Abstract
Purpose
The purpose of this study was to determine if quantifying visceral adipose tissue at CT in asymptomatic adults can predict the likelihood of future cardiac events.
Methods
Subcutaneous and visceral fat volumes were obtained from abdominal CT utilizing a validated semi-automated software tool in 663 asymptomatic adults (mean age 57.3 years, 379F/284M) undergoing colorectal screening. Patients were followed for subsequent cardiac events, defined as myocardial infarction or coronary intervention for a mean follow-up interval of 7.0±1.4 years. Relevant clinical data including Framingham risk score (FRS) were also collected. Statistical analysis included logistic regression, Pearson correlation coefficients, and Welch and Wilcoxon rank sum tests.
Results
Cardiac events were documented in 32 subjects (4.8%) an average 3.0 years after index CT. FRS was predictive of future cardiac events, signified by a higher score (mean score 11.9 versus 7.4; p<0.001). HDL levels were significantly lower in the cardiac event cohort (mean, 52.2 vs. 61.0; p<0.01). None of the other clinical variables were predictive and none of the CT-based fat measurements (visceral, subcutaneous, and total adipose tissue; visceral fat %) correlated with future cardiac events (p=0.561–0.886). Mean visceral fat % in the cardiac event cohort was 38.1% vs. 39.1% for the non-event group.
Conclusion
Quantification of visceral adipose tissue at abdominal CT was not predictive of future cardiac events in this asymptomatic cohort, whereas HDL levels and Framingham risk scores correlated well with risk.
Introduction
Obesity has become a major public health problem with a progressive rise in prevalence. According to the Centers for Disease Control and Prevention, more than one-third of U.S. adults were obese in 2009–2010. 1 Obesity is an established risk factor for heart disease, stroke, type 2 diabetes, and certain types of cancers. 2 Prior to cross-sectional imaging techniques, the primary methods available for determining abdominal obesity were more indirect, including body mass index (BMI) and waist circumference.3 These anthropomorphic methods do not allow for differentiation of subcutaneous and visceral adipose deposition. Such distinction, however, appears to be important, as recent studies indicate that the both the amount and relative distribution of abdominal fat, particularly visceral fat, are associated with risk factors for coronary artery disease, hypertension, impaired fasting glucose, and metabolic syndrome. 4–13 Measurement of visceral (and subcutaneous) fat using abdominal CT is a fairly straightforward task when automated or nearly automated software tools are applied.14,15
As a screening tool, CT colonography (CTC) may be useful for more than just evaluating the large intestine.16–19 Because volumetric imaging of the abdomen and pelvis is performed, all included anatomic structures can potentially be assessed, at least within the limits of a non-contrast, low-radiation dose CT scan. Quantification of abdominal fat is one such measure that is not impacted by this limited technique.15 Because these CTC screening studies are generally performed in healthy, asymptomatic adults, this represents an ideal cohort for population-based risk assessment.
The main purpose of our study was to determine if quantification of visceral adipose tissue at CT in asymptomatic adults can predict future cardiac events.
Material and Methods
As a retrospective review of imaging and the electronic medical record, this study was HIPAA- compliant, approved by our IRB, and the need for signed informed consent was waived.
