Abstract
Globally, greenhouse gas budgets are dominated by natural sources, and aquatic ecosystems are a prominent source of methane (CH4) to the atmosphere. Beaver (Castor canadensis and Castor fiber) populations have experienced human-driven change, and CH4 emissions associated with their habitat remain uncertain. This study reports the effect of near extinction and recovery of beavers globally on aquatic CH4 emissions and habitat. Resurgence of native beaver populations and their introduction in other regions accounts for emission of 0.18–0.80 Tg CH4 year−1 (year 2000). This flux is approximately 200 times larger than emissions from the same systems (ponds and flowing waters that became ponds) circa 1900. Beaver population recovery was estimated to have led to the creation of 9500–42 000 km2 of ponded water, and increased riparian interface length of >200 000 km. Continued range expansion and population growth in South America and Europe could further increase CH4 emissions.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-014-0575-y) contains supplementary material, which is available to authorized users.
Keywords: Beaver ponds, Castor canadensis, Castor fiber, Methane efflux, Population recovery
Introduction
Inland surface waters are important zones of carbon (C) cycling, and freshwater ecosystems are known sites of biogenic greenhouse gas release to the atmosphere (Cole et al. 2007). Wetlands represent the dominant natural CH4 source globally (Solomon et al. 2007), while open freshwaters (lakes and rivers) constitute another major natural emission source (Bastviken et al. 2011). Emission of CH4 from wetlands is subject to anthropogenic pressures that can potentially alter the spatial extent of this habitat, for example through observed widespread increase in rice cultivation, or loss of wetlands due to physical drainage. Natural influences, including climate cycles and ecological succession, can also alter the spatial extent of wetlands over longer time periods. Uncertain estimates of wetland and surface water areas have been highlighted as a challenge for quantifying global CH4 emissions (Bridgham et al. 2013).
Beavers have long been recognized as agents of geomorphic change (Ruedemann and Schoonmaker 1938), owing largely to their dam-building abilities and consequent creation of open-water ponds and associated wetland complexes (Westbrook et al. 2006). For more than three centuries, global beaver (Castor canadensis and Castor fiber) populations have been suppressed by widespread trapping; however, in recent decades, resource management activities, including re-introduction programs, have stimulated population growth. Accordingly, it can be expected that the extent of water impounded by beaver dams has undergone profound changes coincident with the global population fluctuation. This may have important consequences for the global CH4 cycle.
Beavers’ hydrogeomorphic alteration of the landscape, principally via creation of lentic (standing water) environments in place of lotic (flowing water environments), promotes CH4 production. Beaver ponds share properties of both wetlands and open-water systems, but emit more CH4 than other types of wetlands (Roulet et al. 1992) or lotic habitats (Naiman et al. 1986) on an areal basis. Both increased stocks of C per unit area (Naiman et al. 1986) and greater area of anaerobic benthos will contribute to CH4 generation in beaver ponds. Further, while methanotrophy can occur during CH4 movement through oxic water columns (Rudd et al. 1976), beaver ponds are invariably shallow, with dam height generally less than 1.5 m (McComb et al. 1990), and consequently there is little opportunity for CH4 oxidation.
Beavers have long been acknowledged as mediators of CH4 emission to the atmosphere (Nisbet 1989); however, the global significance of this source has not been comprehensively quantified, nor has the effect of population recovery on aquatic and riparian habitat. In this paper, the first reconstruction of global beaver population size is presented. Population estimates for Eurasia, North America, and South America (Tierra del Fuego), combined with ecological information and beaver pond size estimates, are used to determine the area of aquatic habitat and length of riparian interface (between water and land) created during twentieth century beaver population growth. This information is combined with measurements of CH4 efflux rates to estimate recent (year 2000) global beaver-mediated aquatic CH4 emissions and uncertainty associated with these fluxes.
Materials and Methods
Beaver population estimates
Beaver population sizes for the three continents (South America, North America and Eurasia) inhabited by C. canadensis and C. fiber were estimated for the year 2000. For each region a different approach was used to calculate the range in size of the population(s), owing to the constraints associated with the available data. The methods for each population are outlined below and summarized in Electronic Supplementary Material, Table S1.
South America
Beaver (C. canadensis) colonization in Tierra del Fuego (Chile and Argentina) is linked to landscape type (Lizarralde 1993), with more mountainous and forested terrain supporting higher population density. Stream length-based densities for different landscape types and stream network length for colonized islands of the archipelago (Parkes et al. 2008) were used to estimate population size. Reported densities for beaver populations c. 1990 likely reflect population maxima, with small decreases expected to have occurred prior to the end of the twentieth century (Parkes et al. 2008); thus, the population estimate for 2000 was adjusted to reflect a smaller population size than estimated directly from the length-based densities based on earlier data.
North America
To characterize the North American continent, a population estimation approach that allowed existing localized data to be extrapolated across Level I ecoregions (Commission for Environmental Cooperation 1997) after adjusting the ecoregion areas for non-colonized zones (i.e., deserts of the American southwest, peninsular Florida) was used. With this stratified approach, each ecoregion was classified according to probability of beaver occurrence (high, moderate, or low). Average stream length-based densities for the three classes were used to adjust area-based density, available only for high-density ecoregions of Canada (Electronic Supplementary Material, Table S2), such that population size could be calculated according to the land area in each class assuming constant stream network density. Average areal density was 0.6 colony km−2 among sites in ecoregions classified as high density (n = 14; Table S2) although there was considerable variability among density estimates (SD 0.3), which is not unexpected given the range in geographic character within the broad classifications used. High- and low-density estimates for each density class were quantified as the mean plus and minus one standard deviation, respectively. Data used in this analysis span several decades (mostly 1970–1990) and likely reflect recovering (i.e., growing) rather than stable populations. Population recovery has not been uniform across the continent, however, so it was not possible to normalize individual datum from different regions to a single time point. Instead, the available data were assumed to (conservatively) reflect the condition of the population in 2000.
