Abstract
Graminaceous monocots, including most of the world's staple grains (i.e. rice, corn, and wheat) use a chelation strategy (Strategy II) for primary acquisition of iron from the soil. Strategy II plants secrete phytosiderophores (PS), compounds of the mugineic acid family that form stable Fe(III) chelates in soil. Uptake of iron-PS chelates, which occurs through specific transporters at the root surface, thus represents the primary route of iron entry into Strategy II plants. The gene Yellow stripe1 (Ys1) encodes the Fe(III)-PS transporter of maize (Zea mays). Here the physiological functions performed by maize YS1 were further defined by examining the pattern of Ys1 mRNA and protein accumulation and by defining YS1 transport specificity in detail. YS1 is able to translocate iron that is bound either by PS or by the related compound, nicotianamine; thus, the role of YS1 may be to transport either of these complexes. Ys1 expression at both the mRNA and protein levels responds rapidly to changes in iron availability but is not strongly affected by limitation of copper or zinc. Our data provide no support for the idea that YS1 is a transporter of zinc-PS, based on YS1 biochemical activity and Ys1 mRNA expression patterns in response to zinc deficiency. YS1 is capable of transporting copper-PS, but expression data suggest that the copper-PS uptake has limited significance in primary uptake of copper.
Plants use two distinct systems or strategies for uptake of sparingly soluble iron from the soil (for review, see Romheld, 1987; Briat, 1992; Guerinot, 1994; Briat and Lobreaux, 1997; Eide, 1997; Mori, 1999; Curie and Briat, 2003). The reduction strategy, referred to as Strategy I, is characterized by plasma membrane localized ferric reductases coupled with Fe(II) transporters. Graminaceous monocots are distinct from other plant groups as they use a chelation strategy (Strategy II). Strategy II plants secrete phytosiderophores (PS), compounds of the mugineic acid (MA) family that form stable Fe(III) chelates (Tagaki et al., 1984). Specific uptake systems located at the root surface then recognize the Fe(III)-PS, which is used as an iron source by the plant (Romheld and Marchner, 1986).
The gene Yellow stripe1 (Ys1) was predicted to encode a Fe(III)-PS transporter of maize (Zea mays) based on the phenotype of ys1 mutants, which show signs of iron deficiency that can be corrected by application of Fe to the leaves (E.L. Walker, unpublished data) or to cut root tips (Bell et al., 1962). Furthermore, ys1 mutants make normal amounts of PS (von Wiren et al., 1994) but lack the ability to use Fe(III)-PS efficiently (Jolley and Brown, 1991; Hopkins et al., 1992; von Wiren et al., 1994, 1995). We have recently cloned ys1 from maize (Curie et al., 2001) and have shown that the YS1 protein contains multiple putative transmembrane domains, consistent with its being a transporter protein. YS1 functionally complements yeast strains that are defective in iron uptake. Complementation occurs only on medium containing Fe(III)-PS complexes, not on medium containing uncomplexed sources of iron, confirming YS1 as a PS-dependent iron transporter.
Although the role of YS1 in primary iron uptake (i.e. from the soil) is clear, questions remain regarding whether YS1 performs additional physiological functions. One potential physiological role for YS1 is in the uptake and/or translocation of other transition metals. Chaignon et al. (2001) have found that iron deficiency (which causes increased PS release) stimulates copper uptake, while zinc deficiency (which has little effect on PS release), does not, suggesting that increased copper accumulation may occur through uptake of Cu-PS complexes. Moreover, in the calcicole grass Hordelymus europaeus, copper deficiency has been shown to induce PS release (Gries et al., 1998). Several authors have suggested that Zn might enter Strategy II plants as Zn-PS complexes, but direct evidence for this mechanism is lacking (von Wiren et al., 1996; Erenoglu et al., 2000; Chaignon et al., 2001; Tolay et al., 2001), and at least one report has questioned whether the observed increases in PS production under Zn deficiency might be the result of an induced physiological deficiency for Fe (Pedler et al., 2000). In addition, Zn has been clearly demonstrated to enter Strategy II plants via a non-PS-mediated route (von Wiren et al., 1996).
Another important question concerns the role of YS1 in leaves. YS1 mRNA is strongly induced in leaves in response to iron deficiency (Curie et al., 2001), yet the amount of PS in leaves is low relative to PS concentration in roots (Walter et al., 1995). However, PS are found in xylem sap, particularly in response to iron deficiency, suggesting that PS most likely are translocated from root to shoot (Alam et al., 2001), and that significant amounts of PS may accumulate in leaves under some circumstances.
An alternative role for YS1 in leaves could be in transport of Fe-nicotianamine (NA) complexes. NA is the direct biochemical precursor to PS, and, as such, is structurally quite similar. Consistent with this similarity of structure, NA, like PS, is a strong complexor of various transition metals, particularly Fe(II) (Anderegg and Ripperger, 1989) and Fe(III) (von Wiren et al., 1999), as well as Cu(II), Ni(II), Co(II), Mn(II), and Zn(II) (Anderegg and Ripperger, 1989). NA is present in shoots and roots at concentrations ranging between 20 and 500 nmol/g fresh weight (Stephan et al., 1990) and is present in both xylem (approximately 20 μm) and phloem (approximately 130 μm), suggesting that NA is a major complexor of metals throughout the plant (Schmidke and Stephan, 1995; Pich and Scholz, 1996).
We have explored the physiological functions performed by YS1 by examining the pattern of YS1 expression in more detail and by better defining YS1 transport specificity. Here we demonstrate that YS1 can translocate iron that is bound either by PS or by the related compound, NA; thus, the role of YS1 in leaves may be to transport either of these complexes. Interestingly, YS1 is capable of transporting either Fe(III) or Fe(II), depending on the particular chelated form (PS or NA) presented. Our data suggest that YS1 does not have a significant role in the uptake or allocation of zinc. YS1 can translocate copper as Cu-PS complexes, but this activity may be of only limited significance for primary copper uptake by maize.