The study cohort was derived from a consecutive series of generally healthy, asymptomatic adults undergoing colorectal cancer screening with CTC between April 2004 and March 2005. This remote time frame was chosen to allow ample follow-up time for subsequent cardiac events (as defined below). Electronic medical records were reviewed to identify significant cardiac events and for the necessary clinical data to derive the Framingham risk score (FRS). Cardiac events were defined as a documented myocardial infarction (MI) or any coronary artery procedure leading to intervention (eg, coronary artery bypass, stenting, or angioplasty). The FRS was established to predict coronary heart disease; components of the FRS include age, gender, smoking, diabetes, blood pressure, and LDL/HDL/total cholesterol. 20
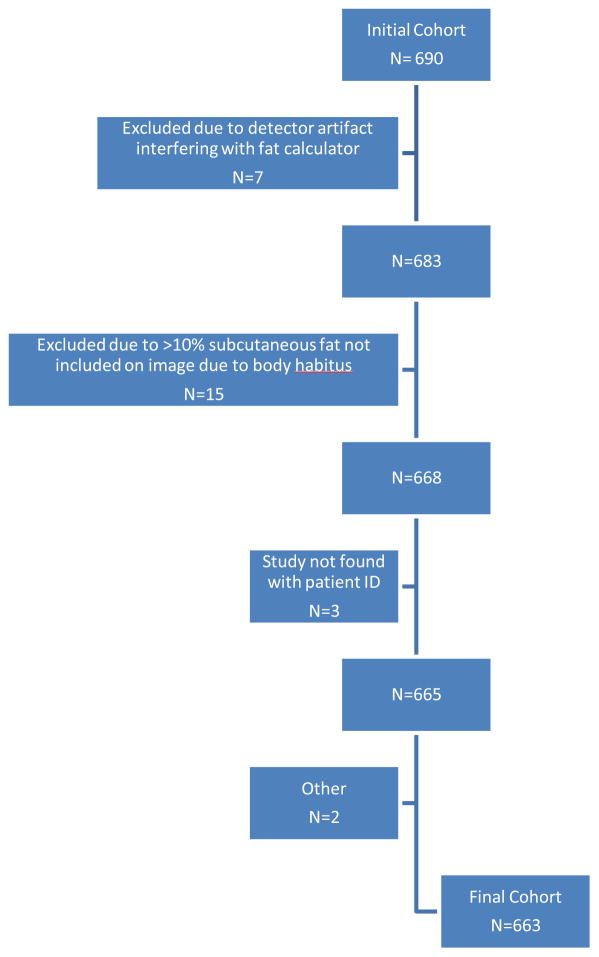
Patients with a history of a cardiac event prior to the index CT or with insufficient data to derive the FRS were excluded. Other reasons for exclusion included a less than two-year period of adequate clinical follow-up and technical reasons related to CT that precluded adequate assessment of abdominal fat (see below). After all exclusions (Fig. 1), the final study cohort consisted of 663 asymptomatic adults (mean age 57.3 years; 379 women, 284 men).
Figure 1.
Flow diagram of study cohort.
CTC was performed as a non-contrast examination of the abdomen and pelvis using a low-dose technique on 8-, 16-, and 64- detector-row MDCT scanners (LightSpeed Series, GE Healthcare).21,22 The scanning protocol consisted of 1.25-mm collimation, 120 kVp, and 50–100 mAs. Although both supine and prone series were acquired for colorectal evaluation, only the supine images were utilized for fat assessment. Specifically, the “extracolonic” series consisting of a 5-mm slice thickness at 3-mm intervals was used for fat quantification analysis.
Abdominal fat quantification
Subcutaneous and visceral abdominal fat compartments were quantified using a validated semi-automated software tool developed by co-authors (RMS, JY) at the Imaging Biomarkers and Computer-Aided Diagnosis Laboratory, Radiology and Imaging Sciences, National Institutes of Health Clinical Center.14,23 The transverse (axial) L2-3 level was used for all abdominal fat measurements,24,25 as verified by a board-certified radiologist (EMR). The adipose tissue software algorithm involves body masking, noise reduction, and adipose tissue labeling prior to visceral and subcutaneous fat quantification (Fig. 2).14,23 A region-growing algorithm first creates the body mask, followed by an anisotropic diffusion filter to reduce noise. Voxels between −274 and −49 HU were then labeled as adipose tissue. The internal and external body contours were created with iterative modifications along the inner boundary of the subcutaneous adipose tissue.
Figure 2. Examples of high and low abdominal visceral fat percentage (VF%). Neither subject experienced a cardiac event during clinical follow-up after CT.