Eurasia
Beaver in Eurasia comprise several overlapping populations of the native C. fiber and introduced C. canadensis, and had been enumerated in many countries by the end of the twentieth century (Halley and Rosell 2003). Population data (both species) for 23 countries were compiled and used to reconstruct the temporal dynamics of the two populations (Table S1). For many countries, population estimates were available for several different years, allowing use of a (best-fit) parametric growth curve such that the population could be established for the year (2000) where only earlier observations were available.
Pond-building activity
Beaver colonies do not always establish ponds through dam building and may instead occupy bank dens in rivers or build lodges in lakes, thus limiting their hydrogeomorphic impact. Pond-building frequency of C. fiber was assumed to be 10–50 % (Zurowski and Kasperczyk 1988; Bluzma 2003). North American beavers are associated with greater dam-building activity (Gurnell 1998; Collen and Gibson 2001), although there are few data quantifying the propensity of dam building for either population. A comparison of the two species in Russia suggested greater damming by C. canadensis (66 % vs. 45 % for C. fiber (Danilov 1995)). A pond-building frequency range of 50–80 % was assumed for all C. canadensis populations.
Among those colonies that do build dams, there is considerable variability in the number of ponds they create. For C. fiber, the number of dams per colony is reported to be as high as 13 (Pupininkas 1999), with an average of 3.1 reported for Lithuania (Ulevičius et al. 2009). In North America, 2–5 dams per colony were suggested as typical (Butler and Malanson 2005). Neither pond longevity nor site use characteristics are sufficiently well known to consider explicitly in this analysis. The number of ponds attributed to each colony was therefore assumed to reflect both active ponds and abandoned ponds that continue to retain water. A Poisson distribution (λ = 2), truncated to reflect a conservative estimate of the number of ponds per colony (2–5), was used in this analysis. This approach ensured that colonies were most frequently described as having fewer ponds (e.g., of the pond establishing colonies, 67 % were attributed only two ponds), and therefore should provide a conservative estimate of the total number of ponds.
Pond size
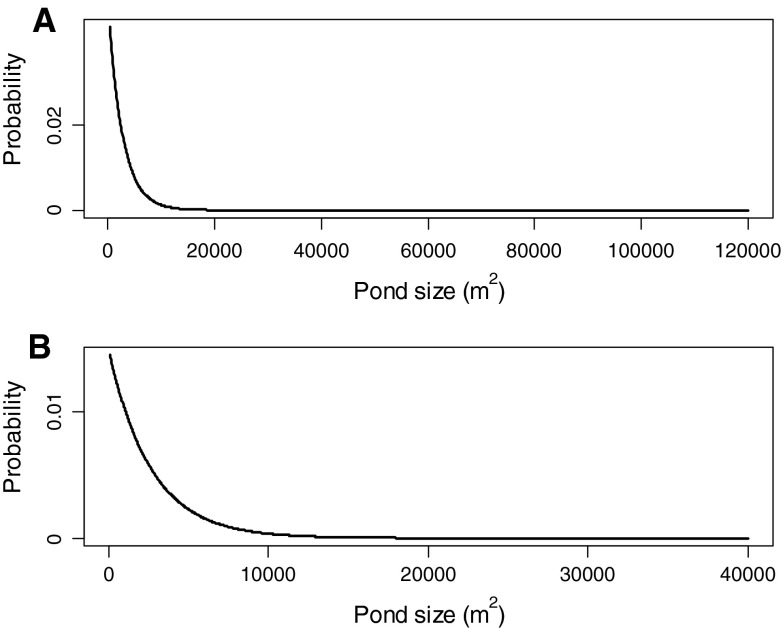
For beaver populations in the northern hemisphere, pond area is rarely described. Pond areas have been reported to reach 54 000 m2 in Europe (Nyssen et al. 2011) and 300 000 m2 in North America (Novak 1999), although average sizes rarely exceed 10 000–20 000 m2 (Naiman et al. 1986; Butler and Malanson 1995; Novak 1999). In contrast, pond surveys in Tierra del Fuego, South America found that the range in upland pond size was 300–16 000 m2 (average 2700 m2) with infrequent occurrence of larger ponds (up to 160 000 m2) in flat valleys (Lizarralde 1993). Exponential distributions, modified to include a small proportion of larger ponds, were used to characterize pond size of both northern and southern hemisphere populations. The resulting average pond size for the northern hemisphere populations was 3300 m2 (range 50–40 000 m2), while for Tierra del Fuego the distribution averaged 3500 m2 (range 300–120 000 m2; Fig. 1).
Fig. 1.