RESULTS
YS1-Specific Antibodies
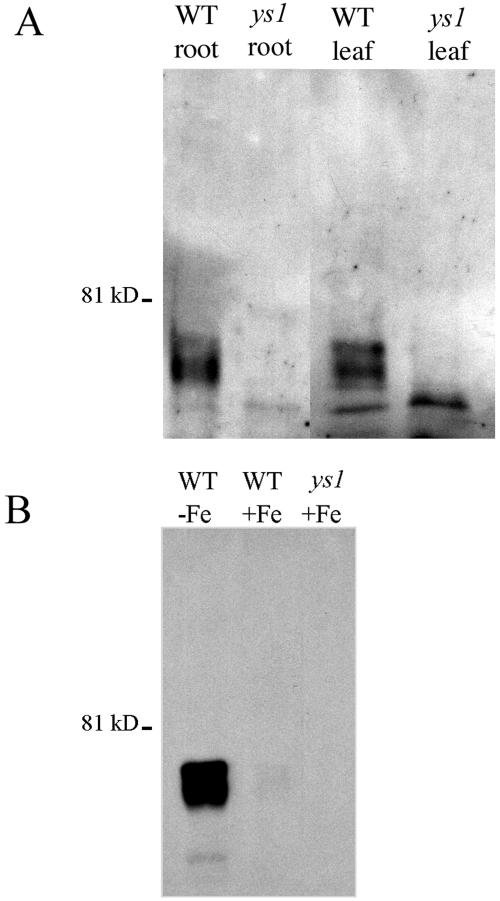
YS1-specific antibodies were generated using the amino terminal 48 amino acids of YS1 (see “Materials and Methods”). To test for specificity of these antibodies, we prepared protein from roots of ys1 mutant plants and wild-type (WT) plants grown in iron-deficient (Fig. 1A) and iron-sufficient (Fig. 1B) conditions. Immunoblots were prepared from these protein samples, and a doublet of 76 kD and 74 kD was observed in WT plants. In WT plants grown under iron deficiency, the intensity of the signal was increased (Fig. 1B). No bands were observed in ys1 mutant plants grown under either condition (Fig. 1, A and B). The ys1-ref allele in these plants bears a large Long Terminal Repeat retrotransposon insertion (Curie et al., 2001) and produces no detectable ys1 mRNA (A.J. Pierson, unpublished data). YS1 has a predicted molecular mass of 74.3 kD, which is consistent with the smaller of the two bands observed. Posttranslational modification of the YS1 protein may account for the second, larger band. The lack of cross reaction to these two strongest bands in the ys1 mutant lanes demonstrates that these antibodies are specific to YS1. Cross reaction of the YS1 antibodies with smaller molecular mass proteins present in both the WT and mutant samples was evident on some blots (see Fig. 1A), but this signal was more often weak or absent (see Fig. 1B.)
Figure 1.
Immunoblot showing the specificity of YS1 antibodies. Blots were probed with affinity-purified anti-YS1 antibodies at a dilution of 1:100. A, Protein samples were prepared from roots and leaves of iron-starved wild-type (WT) and ys1-ref (ys1) mutant plants. B, Protein samples were prepared from roots of fully fed (+Fe) and iron-starved (−Fe) wild-type (WT) plants, and from fully fed (+Fe) ys1-ref mutant plants.
Metal Regulation of Ys1 mRNA and Protein Accumulation
To address whether YS1 might be involved in uptake of metal micronutrients other than iron, we analyzed YS1 gene and protein accumulation under conditions of metal deficiency. Plants were grown hydroponically in modified Hoagland solution (see “Materials and Methods”) that lacked either Fe, Zn, or Cu. Control plants were grown in complete medium. To ensure that the metal starvation regime had caused metal deficiency in plant tissues, the levels of iron, zinc, and copper were measured in leaves of plants grown under each condition (see “Materials and Methods”.) Plants grown in complete nutrient solution contained an average (n = 5) of 65.7 ± 11.2 ppm iron, 122.9 ± 36.8 ppm zinc, and 12.1 ± 0.9 ppm copper. Iron starvation resulted in a 29% decrease (n = 5; P = 0.003 by one-tailed t test) in iron in leaves; zinc starvation resulted in a 35% decrease (n = 5; P = 0.03 by one-tailed t test) in zinc in leaves; and copper starvation resulted in a 29% decrease (n = 5; P = 0.0001 by one-tailed t test) in copper in leaves. Thus, in each case, the tissue levels of the selected metal was significantly decreased by the growth conditions used.
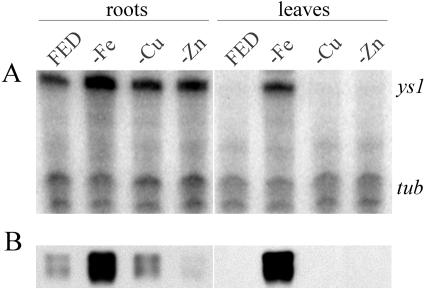
Leaves and roots were harvested from plants grown under each condition, and both RNA and protein were isolated from the same tissue samples. An RNase protection assay was used to analyze Ys1 mRNA levels. The RNase protection method has the advantage of incorporating an internal control (tubulin), which is used to normalize for equal RNA amounts during quantitation. In roots, Ys1 mRNA is detected under all conditions tested but is induced 2.5-fold under conditions of iron starvation (Fig. 2A). A period of 8 d of starvation for Zn and Cu resulted in a level of ys1 mRNA 1.3-fold (Zn) or 1.2-fold (Cu) that of the control value. Thus, the steady-state accumulation of Ys1 mRNA in Cu- or Zn-deficient plants was very similar to the levels present in control plants grown in complete nutrient medium. In leaves, Ys1 mRNA was barely detectable in plants grown with full metal nutrients and in plants grown without Zn or Cu. The relative steady-state levels of ys1 mRNA in Cu- and Zn-deficient plants were 1-fold and 1.2-fold that of the controls, respectively.
Figure 2.
Response of maize seedlings to iron, copper, and zinc deficiency. Protein and RNA were prepared simultaneously from both roots and leaves of maize seedlings grown hydroponically in nutrient-sufficient (FED) or nutrient-deficient (−Fe, −Cu, and −Zn) medium. A, Ribonuclease protection assay. ys1 indicates the position of signal due to specific ribonuclease protection of the Ys1-specific probe; tub indicates the position of signal due to specific ribonuclease protection of the tubulin probe, which serves as an internal loading control. B, Immunoblot using anti-YS1 antibodies.
