiangyuan (“Three Great Rivers”) region, which serves as the headwaters for the Huang (Yellow), Yangtze, and Mekong rivers (Fig. 2). Special consideration was given to site selection. All active colony sites were located in flat bottomland meadow and central to a surrounding large population of pikas in all directions. Poisoned sites were areas which had supported pika colonies before poisoning campaigns and which were physically similar to areas with currently established pika populations. Due to the influence of livestock grazing, vegetative characteristics were similar in structure among the three treatment sites (Fig. 1). As shown by Shi (1983) at the landscape scale, due to livestock grazing there is no significant variation in structure of ground cover (height, percent cover) between areas where pikas have been eliminated and where healthy populations occur. Similarly, Pech et al. (2007) determined experimentally that grazing by livestock appeared to have a stronger influence than plateau pikas on the biomass of standing vegetation in alpine meadows on the QTP.
Fig. 2.
Map of the study area on the Qinghai-Tibetan Plateau, People’s Republic of China. Locations for measurements were broadly spread across the alpine meadows of eastern Qinghai Province (average elevation = 4000 m), and encompassed the drainage systems of the Mekong (Nangqian = map site 1), Yangtze (Chendou = 2, Zhenqin = 3), and Huang He (Dawu = 4; Sendou = 5) rivers
Results
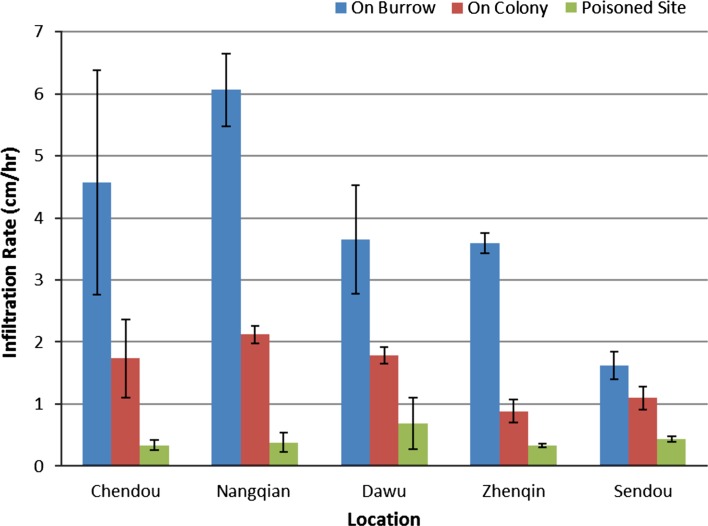
We found that the infiltration rate of water varied significantly across treatment sites (Fig. 3; Blocking-Factor ANOVA (two tailed): F2,8 = 16.992; P < 0.001). The lowest infiltration rate was consistently recorded at poisoned sites (95 % CI, 0.08–0.58 cm h−1). Intermediate infiltration rates were observed at sites within a colony but away from burrows (95 % CI, 1.25–1.88 cm h−1), and the highest rates of infiltration were consistently measured at sites adjacent to burrow openings (95 % CI, 3.01–5.02 cm h−1). See Fig. 3 for Tukey–Kramer comparisons.
Fig. 3.