Distributions of pond sizes associated with the populations in the southern hemisphere (a) and northern hemisphere (b) used in this analysis. Minimum pond sizes were 300 and 50 m2, respectively, while the upper limit of pond size for each distribution is denoted by the largest tick mark (see text)
Methane efflux rates
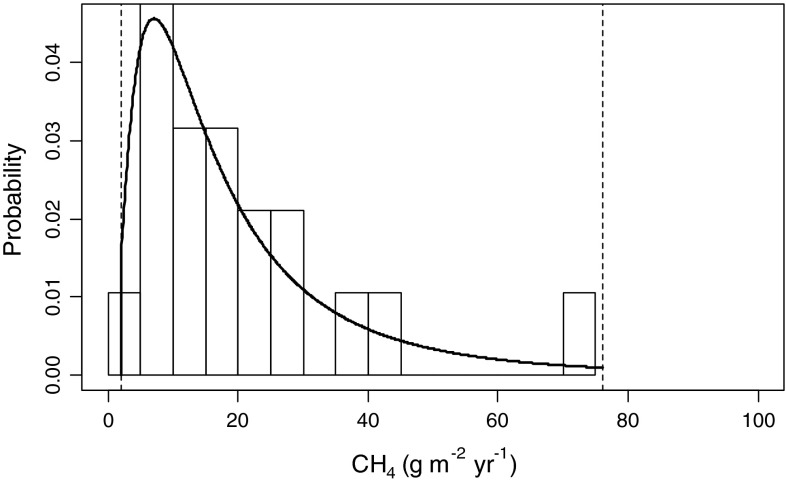
Many studies have quantified CH4 efflux from freshwaters to the atmosphere; however, for the purposes of this analysis, only those studies which quantified annual or open-water periods of 120 or more days were included. Where observations spanned less than a typical ice-free period of 180 days, the efflux estimates were adjusted to this time period. Nonetheless, the CH4 efflux estimates used in this analysis may be conservative as ebullitive and ice-out fluxes are both episodic and difficult to capture, and therefore not always well represented by available measurements. Annual efflux of methane from beaver ponds to the atmosphere has been reported for upward of 20 sites in North America (Table 1). The efflux estimates range from 2 to 76 g CH4 m−2 year−1 (Moore and Roulet 1995) and average approximately 20 g CH4 m−2 year−1 across the ponds where annual emissions have been quantified. A truncated lognormal distribution fit to the available data was used to characterize CH4 efflux (Fig. 2) and was assumed to reflect the nature of emission rates in the other regions. Emissions associated with lotic waters that preceded pond formation (i.e., background emissions) were estimated using CH4 efflux rates for rivers and streams and open-water areas of 0.2–2 % that of ponds (Electronic Supplementary Material, Table S3).
Table 1.
Beaver pond CH4 emission estimates (g m−2 year−1) for sites in different states and provinces of North America
| Location | Ponds | Efflux | Reference |
|---|---|---|---|
| Ontario | 21 | 2.0–76 | Roulet et al. (1992), Bubier et al. (1993), Moore and Roulet (1995), and Weyhenmeyer (1999) |
| Quebec | 2 | 5.9–9.9 | Naiman et al. (1986) and Ford and Naiman (1988) |
| Manitoba | 2 | 11–18 | Dove (1995) and Roulet et al. (1997) |
| New York | 2 | 21–25 | Yavitt et al. (1992) |
| Minnesota | 2 | 12–14 | Naiman et al. (1991) |
Fig. 2.
Truncated lognormal distribution used to describe potential aquatic CH4 emission from beaver ponds (min, mean, and max are 2, 20, and 76 g CH4 m−2 year−1, respectively). Histogram represents observations (original data summarized in Table 1)
Continental CH4 emission from beaver ponds
A Monte Carlo uncertainty analysis was used to characterize CH4 emission associated with each beaver population (year 2000) on the three continents where they are resident. One thousand simulations were conducted for each of the four populations. For each simulation, the total number of ponding colonies was estimated from population size, colony sizes, and pond-building frequency with uniform distributions used to characterize these parameters. Each ponding colony was subsequently attributed a number of ponds, and pond size and efflux rate for each pond were characterized according to the distributions described above (Figs. 1, 2). In total, tens of thousands of iterations were used to characterize individual ponds associated with each population. For each simulation, emissions for each pond were summed to quantify CH4 efflux associated with the population. For years prior to 2000, pond-based CH4 emissions were calculated by scaling emissions for 2000 according to the population size in a given year (Electronic Supplementary Material, Table S1).
A sensitivity analysis was conducted to assess the uncertainty associated with each of the main factors (population size and colony size, ponding frequency, number of ponds established by a colony, pond size, and CH4 efflux rates) used to produce the efflux estimates. In this analysis, 1000 simulations were run for each factor wherein an individual factor was sampled from its distribution (population/colony size was evaluated together using uniform distributions owing to differences in methods for deriving the population estimates; Electronic Supplementary Material, Table S1), and emissions were calculated with the other factors held constant (at mean).
Riparian interface creation
Pond circumference was used to estimate the length of riparian interface (between aquatic and terrestrial habitats) created through beaver pond formation during the twentieth century. Pond morphometry is not well characterized, and is highly dependent on regional and local conditions. Nonetheless, a (minimum) estimate of riparian interface resulting from beaver population growth was approximated by assuming that all ponds are round, and subtracting the pre-impoundment riparian interface (two times stream length).
Results and discussion
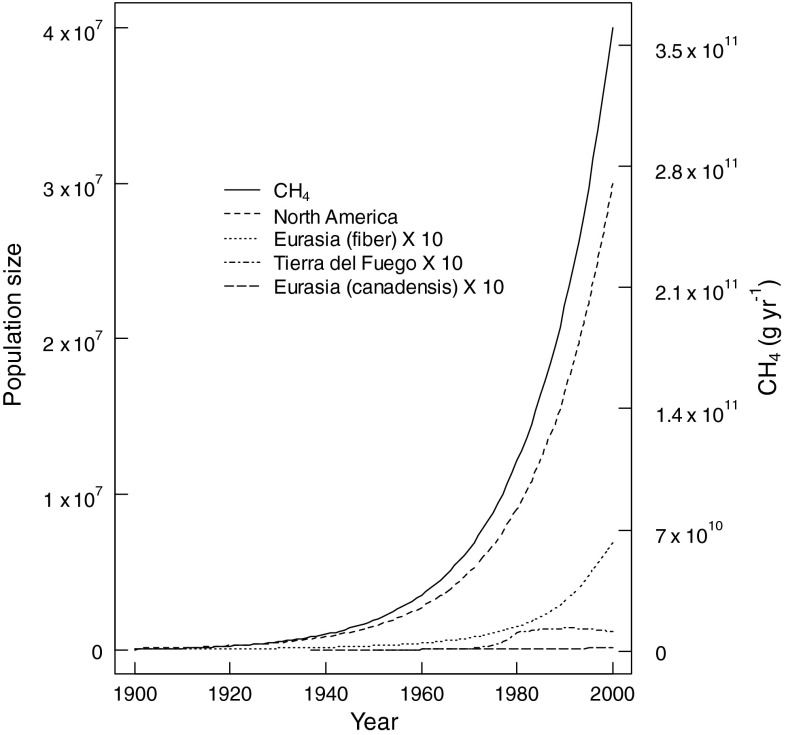
The fur trade of the sixteenth to nineteenth centuries led to near extinction of global beaver populations. In the ensuing period of decreased trapping pressure and assisted introductions, global beaver populations are believed to have grown nearly exponentially, reaching global populations of 3.0 × 107 (Fig. 3). This has led to the creation of ~9500–42 000 km2 of new aquatic pond habitat, and in excess of 200 000 km of riparian interface (Table 2). Given the value of beaver ponds as habitat (Wright 2002), this suggests that significant ecological benefits are associated with beaver recovery.
