Abstract
Immune-based assays are promising tools to help to formulate diagnosis of active tuberculosis. A multiparameter flow cytometry assay assessing T-cell responses specific to Mycobacterium tuberculosis and the combination of both CD4 and CD8 T-cell responses accurately discriminated between active tuberculosis and latent infection.
Keywords: diagnosis, active tuberculosis disease, latent Mtb infection, functional profile, CD8 T cells
Tuberculosis represents a major public health threat with >1.5 million deaths annually. Despite intensive investigations, rapid formulation of diagnosis of active tuberculosis remains a major obstacle to the global control of tuberculosis disease [1]. The recent development of multiplexed assays in proteomics, transcriptomics, or metabolomics may provide the basis for developing highly sensitive and specific tools for active tuberculosis diagnosis [2, 3]. In addition to molecular biology assays, recent observations have indicated that immunological measures may be instrumental in the diagnosis of active tuberculosis. We have recently shown using flow cytometry that the cytokine profile of Mycobacterium tuberculosis (Mtb)-specific CD4 T cells allowed a strong immunological discrimination between patients with active tuberculosis and latent Mtb infection (LTBI) [4]. Furthermore, consistent with a previous study [5], we recently confirmed that Mtb-specific CD8 T-cell responses were predominantly (>70%) found in patients with active tuberculosis compared to those with LTBI (15%) [6]. On the basis of these previous observations, we hypothesized that the combined assessment of Mtb-specific CD4 and CD8 T-cell responses could result in improved diagnosis of active tuberculosis.
To test our working hypothesis, we analyzed both the functional profile of Mtb-specific CD4 T-cell responses and the presence of Mtb-specific CD8 T-cell responses in 194 subjects diagnosed with active tuberculosis or LTBI, and performed multivariate regression analysis to assess their relative or combined capacity to distinguish active tuberculosis from latent infection.
The results show that both individual immunological measures had variable power to discriminate between active tuberculosis and LTBI. However, the combination of both measures greatly improved the power of this flow cytometry–based assay in the diagnosis of active tuberculosis.
METHODS
Study Groups
Of 53 patients with active tuberculosis, 28 have already been described (Supplementary Table 1). Thirty had a diagnosis based on laboratory isolation of Mtb on mycobacterial culture from sputum, bronchoalveolar lavage fluid, or biopsies and/or tuberculin skin test, enzyme-linked immunospot assay (ELISpot), and/or polymerase chain reaction as described elsewhere [4]. Five patients (clinical tuberculosis, culture negative) presented specific symptoms and radiological evidence of lesions suggestive of tuberculosis and responded to treatment, and 16 patients had a diagnosis based on GeneXpert assay (Supplementary Table 1). All 141 LTBI subjects were previously described [4, 6] and were asymptomatic and had T-cell responses specific to ESAT-6 and/or CFP-10 (detected by ELISpot and/or by intracellular cytokine staining [ICS]). Subjects with LTBI were either healthcare workers routinely screened at the Centre Hospitalier Universitaire Vaudois (CHUV) or were investigated for Mtb infection prior to the initiation of anti–tumor necrosis factor alpha (TNF-α) antibody treatment and had chest radiographs negative for lung lesions. Samples from active tuberculosis patients and LTBI subjects were consistently and similarly obtained, processed, stored, and analyzed. These studies were approved by the institutional review board of the CHUV (number 35/09), and all subjects gave written informed consent.
Flow Cytometry Analyses and T-Cell Stimulations
In brief, cryopreserved peripheral blood mononuclear cells were thawed, rested, and stimulated overnight with pools of overlapping peptides encompassing ESAT-6 or CFP-10 and were then labeled (viability dye, CD3, CD4, CD8, interferon gamma [IFN-γ], TNF-α, and interleukin 2 [IL-2]), acquired on a 4-laser flow-cytometer and analyzed as described [4].
