Abstract
Reflectance confocal microscopy (RCM) is a modern, non-invasive diagnostic method that enables real-time imaging of epidermis and upper layers of the dermis with a nearly histological precision and high contrast. The application of this technology in skin imaging in the last few years has resulted in the progress of dermatological diagnosis, providing virtual access to the living skin erasing the need for conventional histopathology. The RCM has a potential of wide application in the dermatological diagnostic process with a particular reference to benign and malignant skin tumors. This article provides a summary of the latest reports and previous achievements in the field of RCM application in the diagnostic process of skin neoplasms. A range of dermatological indications and general characteristics of confocal images in various types of tumors are presented.
Keywords: reflectance confocal microscopy, in vivo biopsy
Introduction
In the recent years several new techniques that enable real-time rapid diagnosis and monitoring of treatment of skin diseases have been developed. These include magnetic resonance [1], high frequency ultrasonography [2], optical coherent tomography (OCT) [3] and reflectance confocal microscopy (RCM) [4, 5]. Compared with other non-invasive techniques that produce images of resolutions allowing to only evaluate architectural changes of the skin, RCM enables imaging of the respective skin layers and even cellular structures, which makes it a method of a great potential [6]. The method allows real-time imaging of the epidermis and upper layers of the dermis with an almost histological accuracy and good contrast [7]. The RCM has a wide range of applications, including the diagnostic process of skin diseases, with a particular reference to benign and malignant skin tumors, non-invasive monitoring of the progress of therapy [8–12] and planning of surgical margins during the pre- and intraoperative procedure [13–15]. Despite the increasing popularity of skin cancer prevention programs, a significant increase in its incidence is observed, especially of basal and squamous cell carcinoma and malignant melanoma. Currently, the histopathological examination is the gold standard in the diagnosis of skin tumors. A skin biopsy is painful, leaves a scar and the final diagnosis sometimes requires multiple interventions. The use of RCM eliminates these drawbacks by enabling quick and painless skin visualization in vivo. The skin is not altered by the procedure, which eliminates the risk of artifacts. All images can be stored electronically, therefore they can be reproduced and compared with successive results, which allows for evaluation of dynamic changes in the skin such as response to external stimuli or response to treatment [7].
Aim
The aim is to present the RCM, its diagnostic applications and the potential in monitoring the efficacy of skin cancer treatment.
Reflectance confocal microscopy of the normal skin
Unlike conventional histological examination, confocal images represent a set of horizontal sections of the individual layers of the epidermis and the dermis. In addition, images are displayed in grayscale, while routine histology requires tissue staining with exogenous dyes. Confocal images are based on the presence of endogenous contrast [16]. In the human skin, melanin is the strongest endogenous contrast source [5]. Other sources include keratin, mitochondria, cytoplasmic organelles, nuclear chromatin and collagen located in the dermis.
The visualization of various tissue structures are based on the optical properties such as the refraction index and the reflectivity of the tissue under investigation [17, 18]. In vivo examination of the skin using RCM starts from the epidermis. The most superficial images correspond to the stratum corneum. In optical sections, large (10–30 µm), very bright, anucleated and polygonal corneocytes are observed. These cells are grouped in “islands” separated by skin folds, which appear very dark [7].
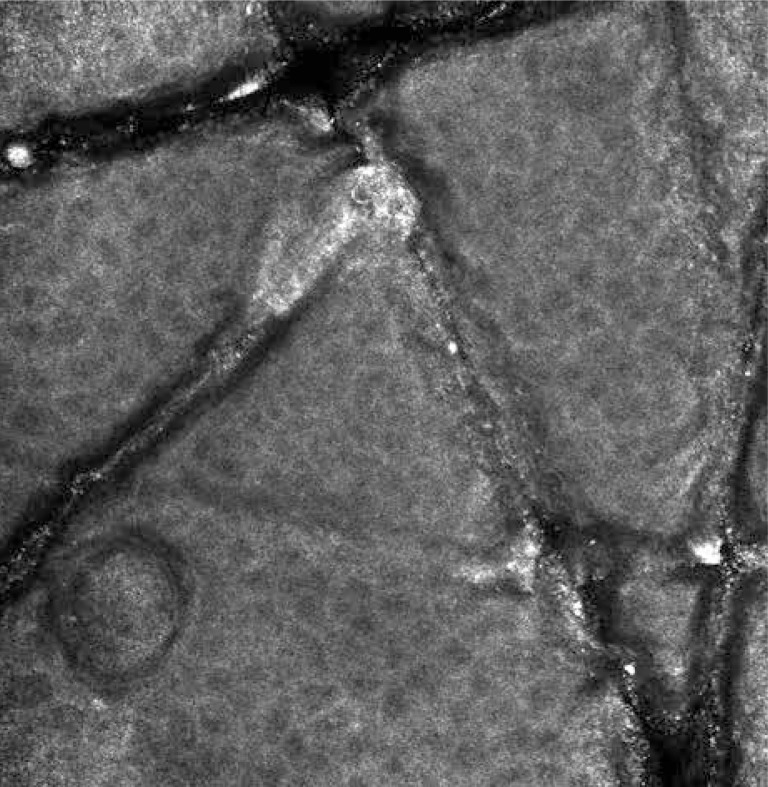