Fig. 3.
Reconstructed populations for the twentieth century, according to median (best) estimates of population size for C. canadensis in Tierra del Fuego, Eurasia and North America, and C. fiber in Eurasia. Three of the populations were scaled (factor of 10) for presentation purposes. Total CH4 emissions for the estimated (median) global population are also shown
Table 2.
Estimates of population size, number of colonies, total surface area of beaver ponds (km2), and length of riparian interface created (km) through beaver pond formation for the different species of beaver and geographic regions as of 2000. Where only a minimum is provided, it represents a single (best) estimate for the population
| Region | Species | Population | Colonies | Pond area | Riparian interfacea | |||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Range | ||
| Tierra del Fuego | C. canadensis (introduced) | 95 000 | 168 000 | 26 600 | 100 | 186 | 2160–4010 | |
| North America | C. canadensis (native) | 9 600 000 | 50 400 000 | 2 400 000 | 7 200 000 | 9280 | 41 210 | 204 000–908 000 |
| Eurasia | C. canadensis (introduced) | 13 000 | 2160 | 3240 | 8 | 19 | 180–420 | |
| Eurasia | C. fiber (native) | 683 000 | 137 000 | 228 000 | 106 | 821 | 2340–18 900 | |
aEstimates for riparian interface are constrained according to minimum and maximum estimates of other parameters but assume a circular pond shape and, therefore, represent a minimum range
Parallel with this increase in beaver populations is an increase in beaver-mediated CH4 emissions (Fig. 3). Our results indicate that beaver-mediated aquatic CH4 emissions have increased ~400-fold since the early twentieth century, reaching 0.18–0.80 Tg CH4 year−1 in 2000. Comparing these figures to background emissions from lotic waters (systems which were dammed during pond formation) suggests that emissions have increased 200-fold as a result of beaver activity during this period.
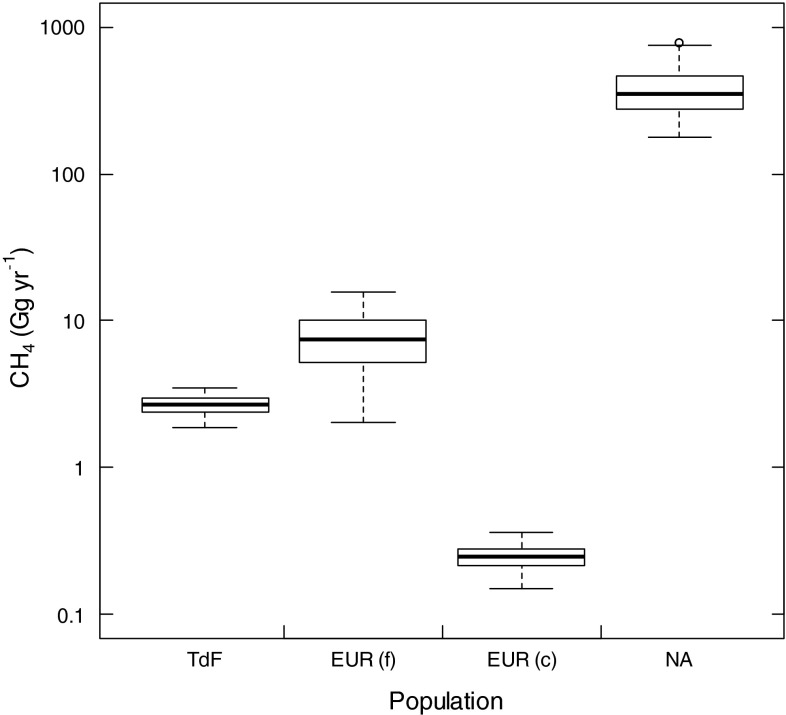
Population growth during the twentieth century is evident on all continents where beavers are resident (Fig. 3). In South America, beaver-mediated CH4 emissions represent an anthropogenic greenhouse gas source, because this exotic-invasive population resulted from introduction of C. canadensis in Argentinian Tierra del Fuego (Lizarralde 1993). The size of this emission source is estimated to be 2.7 Gg year−1 (median; Fig. 4). Although minor on a global scale, this represents a major regional source, given this emission estimate was derived from a beaver population restricted to the archipelago of Tierra del Fuego, an area of ~60 000 km2 with low human population and relatively undisturbed natural environment. Eurasia features both the introduced C. canadensis and the native C. fiber. Methane release from the C. fiber population in Eurasia was comparable to that for C. canadensis in Tierra del Fuego (Fig. 4), despite the Eurasian population being much larger, owing largely to the less-frequent pond-building activity of C. fiber (Danilov 1995). The population size of introduced C. canadensis in Eurasia remains small (approximately 13 000 individuals), and they are a minor contributor to the global beaver pond CH4 efflux. Emissions for North American beaver populations were more than an order of magnitude larger than emissions from other continents (Fig. 4). This is because of the large number of C. canadensis in North America (Table 2), and their greater propensity for dam building.