Statistical Analyses
Comparisons of categorical variables were performed using Fisher exact test. Statistical significance of the magnitude of ICS responses was calculated by unpaired 2-tailed Student t test using GraphPad Prism version 6. Logistic regression followed by receiver operating characteristic (ROC) curve analysis was used to evaluate the performances of each parameter (presence of Mtb-specific CD8T cells and the frequency of single TNF-α–producing CD4 T cells) to identify active tuberculosis [7]. A linear logistic regression model was used to assess the potential benefit of the combination of both covariates. This model estimated the log odds of active tuberculosis disease probability as a function of both variables. Results for the distinct variables were summarized as a contingency table giving sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). These analyses were performed using MedCalc 13.3.3 and was confirmed using R-Cran associated with the “pROC” and “ROC” packages in addition to in-house coding.
RESULTS
We enrolled 141 patients with LTBI and 53 patients with untreated active tuberculosis (Supplementary Table 1). Mtb-specific CD4 and CD8 T-cell responses were assessed using polychromatic flow cytometry following stimulation with ESAT-6 and CFP-10 peptide pools and labeled with a viability marker and anti-CD3, -CD4, -CD8, -IFN-γ, -TNF-α, and -IL-2 antibodies as described [4].
Functional Profile of Mtb-Specific CD4 T-Cell Responses and Identification of Mtb-Specific CD8T Cells in Patients With Active or Latent Tuberculosis
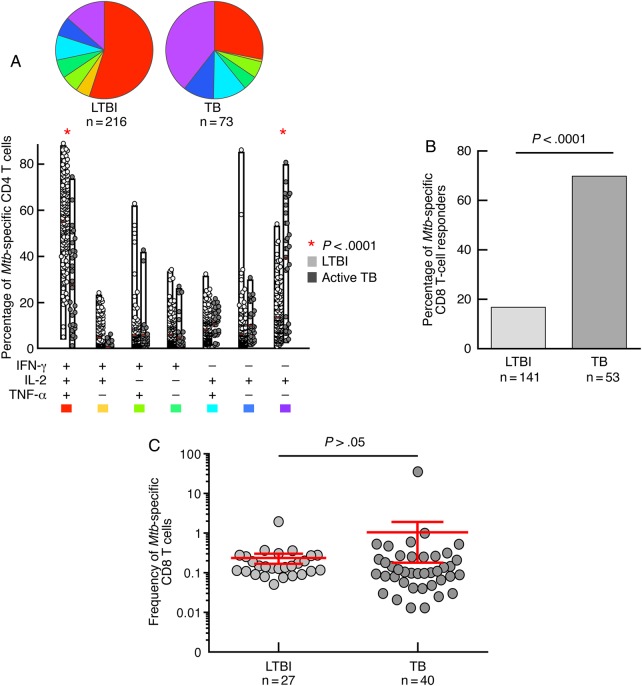
Consistent with our previous observation [4], the cytokine profile of Mtb-specific CD4 T-cell response was distinct between active tuberculosis patients and LTBI subjects, and a significantly (P < .00001) higher proportion of single TNF-α–producing CD4 T cells was found in patients with active tuberculosis (Figure 1A).
Figure 1.
Mycobacterium tuberculosis (Mtb)-specific CD4 and CD8 T-cell responses. A, Analysis of the functional profile of Mtb-specific CD4 T cells on the basis of interferon gamma (IFN-γ), interleukin 2 (IL-2), and/or tumor necrosis factor alpha (TNF-α) production. All 194 individuals had Mtb-specific CD4 T-cell responses, and 216 and 73 Mtb-specific CD4 T-cell responses against ESAT-6 or CFP-10 were analyzed in the 141 patients with latent tuberculosis (LTBI) and 53 patients with tuberculosis (TB), respectively. The combinations of the different functions are shown on the x-axis, and the percentages of the distinct cytokine-producing cell subsets within Mtb-specific CD4 T cells are shown on the y axis. The pie charts summarize the data. Comparisons of marker distribution were performed using Student t test and a partial permutation test as described elsewhere [8]. B, Proportion of LTBI subjects and TB patients with detectable Mtb-specific CD8 T-cell responses. Mtb-specific CD8 T-cell responses were defined by the presence of IFN-γ–producing CD8+CD4–CD3+ T cells following stimulation with ESAT-6 and/or CFP-10 peptide pools. Statistical significance was calculated using 2-tailed Fisher exact test. C, Magnitude (mean with 95% confidence interval) of Mtb-specific CD8 T-cell responses (against ESAT-6 and/or CFP-10) in the 21 LTBI and 37 TB patients with detectable Mtb-specific CD8 T-cell responses. An unpaired 2-tailed Student t test was performed.