Protein accumulation of YS1 generally corresponded with mRNA accumulation in that only Fe-starved tissues contained strongly elevated levels of Ys1 mRNA and protein (Fig. 2B). YS1 protein expression was strong in both leaves and roots of Fe-deficient plants. Just as was observed for mRNA, leaves of control, Zn-starved, and Cu-starved plants did not contain detectable levels of YS1 protein. In roots, subtle differences between protein and mRNA accumulation were noted. The root levels of YS1 protein accumulation in control and Cu-starved plants were similar and were low relative to the level present in Fe-starved roots; thus, for these conditions, there was a general correspondence of protein and mRNA levels. However, the level of YS1 protein in Zn-deficient roots was lower than that for roots grown in complete nutrients, although the Ys1 mRNA level in Zn-starved roots was similar to the level from roots grown in complete medium. This difference may indicate independent regulation of mRNA and protein levels. The trend that Zn-starvation causes a decrease in YS1 protein accumulation is opposite to the expectation if YS1 is responsible for primary zinc uptake. Up-regulation of Ys1 in response to iron limitation is understood as an attempt to garner more iron from the environment under conditions of insufficiency. Failure of Ys1 expression to increase under conditions of Cu or Zn limitation may therefore be taken as suggesting that YS1 is not involved in the primary acquisition of these metals.
Pattern of Ys1 mRNA and Protein Accumulation in Leaves and Roots
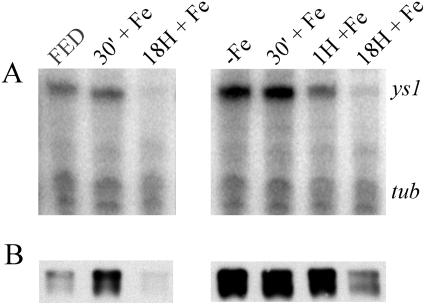
Previously, we demonstrated that Ys1 mRNA accumulation in roots begins to increase after only a single day of iron depletion of the medium (Curie et al., 2001). We sought to understand the dynamics of Ys1 mRNA accumulation following iron resupply to previously starved plants. To do this, we grew plants in the absence of iron for 8 d, then resupplied iron at normal levels, and took tissue samples at several time points following resupply. Control plants were grown in medium containing iron throughout the experiment and were supplied with fresh medium at the same times as the iron-starved samples. The results of these analyses are shown in Figures 3 and 4. In roots, a 2-fold decrease in steady-state Ys1 mRNA level was apparent after only 1 h of iron resupply (Fig. 3A). No change was apparent 30 min following iron resupply (Fig. 3A). Eighteen hours following iron resupply, Ys1 mRNA levels had decreased 8.5-fold (Fig. 3A).
Figure 3.
Response to iron resupply in roots. Protein and RNA were prepared simultaneously from roots of maize seedlings grown hydroponically in iron-sufficient (+Fe) or iron-insufficient (−Fe) medium for 8 d, and then shifted to iron-sufficient medium for 30 min (30′), 1 h (1H), or 18 h (18H). A, Ribonuclease protection assay. ys1 indicates the position of signal due to specific ribonuclease protection of the Ys1-specific probe; tub indicates the position of signal due to specific ribonuclease protection of the tubulin probe, which serves as an internal loading control. B, Immunoblot using anti-YS1 antibodies.
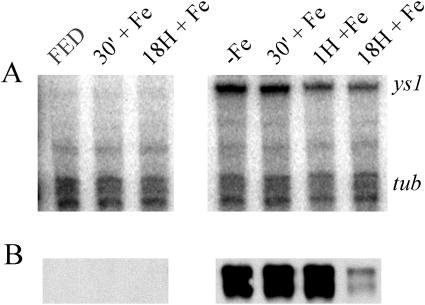
Figure 4.
Response to iron resupply in leaves. Protein and RNA were prepared simultaneously from leaves of maize seedlings grown hydroponically in iron-sufficient (+Fe) or iron-insufficient (−Fe) medium for 8 d, and then shifted to iron-sufficient medium for 30 min (30′), 1 h (1H), or 18 h (18H). A, Ribonuclease protection assay. ys1 indicates the position of signal due to specific ribonuclease protection of the Ys1-specific probe; tub indicates the position of signal due to specific ribonuclease protection of the tubulin probe, which serves as an internal loading control. B, Immunoblot using anti-YS1 antibodies.
We noted that, even in control plants, Ys1 mRNA levels decrease in response to fresh Fe-containing medium. This probably reflects slight iron depletion of the medium that occurs during normal growth, which is reversed when fresh medium is provided. At 18 h following iron resupply, even the control plants exhibited a 4-fold decrease in the steady-state level of Ys1 mRNA. No change was apparent 30 min following iron resupply.
In Fe-resupplied roots, YS1 protein levels did not decrease detectably until 18 h after iron resupply (Fig. 3B). Interestingly, YS1 levels in control plants growing in complete medium actually increased in response to freshly supplied medium, and then declined by the 18-h time point. This pattern of protein accumulation is markedly different from the pattern observed for mRNA accumulation in the same samples, suggesting that posttranscriptional mechanisms control protein accumulation in roots. The rapid decline in mRNA (after 1 h) and slower decline in protein (18 h) also suggest independent regulation of protein and mRNA accumulation in roots.
In the control plants, which were continuously supplied with iron in the medium, but nevertheless responded to fresh medium application, as described above, neither Ys1 mRNA nor protein was detected in leaves (Fig. 4, A and B). In the plants that had been subjected to 8 d of Fe-starvation prior to resupply of Fe, no decrease in Ys1 mRNA or protein levels was apparent at the 30-min time point and only a slight decrease in Ys1 steady-state mRNA level (1.4-fold) was observed after 1 h of iron resupply. Eighteen hours following iron resupply, Ys1 mRNA levels in these leaves were still high compared both to control leaves and to roots at the same time point, but had decreased 1.8-fold relative to the initial time point. The continued abundance of Ys1 mRNA at the 18-h time point is the most obvious difference between roots and shoots following iron resupply, although quantitative differences in the level of response were apparent at each time point. As observed for roots, YS1 protein levels in leaves were regulated somewhat differently from mRNA levels. YS1 protein levels in leaves were equally high at the 0-min, 30-min, and 1-h time points, but declined markedly at 18 h following iron resupply. The strong decrease in protein accumulation in 18-h iron resupplied leaves is particularly striking considering that Ys1 mRNA is still readily detectable.