Fig. 4.
Calculated annual methane (CH4) emissions associated with the C. canadensis populations in Tierra del Fuego (TdF), Eurasia (EUR (c)) and North America (NA), and the Eurasian C. fiber population (EUR (f)) in the year 2000. For each population, the boxplot represents the results from 1000 Monte Carlo simulations used to quantify CH4 efflux
This analysis indicated that aquatic emissions of CH4 to the atmosphere associated with beaver ponds are notable; the maximum annual efflux estimate (year 2000) of 0.80 Tg CH4 is equal to approximately 15 % of the production from wild ruminants (Lelieveld et al. 1998). The global estimate of annual CH4 emissions from lakes (72 Tg CH4 year−1) is considerably higher (Bastviken et al. 2011), consistent with the much higher global surface area of lakes. Beaver-mediated emissions originate predominantly from higher than 45° north latitude, a region that also features significant CH4 sources from lakes as approximately half of global lake surface area is above this latitude (Verpoorter et al. 2014). Moreover, aquatic CH4 emissions from beaver ponds represent 1 % or less of total global wetland emissions (55–231 Tg year−1; Turetsky et al. (2014)). Additionally, while beaver pond CH4 emissions have increased considerably during the last century, particularly in northern Canada, thermokarst pond area also increased dramatically in the Hudson Bay Lowland of northern Canada, representing a large CH4 source (1–7 Tg year−1: Packalen et al. (2014)) during this period (Payette et al. 2004). Consequently, it is reasonable to expect that parallel increases in CH4 emission from other natural systems may represent a comparable or larger change than predicted here for beaver ponds. Thus while beavers, through pond-building activities, can be important local sources of CH4 to the atmosphere, these aquatic emissions do not play a major role in the global CH4 cycle.
Uncertainty associated with aquatic CH4 emission from beaver ponds was quantified in this study using Monte Carlo analyses. A conservative approach for quantifying the number of ponds was used, as this aspect of ecology is not well documented. Distributions representing pond size and CH4 efflux rates (Figs. 1, 2) characterize the uncertainty in these parameters according to reported values from the literature. Ultimately, the uncertainty associated with each aspect of the calculations is translated into uncertainty around the CH4 efflux estimates for each population (Fig. 4). Sensitivity analysis indicated that the variability in efflux estimates can be principally attributed to the uncertainty in the number of colonies (population size) and also to the range in pond-building activity. Uncertainty in the North American efflux estimates associated with these factors was 0.58 and 0.3 Tg CH4 year−1, respectively. For the European populations, data on the number of beavers were used to generate a single estimate for each species (Table 2). Thus for these populations, the distribution for pond-building activity contributed the greatest uncertainty, about double that due to the effect of colony size. Uncertainty attributed to CH4 emission rates, pond size, and the number of established ponds was minor for all populations. Improved estimates of population size, particularly for North America (range 9.6–50 million individuals) coupled with better knowledge of beaver ecology, including the propensity to establish ponds, would prove useful for refining understanding of the role beavers play in promoting aquatic CH4 release to the atmosphere, as well as the extent of habitat modified by their dam building.
Investigations that seek to quantify aquatic emissions of biogenic greenhouse gases across large spatial scales represent a tremendous challenge. Because efflux estimates are often available in limited geographic regions, and can be biased to certain types of systems, exercises in scaling necessarily invoke assumptions in order to scale up from small regions of more plentiful data to larger regions of sparse data availability. In the current study, efflux estimates for North American beaver ponds were extrapolated to other regions. Methane efflux rate uncertainty was characterized through a distribution representing available measurements (Fig. 2); while it is the most detailed depiction of annual emissions from these systems to date, it is unknown how well it reflects the behavior of comparable systems in other regions.
Further, global estimates of aquatic gas emission are contingent on estimates of areal coverage of water. In recent years, steady progress has been made toward quantifying coverage of lentic and lotic systems globally (Downing et al. 2012), but characterization of small water bodies remains a challenge. In the absence of a robust method for quantifying the size and global abundance of beaver ponds, a simple approach that related distributions of pond size and number to population size was used. The population estimate for North America assumed constant stream density. This assumption was tested by recalculating population size using stream network coverage in moderate and low beaver density regions that are 75 and 50 % that of the highest density area, respectively. This adjustment for differential stream density is based on evidence that at large geographic scales, coverage of streams varies only by a factor of about two (Downing et al. 2012). Adjusted stream density had little effect on the population estimate (variable stream density: 8.5–44 million; constant stream density: 9.6–50 million) suggesting the constant stream density assumption used is reasonable. As indicated in the sensitivity analysis, uncertainty associated with the number of ponding colonies contributed the largest error to the global efflux estimate. This suggests that direct enumeration of beaver pond area could stand to greatly enhance the efflux estimate. As remote sensing techniques evolve to enable numerical estimation of coverage of beaver ponds (or other small aquatic features), scaling exercises for these systems can be expected to experience notable improvements in precision and reductions in uncertainty.