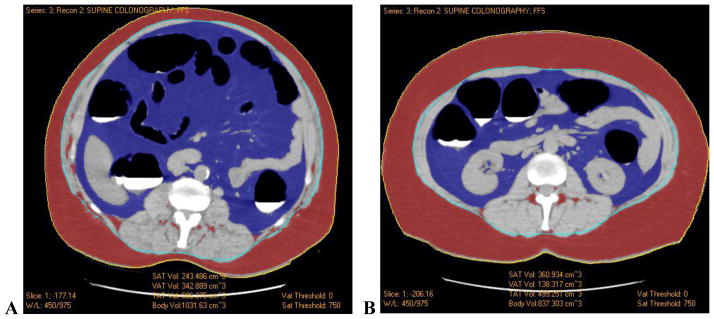
A. CT image at the L2-3 level in a 50-year-old male shows the segmentation of visceral (blue) and subcutaneous (red) adipose tissue by the automated tool. Measurements of subcutaneous (243.5 cm3) and visceral (342.9 cm3) adipose tissue volumes result in a VF% of 58.5%.
B. CT image at the L2-3 level in a 66-year-old male shows similar fat segmentation. Subcutaneous (360.9 cm3) and visceral (138.3 cm3) adipose tissue volumes result in a VF% of 27.7%.
Visceral adipose tissue (VAT) volume quantification (in cm3) was obtained by multiplying voxel counts by the voxel volumes (i.e., the pixel area times the slice thickness). Visceral adipose tissue volume was defined as the volume of all adipose tissue voxels inside of the internal contour, except for fat interspersed within abdominal wall musculature. Subcutaneous adipose tissue (SAT) and total adipose tissue (TAT) volumes were also quantified. The visceral fat percentage (VF%) was defined as the percentage of total adipose tissue that is visceral in location. Each automated color-coded segmented image was reviewed for any areas of misregistration and fixed as necessary; very few cases required any substantial manipulation. Seventeen cases where subcutaneous fat extended beyond displayed FOV were excluded due to imaging artifact and/or exclusion of at least 10% of the subcutaneous fat. None of these excluded patients had a subsequent cardiac event.
Statistical Analysis
Statistical analysis included logistic regression, Pearson correlation coefficients, and Welch, and Wilcoxon rank sum tests. Multiple variables were evaluated for correlation within our cohort for association with a cardiac event. Fat-based variables included VAT, SAT, TAT, VF%, and BMI. Clinical variables included Framingham Risk Score (FRS), cholesterol, HDL, LDL, systolic blood pressure, diastolic blood pressure, and blood pressure ratio. Analyses for the likelihood of cardiac events were performed according to the total cohort, men only, and women only.
Results
In the final cohort of 663 adults, the mean clinical follow-up interval after CT was 7.0 ± 1.4 years. Cardiac events were documented in 32 subjects (4.8%) an average of 3.0 years after CT examination. Among the cardiac events, there were 2 deaths due to coronary heart disease. Cardiac events were documented in 6.7% of men (19 of 284) and 3.4% of women (13 of 379). Mean patient age at the time of index CT was 62.9 years in the cardiac event group and 57.0 years in the non-event group.
Mean, median, and the range of values for the fat-based measurements for both the cardiac event and non-event cohorts are shown in Table 1. Neither BMI nor any of the CT-based fat quantification measurements correlated with an increased risk for future cardiac events (p=0.561–0.886). Mean visceral fat % in the cardiac event cohort was 38.1% (IQR, 25.6–46.9%) vs. 39.1% (IQR, 28.2–49.2%) for the non-cardiac group. Values of all the fat-based measurements according to gender are shown in Table 2.