At a depth of approximately 15–20 µm below the stratum corneum, stratum granulosum built of two to four layers of corneocytes with a diameter of 25–35 µm is visualized. The stratum granulosum cells are characterized by the presence of centrally located dark, oval nuclei and bright grainy cytoplasm [7] (Figure 1). The stratum spinosum is located 20–100 µm below the stratum corneum. It consists of smaller (15–25 µm), tightly packed corneocytes with distinct borders, dark nuclei and clear, homogeneous cytoplasm [7]. On an RCM image, both the stratum granulosum and spinosum cells are arranged in a characteristic “honeycomb pattern” [19]. The deepest layer of epidermis – the basal layer – is located 50–100 µm below the stratum corneum. The diameter of the basal layer cells is 7–12 µm [7]. Basal cells are very bright due to “melanin hats”, which are supranuclear melanin aggregates [20]. The melanocytes are visualized as nests of bright, round, oval and spindle cells or dendritic cells, located in the dermo-epidermal junction [21]. The other melanin-containing cells are melanophages that can be identified as large (5–25 µm), bright, nucleated cells, with poorly marked borders, located in the surroundings of blood vessels in the upper layers of the dermis [22]. The papillary dermis is visualized at the level of dermo-epidermal junction as “edged papillae” that are dark, round areas surrounded by bright rings of the basal layer cells [22]. In the central part of the dermal papilla, capillary loops with a clearly visible blood flow are observed. Below the dermo-epidermal junction in the papillary dermis, a network of collagen fibers with a diameter of 1–5 µm can be observed. The collagen fibers with a diameter of 5–25 µm located in the reticular dermis are characterized by a parallel arrangement [23]. Other structures such as eccrine ducts can be visualized in optical sections as bright, centrally hollow structures, penetrating out through the epidermis and dermis. Hair shafts associated by pilo-sebaceous units can be visualized as centrally hollow structures with elliptical, elongated cells at the circumference and a central refractile hair shaft [7, 24]. The abovementioned description of skin morphology can differ depending on its location, phototype, sex, age, and the degree of post-sun damage of the skin [25]. For example, the skin of palms and soles has a clearly visible greater thickness of the stratum corneum and a higher amount of eccrine glands [19].
Figure 1.
Normal skin – stratum granulosum
The use of reflectance confocal microscopy for examination of benign and malignant skin tumors
Pigmented lesions
The RCM is a particularly useful diagnostic tool for melanocytic lesions imaging, since the high content of melanin provides excellent contrast [5]. We use the function called “mosaic” for nevi examination, usually performed at three levels: the upper layers of the epidermis (stratum granulosum and spinosum), at the level of the dermo-epidermal junction and in the upper layers of the dermis.
Benign melanocytic nevi
Benign melanocytic nevi exhibit unaltered stratum granulosum and spinosum of the epidermis, composed of homogeneous, well-demarcated, bright keratinocytes, presenting a “honeycomb” or “cobblestone” pattern [26, 27]. Dermal papillae are uniformly distributed, circumscribed (“edge papillae”) and brighter than dermal papillae of unchanged skin in nevi surroundings. The presence of a homogeneous population of monomorphous, oval, bright cells with centered nuclei (nevomelanocytes) is a common feature [21, 26]. In junctional nevi nevomelanocytes localize only at the dermo-epidermal junction, usually situated in the dermal papillae surroundings. In compound nevi nevomelanocyte nests localize both at the dermo-epidermal junction (especially around vessels) and in the papillary and reticular dermis. In both types of nevi, single nevomelanocytes may also be observed in the epidermis and, as previously described, it does not change its architecture. Dermal nevi are characterized by the presence of nevomelanocyte nests within the papillary and reticular dermis [26, 28, 29].
Dysplastic nevi
The RCM features of dysplastic nevi are characterized by the presence of a population of heterogeneous cells of different size, shape and brightness. However, these cells are round or oval, similar to benign melanocytic nevi, unlike the dendritic cells that are characteristic ofmalignant melanoma [21, 26]. Most of nevomelanocytes have attenuated brightness, elliptical shape and peripherally located nucleus. In the stratum granulosum the loss of demarcation between keratinocytes and the presence of highly refractive granules, probably representing melanin dust, can be observed. Dysplastic nevi, especially those located on the face, may exhibit a structure disorder at the level of dermo-epidermal junction and a local presence of non-edged papillae [28, 29].