Going forward, the capacity to estimate the full impacts of beaver on greenhouse gas budgets is contingent on obtaining estimates of CO2 and N2O emissions from these aquatic habitats. It should also be cautioned that the figures reported herein likely underestimate the true impact of beaver on global CH4 emission, as they do not include plant-mediated CH4 fluxes, which can be important in shallow-water habitats (Dacey and Klug 1979). In addition, the effects of damming activity on CH4 emission from waterlogged soils (Le Mer and Roger 2001) of flooded riparian habitat around the ponds were not accounted for. Beaver ponding activity can raise the local water table across an area of comparable size to the pond proper (Westbrook et al. 2006), and elevated water table due to beaver ponding is associated with increased CH4 emission from adjacent soil (Moore et al. 2011). As a result, the total global CH4 emission to the atmosphere associated with beaver activity is likely larger than our pond-based aquatic efflux estimate suggests. As was highlighted above, the emissions beavers mediate through pond formation greatly exceed the estimated (background) stream-based CH4 efflux prior to the establishment of beaver ponds. Stream emissions are minor components of the overall budget due to their small surface area, and lower emission rates relative to ponds (Bubier et al. 1993; Bastviken et al. 2011). Emissions from the area of streams lost due to damming and pond formation during the twentieth century were estimated to total ~1 Gg year−1 globally in 1900.
The future of the global beaver populations, while unlikely to return to historic low levels, remains uncertain. In light of ongoing efforts to re-establish this keystone species in Europe and Asia, it seems likely that the C. fiber population will continue to grow in the short term. Indeed, an independent estimate of the C. fiber population suggests that the population was 9 % larger in 2010 (Halley et al. 2012) than when estimated for the same year using our growth curve (based on data for the twentieth century). Few countries have reached the density of 0.2 colonies km−2 described as the carrying capacity for C. fiber in Sweden (Hartman 1994), and re-introductions in additional regions (e.g., Scotland) are ongoing. Assuming this density is viable across the continent, future population size may exceed 5 million, which could increase beaver-mediated European emissions sevenfold. In Poland, damming activity increased coincident with population growth (Zurowski and Kasperczyk 1986), presumably as a means of creating territories with suitable hydrologic conditions where the availability of such naturally occurring sites was becoming more limited. If this pattern holds elsewhere in Eurasia, the growth rate of beaver-mediated CH4 emission may exceed that of the beaver population itself. In South America, recent migration of beaver to the mainland have renewed concerns that the adverse impacts of introduced C. canadensis observed on Nothofagus forests typical of the Tierra del Fuegan archipelago (Anderson et al. 2006) could spread northward across the continent; however, the capacity for this habitat to support beaver over the long-term remains questionable. In North America, population expansion has stimulated beaver management programs in many jurisdictions; a bounty program in the Canadian province of Saskatchewan eliminated 35 000 beaver in 2011 alone. Northward range expansion is also expected as the climate warms (Jarema et al. 2009), although globally the net effect of future climatic changes on species range and density is uncertain.
Conclusions
The effects of beaver on hydrology, habitat complexity, biodiversity, and nutrient cycling are well documented (Naiman et al. 1986; Wright 2002). Establishment of large areas of pond habitat and extension of the riparian interface, reported herein for the first time on a global and continental scale, highlight the ecological importance of beaver recovery, most significantly in North America.
Beavers mediate aquatic emissions of CH4 to the atmosphere (up to 0.80 Tg year−1 globally) by means of water impounded behind their dams, although their impact on CH4 cycling may be greater than estimated herein due to the large areas with elevated water table that surround these ponds. This source of CH4 to the atmosphere is, however, not static. Instead, it is expected to fluctuate in concert with the beaver population. Thus, while emissions appear to have grown tremendously during the twentieth century, they have likely not yet reached the level of several centuries ago when the global beaver population may have been larger.
The dynamic nature of beaver-mediated CH4 emissions in recent years may portend the potential for future changes in this component of the global CH4 budget. Continued range expansion associated with exotic-invasive populations, regional re-introductions, and climate change, coupled with changes in population and pond densities may dramatically increase the amount of water impounded by beaver. This, in combination with anticipated increases in surface water temperatures, and likely effects on rates of methanogenesis (van Hulzen et al. 1999), suggests that the contribution of beaver activity to global CH4 emissions may continue to grow.
Electronic supplementary material
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship awarded to the lead author. Additional support was provided by a Natural Sciences and Engineering Research Council of Canada Discovery Grant and by the Global Institute for Water Security. Comments on an earlier version of the paper from Shaun Watmough and three anonymous reviewers helped improve the final version and were greatly appreciated.