Average infiltration rate of water by treatment and location. Error bars represent 1 SEM. Blocking-factor ANOVA was used to test for significant variation in mean infiltration rate of all three treatments across localities. Treatments included measurements on burrow (adjacent to an active pika burrow), On Colony (at least 1 m from active burrows, but within an active pika colony), and Poisoned Site (a location where pikas had been poisoned and old burrows had collapsed). Total sample size for the project was 54 trials with sample sizes varying from nine (three per treatment) to 15 (five per treatment) by locality. Blocking-factor ANOVA (two tailed): F 2,8 = 16.992; P < 0.001. Tukey–Kramer comparisons between sites: Poisoned Site versus On Burrow = P < 0.001; Poisoned Site versus On Colony = P < 0.004; On Colony versus On Burrow = P < 0.001
Discussion
These data confirm that through its burrowing activity, the plateau pika is an ecosystem engineer; the infiltration rate of water was consistently higher in areas occupied by pikas. Hogan (2010), using a more primitive single-ring infiltrometer protocol which did not control for initial soil moisture, similarly determined that infiltration rates were higher in areas on the QTP with active pika colonies than areas where they had been poisoned and all burrows had collapsed. Li and Zhang (2006) investigated moisture content of soil in alpine meadows on the QTP by comparing a medium density pika population with an area from which pikas had been eliminated 18 years previously. They found increased soil moisture in the top 10 cm of soil, but similar soil moisture content in deeper soil horizons (to 50 cm in depth). In this respect the biopedturbation of plateau pikas leading to increased rates of infiltration is similar to that of burrowing mammals in other ecosystems (Whitford and Kay 1999; Eldridge and James 2009).
Increased infiltration rates on pika-occupied sites (compared with poisoned sites) could lead to less local runoff during the intense summer monsoonal rains on the plateau. This effect, in turn, should minimize the potential for down-slope water erosion. However, it has become a shibboleth in much of the literature on plateau pikas that their presence, hence their burrowing activity, leads to increased erosion (Fan et al. 1999; Limbach et al. 2000; Zhou et al. 2005; Wei et al. 2007; Dong et al. 2013; Li et al. 2013). The assumption of increased erosion is then given as a further justification for controlling plateau pikas. In none of these cited papers is erosion defined, and none of them offers any experimental evidence for the claim that the presence of pikas leads to increased erosion. Fan et al. (1999, p. 286) state: “Rodents [n.b. pikas are lagomorphs, not rodents] also dig and destroy vegetation causing many serious problems such as soil erosion, and reductions in livestock carrying capacity and ecosystem diversity” [n.b. the later claim is clearly contravened by Smith and Foggin (1999); Lai and Smith (2006); Delibes-Mateos et al. (2011)]. Wei et al. (2007) cite only reports by “local herdsmen” for their contention that pikas cause erosion. Li et al. (2013) cite Limbach et al. (2000) and Zhou et al. (2005); and Zhou et al. (2005) cite Limbach et al. (2000) to support this claim. Limbach et al. (2000, p. 515) present no experimental evidence, and present only the following unsupported narrative concerning the plateau pika: “…its burrowing activity exacerbates erosion by loosening the Kobresia sod and killing its roots, its burrows form paths of preferential flow of snowmelt, runoff, and storm waters thereby exacerbating these erosive forces…” While we also did not measure erosion directly, our controlled experiments conducted across much of the range of the plateau pika consistently showed an increase in infiltration rate in active pika colonies compared with poisoned sites, and all water has to go somewhere. The observed increase in infiltration rates on occupied sites does not support a hypothesis of increased water erosion potential caused by the burrowing of plateau pikas, and it is highly likely that runoff and the potential for downslope erosion is higher on poisoned sites.
It seems unlikely that pikas “choose” sites with the potential for higher infiltration rates, as each poisoned site had, in the recent past, supported a pika population. Further, as noted above, the natural history of plateau pikas indicates that their distribution includes all open habitat types across the QTP, thus indicating that they do not select areas with a high infiltration potential. These trends are particularly relevant when the lack of confounding processes is considered. Previous studies have shown that ground cover on and off pika colonies varies little (Shi 1983; Pech et al. 2007), eliminating possible interactions between ground cover and infiltration rates, groundwater recharge, and surface runoff (compare Fig. 1b and 1c). Thus our observed variation in infiltration rates appears representative of local-level ecohydrological processes.