Spitz nevi
In an RCM examination of Spitz nevi, Pellacani et al. [30] showed in stratum granulosum and spinosum a prevalence of the cobblestone pattern, but also the honeycomb pattern and a partial or complete disarrangement of the epidermis. The presence of single dendritic or oval cells with bright, granular cytoplasm, eccentric dark nucleus, corresponding to pagetoid proliferation of nevomelanocytes in the epidermis was observed. In the dermo-epidermal junction the presence of edged and non-edged papillae was detected. The presence of dense and loose melanocytic nests located both in the basal layer and in the dermis was also shown. Within the papillary dermis a number of bright, plump cells corresponding to melanophages as well as an increased vascularity were revealed. The most important features that provide a differential diagnosis between Spitz nevi and malignant melanoma include sharp demarcation of the lesion, melanocytic nests associated with the basal layer and the presence of melanophages in the papillary dermis.
Malignant melanoma
The RCM is a useful tool for the diagnosis of malignant melanoma. It supports an early diagnosis when the dermatoscopy does not allow for definitive diagnosis [31, 32]. Pellacani et al. [27] postulate that the presence of at least three pagetoid cells of > 20 µm diameter within a single optical section is characteristic of melanoma. The presence of diffuse pagetoid infiltrations and of polymorphic pagetoid cells constitute a specific, but less sensitive marker of malignancy.
Superficial spreading melanoma
Superficial spreading melanoma (SSM) is characterized by a radial growth in the initial phase, followed by skin invasion with vertical proliferation. As the tumor proliferates, its architecture changes. In superficial lesions the presence of pagetoid cells, features of mild to moderate cytological atypia, the presence of non-edged papillae and less single melanocyte nests located in the epidermis and in the dermo-epidermal junction can be observed. As the thickness of the tumor increases, disarrangement of stratum granulosum and spinosum with a disorder of honeycomb pattern, increased cell pleomorphism, gradual infiltration of papillary dermis, with single bright cells or cerebriform melanocytes surrounded by melanin dust occurs [28].
Nodular melanoma
Nodular melanoma (NM) is the most aggressive histological type of melanoma, which accounts for approximately 15% of all melanomas [33]. In an RCM examination, Segura et al. [34] observed flattened epidermis, with occasional pagetoid cells. Within the stratum granulosum and spinosum of the epidermis, polygonal cells with demarcated, bright borders and black nuclei, forming a broadened honeycomb pattern were observed. There was no presence of typical dermal papillae within the dermo-epidermal junction and the basal layer was composed of pleomorphic cells with bright cytoplasm and dark nuclei (atypical melanocytes), arranged in “sheet-like structures”. Within the dermis, numerous single atypical melanocytes and cerebriform melanocyte nests were revealed. In the papillary dermis of the majority of patients, the presence of bright, a nucleated plump cells, corresponding to melanophages was observed.
Lentigo maligna melanoma
In the RCM examination of lentigo maligna melanoma (LMM), a significant similarity to superficial spreading melanoma was noticed. Langley et al. [35] reported an architectonic disarray of the epidermis with the presence of numerous round or dendritic pleomorphic cells, distributed in all of its layers, suggesting a pagetoid spread of atypical melanocytes. The presence of bright, atypical cells in the hair follicle surroundings was also noticed. A tendency of atypical cells to group around the hair follicle is an important diagnostic clue [35, 36]. In the papillary dermis, the presence of numerous a nucleated, plump melanophages and reticulated bright collagen bundles – characteristic of solar damage – was observed. In invasive lesion, nests of atypical, nucleated melanocytes could also be observed. There are few publications discussing the application of RCM for preoperative margin mapping and as a tool for noninvasive monitoring of topical therapy of LMM [37, 38].
Amelanotic melanoma
Typical diagnostic features of malignant melanoma can also be observed in an optical section of a melanotic melanoma due to the residual presence of melanin and melanosomes which create a source of contrast in the RCM. In 2001, Busam et al. [39] showed a complete disarray of epidermal architecture manifested as a blurring in the pattern of keratinocytes as well as the presence of bright, round, oval, or fusiform cells dispersed in an asymmetrical manner in the stratum spinosum and along the dermo-epidermal junction or grouped in irregular nests corresponding with atypical melanocytes.
Reflectance confocal microscopy-based diagnosis of melanoma
In 2005, Pellacani et al. [40] proposed an RCM-based diagnostic algorithm consisting of 2 major and 4 minor criteria to evaluate severity of melanocytic lesions. The major criteria (scoring two points each) are poorly delineated papillae (non-edged papillae) and the presence of atypical melanocytes. The minor criteria (scoring 1 point each) include pagetoid cells in the epidermis, the disseminated infiltration of pagetoid cells, cerebriform melanocytic clusters, and individual, nucleated melanocytes within the dermal papilla. A score of ≥ 3 yields am RCM-based diagnosis of melanoma. In 2009, Guitera et al. [41] compared sensitivity and specificity of RCM and dermoscopy in the diagnosis of melanoma. The study showed a greater specificity of RCM in diagnosing melanoma as compared to dermoscopy (68%, 95% CI: 61.1–74.3 for RCM; 32%, 95% CI: 25.9–38.7 for dermoscopy), while the use of RCM is comparable to dermoscopy when it comes to sensitivity (91%, 95% CI: 84.6–95.5 for RCM; 88%, 95% CI: 80.7–92.6 for dermoscopy).