YS1 Transports Fe(II)-NA
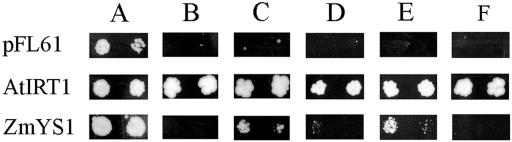
Previously, we have shown that YS1 functionally complements the iron uptake-defective, fet3fet4 strain of yeast (Saccharomyces cerevisiae, DEY1453) when iron is provided as Fe-MA complex, but not when iron citrate is provided (Curie et al., 2001). We tested whether YS1 is also capable of transporting Fe-NA using a similar complementation assay (Fig. 5). In this assay, fet3fet4 yeast were transformed with the YS1-expressing plasmid pYS34 (Curie et al., 2001); with the pFL61 vector, which serves as a negative control; or with Arabidopsis IRT1 (AtIRT1)-expressing plasmid, which serves as a positive control, since it is known to complement growth of this strain (Eide et al., 1996). Five assay conditions were tested, and the positive and negative control strains behaved as expected under all conditions tested. To demonstrate viability of all strains, each was grown under permissive conditions (50 μm iron citrate; Fig. 5A). To demonstrate the YS1 requirement for Fe(III)-MA, the strains were grown on 10 μm FeCl3 (Fig. 5B), and on 10 μm FeCl3 plus 10 μm MA (Fig. 5C). YS1 complements growth in the presence of MA, but not on FeCl3, as expected. When Fe(II) is provided (as 4 μm FeSO4; Fig. 5D), YS1 fails to restore growth, but when NA is provided along with Fe(II) (4 μm FeSO4 with 5 μm NA; Fig. 5E), YS1 complementation of the fet3fet4 growth defect was observed. This result indicates that YS1 is capable of utilizing both Fe(II)-NA and Fe(III)-MA as substrates for transport. The finding that Fe(II)-NA is a potential substrate for transport by YS1 lends additional support to the hypothesis that the role of YS1 in leaves is to transport Fe(II)-NA. The final substrate tested was Fe(III)-NA (10 μm FeCl3 plus 10 μm NA; Fig. 5F). There has been some debate about whether the Fe(III)-NA complex would be stable in vivo (von Wiren et al., 1999; Reichman and Parker, 2002). The failure of YS1 to rescue growth of fet3fet4 yeast on Fe(III)-NA suggests that YS1 is incapable of using this complex as a substrate, in spite of its ability to use the structurally similar complex Fe(III)-MA.
Figure 5.
Functional complementation of fet3fet4 yeast. DEY1453-derived yeast strains transformed with constructs expressing maize Ys1, AtIRT1, or the empty vector, pFL61, were grown on synthetic defined medium containing: A, 50 μm iron citrate; B, 10 μm Fe(III)Cl3; C, 10 μm Fe(III)Cl3 with 10 μm MA; D, 4 μm Fe(II)SO4; E, 4 μm Fe(II)SO4 with 5 μm NA; F, 10 μm Fe(III)Cl3 with 10 μm NA. Pairs of spots correspond to 10-fold and 100-fold dilutions of the original cultures.
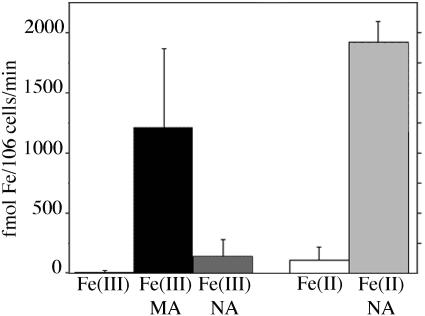
To confirm the results obtained using yeast growth assays, uptake assays using 55Fe were performed. In these assays, fet3fet4 yeast transformed with the Ys1 cDNA expression construct pYS34 were allowed to take up Fe from a simple, defined buffer (see “Materials and Methods” for details). Several transport substrates were tested: Fe(III), Fe(II), Fe(III)-MA, Fe(III)-NA, and Fe(II)-NA. Each uptake condition was replicated a minimum of three times, using three separate cultures of the YS1-expressing strain. As shown in Figure 6, little or no Fe uptake was measured when Fe was provided in the absence of MA or NA, regardless of the valence of the provided iron. Iron provided as Fe(III)-MA was readily taken up with an average rate of 1,209 fmol Fe/106 cells/min, confirming the conclusion from the growth assays that YS1 transports iron from Fe(III)-PS complexes. Fe(II)-NA was also a good substrate for YS1, with an average uptake rate of 1,924 fmol Fe/106 cells/min, again confirming the conclusions from growth assays that YS1 is capable of using Fe(II)-NA as a substrate for transport. Uptake of iron from Fe(III)-NA complexes was significantly slower, with an average rate of only 139 fmol Fe/106 cells/min, which is similar to the control rate in the absence of NA or PS. So, as suggested by the negative result from the yeast growth assays, Fe(III)-NA does not appear to be an optimal substrate for iron transport via YS1.
Figure 6.
Iron uptake assay of YS1 activity. Yeast strain DEY1453 transformed with the YS1 expression construct was grown to exponential phase in synthetic defined medium supplemented with 50 μm iron citrate at pH 4.2. Cells were harvested, washed, and assayed for iron uptake in the presence of 55Fe for a period of 10 min. The values shown are the YS1-dependent rates of Fe uptake, derived as described in “Materials and Methods”.
Transport of Other Metals by YS1
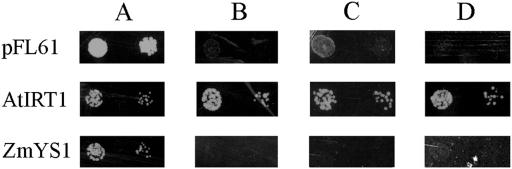
To test whether YS1 can transport metals other than iron, we attempted complementation of additional yeast metal uptake defective strains. The strain, ZHY3 (zrt1zrt2), lacks both high- and low-affinity zinc transporters, and thus has a growth limitation that is due to failure of zinc uptake. We transformed this strain with the YS1-expressing plasmid pYS34 (Curie et al., 2001); with the pFL61 vector, which serves as a negative control; and with an AtIRT1-expressing plasmid, which serves as a positive control, since AtIRT1 is known to mediate uptake of zinc in this functional complementation assay (Korshunova et al., 1999). To demonstrate viability of the strains, each was grown on medium containing 1.4 mm ZnSO4. All three strains grew well under these permissive conditions (Fig. 7A). Growth limitation of zrt1zrt2 strains was observed on medium containing 1.4 μm Zn ZnSO4 (Fig. 7B). The Zn uptake defect was complemented by AtIRT1, but YS1 did not restore growth under Zn limiting conditions. To test for Zn-MA transport and Zn-NA transport, 10 μm MA or 5 μm NA was added to medium containing 1.4 μm Zn ZnSO4. Under all conditions tested, AtIRT1 was able to restore growth, but YS1 failed to complement. These results do not support a Zn uptake function for YS1.
Figure 7.