Furthermore, as previously shown [5, 6], Mtb-specific CD8 T-cell responses were detected in the majority of active tuberculosis patients (69.8%) and in few (15%) LTBI subjects (P < .0001; Figure 1B). In contrast, the magnitude of Mtb-specific CD8 T-cell responses, as determined by the frequency of IFN-γ–producing CD8 T cells, was not significantly different between LTBI and active tuberculosis subjects (Figure 1C).
Potency of the Individual Immunological Measures in the Diagnosis of Active Tuberculosis
We then calculated the capacity for each parameter (ie, the cytokine profile of Mtb-specific CD4 T cells or the detection of Mtb-specific CD8 T-cell responses) to distinguish active tuberculosis patients from LTBI subjects.
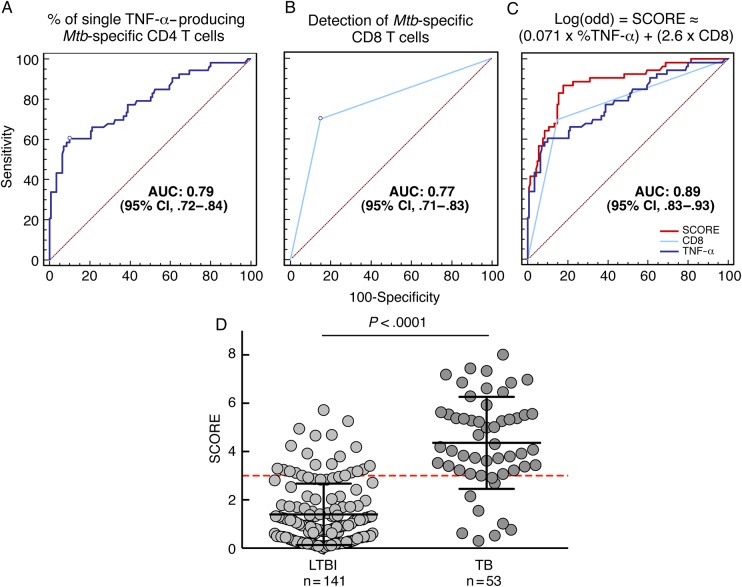
On the basis of a logistic regression analysis, we confirmed that the cytokine profile of Mtb-specific CD4 T cells was a strong predictor of active tuberculosis or latent infection (area under the curve [AUC] = 0.79 [95% confidence interval {CI}, .72–.84]; Figure 2A). Using the predefined threshold of 37.4% of single TNF-α–producing CD4 cells [4], the odds ratio (OR) was 17.7, the specificity was 93.6%, the sensitivity was 60.4%, and PPV and NPV were 76.3% and 84.6%, respectively.
Figure 2.
Individual and combined performances of the distinct components of the Mycobacterium tuberculosis (Mtb)–specific T-cell response to diagnose active tuberculosis (TB). A, Logistic regression analysis showing the association between the proportion of single tumor necrosis factor alpha (TNF-α)–producing CD4 T cells with the ability to discriminate between active TB and latent Mtb infection (LTBI) (area under the curve [AUC] = 0.79 [95% confidence interval {CI}, .72–.84]). B, Logistic regression analysis showing the association between the presence of a detectable Mtb-specific CD8 T-cell response with the ability to discriminate between active TB and LTBI (AUC = 0.77 [95% CI, .71–.83]). C, Logistic regression analysis showing the association between the SCORE (integrated combination of the proportion of single TNF-α–producing CD4 T cells and the presence of a detectable Mtb-specific CD8 T-cell response) with the ability to discriminate between active TB and LTBI (AUC = 0.89 [95% CI, .83–.93]). D, Analysis of the distribution of SCORE results on the 141 LTBI subjects and the 53 active TB patients from this study.
The detection of Mtb-specific CD8 T cells was also a strong predictor of discrimination between active and latent tuberculosis, although it was less accurate than the CD4 T-cell cytokine profile (AUC = 0.77 [95% CI, .71–.83]; OR = 13.1; specificity = 85%; sensitivity = 69.8%; PPV = 63.8%; NPV = 88.1%; Figure 2B).