Benign and malignant non-melanoma skin tumors
Dermatofibroma
Dermatofibromas, benign tumors of connective tissue, originate from proliferation of healthy fibroblasts, that occur most often in young women. These papules, due to their dark, bluish brown color, sometimes require differentiation from dysplastic nevus and malignant melanoma. Scope et al. [42] showed on an RCM image, the presence of normally structured epidermis in dermatofibroma packed with bright rings composed of basal keratinocytes around dermal papillae. The rings were separated by bright, thick fibrillar structures, corresponding to collagen fibres located in the upper layers of the dermis. The RCM image analysis of dermatofibroma is very difficult, due to the location of histiocytes and fibroblasts and stromal fibrosis rising up to the reticular layer of the dermis. However, the test may find its application in the differential diagnosis of dark-colored dermatofibromas and melanocytic lesions.
Seborrheic keratosis/seborrheic verruca
Seborrheic keratosis (SK) is a common, benign proliferation of epidermal cells. The RCM highlights significant epidermal acanthosis with the presence of normal keratinocytes and elongated, parallel edged papillae. The correct honeycomb structure is disturbed by bright, cylindrical structures corresponding to keratinous cysts [43]. Busam et al. [21] demonstrated in the dark-colored and inflammatory SK foci, the presence of bright cellular clusters within the papillae, similar to melanophages as well as dark channels corresponding to blood vessels.
Actinic keratosis
Actinic keratosis (AK) is the most common premalignant condition occurring among older people of light phototype subjected to chronic exposure to sunlight. These changes are difficult to evaluate by means of RCM due to the distinctly marked hyperkeratosis greatly limiting the depth of the test. Features of hyperkeratosis and parakeratosis can be observed in the optical sections of the cortical epidermis. The structure of the stratum granulosum remains almost unchanged compared to healthy skin. The most sensitive and distinct characteristics of AK include the architectural disorder within the prickle and basal layers together with a significant cellular pleomorphism [44–46]. Expanded blood vessels and features of the elastosis can be observed in the upper layers of the dermis [44, 46]. The presence of dysplastic features throughout the depth of the epidermis suggests the transformation of lesions into the squamous-cell carcinoma [20].
Squamous-cell carcinoma