Functional complementation of zrt1zrt2 yeast. ZHY3-derived yeast strains transformed with constructs expressing maize Ys1, AtIRT1, or the empty vector, pFL61, were grown on synthetic defined medium containing: A, 1.4 mm ZnSO4; B, 1.4 μm ZnSO4; C, 1.4 μm ZnSO4 with 10 μm MA; D, 1.4 μm ZnSO4 with 5 μm NA. Pairs of spots correspond to 10-fold and 100-fold dilutions of the original cultures.
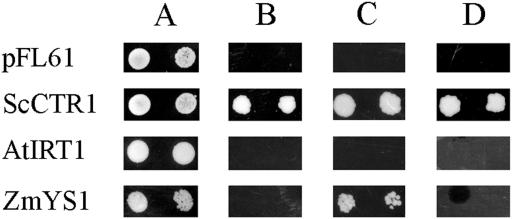
To test for copper uptake by YS1, yeast strain YSC5 (ctr1) was used. The YSC5 strain is unable to take up adequate amounts of copper due to deletion of the CTR1 gene encoding the yeast high-affinity copper transporter. The growth assay to test copper uptake is complicated by the fact that iron uptake in yeast depends on adequate intracellular levels of copper (Askwith et al., 1994; Dancis et al., 1994b). Because of this, under ordinary conditions, the growth defect exhibited by ctr1 yeast is due to secondary failure to take up iron, not the primary copper uptake defect. Growth defects related to inadequate iron uptake could potentially be reversed through expression of the YS1 iron transporter. To test directly for copper uptake, glycerol was substituted for Glc in the medium, requiring the cells to respire, and creating an absolute requirement for copper (Dancis et al., 1994a; Zhou and Gitschier, 1997). Thus, inadequate copper uptake restricts growth of the ctr1 strain on medium containing glycerol as the sole carbon source.
Yeast ctr1 cells transformed with the Ys1 cDNA were grown on plates supplemented with 1 μm CuSO4, 1 μm Cu-MA, or with 1 μm Cu-NA. The positive control was the gene encoding the yeast high-affinity copper transporter, CTR1. IRT1, which does not transport Cu but does transport Fe (Korshunova et al., 1999), is used here as a negative control that would reveal restoration of growth due to alleviation of iron deficiency. AtIRT1 did not complement the YSC5 growth defect under any of the conditions tested. All strains grew on Glc-containing medium (Fig. 8A), demonstrating that all were viable. Under the growth limiting conditions (glycerol with 1 μm Cu), only the CTR1 positive control was able to restore growth (Fig. 8B). Growth restoration by YS1 required Cu-MA in the medium; YS1 failed to complement on Cu-NA containing medium (Fig. 8, C and D). These results suggest that, in addition to being a transporter of iron, YS1 is a copper transporter that requires Cu-PS as its substrate.
Figure 8.
Functional complementation of ctr1 yeast. YSC5-derived yeast strains transformed with constructs expressing maize Ys1, AtIRT1, ScCTR1, or the empty vector, pFL61, were grown on synthetic defined medium containing: A, Glc and 1 μm CuSO4; B, Glycerol and 1 μm CuSO4; C, Glycerol and 1 μm CuSO4 with 10 μm MA; D, Glycerol and 1 μm CuSO4 with 5 μm NA. Pairs of spots correspond to 10-fold and 100-fold dilutions of the original cultures.
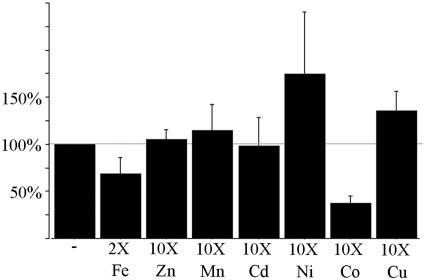
An additional means of assessing the metal selectivity of YS1 is to inhibit uptake of radiolabeled iron by the addition of excess competing metals. The conditions for competition assays were carefully controlled so that MA was present in 10-fold excess over total metal, to ensure that we were observing competition for YS1 transport of metals rather than competition for metal binding by MA. In most cases competing metal was present in 10-fold excess over iron; however, for the control using unlabeled iron as competitor, a 2-fold excess was used. This is due to the technical complication that high concentrations of iron in the uptake medium leads to iron precipitation, which prevents accurate determination of cell-associated radiolabel. We tested for competition by six metals: zinc, manganese, cadmium, nickel, cobalt, and copper. The results, expressed as percent of control, are shown in Figure 9. Among the metals tested, only iron and cobalt significantly inhibited iron uptake. This suggests that Co-MA is a substrate for transport by YS1. Mn, Cd, and Ni fail to compete with iron uptake, suggesting that these metals are not transported by YS1. The lack of competition observed using excess zinc is consistent with the results from yeast complementation assays, which showed that YS1 has little or no Zn transport activity. However, failure to observe competition by excess copper is inconsistent with the observations from the yeast growth assays, which had indicated that YS1 transports copper when it is provided as Cu-MA complex. This may be due to a low affinity of YS1 for Cu-MA, as discussed below.
Figure 9.
Inhibition of YS1 iron uptake by competing metals. Yeast strain DEY1453 transformed with the YS1 expression construct was grown to exponential phase in sd-Glc supplemented with 50 μm iron citrate at pH 4.2. Cells were harvested, washed, and assayed for iron uptake in the presence of 1 μm 55Fe with 100 μm MA, for a period of 10 min. In each assay, a 10-fold excess of competing metal was also added, with the exception of the control unlabeled iron, which was present in only a 2-fold excess over radiolabeled iron. Results are expressed as percent of control.
DISCUSSION
Several authors have suggested that Strategy II plants may acquire metal micronutrients other than iron by uptake of metal-PS complexes. If this is the case, the iron-PS transporter, YS1, may be responsible for moving these complexes into cells. We investigated the possibility that maize YS1 transports metals other than iron using two approaches. In the first study, we demonstrated that YS1 accumulation does not change in response to deficiency for copper, although YS1 levels in maize are markedly increased under conditions of iron deficiency. In Zn-starved plants, the level of YS1 actually decreases in roots. This result suggests that YS1 is unlikely to be an important determinant of primary uptake of Zn and Cu in maize. This suggestion is further substantiated by tests of protein function using yeast functional complementation and radioactive isotope uptake assays. YS1 does not complement a zinc uptake defective yeast strain, and zinc does not compete with iron for transport by YS1. Together, these results show that YS1 does not transport zinc. It is important to point out, however, that Zn-PS transport could occur via another transporter molecule, e.g. one of the other YSL family members in maize. Recent work by Schaaf et al. (2004) demonstrated that YS1 is capable of functional complementation of yeast on Zn-PS when the complex is provided at high (700 μm) concentrations. That the studies presented here used substantially lower amounts of Zn-PS in functional complementation and competition assays provides a likely explanation for these different observations.