Combination of Mtb-Specific CD4 and CD8 T-Cell Responses to Diagnose Active Tuberculosis
We then combined both components of the Mtb-specific T-cell response (ie, the cytokine profile of Mtb-specific CD4 T cells and the detection of Mtb-specific CD8 T-cell responses). A logistic regression model was used to assess the potential benefit of the combination of both covariates. This model estimated the log odds of active tuberculosis probability as a function of both variables, identifying a new covariate, termed “SCORE,” which was defined as follows:
where %TNF-α refers to the percentage of Mtb-specific single TNF-α–producing CD4 T cells and CD8 refers to 0 (zero) or 1 according to the absence or presence of an Mtb-specific CD8 T-cell response, respectively (P < .0001 for both coefficients). With an AUC of 0.89 (95% CI, .83–.93; Figure 2C), the SCORE was a significantly improved predictor measure of discrimination between active and latent tuberculosis compared with both individual variables analyzed independently (both P < .004). Analysis of the distribution of SCORE results from tuberculosis and LTBI subjects showed a significant difference between the groups (P < .0001; Figure 2D). On the basis of the logistic regression analysis, an optimal cutoff of SCORE of 3 was determined (Figure 2D), and assay performances were as follows: OR = 26.2, specificity = 86.5%, sensitivity = 81.1%, PPV = 66.7%, and NPV = 93%. Compared with the individual use of the percentage of single TNF-α–producing CD4 T cells, the combined use of CD4 and CD8 T-cell responses was associated with substantial improvement in the NPV (10% increase), PPV (10% increase), OR (48% increase), and sensitivity (34% increase) but a minor decrease in specificity (7%).
Of interest, assay performance was not significantly different between culture-positive tuberculosis and culture-negative tuberculosis or by tuberculosis clinical status (pulmonary vs extrapulmonary tuberculosis; Supplementary Figure 1), nor by the geographical origin of participants.
DISCUSSION
Cellular immune responses are involved in the control of Mtb infection [9], and both CD4 and CD8 T cells play a key role in granuloma formation [10]. As opposed to CD4 T cells, the function of Mtb-specific CD8 T cells remains unclear, even though they were described in humans as well as in animal models [6, 11–13] and were associated with the pathogen load [14] or with better disease control [15].
T-cell–based assays such as IFN-γ release assays have substantially improved the diagnosis of Mtb infection but have failed to discriminate between active and latent tuberculosis [16, 17]. Recently, the functional profile of Mtb-specific CD4 T cells has shown to be instrumental in discriminating between patients with LTBI and those with tuberculosis [4].
It was previously shown that Mtb-specific CD8 T-cell responses are generated in response to high bacillary load and may be able to distinguish children with active tuberculosis from LTBI [14]. Here, we demonstrate that the detection of CD8 T-cell responses can discriminate between active tuberculosis and LTBI also in adults, but is substantially less powerful than the Mtb-specific CD4 T-cell response (ie, single TNF-α–producing CD4 T cells). More importantly, the multivariate analysis combining both the functional profile of CD4 T cells and the presence of Mtb-specific CD8 T cells led to the identification of a new variable (SCORE) and to a significantly improved discrimination between patients with active vs latent tuberculosis.
The 2 immunological measures, that is, increase in the percentage of single Mtb-specific TNF-α–producing CD4 T cells and the detection of Mtb-specific CD8 T cells, when used in combination have a sensitivity (81.1%) and a specificity (86.5%) in the same range of other immunological assays measuring only IFN-γ such as the T.SPOT.TB and the QuantiFERON-TB Gold In-Tube test while largely superior to the tuberculin skin test [18]. However, the present flow cytometry–based assay has the advantage of discriminating between active and latent tuberculosis, which is not the case for the 2 assays measuring only Mtb-specific T cells producing IFN-γ. In this regard, recent studies have identified other immunological markers that may discriminate between active and latent tuberculosis. The new markers include IFN-γ and IL-2 single T-cell responses [19] and the decrease of CD27 expression in Mtb-specific CD4 T cells [20]. However, the former markers were analyzed in a very small number (<20) of patients, and the latter has been shown to discriminate between active and chronic tuberculosis only in pediatric patients [21] and to be associated with persistent active tuberculosis in adult patients [22]. Therefore, the flow cytometry–based assay and the combined use of the 2 immunological measures described in the present study represent the most instrumental tool for discriminating between active and latent tuberculosis in adult patient populations.