Squamous-cell carcinoma (SCC) is the second most common skin cancer which often originates from actinic keratosis. Optical sections of the SCC are characterized by the presence of architectural disorder, pleomorphic keratinocytes with enlarged cell nuclei within the stratum granulosum and stratum spinosum of the epidermis and the presence of a modified vascular pattern and keratin pearls [44, 47]. Due to the limited depth of penetration and impaired visualization of the dermo-epidermal junction, RCM does not allow for differential diagnosis between hyperkeratotic AK and SCC as well as between invasive SCC and Bowen's disease (SCC in situ) [48].
Basal cell carcinoma
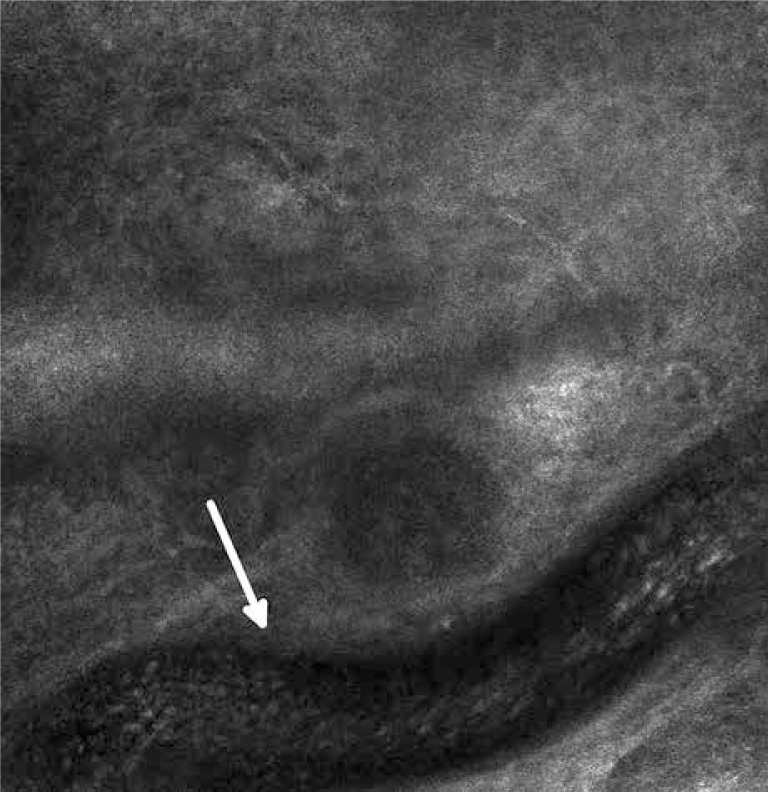
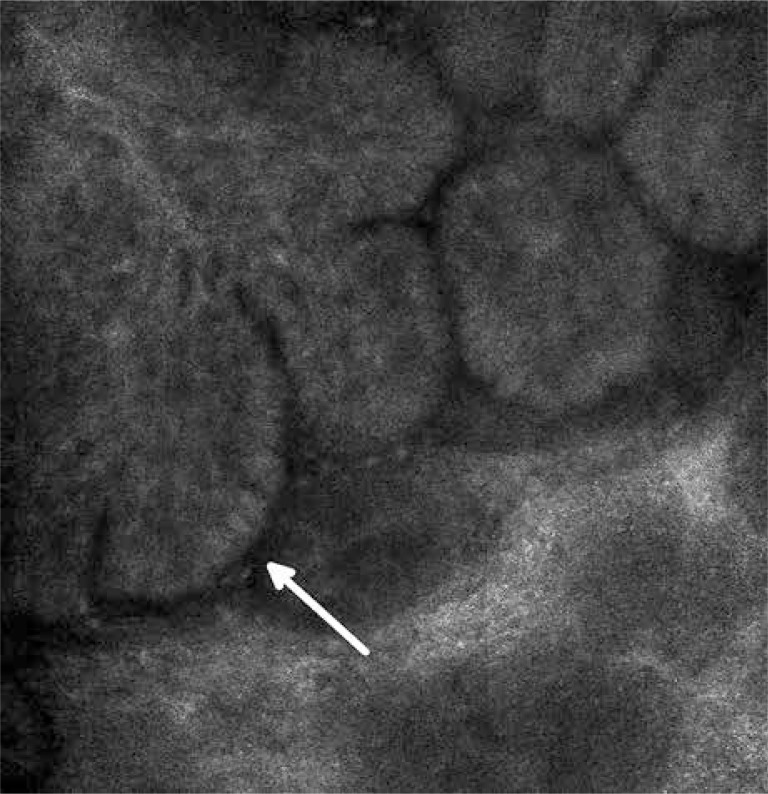
Basal cell carcinoma (BCC) is the most common skin cancer. The characteristic features of BCC include islands of bright, elongated, monomorphic tumor cells which appear to be elongated with their dark nuclei oriented along the same axis (basal cells). A line of tightly packed malignant cells with elongated nuclei arranged perpendicularly to the remaining cells (the palisade arrangement of cancer cells) (Figure 2) can be observed on the perimeter of tumor islands. The islands are surrounded by dark, slit-like spaces filled with mucin [49]. Atypical keratinocytes, characterized by cellular pleomorphism, nuclear size variation, architectural disorder and parakeratosis, are observed within the epidermis. These phenomena lead to the disturbance of the honeycomb pattern and the loss of the typical structure of the papillae. Furthermore, an increased amount and width of blood vessels is observed with a sporadically evident blood flow (leukocytes roll along the course of vascular endothelial cells) [50] (Figure 3). Both in the pattern and the stroma of the tumor, inflammatory infiltrations are visible in the form of small, round cells with bright cytoplasm. In the optical sections of pigmented BCC, apart from the above described characteristics, bright dendritic cells, similar to melanocytes and melanin dust can be observed within the tumor islands [49]. In addition, between the tumor islands big, bright, oval cells with poorly defined borders are visible [49, 51]. The RCM images of the nodular BCC, in addition to the standard characteristics, revealed nests of epithelial cells within the papillary layer. The image of fibroepithelial tumor (Pinkus tumor) showed large tumor cells and clusters of twisted collagen fibers corresponding to the tumor stroma [52].
Figure 2.
Basal cell carcinoma – a blood vessel with visible morphotic elements
Figure 3.