The case for copper is more complicated. YS1 does complement a yeast strain that has a block in the uptake of copper, and this complementation occurs specifically when copper is presented as copper-PS complex. Complementation did not occur with other copper complexes, e.g. with copper-NA. Although YS1 transports copper according to the yeast growth assay, biochemical evidence for copper transport was lacking. Copper did not compete with iron for transport via YS1. Failure to observe competition by copper might be due to a low rate of copper uptake via YS1. Conceivably, this low rate could be enough to support yeast growth, yet be inadequate for efficient competition by copper. Plants' requirement for copper is quite low compared to their requirement for other essential metal micronutrients. Thus it is possible that even a low rate of transport of Cu-PS complexes by YS1 is significant. Furthermore, if overexpression of YS1 were used to boost acquisition of iron in cereals, as has been suggested (Mori, 1999), copper transport by YS1 could lead to undesired acquisition of copper. However, the finding that Ys1 mRNA and protein accumulation do not change in response to Cu deficiency suggests that YS1 may not be a major route of primary copper acquisition for maize.
PS-mediated transport of toxic heavy metals has also been postulated, and the use of the PS transporter YS1 is an attractive candidate for engineering heavy metal uptake for the purposes of phytoremediation. Here we tested the ability of YS1 to transport heavy metals using a competition assay, and showed that cadmium and nickel are not able to compete with iron for transport by YS1. Among the heavy metals tested, only cobalt competes for iron uptake; thus, YS1 does appear to be able to transport this metal.
The realization that YS1 is expressed in leaves led to the idea that this transporter must have additional functions that are not strictly related to primary metal acquisition from soil. A key question has been whether YS1 is transporting iron-PS complexes in leaves, or the related complex, iron-NA. YS1 is only found in leaves of plants that are experiencing iron deficiency, and are thus producing PS. In these iron-starved plants, Fe-PS substrates for YS1 could be present in leaves, having arrived there following transport of PS from the roots. EST data indicate that the maize genome encodes at least four YSLs in addition to the YS1 gene. The function of these proteins is likely to transport metal-NA complexes, and presumably these YSL transporters would be responsible for movement of Fe-NA complexes under iron-replete conditions, when YS1 is absent from leaves. In this study, we have shown that YS1 is able to transport iron provided as Fe-NA complexes, based on yeast complementation and on uptake assays. Thus the function of YS1 in shoot tissues may be to transport Fe-NA complexes, Fe-PS complexes, or both during times of iron deficiency.
We found that YS1 cannot use Fe(III)-NA as a substrate for transport; instead it requires Fe(II)-NA. This valence requirement is particularly noteworthy given that Fe(III)-PS complexes are readily accepted by YS1, and indeed are the predominant species expected in soils. Thus, we conclude that YS1 appears to be capable of transporting either Fe(III) or Fe(II), depending on the particular chelated form presented. In a recent report (Schaaf et al., 2004), YS1 was shown to transport Fe(III)-NA, but the efficiency of Fe(III)-NA transport was low compared to transport of Fe(II)-NA. Differences in experimental procedures, particularly in the concentrations of substrates used in the two studies, may account for our failure to detect low rates of Fe(III)-NA transport by YS1.
MATERIALS AND METHODS
Plant Growth Conditions
Maize (Zea mays) plants of the W23 inbred strain were grown hydroponically in modified Hoagland number 1 medium, with 25% of the nitrogen supplied as ammonium (50 mm MES; 1 mm KH2PO4; 3.75 mm KOAc; 5 mm Ca(NO3)2; 1.25 mm KNO3; 2 mm MgSO4; 3.75 m NH4OAc; 46 μm H3BO3; 9.1 μm MnCl2; 0.77 μm ZnSO4; 0.32 μm CuSO4; 0.83 μm H2Mo4; 100 μm FeSO4; 100 μm EDTA). Plants were grown in the greenhouse in March and April, with 14 h of supplemental light provided by two high pressure sodium lamps (GE Lucalox Lamp Lu 400; General Electric, Fairfield, CT). Plants designated as fed were grown on complete medium, containing all macro- and micronutrients, while plants designated −Fe, −Cu, and −Zn were grown in medium lacking iron, copper, or zinc, respectively. Plants were grown for 8 d after emergence of coleoptiles for each experimental condition, and medium was changed every 7 d after planting.
Inductively Coupled Plasma-Mass Spectrometry
Individual leaves were dried at 60°C for 3 d and were then weighed. Dried material was digested in 1.5 mL concentrated. HNO3 at 116°C for 5 h in Pyrex tubes and then diluted to 10.0 mL with 18 MΩ water. Samples were run on a VG PQ Excell inductively coupled plasma-mass spectrometer (ICP-MS; Thermo Electron Corporation, Houston) using a microconcentric nebulizer drawing about 1 mL/min. Iron was run separately using a cool plasma.
RNA Isolation and Ribonuclease Protection Assay
RNA was prepared using QIAgen RNeasy Plant (Qiagen, Valencia, CA). Either 5 or 10 μg of total RNA were used in each protection reaction. Ys1 and tubulin probe fragments were amplified from maize genomic DNA using primers which concurrently added T7 promoter sequences at the 3′ ends. Primers for Ys1 were: oYS.3365..3386: GTGTTCATGGTGCCTGCGGTTG and oYS_T7.3879..3857 TAATACGACTCACTATAGGTGCACCGAAGGCAAAAAGTTGT, fragment length 514 bp. The 3′ primer is 134 bp downstream from the Ys1 cDNA end; thus, the expected length of the protected Ys1 fragment is 380 nt. The gene specificity of this probe is ensured because it is derived from sequence that includes the unique 3′ noncoding region of Ys1. Primers for alpha1-tubulin were: oTUB1.661..683: CCATGCCCACAATGCATCCATTC and oTUB1_T7.1267..1245: TAATACGACTCACTATAGGGCAAAGTTGTTGGCTGCATCC, fragment length 606 bp. The 5′ tubulin primer is within an intron so that the expected length of the protected fragment for alpha1-tubulin is 193 nt. Other, closely related α-tubulins are also detected by this probe. RNA copies of the amplified fragments were transcribed and labeled using T7 RNA polymerase (Invitrogen, Carlsbad, CA) and α32P-UTP. Full-length probes were gel purified and used with the RPA III kit (Ambion, Austin, TX). Tubulin was used as a control in each reaction for the purpose of lane loading standardization. Gels were imaged using Molecular Dynamics Storm phosphorimager and software. Quantitation of ys1 mRNA was performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Briefly, the intensity of the protected tubulin fragments was quantified and expressed as a fraction of the level present in control plants. The levels of ys1 mRNA for each sample were normalized using these values. Quantifications are expressed relative to the levels of ys1 mRNA present in control plants grown in complete nutrient solution.