Though impossible to quantify accurately due to gaps in geographical data (i.e., maps of now-contracted pika ranges, fine-grained precipitation data, fine-grained soil moisture data) and the extremely complex geology of the QTP, the additive impacts of an increased infiltration rate across the range of the plateau pika (nearly the entire QTP; Smith et al. 1990; Smith and Xie 2008) on both groundwater retention and runoff control could be large and should be taken into consideration by policy-makers. Many contemporary factors enter into the hydrological profile on the QTP, including changes in grazing intensity, fencing, “ecological migration,” and climate change (Bauer 2005; Yan et al. 2005; Yeh 2005; Foggin 2008; Xu et al. 2009; Immerzeel et al. 2010; Liang et al. 2013; Yang et al. 2014). The difference in runoff potential between poisoned and un-poisoned areas should be considered contributory to these factors. However, to the best of our knowledge, the negative consequences of an increased potential for overland flow, including flooding in downstream watersheds, due to the poisoning of pikas, has not been considered by Chinese policy-makers.
We argue that the policy of poisoning plateau pikas should be reconsidered. Not only does this policy lead to critical losses of biodiversity on the QTP (Smith and Foggin 1999; Lai and Smith 2003; Badingqiuying 2008; Hogan 2010, Delibes-Mateos et al. 2011), but it ignores the ecosystem services pikas provide. Our precise experiments using the infiltrometer approach conducted across much of the range of the plateau pika demonstrate that the radical reduction in infiltration rates that accompanies pika poisoning exhibits the potential to alter the hydrologic regime of this headwaters region. Future research should focus on closing the data gaps necessary for directly quantifying these risks; however, in the absence of such data, these results are compelling evidence that pikas play a key role not only in biodiversity on the QTP, but in the flow of the rivers that originate throughout their geographic range.
Acknowledgments
Funding for this research was provided by the Phoenix Zoo Conservation and Science Grants program, the Cleveland Metroparks Zoo Asia Seed Fund, and the US NSF Dynamics of Coupled Natural and Human Systems Program (DBCS-0814794). We thank Badingqiuying and Nicholas Whipps for their assistance in providing on-the-ground support. Brigitte Hogan and Zhao Qingling assisted in the initial design of this experiment, and Crystal Palmer drafted the map. We thank Ana Davidson, Ed Grumbine, Sharon Hall, Harriet Smith, Cherie Westbrook, and Jingle Wu for their conscientious and insightful reviews of an earlier draft of this manuscript.
Biographies
Maxwell C. Wilson
is a doctoral candidate in the School of Life Sciences at Arizona State University. His research interests include ecohydrology and landscape sustainability.
Andrew T. Smith
is a President’s Professor, Parents Association Professor and Distinguished Sustainability Scientist in the School of Life Sciences and Global Institute of Sustainability at Arizona State University. His research interests include human dimensions of wildlife conservation, ecosystem services, behavioral ecology, and metapopulation dynamics.
Contributor Information
Maxwell C. Wilson, Email: mcwilso2@gmail.com
Andrew T. Smith, Email: a.smith@asu.edu
References
- An, B. 2008. Prospective and review for integrative rodent control measure in the grasslands of Qinghai. Prataculture and Animal Husbandry 5: 46–47, 62 (In Chinese).
- Badingqiuying. 2008. Effect of elimination of plateau pikas on the alpine meadow grassland ecosystem of Santu nomadic community. Master’s Thesis. Quezon City, Philippines: Miriam College.
- Bagchi S, Namgail T, Ritchie ME. Small mammalian herbivores as mediators of plant community dynamics in the high-altitude arid rangelands of Trans-Himalaya. Biological Conservation. 2006;127:438–442. doi: 10.1016/j.biocon.2005.09.003. [DOI] [Google Scholar]
- Bauer K. Development and the enclosure movement in pastoral Tibet since the 1980s. Nomadic Peoples. 2005;9:53–81. doi: 10.3167/082279405781826119. [DOI] [Google Scholar]
- Bodhinayake, W., B.C. Si, and K. Noborio. 2004. Determination of hydraulic properties in sloping landscapes from tension and double-ring infiltrometers. Vadose Zone Journal 3: 964–970.