Table 1.
| Cardiac event cohort (n=32) | Non-event (n=631) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | p-value* | |
| CT-based Adiposity Measures | |||||
| VAT (cm3) | 140.6 ± 112.5 | 108.5 (13.6 – 399.5) | 131.3 ± 86.0 | 117.1 (9.2 – 515.7) | 0.8655 |
| SAT (cm3) | 192.7 ± 107.5 | 186.1 (30.2 – 568.0) | 190.1 ± 99.1 | 171.5 (16.3 – 645.9) | 0.9419 |
| TAT (cm3) | 333.2 ± 193.2 | 286.3 (45.0 – 790.0) | 321.4 ± 158.0 | 296.1 (29.9 – 1011.0) | 0.9145 |
| VF% (%) | 38.1 ± 16.1 | 36.0 (13.5 – 75.6) | 39.1 ± 14.2 | 37.2 (11.6 – 83.6) | 0.5373 |
| Clinical Measurements | |||||
| FRS | 11.9 ± 7.7 | 9.0 (2.0 – 33.0) | 7.1 ± 4.7 | 6.0 (1.0 – 31.0) | 0.000119 |
| HDL | 52.2 ± 19.6 | 48.0 (14.0 – 114.0) | 61.0 ± 17.8 | 59.0 (10.0 – 130.0) | 0.003356 |
| LDL | 120.5 ± 34.0 | 122.0 (59.0 – 188.0) | 119.9 ± 32.6 | 119.0 (34.0 – 269.0) | 0.9310 |
| Total Cholesterol | 200.5 ± 38.9 | 209.0 (120.0 – 269.0) | 203.3 ± 35.5 | 202.5 (93.0 – 389.0) | 0.8691 |
| Systolic BP | 129.0 ± 18.1 | 125.5 (108.0 – 172.0) | 124.1 ± 15.3 | 122.0 (82.0 – 180.0) | 0.2644 |
| Diastolic BP | 77.5 ± 12.2 | 77.0 (54.0 – 106.0) | 75.9 ± 9.2 | 77.5 (42.0 – 110.0) | 0.7336 |
| BP ratio | 1.7 ± 0.2 | 1.6 (1.4 – 2.2) | 1.6 ± 0.2 | 1.6 (1.2 – 2.6) | 0.6070 |
| BMI (kg/m2) | 27.2 ± 5.6 | 26.5 (18.8 – 40.9) | 26.6 ± 4.7 | 25.8 (16.6 – 50.4) | 0.9104 |
Using Wilcoxon Rank Sum test; similar values were derived with the Welch t-test
Clinical values for the cardiac event and non-event cohorts are shown in Table 3. The Framingham Risk Score (FRS) was predictive of future cardiac events (p<0.001); mean FRS was 11.9 in cardiac event cohort vs. 7.4 in non-cardiac group. The FRS was predictive of future cardiac events in females (p=0.0271), with a mean FRS of 7.2 among the cardiac event cohort, versus 5.8 among the non-event group. For males, the FRS was also predictive of future cardiac events (p=0.0056), with a mean FRS of 15.2 in cardiac event cohort versus. 9.7 in non-events group. HDL levels were significantly lower in cardiac event cohort (mean, 52.2 vs. 61.0; p<0.01). None of the other clinical values were predictive of future cardiac events.
Discussion
Obesity is generally defined as a body mass index (BMI) of at least 30 kg/m2 and is clearly on the rise.1,4 Obesity is an established risk factor for hypertension, dyslipidemia, metabolic syndrome, and type 2 diabetes. 2,4 All of these are risk factors for cardiovascular disease, among others diseases. Previous research has also found that the specific location of abdominal fat within the body (ie, visceral and subcutaneous) can influence the degree of risk. Other studies have demonstrated an association between visceral adipose tissue and insulin resistance, hyperinsulinemia, and insulin-like growth factors, among other things.8–13
CT can readily distinguish subcutaneous and visceral fat, whereas anthropometric measurements such as BMI and waist circumference do not provide this information. 26 Fox et al evaluated the correlation of visceral and subcutaneous fat with risk factors for metabolic syndrome and coronary artery disease and found that visceral fat had a stronger association than subcutaneous fat with elevated fasting triglycerides and HDL cholesterol, among others.4 They also found that visceral fat was more strongly associated with all risk factors in women compared with men. Pickhardt et al found that visceral fat in women correlated with metabolic syndrome, but that in men subcutaneous fat was the better predictor of metabolic syndrome.15 Given the association of metabolic syndrome with coronary artery disease, it would stand to reason that quantification of visceral fat might yield a predictive measure for cardiac events.