Biographies
Colin J. Whitfield
is an applied biogeochemist with an interest in both terrestrial and aquatic systems. He has lead projects investigating greenhouse gas dynamics of aquatic systems in Canada, Ireland, and Argentina. He also has expertise in modeling the chemical response of aquatic and terrestrial ecosystems to stressors including acidic deposition
Helen M. Baulch
is a biogeochemist and ecologist with experience in the study of lakes, streams, and watersheds. Her research focuses on nitrogen biogeochemistry and climate change. Her work has used field and model-based approaches for understanding current conditions and assessing future climate scenarios. She is a member of the Global Institute for Water Security, and the School of Environment and Sustainability at the U of S
Kwok P. Chun
is a hydrologist with experience in the application of non-stationary hydrology and uncertainty analysis to disciplines such as toxicology, limnology, and ecology. His research is currently on the topics of climate variability, hydrological change, and geomorphology. He is a member of the Global Institute for Water Security, and an instructor in the School of Environment and Sustainability at the U of S
Cherie J. Westbrook
is a hydrologist with research experience on issues of water quality and ecohydrological processes. She has lead research projects in the boreal plains ecozone and the Rocky Mountains of Canada and is interested in hydrochemistry, groundwater-surface water interactions, and applied hydrological modeling. She is a member of the Centre for Hydrology at the U of S
References
- Anderson CB, Griffith CR, Rosemond AD, Rozzi R, Dollenz O. The effects of invasive North American beavers on riparian plant communities in Cape Horn, Chile—Do exotic beavers engineer differently in sub-Antarctic ecosystems? Biological Conservation. 2006;128:467–474. doi: 10.1016/j.biocon.2005.10.011. [DOI] [Google Scholar]
- Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. Freshwater methane emissions offset the continental carbon sink. Science. 2011;331:50. doi: 10.1126/science.1196808. [DOI] [PubMed] [Google Scholar]
- Bluzma P. Beaver abundance and beaver site use in a hilly landscape (eastern Lithuania) Acta Zoologica Lituanica. 2003;13:8–14. doi: 10.1080/13921657.2003.10512537. [DOI] [Google Scholar]
- Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Global Change Biology. 2013;19:1325–1346. doi: 10.1111/gcb.12131. [DOI] [PubMed] [Google Scholar]
- Bubier JL, Moore TR, Roulet NT. Methane emissions from wetlands in the midboreal region of northern Ontario, Canada. Ecology. 1993;74:2240–2254. doi: 10.2307/1939577. [DOI] [Google Scholar]
- Butler DR, Malanson GP. Sedimentation rates and patterns in beaver ponds in a mountain environment. Geomorphology. 1995;13:255–269. doi: 10.1016/0169-555X(95)00031-Y. [DOI] [Google Scholar]
- Butler DR, Malanson GP. The geomorphic influences of beaver dams and failures of beaver dams. Geomorphology. 2005;71:48–60. doi: 10.1016/j.geomorph.2004.08.016. [DOI] [Google Scholar]
- Cole JJ, Prairie YT, Caraco NF, McDowell WH, Tranvik LJ, Striegl RG, Duarte CM, Kortelainen P, et al. Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems. 2007;10:171–184. doi: 10.1007/s10021-006-9013-8. [DOI] [Google Scholar]
- Collen P, Gibson RJ. The general ecology of beavers (Castor spp.), as related to their influence on stream ecosystems and riparian habitats, and the subsequent effects on fish—A review. Reviews in Fish Biology and Fisheries. 2001;10:439–461. doi: 10.1023/A:1012262217012. [DOI] [Google Scholar]
- Commission for Environmental Cooperation . Ecological regions of North America. Montreal: Commission for Environmental Cooperation; 1997. [Google Scholar]
- Dacey JWH, Klug MJ. Methane efflux from lake sediments through water lillies. Science. 1979;203:1253–1255. doi: 10.1126/science.203.4386.1253. [DOI] [PubMed] [Google Scholar]
- Danilov, P.I., 1995. Canadian and European beavers in Russian Northwest. In The third Nordic beaver symposium, 10–16.
- Dove AE. Methane dynamics of a northern boreal beaver pond. MSc. Montreal: McGill University; 1995. [Google Scholar]
- Downing JA, Cole JJ, Duarte CM, Middelburg JJ, Melack JM, Prairie YT, Kortelainen P, Striegl RG, et al. Global abundance and size distribution of streams and rivers. Inland Waters. 2012;2:229–236. doi: 10.5268/IW-2.4.502. [DOI] [Google Scholar]
- Ford TE, Naiman RJ. Alteration of carbon cycling by beaver—Methane evasion rates from boreal forest streams and rivers. Canadian Journal of Zoology. 1988;66:529–533. doi: 10.1139/z88-076. [DOI] [Google Scholar]
- Gurnell AM. The hydrogeomorphological effects of beaver dam-building activity. Progress in Physical Geography. 1998;22:167–189. [Google Scholar]
- Halley DJ, Rosell F. Population and distribution of European beavers (Castor fiber) Lutra. 2003;46:91–101. [Google Scholar]
- Halley DJ, Rosell F, Saveljev A. Population and distribution of Eurasian beaver (Castor fiber) Baltic Forestry. 2012;18:168–175. [Google Scholar]
- Hartman G. Long-term population development of a reintroduced beaver (Castor fiber) population in Sweden. Conservation Biology. 1994;8:713–717. doi: 10.1046/j.1523-1739.1994.08030713.x. [DOI] [Google Scholar]
- Jarema SI, Samson J, McGill BJ, Humphries MM. Variation in abundance across a species’ range predicts climate change responses in the range interior will exceed those at the edge: A case study with North American beaver. Global Change Biology. 2009;15:508–522. doi: 10.1111/j.1365-2486.2008.01732.x. [DOI] [Google Scholar]
- Le Mer J, Roger P. Production, oxidation, emission and consumption of methane by soils: A review. European Journal of Soil Biology. 2001;37:25–50. doi: 10.1016/S1164-5563(01)01067-6. [DOI] [Google Scholar]
- Lelieveld J, Crutzen PJ, Dentener FJ. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus. 1998;50B:128–150. doi: 10.1034/j.1600-0889.1998.t01-1-00002.x. [DOI] [Google Scholar]
- Lizarralde MS. Current status of the introduced beaver (Castor canadensis) population in Tierra del Fuego, Argentina. AMBIO. 1993;22:351–358. [Google Scholar]
- McComb WC, Sedell JR, Buchholz TD. Dam-site selection by beavers in an eastern Oregon basin. Great Basin Naturalist. 1990;50:273–281. [Google Scholar]
- Moore, T.R., and N.T. Roulet. 1995. Methane emission from Canadian peatlands. In Soils and global change, R. Lal, J. Kimble, E. Levine and B. A. Stewart, 153–164. Boca Raton: CRC Press.