- Ci HX, Lin GH, Cai ZY, Tang LZ, Su JP, Liu JQ. Population history of the plateau pika endemic to the Qinghai-Tibetan Plateau based on mtDNA sequence data. Journal of Zoology. 2009;279:396–403. doi: 10.1111/j.1469-7998.2009.00635.x. [DOI] [Google Scholar]
- Davidson AD, Detling JK, Brown JH. Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Frontiers in Ecology and the Environment. 2012;10:477–486. doi: 10.1890/110054. [DOI] [Google Scholar]
- Delibes-Mateos M, Smith AT, Slobodchikoff CN, Swenson JE. The paradox of keystone species persecuted as pests: A call for the conservation of abundant small mammals in their native range. Biological Conservation. 2011;144:1335–1346. doi: 10.1016/j.biocon.2011.02.012. [DOI] [Google Scholar]
- Dobson FS, Smith AT, Wang XG. Social and ecological influences on dispersal and philopatry in the plateau pika (Ochotona curzoniae) Behavioral Ecology. 1998;10:622–635. doi: 10.1093/beheco/9.6.622. [DOI] [Google Scholar]
- Dobson FS, Smith AT, Wang XG. The mating system and gene dynamics of plateau pikas. Behavioural Processes. 2000;51:101–110. doi: 10.1016/S0376-6357(00)00122-4. [DOI] [PubMed] [Google Scholar]
- Dong QM, Zhao XQ, Wu GL, Shi JJ, Ren GH. A review of formation mechanism and restoration measures of “black-soil-type” degraded grassland in the Qinghai-Tibetan plateau. Environmental Earth Science. 2013;70:2359–2370. doi: 10.1007/s12665-013-2338-7. [DOI] [Google Scholar]
- Ekvall, R.B. 1968. Fields on the hoof: nexus of Tibetan nomadic pastoralism. Re-issued 1983 by Prospect Heights, Illinois: Waveland Press.
- Eldridge DJ, James AI. Soil-disturbance by native animals plays a critical role in maintaining healthy Australian landscapes. Ecological Management and Restoration. 2009;10(S1):S27–S34. doi: 10.1111/j.1442-8903.2009.00452.x. [DOI] [Google Scholar]
- Fan N, Zhou W, Wei W, Wang Q, Jiang Y. Rodent pest management in the Qinghai-Tibet alpine meadow ecosystem. In: Singleton GR, Hinds LA, Leirs L, Zhang Z, editors. Ecologically-based rodent management. Canberra: Australian Centre for International Agricultural Research; 1999. pp. 285–304. [Google Scholar]
- Foggin JM. Depopulating the Tibetan grasslands: National policies and perspectives for the future of Tibetan herders in Qinghai Province, China. Mountain Research and Development. 2008;28:26–31. doi: 10.1659/mrd.0972. [DOI] [Google Scholar]
- Gan, R. 2014. Killing the pika won’t save the Tibetan grasslands. In Chinadialogue. Retrieved 10 June, 2014, from https://www.chinadialogue.net/article/show/single/en/6983-Killing-the-pika-won-t-save-the-Tibetan-grasslands.
- Harris RB. Wildlife conservation in China: Preserving the habitat of China’s wild west. Armonk, NY: M.E. Sharpe; 2008. [Google Scholar]
- Harris RB. Rangeland degradation on the Qinghai-Tibetan plateau: A review of the evidence of its magnitude and causes. Journal of Arid Environments. 2010;74:1–12. doi: 10.1016/j.jaridenv.2009.06.014. [DOI] [Google Scholar]
- Hogan, B.W. 2010. The plateau pika: A keystone engineer on the Tibetan Plateau. Doctoral dissertation. Tempe, AZ: Arizona State University.