Basal cell carcinoma – the palisade arrangement of cancer cells
Reflectance confocal microscopy-based diagnosis of basal cell carcinoma
In 2004, Nori et al. [53] developed an RCM-based diagnostic algorithm for the diagnosis of BCC. Confocal images of 152 lesions were analyzed in a series of multi-center studies and five most characteristic features were distinguished for the diagnosis of BCC. These include: (1) epidermal pleomorphism, (2) the presence of elongated, monomorphic nuclei in the basal layer of the epidermis, (3) the orientation of the tumor cell nuclei along the same axis, (4) prominent inflammatory cell infiltrate and (5) increase of capillary vessels in the upper layers of the dermis. The presence of 4 out of 5 of these criteria allows for the diagnosis of BCC with specificity equal to 95.7% and a sensitivity of 82.9%. Fulfilling five of these criteria increases the specificity of the diagnosis to 100% while reducing the sensitivity to 48.8%. The most sensitive (91.6%) and specific (97.0%) of the above criteria is the orientation of tumor cell nuclei along the same axis.
Reflectance confocal microscopy-assisted tumor margin assessment
The RCM can be used to determine the borders of the pre-neoplastic lesions prior to both surgical and non-invasive treatment. This test is particularly useful in the evaluation of the margins in the horizontal growth pattern. The basic drawback of the method is the limited ability of penetration into the skin which prevents the accurate assessment of the deep margin of the lesions below the superficial layers of the dermis. In addition, RCM can be used in the Mohs microsurgery enabling an immediate assessment of the tissue specimen without the need for conventional histopathology [13, 14]. The cancer cells can be detected ex vivo by means of a 5% acetic acid which enhances the contrast of the tumor cell nuclei. Acetic acid concentrates the nuclear chromatin, making the nuclei clearer as a result of increased light scattering as opposed to the standard confocal image. The RCM has also been applied to establish margins of BCC in vivo during Mohs microsurgery, however, limited depth of visualization on non-planar surfaces of wounds makes this test is very difficult [15].
Reflectance confocal microscopy-assisted skin cancer treatment monitoring
The RCM has a considerable potential to become a useful tool in monitoring the course of treatment of skin diseases. Numerous reports discussing the monitoring of therapeutic outcomes have emerged. Torres et al. [11] applied RCM to control therapeutic effects of 5% imiquimod in the treatment of BCC. It has been shown that RCM-based results fully correlate with the response to the therapy (subsequently confirmed by a conventional histopathological examination), which suggests that RCM may help to identify the necessity of a surgical intervention. Longo et al. [12] studied the role of RCM in evaluating the effectiveness of photodynamic therapy (PDT). Ten patients with a total of 12 BCCs were treated with PDT. The RCM and dermatoscopy were performed before and after PDT (7 days, 30 days and 18 months). Conventional histopathological examination was performed at baseline and in the case of BCC persistence. After 1 month, RCM revealed the persistence of two BCCs, which evaded the clinical and dermoscopic diagnosis. Ahlgrimm-Siess et al. [8] monitored by means of RCM the efficacy of cryotherapy for treating superficial BCC (SBCC). Five patients with a total of 10 SBCCs were observed. The RCM was performed at baseline and after the cryotherapy (5 h, 24 h and 3 months). The SBCCs were frozen two times using a spray nitrogen cryoprobe. It has been shown that the RCM results correlated with the results obtained in the conventional histopathological examination. These examples confirm that RCM is not only a useful diagnostic tool but is also helpful in monitoring the therapeutic outcomes in the field of dermatological oncology.
Summary and conclusions
Although biopsy remains the gold standard in the diagnosis of skin diseases, its invasive nature poses many limitations in the examination of the physiology and pathology of the skin. The RCM is the only noteworthy alternative allowing for non-invasive visualization of the tissue while maintaining high resolution and good contrast. The method is used both in in vivo and ex vivo scientific research and in clinical practice. Introducing this technology into the everyday dermatological practice can reduce the number of invasive diagnostic biopsies. The RCM is currently in the early stages of development which renders the method imperfect. Still, further research and far-ranging use will in the near future create the impulse for the technological development and the emergence of clearly defined diagnostic criteria. Interpretation of the grayscale horizontal optical sections of tissue poses a considerable challenge for clinicians skilled in conventional microscopy, therefore, a thorough training process is necessary to master this diagnostic method. Teledermatological platforms through skill development and exchange of experiences can prove a valuable support for the beginners in this field.