The sensitivity of immunologically based assays in discriminating between active and latent tuberculosis is inferior to that of molecular assays such as the Xpert MTB/RIF test, which is based on DNA amplification and has a sensitivity of 99.8% in smear-positive and culture-positive cases and 90.2% in smear-negative and culture-positive cases. However, the limitation of this test is that it can be performed only on sputum samples, which are difficult to collect in children.
With regard to the feasibility of implementing this technology in developing countries with high tuberculosis prevalence, the antibody panel to measure these Mtb-specific CD4 and CD8 T-cell responses has been validated in our laboratory for diagnostic use and routinely performed on a Facscanto flow cytometer at the Lausanne University Hospital. The implementation of the present test in laboratories in developing countries is certainly feasible if the appropriate flow cytometry equipment is available. However, the assay is currently performed on blood mononuclear cell preparations, and the possibility of performing the assay on total blood will facilitate substantially the implementation of the test in laboratories in developing countries.
In conclusion, the present flow cytometry–based assay and the combination of 2 immunological measures—Mtb-specific CD4 T cells producing TNF-α and the detection of Mtb-specific CD8 T-cell responses—represent a powerful diagnostic tool to discriminate between active tuberculosis and latent infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Delphine Gani for logistic coordination. The authors also thank many additional members of the Centre Hospitalier Universitaire Vaudois and South African Tuberculosis Vaccine Initiative teams who helped with enrollment and evaluation of participants, and finally, the participants themselves.
Financial support. The research leading to these results has received funding from the Swiss Vaccine Research Institute and the European Community's Seventh Framework Programme (FP7/2007-2013 and FP7/2007-2011) under European Commission Grant Agreement (number 241642).
Potential conflicts of interest. G. P., M. P., and A. H. are inventors on patent PCT/IB2011/003145, “Methods for differentiating between disease states of Mycobacterium tuberculosis infection.” All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis control: WHO (World Health Organization) report 2012. Available at: http://www.who.int/tb/country/en/ . Accessed 1 March 2013.
- 2.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138–41. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harari A, Rozot V, Enders FB, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–6. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CL, Abrahams DA, Lerumo L, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187:2222–32. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozot V, Vigano S, Mazza-Stalder J, et al. Mycobacterium tuberculosis-specific CD8 T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43:1568–77. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 8.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper AM. Cell-mediated immune responses in tuberculosis. Ann Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JL, Chan J. Immunology of tuberculosis. Ann Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzel AS, Grotzke JE, Lines RA, et al. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196:1473–81. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol. 2006;26:317–52. doi: 10.1615/critrevimmunol.v26.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancioni C, Nyendak M, Kiguli S, et al. CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med. 2012;185:206–12. doi: 10.1164/rccm.201107-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruns H, Meinken C, Schauenberg P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–77. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24:529–36. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 17.Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002;347:1860–6. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 18.De Keyser E, De Keyser F, De Baets F. Tuberculin skin test versus interferon-gamma release assays for the diagnosis of tuberculosis infection. Acta Clin Belg. 2014;69:358–66. doi: 10.1179/2295333714Y.0000000043. [DOI] [PubMed] [Google Scholar]
- 19.Essone PN, Kalsdorf B, Chegou NN, et al. Bifunctional T-cell-derived cytokines for the diagnosis of tuberculosis and treatment monitoring. Respiration. 2014;88:251–61. doi: 10.1159/000365816. [DOI] [PubMed] [Google Scholar]
- 20.Schuetz A, Haule A, Reither K, et al. Monitoring CD27 expression to evaluate Mycobacterium tuberculosis activity in HIV-1 infected individuals in vivo. PLoS One. 2011;6:e27284. doi: 10.1371/journal.pone.0027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portevin D, Moukambi F, Clowes P, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect Dis. 2014;14:931–8. doi: 10.1016/S1473-3099(14)70884-9. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Wang X, Wang X, et al. Reduced CD27 expression on antigen-specific CD4+ T cells correlates with persistent active tuberculosis. J Clin Immunol. 2010;30:566–73. doi: 10.1007/s10875-010-9418-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.