We found no correlation between abdominal fat-based measures in asymptomatic adults at CT and future cardiac events. Although these results were somewhat surprising given the link between visceral fat and the metabolic syndrome, the strong association between the FRS (and HDL levels) and future cardiac events lends credence to the overall veracity of this cohort. There are a paucity of other studies looking at the association between visceral (and subcutaneous) adiposity and future cardiac events. Kamimurra et al found visceral adiposity to be a predictor of cardiovascular events in non-dialyzed chronic kidney patients. In their study cardiovascular events were three times higher (22%) in patients with visceral adipose to subcutaneous tissue ratio of >0.55, compared to patients with lower ratios.27 Nakagawa et al found that patients with sleep disordered breathing and visceral adiposity with dysregulation of adiponectin correlated with night time acute coronary syndrome requiring percutaneous intervention, but did not correlate with non-night time acute coronary syndrome. In their study patients with visceral fat accumulations (VFA) >100cm2 had lower adiponectin, higher plasminogen activator inhibitor-1 levels, and higher prevalence of sleep disordered breathing than patients with <100 cm2 visceral fat. Acute coronary syndrome occurred at night (12–7am) in 68% of patients and non-night time (7am–12) in 56% with >100 cm2 of VFA. 28 Kim et al found no association between visceral adiposity and CT measurements of coronary artery calcification, which is an indirect measure of cardiac risk but aligns more closely with our findings. In this study the odds ratio of visceral fat mass in men was 1.03 (CI 95% 0.78– 1.36) and in women 1.02 (CI 95% 0.76–1.37) with p-value of 0.85 and 0.88 respectively.29
At our institution, we have a large screening CTC database of asymptomatic adults, which is uniquely suited for questions such as the ones posed in this study. Performing a prospective CT trial to solely evaluate adiposity measures in asymptomatic adults would likely be cost-prohibitive and require many years of follow-up before any useful information would be available. We specifically chose to assess the most remote CTC screening examination in our database in order to maximize the surveillance interval for cardiac events.
Our study did have certain limitations. Although the overall size of the study cohort provides reasonable power, it is limited somewhat by the small number of cardiac events (32 out of 663 patients). Another potential limitation is the use of one transverse CT image to generate the volumetric analysis for fat quantification. However, prior studies have not found a difference between full volumetric analysis and single-slice analysis.3 Additional studies have validated this specific technique and have found that evaluation near the L3 level can provide a good measure of overall visceral fat.24 Another potential study limitation includes the sample population itself. The fact that this was an asymptomatic population actively seeking out a screening study may suggest that this cohort may be inherently different from the overall general population in some way. It is also possible that some individuals may have had a cardiac event elsewhere that was not recorded in our electronic medical record.
In conclusion, quantification of visceral and subcutaneous adipose tissue at abdominal CT was not predictive of future cardiac events in this asymptomatic cohort, whereas both the Framingham Risk Score and HDL levels were predictive.