YS1 Antibodies
A peptide consisting of the 48 N-terminal amino acids of YS1 (MDLARRGGAAGADDEGEIERHEPAPEDMESDPAAAREKELELERVQSW) was expressed as a 6×-His fusion protein from vector pET30LIC (Novagen, Madison, WI) in Escherichia coli BL21(DE3) pLysS (Novagen). The expressed peptide was purified over Ni-agarose (Ni-NTA, Qiagen), and then conjugated to keyhole limpet hemocyanin. The conjugated protein was used to immunize New Zealand white rabbits. One rabbit showed an apparent YS1-specific reaction, and the serum from this rabbit was affinity-purified against the same peptide bound to Ni-NTA (Qiagen) as described (Gu et al., 1994). Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Protein Preparation and Immunoblot Analysis
Tissue was ground using mortar and pestle in liquid nitrogen, and the grindate was added 1:2 (w/v) to 2× Sample Buffer (Sambrook et al., 1989). Samples were vortexed thoroughly and incubated on ice for 10 min before being centrifuged at 10,000g, 10 min at 4°C. Two identical gels were prepared for each experiment. One was used to prepare blots; the other was stained with Coomassie Blue and used as a control for equal protein loading. Blots were stripped (per Amersham's ECL+ kit instructions; Amersham, Buckinghamshire, UK) for 2 h at 70°C to remove residual endogenous peroxidase activity, prior to incubation with primary and secondary antibodies. Affinity-purified YS1 antibodies were used at a dilution of 1:100; donkey anti-rabbit conjugated to HRP (Amersham) was used at a dilution of 1:5,000. Detection was performed using ECL+ (Amersham) and Kodak MS autoradiography film.
Yeast Functional Complementation
Saccharomyces cerevisiae strains DEY1453 (MATa/MATα ade2/ADE2 can1/can1 his3/his3 leu2/leu2 trp1/trp1 ura3/ura3 fet3-2::HIS3/fet3-2::HIS3 fet4-1::LEU2/fet4-1::LEU2, described in Eide et al. [1996]) and ZHY3 (MATα ade6 can1 his3 leu2 trp1 ura3 zrt1::LEU2 zrt2::HIS3, described in Zhao and Eide [1996a, 1996b]) were a generous gift of David Eide (University of Missouri, Columbia, MO) and are defective in specific uptake of Fe and Zn, respectively. The DEY1453 strain has intact systems for uptake of iron chelated by bacterial siderophores (Lesuisse et al., 2001) but does not take up PS-bound Fe (Curie et al., 2001). Yeast strain YSC5 (MATα, ura3-52 lys2-801 ade2-101 trp1Δ1 his3-Δ200 leu2-2, 112 Δctr1::LEU2) is defective in specific uptake of copper and was generously provided by Andy Dancis (University of Pennsylvania, Philadelphia). All strains were transformed with URA3-containing plasmids expressing the specified genes under the yeast PGK promoter, using a modified lithium chloride transformation method (Elble, 1992). The fet3fet4 strain required the addition of 50 μm Fe citrate, pH = 4.2 to the growth medium.
Yeast functional complementation plate assays were performed essentially as described (Curie et al., 2001). All plate assays utilized specially-prepared metal-free yeast nitrogen base (lacking the metal nutrients Cu, Zn, Mn, and Fe; U.S. Biological, Swampscott, MA), which was then supplemented with appropriate levels of each metal depending on the assay being performed. Media were prepared using reverse osmosis purified water and were buffered using MES to appropriate pH. Functional complementation of the fet3fet4 mutant was performed in the presence of 4 μm Fe(II) or 10 μm Fe(III), ± MA and NA. Although deoxymugineic acid is the dominant PS produced by maize plants, it is well established that Strategy II plants are capable of using both hydroxylated and unhydroxylated PS species (Römheld and Marschner, 1990); thus, we used MA in these assays because of its availability, thanks to the kind gift of Satoshi Mori (University of Tokyo, Tokyo). Fe(II) transport assays were conducted in the presence of 1 mm ascorbate. The copper transport deficient strains were tested on synthetic glycerol medium (SG-ura), pH 5.7, in which the Glc was replaced with glycerol to force cells to undergo oxidative phosphorylation.
Radioactive Uptake Assays
Radioactive uptake of 55Fe was performed essentially as described (Eide et al., 1996). Briefly, yeast strains transformed with indicated plasmids were grown in selective medium to an optical density (OD600) of 0.5 to 1.0, pelleted, washed twice in reverse osmosis water, and resuspended at a final density of approximately 2 × 107 cells/mL. At time zero, 50 μL of cell suspension was added to 450 μL assay buffer (25 mm MES 2% Glc, pH = 5.5) containing 1 μm of metal to be transported. 55FeCl3 (used as a tracer at 40 nm) was obtained from Perkin-Elmer Life Sciences (Shelton, CT). Transport of reduced Fe was assayed in the presence of 1 mm ascorbate. In all experiments the uptake period was 10 min and was performed for each sample at 30°C and 0°C. Metal transport was measured in the presence and absence of 10 μm MA or NA. Competition assays were conducted in the presence of 10-fold excess competing metal (prepared as 10 mm stocks from CuSO4, ZnSO4, MnSO4, CdSO4, CoCl2, and NiSO4), except for Fe, which was performed with only a 2-fold excess of FeCl3 to minimize precipitation of insoluble ferric hydroxides. Radioactive counts were obtained by liquid scintillation counting and were converted into transport rates expressed as fmol Fe/106 cells/min. Transport rates for each metal were calculated by subtracting the rate at 0°C from the rate at 30°C for each sample, and then subtracting the nonspecific metal transport rate obtained for the vector-only negative control.
Acknowledgments
We thank David Salt and Brett Lahner for performing ICP-MS measurements, David Eide and Andy Dancis for generously sharing strains and advice, Satoshi Mori for his very kind gifts of MA and NA, and Teddi Bloniarz for expert assistance in the greenhouse.
This work was supported by the U.S. Department of Agriculture (grant no. 99–35100–7601) and the National Science Foundation (grant no. MCB0114748).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.037572.