- Holzner W, Kreichbaum M. Pastures in south and central Tibet (China) II. Probable causes of pasture degradation. Die Bodenkultur. 2001;52:37–44. [Google Scholar]
- Immerzeel WW, van Beek LPH, Bierkens MFP. Climate change will affect the Asian water towers. Science. 2010;328:1382–1385. doi: 10.1126/science.1183188. [DOI] [PubMed] [Google Scholar]
- Lai CH, Smith AT. Keystone status of plateau pikas (Ochotona curzoniae): Effect of control on biodiversity of native birds. Biodiversity and Conservation. 2003;12:1901–1912. doi: 10.1023/A:1024161409110. [DOI] [Google Scholar]
- Li W, Zhang Y. Impacts of plateau pikas on soil organic matter and moisture content in alpine meadow. Acta Theriologica Sinica. 2006;26:331–337. [Google Scholar]
- Li XL, Perry PLW, Brierley G, Gao J, Zhang J, Yang YW. Restoration prospects for heitutan degraded grassland in the Sanjiangyuan. Journal of Mountain Science. 2013;10:687–698. doi: 10.1007/s11629-013-2557-0. [DOI] [Google Scholar]
- Liang LQ, Li LJ, Liu CM, Cuo L. Climate change in the Tibetan plateau three rivers source region: 1960–2009. International Journal of Climatology. 2013;33:2900–2916. doi: 10.1002/joc.3642. [DOI] [Google Scholar]
- Limbach WE, Davis JB, Bao T, Shi D, Wang C. The introduction of sustainable development practices of the Qinghai Livestock Development Project. In: Zheng D, editor. Formation and evolution, environmental changes and sustainable development on the Tibetan plateau. Beijing: Academy Press; 2000. pp. 509–522. [Google Scholar]
- Ma, L. 2006. Environment fund targets rats. China Daily. Retrieved 10 June, 2014, from http://www.chinadaily.com.cn/english/doc/2006-03/03/content_525780.htm.
- Pech, R.P., Jiebu, A.D. Arthur, Y. Zhang, and L. Hui. 2007. Population dynamics and responses to management of plateau pikas Ochotona curzoniae. Journal of Applied Ecology 44: 615–624.
- Prejevalsky, N. 1876. Mongolia, the Tangut country and the solitudes of northern Tibet. Re-issued 1991 by New Delhi: Asian Educational Services.
- Qu JP, Li KX, Yang M, Li WJ, Zhang YM, Smith AT. Seasonal dynamic pattern of spatial territory in social groups of plateau pikas (Ochotona curzoniae) Acta Theriologica Sinica. 2007;27:215–220. [Google Scholar]
- Qu JP, Yang M, Li W, Li K, Zhang YM, Smith AT. Seasonal variation of family group structure of plateau pikas (Ochotona curzoniae) Acta Theriologica Sinica. 2008;28:144–150. [Google Scholar]
- Qu JP, Li WJ, Yang M, Ji W, Zhang YM. Life history of the plateau pika (Ochotona curzoniae) in alpine meadows of the Tibetan plateau. Mammalian Biology. 2013;78:68–72. [Google Scholar]
- Schaller GB. Wildlife of the Tibetan steppe. Chicago: University of Chicago Press; 1998. [Google Scholar]
- Shi, Y. 1983. On the influence of rangeland vegetation to the density of plateau pikas (Ochotona curzoniae). Acta Theriologica Sinica 3: 181–187. (In Chinese; English abstract).