- Moore TR, De Young A, Bubier JL, Humphreys ER, Lafleur PM, Roulet NT. A multi-year record of methane flux at the Mer Bleue Bog, southern Canada. Ecosystems. 2011;14:646–657. doi: 10.1007/s10021-011-9435-9. [DOI] [Google Scholar]
- Naiman RJ, Melillo JM, Hobbie JE. Ecosystem alteration of boreal forest streams by beaver (Castor canadensis) Ecology. 1986;67:1254–1269. doi: 10.2307/1938681. [DOI] [Google Scholar]
- Naiman RJ, Manning T, Johnston CA. Beaver population fluctuations and tropospheric methane emissions in boreal wetlands. Biogeochemistry. 1991;12:1–15. doi: 10.1007/BF00002623. [DOI] [Google Scholar]
- Nisbet EG. Some northern sources of atmospheric methane: production, history, and future implications. Canadian Journal of Earth Sciences. 1989;26:1603–1611. doi: 10.1139/e89-136. [DOI] [Google Scholar]
- Novak, M. 1999. Beaver. In Wild furbearer management and conservation in North America, M. Novak, J. A. Baker, M. E. Obbard, and B. Malloch, 282–312. Queen’s Printer for Ontario.
- Nyssen J, Pontzeele J, Billi P. Effect of beaver dams on the hydrology of small mountain streams: Example from the Chevral in the Ourthe Orientale basin, Ardennes, Belgium. Journal of Hydrology. 2011;402:92–102. doi: 10.1016/j.jhydrol.2011.03.008. [DOI] [Google Scholar]
- Packalen MS, Finkelstein SA, McLaughlin JW. Carbon storage and potential methane production in the Hudson Bay Lowlands since mid-Holocene peat initiation. Nature Communications. 2014;5:1–8. doi: 10.1038/ncomms5078. [DOI] [PubMed] [Google Scholar]
- Parkes, J.P., J. Paulson, C.J. Donlan, and K. Campbell. 2008. Control of North American beavers in Tierra del Fuego: Feasibility of eradication and alternative management options. Landcare Research, LC0708/084, Lincoln, NZ.
- Payette S, Delwaide A, Caccianiga M, Beauchemin M. Accelarated thawing of subarctic peatland permafrost over the last 50 years. Geophysical Research Letters. 2004;31:L18208. doi: 10.1029/2004GL020358. [DOI] [Google Scholar]
- Pupininkas S. The state of the beaver (Castor fiber) population and characteristics of beaver sites in eastern Lithuania. Acta Zoologica Lituanica. 1999;9:20–26. doi: 10.1080/13921657.1999.10512258. [DOI] [Google Scholar]
- Roulet NT, Ash R, Moore TR. Low boreal wetlands as a source of atmospheric methane. Journal of Geophysical Research-Atmospheres. 1992;97:3739–3749. doi: 10.1029/91JD03109. [DOI] [Google Scholar]
- Roulet NT, Crill PM, Comer NT, Dove A, Boubonniere RA. CO2 and CH4 flux between a boreal beaver pond and the atmosphere. Journal of Geophysical Research-Atmospheres. 1997;102:29313–29319. doi: 10.1029/97JD01237. [DOI] [Google Scholar]
- Rudd JWM, Furutani A, Flett RJ, Hamilton RD. Factors controlling methane oxidation in shield lakes: the role of nitrogen fixation and oxygen concentration. Limnology and Oceanography. 1976;21:357–364. doi: 10.4319/lo.1976.21.3.0357. [DOI] [Google Scholar]
- Ruedemann R, Schoonmaker WJ. Beaver dams as geologic agents. Science. 1938;88:523–525. doi: 10.1126/science.88.2292.523. [DOI] [PubMed] [Google Scholar]
- Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor, and H.L. Miller. 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
- Turetsky MR, Kotowska A, Bubier JL, Dise NB, Crill PM, Hornibrook ERC, Minkkinen K, Moore TR, et al. A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Global Change Biology. 2014;20:2183–2197. doi: 10.1111/gcb.12580. [DOI] [PubMed] [Google Scholar]
- Ulevičius A, Jasiulionis M, Jakštienė N, Žilys V. Morphological alteration of land reclamation canals by beavers (Castor fiber) in Lithuania. Estonian Journal of Ecology. 2009;58:126–140. doi: 10.3176/eco.2009.2.06. [DOI] [Google Scholar]
- van Hulzen JB, Segers R, van Bodegom PM, Leffelaar PA. Temperature effects on soil methane production: an explanation for observed variability. Soil Biology & Biochemistry. 1999;31:1919–1929. doi: 10.1016/S0038-0717(99)00109-1. [DOI] [Google Scholar]
- Verpoorter, C., T. Kutser, D.A. Seekell, and L.J. Tranvik. 2014. A global inventory of lakes based on high-resolution satellite imagery. Geophysical Research Letters 41: 6396–6402.
- Westbrook CJ, Cooper DJ, Baker BW. Beaver dams and overbank floods influence groundwater–surface water interactions of a Rocky Mountain riparian area. Water Resources Research. 2006;42:1–12. doi: 10.1029/2005WR004560. [DOI] [Google Scholar]
- Weyhenmeyer CE. Methane emissions from beaver ponds: Rates, patterns, and transport mechanisms. Global Biogeochemical Cycles. 1999;13:1079–1090. doi: 10.1029/1999GB900047. [DOI] [Google Scholar]
- Wright JP. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologica. 2002;132:96–101. doi: 10.1007/s00442-002-0929-1. [DOI] [PubMed] [Google Scholar]
- Yavitt JB, Angell LL, Fahey TJ, Cirmo CP, Driscoll CT. Methane fluxes, concentrations, and production in two Adirondack beaver impoundments. Limnology and Oceanography. 1992;37:1057–1066. doi: 10.4319/lo.1992.37.5.1057. [DOI] [Google Scholar]
- Zurowski W, Kasperczyk B. Characteristics of a European beaver population in the Suwalki Lakeland. Acta Theriologica. 1986;31:311–325. doi: 10.4098/AT.arch.86-29. [DOI] [Google Scholar]
- Zurowski W, Kasperczyk B. Effects of reintroduction of European beaver in the lowlands of the vistula basin. Acta Theriologica. 1988;33:325–338. doi: 10.4098/AT.arch.88-26. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.