References
- Alam S, Kamei S, Kawai S (2001) Effect of iron deficiency on the chemical composition of the xylem sap of barley. Plant Soil 47: 643–649 [Google Scholar]
- Anderegg G, Ripperger H (1989) Correlation between metal complex formation and biological activity of nicotianamine analogues. J Chem Soc Chem Commun 10: 647–650 [Google Scholar]
- Askwith C, Eide D, Ho AV, Bernard PS, Li L, Davis-Kaplan S, Sipe DM, Kaplan J (1994) The Fet3p gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76: 403–410 [DOI] [PubMed] [Google Scholar]
- Bell WD, Bogorad L, McIlrath WJ (1962) Yellow-stripe phenotype in maize. I. Effects of ys1 locus on uptake and utilization of iron. Bot Gaz 124: 1–8 [Google Scholar]
- Briat J-F (1992) Iron assimilation and storage in prokaryotes. J Gen Microbiol 138: 2475–2483 [DOI] [PubMed] [Google Scholar]
- Briat J-F, Lobreaux S (1997) Iron transport and storage in plants. Trends Plant Sci 2: 187–193 [Google Scholar]
- Chaignon V, Di Malta D, Hinsinger P (2001) Fe-deficiency increases Cu acquisition by wheat cropped in a Cu-contaminated vineyard soil. New Phytol 154: 121–130 [Google Scholar]
- Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409: 346–349 [DOI] [PubMed] [Google Scholar]
- Dancis A, Haile D, Yuan DS, Klausner RD (1994. a) The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem 269: 25660–25667 [PubMed] [Google Scholar]
- Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD (1994. b) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76: 393–402 [DOI] [PubMed] [Google Scholar]
- Eide D (1997) Molecular biology of iron and zinc uptake in eukaryotes. Curr Opin Cell Biol 9: 573–577 [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93: 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R (1992) A simple and efficient procedure for transformation of yeasts. Biotechniques 13: 18–20 [PubMed] [Google Scholar]
- Erenoglu B, Eker S, Cakmak I, Derici R, Romheld V (2000) Effect of iron and zinc deficiency on release of phytosiderophores in barley cultivars differing in zinc efficiency. J Plant Nutr 23: 1645–1656 [Google Scholar]
- Gries D, Klatt S, Runge M (1998) Copper-deficiency-induced phytosiderophore release in the calcicole grass Hordelymus europaeus. New Phytol 140: 95–101 [Google Scholar]
- Gu J, Stephenson CG, Iadarola MJ (1994) Recombinant proteins attached to a nickel-NTA column: use in affinity purification of antibodies. Biotechniques 17: 257–262 [PubMed] [Google Scholar]
- Guerinot ML (1994) Microbial iron transport. Annu Rev Microbiol 48: 743–772 [DOI] [PubMed] [Google Scholar]
- Hopkins BG, Jolley VD, Brown JC (1992) Plant utilization of iron solubilized by oat phytosiderophore. J Plant Nutr 15: 1599–1612 [Google Scholar]
- Jolley VD, Brown JC (1991) Differential response of Fe-efficient corn and Fe-inefficient corn and oat to phytosiderophore released by Fe-efficient Coker 227 oat. J Plant Nutr 14: 45–58 [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40: 37–44 [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Blaiseau PL, Dancis A, Camadro JM (2001) Siderophore uptake and use by the yeast Saccharomyces cerevisiae. Microbiology 147: 289–298 [DOI] [PubMed] [Google Scholar]
- Mori S (1999) Iron acquisition by plants. Curr Opin Plant Biol 2: 250–253 [DOI] [PubMed] [Google Scholar]
- Pedler JF, Parker DR, Crowley DE (2000) Zinc deficiency-induced phytosiderophore release by the Triticaceae is not consistently expressed in solution culture. Planta 211: 120–126 [DOI] [PubMed] [Google Scholar]
- Pich A, Scholz G (1996) Translocation of copper and other micronutrients in tomato plants (Lycopersicon esculentum Mill.): nicotianamine-stimulated copper transport in the xylem. J Exp Bot 47: 41–47 [Google Scholar]
- Reichman SM, Parker DR (2002) Revisiting the metal-binding chemistry of nicotianamine and 2′-deoxymugineic acid. Implications for iron nutrition in Strategy II plants. Plant Physiol 129: 1435–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romheld V (1987) Different strategies for iron acquisition in higher plants. Physiol Plant 70: 231–234 [Google Scholar]
- Romheld V, Marchner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80: 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Marschner H (1990) Genotypical differences among graminaceous species in release of phytosiderophores and uptake of iron phytosiderophores. Plant Soil 123: 147–153 [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning. Cold Spring Harbor Laboratory Press, New York
- Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279: 9091–9096 [DOI] [PubMed] [Google Scholar]
- Schmidke I, Stephan UW (1995) Transport of metal micronutrients in the phloem of castor bean (Ricinus communis) seedlings. Physiol Plant 95: 147–153 [Google Scholar]
- Stephan UW, Scholz G, Rudolph A (1990) Distribution of nicotianamine, a presumed symplast iron transporter, in different organs of sunflower and of a tomato wild type and its mutant chloronerva. Biochem Physiol Pflanz 186: 81–88 [Google Scholar]
- Tagaki S, Nomoto K, Takemoto T (1984) Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutr 7: 469–477 [Google Scholar]
- Tolay I, Erenoglu B, Romheld V, Braun HJ, Cakmak I (2001) Phytosiderophore release in Aegilops tauschii and Triticum species under zinc and iron deficiencies. J Exp Bot 52: 1093–1099 [DOI] [PubMed] [Google Scholar]
- von Wiren N, Klair S, Bansal S, Briat J-F, Khodr H, Shioiri T, Leigh RA, Hider RC (1999) Nicotianamine chelates both Fe[III] and Fe[II]. Implications for metal transport in plants. Plant Physiol 119: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Marchner H, Romheld V (1995) Uptake kinetics of iron-phytosiderophores in two maize genotypes differing in iron efficiency. Physiol Plant 93: 611–616 [Google Scholar]
- von Wiren N, Marchner H, Romheld V (1996) Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol 111: 1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Mori S, Marschner H, Romheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Pich A, Scholz G, Marschner H, Romheld V (1995) Effects of iron nutritional-status and time of day on concentrations of phytosiderophores and nicotianamine in different root and shoot zones of barley. J Plant Nutr 18: 1577–1593 [Google Scholar]
- Zhao H, Eide D (1996. a) The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci USA 93: 2454–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Eide D (1996. b) The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem 271: 23203–23210 [DOI] [PubMed] [Google Scholar]
- Zhou B, Gitschier J (1997) hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA 94: 7481–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]