- Smith AT, Foggin JM. The plateau pika (Ochotona curzoniae) is a keystone species for biodiversity on the Tibetan plateau. Animal Conservation. 1999;2:235–240. doi: 10.1111/j.1469-1795.1999.tb00069.x. [DOI] [Google Scholar]
- Smith AT, Wang XG. Social relationships of adult black-lipped pikas (Ochotona curzoniae) Journal of Mammalogy. 1991;72:231–247. doi: 10.2307/1382094. [DOI] [Google Scholar]
- Smith AT, Xie Y, editors. A guide to the mammals of China. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- Smith AT, Smith HJ, Wang XG, Yin X, Liang J. Social behavior of the steppe dwelling black-lipped pika. National Geographic Research. 1986;2:57–74. [Google Scholar]
- Smith AT, Formozov NA, Hoffmann RS, Zheng C, Erbajeva MA. The pikas. In: Chapman JA, Flux JAC, editors. Rabbits, hares and pikas: Status survey and conservation action plan. Gland: International Union for Conservation of Nature and Natural Resources; 1990. pp. 14–60. [Google Scholar]
- Smith, A.T., P. Zahler, and L.A. Hinds. 2006. Poisoning of native small mammals in central Asia is an undesirable and unsustainable activity. In Biodiversity conservation in Asia, ed. J. McNeely, T.M. McCarthy, A.T. Smith, L. Olsvig-Whittaker, and E.D. Wikramanayake, 285–293. Kathmandu, Nepal: Society for Conservation Biology, Asian Section and Resources Himalaya Foundation.
- Wei X, Li S, Yang P, Cheng H. Soil erosion and vegetation succession in alpine Kobresia steppe meadow caused by plateau pika case study of Nagqu County. Tibet. Chinese Geographical Science. 2007;17:75–81. doi: 10.1007/s11769-007-0075-0. [DOI] [Google Scholar]
- Whitford WG, Kay FR. Biopedturbation by mammals in deserts: A review. Journal of Arid Environments. 1999;41:203–230. doi: 10.1006/jare.1998.0482. [DOI] [Google Scholar]
- Worthy FR, Foggin JM. Conflicts between local villagers and Tibetan brown bears (Ursus arctos) threaten conservation of bears in a remote region of the Tibetan Plateau. Human-Wildlife Conflicts. 2008;2:200–205. [Google Scholar]
- Wu L, Pan L, Roberson MJ, Shouse PJ. Numerical evaluation of ring infiltrometers under various soil conditions. Soil Science. 1997;162:771–777. doi: 10.1097/00010694-199711000-00001. [DOI] [Google Scholar]
- Xu A, Jiang Z, Li C, Guo J, Wu G, Cai P. Summer food habits of brown bears in Kekexili Nature Reserve, Qinghai-Tibetan Plateau, China. Ursus. 2006;17:132–137. doi: 10.2192/1537-6176(2006)17[132:SFHOBB]2.0.CO;2. [DOI] [Google Scholar]
- Xu J, Grumbine RE, Shrestha A, Eriksson M, Yang X, Wang Y, Wilkes A. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conservation Biology. 2009;23:520–530. doi: 10.1111/j.1523-1739.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- Yan Z, Wu N, Yeshi D, Ru J. A review of rangeland privatization and its implications in the Tibetan plateau, China. Nomadic Peoples. 2005;9:31–51. doi: 10.3167/082279405781826155. [DOI] [Google Scholar]
- Yang, K., H. Wu, J. Qin, C. Lin, W. Tang, and Y. Chen. 2014. Recent climate changes over the Tibetan Plateau and their impacts on energy and water cycle: A review. Global and Planetary Change 112: 79–91.
- Yeh ET. Green governmentality and pastoralism in western China: “converting pastures to grasslands”. Nomadic Peoples. 2005;9:9–30. doi: 10.3167/082279405781826164. [DOI] [Google Scholar]
- Yu F, Li S, Kilpatrick WC, McGuire PM, He K, Wei W. Biogeographical study of plateau pikas Ochotona curzoniae (Lagomorpha, Ochotonidae) Zoological Science. 2012;29:518–526. doi: 10.2108/zsj.29.518. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhao X, Tang Y, Gu S, Zhou L. Alpine grassland degradation and its control in the source region of the Yangtze and Yellow rivers, China. Grassland Science. 2005;51:191–203. doi: 10.1111/j.1744-697X.2005.00028.x. [DOI] [Google Scholar]