References
- 1.Markisz JA, Aquilia MG. Technical magnetic resonance imaging. Standford: Appleton & Lange; 1996. [Google Scholar]
- 2.Mansotti L. Basic principles and advanced technical aspects of ultrasound imaging. In: Gazzardi R, editor. Physics and engineering of medical imaging. Netherlands, Boston: RE Springer; 1987. pp. 263–317. [Google Scholar]
- 3.Tearney GT, Brezinski ME, Southern JF, et al. Determination of the refractive index of highly scattering human tissue by optical coherence tomography. Opt Lett. 1995;20:2258–60. doi: 10.1364/ol.20.002258. [DOI] [PubMed] [Google Scholar]
- 4.New KC, Petroll WM, Boyde A, et al. In vivo imaging of human teeth and skin using real-time confocal microscopy. Scanning. 1991;13:369–72. [Google Scholar]
- 5.Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–52. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 6.Rallan D, Harland CC. Skin imaging: is it clinically useful? Clin Exp Dermatol. 2004;29:453–9. doi: 10.1111/j.1365-2230.2004.01602.x. [DOI] [PubMed] [Google Scholar]
- 7.Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: Advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahlgrimm-Siess V, Horn M, Koller S, et al. Monitoring efficacy of cryotherapy for superficial basal cell carcinomas with in vivo reflectance confocal microscopy: a preliminary study. J Dermatol Sci. 2009;53:60–4. doi: 10.1016/j.jdermsci.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 9.González S. Clinical applications of reflectance confocal microscopy in the management of cutaneous tumors. Actas Dermosifiliogr. 2008;99:528–31. [PubMed] [Google Scholar]
- 10.Aghassi D, Anderson RR, González S. Time-sequence histologic imaging of laser-treated cherry angiomas with in vivo confocal microscopy. J Am Acad Dermatol. 2000;43:37–41. doi: 10.1067/mjd.2000.105560. [DOI] [PubMed] [Google Scholar]
- 11.Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30:1462–9. doi: 10.1111/j.1524-4725.2004.30504.x. [DOI] [PubMed] [Google Scholar]
- 12.Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of basal cell carcinoma to photodynamic therapy. Dermatology. 2012;225:264–70. doi: 10.1159/000345106. [DOI] [PubMed] [Google Scholar]
- 13.Rajadhyaksha M, Menaker G, Flotte T, et al. Confocal examination of nonmelanoma cancers in thick skin excisions to potentially guide Mohs micrographic surgery without frozen histopathology. J Invest Dermatol. 2001;117:1137–43. doi: 10.1046/j.0022-202x.2001.01524.x. [DOI] [PubMed] [Google Scholar]
- 14.Chung VQ, Dwyer PJ, Nehal KS, et al. Use of ex vivo confocal scanning laser microscopy during Mohs surgery for nonmelanoma skin cancers. Dermatol Surg. 2004;30:1470–8. doi: 10.1111/j.1524-4725.2004.30505.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027. doi: 10.1117/1.2750294. [DOI] [PubMed] [Google Scholar]
- 16.Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin microcirculation. J Biomed Opt. 2004;9:323–33. doi: 10.1117/1.1646175. [DOI] [PubMed] [Google Scholar]
- 17.Carlsson K. The influence of specimen refractive index, detector signal integration, and non-uniform scan speed on the imaging properties in confocal microscopy. J Microsc. 1991;163:167–78. [Google Scholar]
- 18.Wan DS, Rajadhyaksha M, Webb RH. Analysis of spherical aberration of a water immersion objective: application to specimens with refractive indices 1.33-1.40. J Microsc. 2000;197:274–84. doi: 10.1046/j.1365-2818.2000.00635.x. [DOI] [PubMed] [Google Scholar]
- 19.González S, Swindells K, Rajadhyaksha M, et al. Changing paradigms in dermatology: confocal microscopy in clinical and surgical dermatology. Clin Dermatol. 2003;21:359–69. doi: 10.1016/j.clindermatol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.González S, Gilaberte-Calzada Y. In vivo reflectance mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci. 2008;30:1–17. doi: 10.1111/j.1468-2494.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 21.Busam KJ, Charles C, Lee G, et al. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14:862–8. doi: 10.1038/modpathol.3880402. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann-Wellenhof R, Wurm EM, Ahlgrimm-Siess V, et al. Reflectance confocal microscopy-state-of-art and research overview. Semin Cutan Med Surg. 2009;28:172–9. doi: 10.1016/j.sder.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology. Lasers Med Sci. 2007;22:73–82. doi: 10.1007/s10103-006-0416-8. [DOI] [PubMed] [Google Scholar]
- 24.Rajadhyaksha M, Anderson RR, Webb RH. Video-rate confocal scanning laser microscope for imaging human tissues in vivo. Appl Opt. 1999;38:1–12. doi: 10.1364/ao.38.002105. [DOI] [PubMed] [Google Scholar]
- 25.Huzaira M, Rius F, Rajadhyaksha M, et al. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001;116:846–52. doi: 10.1046/j.0022-202x.2001.01337.x. [DOI] [PubMed] [Google Scholar]
- 26.Langley RG, Rajadhyaksha M, Dwyer PJ, et al. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. J Am Acad Dermatol. 2001;45:365–76. doi: 10.1067/mjd.2001.117395. [DOI] [PubMed] [Google Scholar]