Acknowledgments
This research was support in part by the National Institutes of Health Clinical Center. We thank Jianhua Yao, PhD, for providing the visceral fat software.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. Jama-Journal of the American Medical Association. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, 2009–2010. National Center for Health Statistics Data Brief. 2012;82:8. [PubMed] [Google Scholar]
- 3.Preis SR, Massaro JM, Robins SJ, et al. Abdominal Subcutaneous and Visceral Adipose Tissue and Insulin Resistance in the Framingham Heart Study. Obesity. 2010;18:2191–8. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments - Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 5.Kissebah AH, Vydelingum N, Murray R, et al. RELATION OF BODY-FAT DISTRIBUTION TO METABOLIC COMPLICATIONS OF OBESITY. Journal of Clinical Endocrinology & Metabolism. 1982;54:254–60. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. American Journal of Clinical Nutrition. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 7.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. ABDOMINAL ADIPOSE-TISSUE DISTRIBUTION, OBESITY, AND RISK OF CARDIOVASCULAR-DISEASE AND DEATH - 13 YEAR FOLLOW UP OF PARTICIPANTS IN THE STUDY OF MEN BORN IN 1913. British Medical Journal. 1984;288:1401–4. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: Evidence for participation of insulin resistance. Clinical Cancer Research. 2005;11:3642–6. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 9.Kissebah AH, Krakower GR. REGIONAL ADIPOSITY AND MORBIDITY. Physiological Reviews. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. Journal of Nutrition. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappa B signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 12.Paz G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Frontiers in Bioscience-Landmark. 2011;16:1634–50. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proceedings of the Japan Academy Series B-Physical and Biological Sciences. 2010;86:131–41. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summers RM, Liu JM, Sussman DL, et al. Association Between Visceral Adiposity and Colorectal Polyps on CT Colonography. American Journal of Roentgenology. 2012;199:48–57. doi: 10.2214/AJR.11.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Jee Y, O’Connor SD, del Rio AM. Visceral Adiposity and Hepatic Steatosis at Abdominal CT: Association With the Metabolic Syndrome. American Journal of Roentgenology. 2012;198:1100–7. doi: 10.2214/AJR.11.7361. [DOI] [PubMed] [Google Scholar]
- 16.Hassan C, Pickhardt P, Laghi A, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm. Arch Intern Med. 2008;168:696–705. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 17.Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249:151–9. doi: 10.1148/radiol.2491072148. [DOI] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, Kim DH, Meiners RJ, et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology. 2010;255:83–8. doi: 10.1148/radiol.09090939. [DOI] [PubMed] [Google Scholar]
- 19.Pickhardt PJ, Lee LJ, del Rio AM, et al. Simultaneous Screening for Osteoporosis at CT Colonography: Bone Mineral Density Assessment Using MDCT Attenuation Techniques Compared With the DXA Reference Standard. Journal of Bone and Mineral Research. 2011;26:2194–203. doi: 10.1002/jbmr.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 23.Yao J, Sussman DL, Summers RM. Fully Automated adipose tissue measurement on abdominal CT. SPIE Medical Imaging. 2011:7965. [Google Scholar]
- 24.Demerath EW, Shen W, Lee M, et al. Approximation of total visceral adipose tissue with a single magnetic resonance image. American Journal of Clinical Nutrition. 2007;85:362–8. doi: 10.1093/ajcn/85.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balentine CJ, Marshall C, Robinson C, et al. Validating Quantitative Obesity Measurements in Colorectal Cancer Patients. Journal of Surgical Research. 2010;164:18–22. doi: 10.1016/j.jss.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 26.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. ASSESSMENT OF ABDOMINAL FAT-CONTENT BY COMPUTED-TOMOGRAPHY. American Journal of Clinical Nutrition. 1982;36:172–7. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 27.Kamimura MA, Carrero JJ, Canziani MEF, Watanabe R, Lemos MM, Cuppari L. Visceral obesity assessed by computed tomography predicts cardiovascular events in chronic kidney disease patients. Nutrition Metabolism and Cardiovascular Diseases. 2013;23:891–7. doi: 10.1016/j.numecd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa Y, Kishida K, Mazaki T, et al. Impact of Sleep-Disordered Breathing, Visceral Fat Accumulation and Adiponectin Levels in Patients with Night-Time Onset of Acute Coronary Syndrome. American Journal of Cardiology. 2011;108:1266–71. doi: 10.1016/j.amjcard.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 29.Kim DJ, Bergstrom J, Barrett-Connor E, Laughlin GA. Visceral adiposity and subclinical coronary artery disease in elderly adults: Rancho Bernardo study. Obesity. 2008;16:853–8. doi: 10.1038/oby.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]