- 27.Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759–65. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 28.Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18:469–74. doi: 10.1038/modpathol.3800330. [DOI] [PubMed] [Google Scholar]
- 29.Pellacani G, Cesinaro AM, Longo C, et al. Microscopic in vivo description of cellular architecture of dermascopic pigment network in nevi and melanomas. Arch Dermatol. 2005;141:147–54. doi: 10.1001/archderm.141.2.147. [DOI] [PubMed] [Google Scholar]
- 30.Pellacani G, Longo C, Ferrara G, et al. Spitz nevi: in vivo confocal microscopic features, dermatoscopic aspects, histopathologic correlates, and diagnostic significance. J Am Acad Dermatol. 2009;60:236–47. doi: 10.1016/j.jaad.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 31.Scope A, Zalaudek I, Ferrara G, et al. Remodeling of the dermoepidermal junction in superficial spreading melanoma: insights gained from correlation of dermoscopy, reflectance confocal microscopy, and histopathologic analysis. Arch Dermatol. 2008;144:1644–9. doi: 10.1001/archdermatol.2008.504. [DOI] [PubMed] [Google Scholar]
- 32.Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216–29. doi: 10.1016/j.jaad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Chang AE, Karnell LH, Menck HR. American College of Surgeons Commission on Cancer and the American Cancer Society, The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Segura S, Pellacani G, Puig S, et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol. 2008;144:1311–20. doi: 10.1001/archderm.144.10.1311. [DOI] [PubMed] [Google Scholar]
- 35.Langley RG, Burton E, Walsh N, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55:88–97. doi: 10.1016/j.jaad.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260–3. doi: 10.1067/mjd.2002.118345. [DOI] [PubMed] [Google Scholar]
- 37.Chen CS, Elias M, Busam K, et al. Multimodal in vivooptical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153:1031–6. doi: 10.1111/j.1365-2133.2005.06831.x. [DOI] [PubMed] [Google Scholar]
- 38.Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2009;36:177–84. doi: 10.1111/j.1524-4725.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 39.Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923–9. [PubMed] [Google Scholar]
- 40.Pellacani G, Cesinaro AM, Seidenai S. Reflectance mode confocal microscopy of pigmented skin lesions – improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979–85. doi: 10.1016/j.jaad.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131–8. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]
- 42.Scope A, Ardigo M, Marghoob AA. Correlation of dermoscopic globule-like structures of dermatofibroma using reflectance confocal microscopy. Dermatology. 2008;216:81–2. doi: 10.1159/000109364. [DOI] [PubMed] [Google Scholar]
- 43.Kopf AW, Rabinovitz H, Marghoob A, et al. “Fat fingers’’: a clue in the dermoscopic diagnosis of seborrheic keratoses. J Am Acad Dermatol. 2006;55:1089–91. doi: 10.1016/j.jaad.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Aghassi D, Anderson RR, González S. Confocal laser microscopic imaging of actinic keratoses in vivo: a preliminary report. J Am Acad Dermatol. 2000;43:42–8. doi: 10.1067/mjd.2000.105565. [DOI] [PubMed] [Google Scholar]
- 45.Horn M, Gerger A, Ahlgrimm-Siess V, et al. Discrimination of actinic keratoses from normal skin with reflectance mode confocal microscopy. Dermatol Surg. 2008;34:620–5. doi: 10.1111/j.1524-4725.2008.34195.x. [DOI] [PubMed] [Google Scholar]
- 46.Rishpon A, Kim N, Scope A, et al. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766–72. doi: 10.1001/archdermatol.2009.134. [DOI] [PubMed] [Google Scholar]
- 47.Al-Arashi M, Salomatina E, Yaroslavsky A. Multimodal confocal microscopy for diagnosing nonmelanoma skin cancers. Lasers Surg Med. 2007;39:696–705. doi: 10.1002/lsm.20578. [DOI] [PubMed] [Google Scholar]
- 48.Gonzáles S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(Suppl. 2):59–69. doi: 10.1016/s0001-7310(09)73380-0. [DOI] [PubMed] [Google Scholar]
- 49.Agero AL, Busam KJ, Benvenuto-Andrade C, et al. Reflectance confocal microscopy of pigmented basal cell carcinoma. J Am Acad Dermatol. 2006;54:638–43. doi: 10.1016/j.jaad.2005.11.1096. [DOI] [PubMed] [Google Scholar]
- 50.González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869–74. doi: 10.1067/mjd.2002.124690. [DOI] [PubMed] [Google Scholar]
- 51.Charles CA, Marghoob AA, Busam KJ, et al. Melanoma or pigmented basal cell carcinoma: a clinical-pathologic correlation with dermoscopy, in vivo confocal scanning laser microscopy, and routine histology. Skin Res Technol. 2002;8:282–7. doi: 10.1034/j.1600-0846.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 52.Sauermann K, Gambichler T, Wilmert M, et al. Investigation of basal cell carcinoma [correction of carcionoma] by confocal laser scanning microscopy in vivo. Skin Res Technol. 2002;8:141–7. doi: 10.1034/j.1600-0846.2002.20345.x. [DOI] [PubMed] [Google Scholar]
- 53.Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51:923–30. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]