Abstract
The functional role of Lys catabolism in balancing Lys levels in plants has only been directly demonstrated in developing seeds. Seed-specific expression of a bacterial feedback-insensitive dihydrodipicolinate synthase (DHPS) in an Arabidopsis knockout mutant of the AtLKR/SDH gene that regulates Lys catabolism synergistically boosted Lys accumulation in mature seeds, but it also severely reduced the growth of seedlings derived from them. Here we further tested whether the inhibition of seedling growth was due to a negative physiological effect of excess Lys on seed maturation or to defective postgermination catabolism of Lys, which accumulated in the mature seeds. To address these questions, we coexpressed a bacterial DHPS gene with an RNAi construct of AtLKR/SDH, both under control of the same seed-specific promoter, to restrict Lys synthesis and catabolism to the developing seeds. Coexpression of these genes boosted seed Lys content and caused a significant, metabolically unanticipated increase in Met content, similarly to our previous report using plants expressing the bacterial DHPS on an AtLKR/SDH knockout background. However, postgermination seedling growth was significantly improved when the reduction of Lys catabolism was restricted to seed development, suggesting that defective postgermination Lys catabolism was responsible for inhibition of seedling growth in the AtLKR/SDH knockout plants expressing the bacterial DHPS gene in a seed-specific manner. Constitutive expression of the bacterial DHPS in the AtLKR/SDH knockout mutant boosted Lys levels in vegetative tissues in a similar manner to that observed in seeds, further demonstrating that Lys catabolism plays an important regulatory role in balancing Lys levels.
Lys is an essential amino acid that is present in limiting amounts in seeds of many crop plants and is therefore considered to be of immense nutritional importance in animal feeds and human foods (Bright and Shewry, 1983; Galili et al., 1994). In plants, Lys strongly regulates its own synthesis by feedback inhibition of dihydrodipicolinate synthase (DHPS; Galili, 1995; Fig. 1), but Lys catabolism can also play an important regulatory role in balancing Lys levels (Arruda et al., 2000; Galili et al., 2001). So far, the metabolic significance of Lys catabolism for regulating steady state Lys levels has been documented only in developing seeds. Seed-specific expression of feedback-insensitive bacterial DHPS enzymes in a number of plant species caused various degrees of Lys overaccumulation, but in many cases this was also associated with enhanced levels of Lys catabolic products (Falco et al., 1995; Mazur et al., 1999). In addition, seed-specific expression of a bacterial DHPS in an Arabidopsis knockout mutant of the AtLKR/SDH gene, which encodes the first two linked enzymes of Lys catabolism, namely Lys-ketoglutarate reductase (LKR) and saccharopine dehydrogenase (SDH; Fig. 1), caused a synergistic increase in Lys levels in mature seeds, compared to transgenic plants expressing the bacterial DHPS and possessing an active Lys catabolism pathway (Zhu and Galili, 2003). Notably, upon germination, the excessive Lys accumulation during seed development in AtLKR/SDH knockout plants expressing the bacterial DHPS impeded seedling growth (Zhu and Galili, 2003). However, it was not determined whether this was due to a negative physiological effect of the excess Lys on seed maturation or defective postgermination catabolism of the Lys that overaccumulated in the mature seeds.
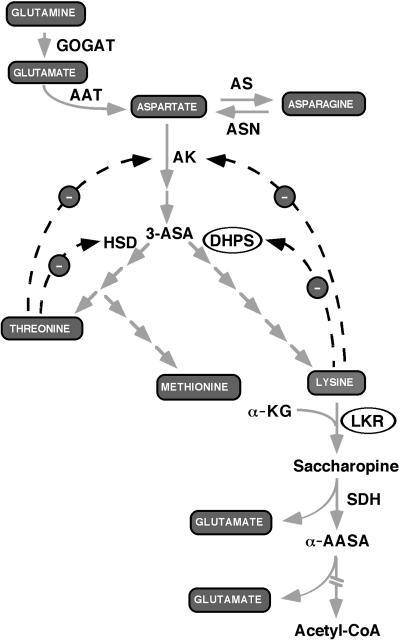
Figure 1.
Schematic diagram of amide amino acid metabolism (top), the Asp-family pathway (center), and the pathway of Lys catabolism (bottom). Dashed arrows with a “-” sign represent end-product feedback inhibition loops. GOGAT, Glu synthase; AAT, Asp aminotransferase; AS, Asn synthase; ASN, asparaginase; AK, Asp kinase; DHPS, dihydrodipicolinate synthase; HSD, homoserine dehydrogenase; LKR, Lys-ketoglutarate reductase; SDH, saccharopine dehydrogenase; 3-ASA, 3-aspartic semialdehyde; α-KG, α-ketoglutarate; α-AASA, α-amino adipic semialdehyde. Broken arrow at the bottom indicates several enzymatic steps leading to the production of acetyl-CoA. The enzymatic steps manipulated in this report (DHPS and LKR) are circled.
In this study, we wished to investigate whether Lys catabolism also plays a significant role in regulating Lys metabolism during vegetative growth, and whether defective Lys catabolism is responsible for the inhibition of seedling growth in the Arabidopsis plants expressing the bacterial DHPS in a seed-specific manner but also lacking Lys catabolism. To address these questions we specifically and coordinately enhanced Lys synthesis and reduced its catabolism during Arabidopsis seed development by coexpressing the bacterial DHPS and an AtLKR/SDH RNAi construct, both under control of the seed-specific phaseolin promoter. In addition, we created plants that constitutively express the bacterial DHPS (using the 35S promoter) in a AtLKR/SDH knockout mutant. Our results suggest that Lys catabolism plays an important role in balancing Lys levels in both developing seeds and in vegetative tissues.
RESULTS
Creation of Transgenic Arabidopsis Plants Coordinately Expressing a Bacterial DHPS and an RNAi Construct of AtLKR/SDH in a Seed-Specific Manner
To coordinately increase Lys synthesis and reduce its catabolism specifically during seed maturation, we constructed a recombinant RNAi construct (termed phas::dsLKR) fused to the bean phaseolin promoter. This construct contains inverse DNA sequence repeats derived from the coding region of the LKR domain of an AtLKR/SDH cDNA, flanking a 1.3-kb spacer fragment (see “Materials and Methods”). This construct was subcloned into a Ti plasmid containing a hygromycin-resistance gene and used to transform Arabidopsis plants. Two genotypes were transformed with this construct. One was wild-type Arabidopsis, and the second was a previously described, kanamycin-resistant transgenic Arabidopsis plant, homozygous for a chimeric phas::DHPS gene encoding a bacterial DHPS under the control of the same phaseolin promoter (Zhu and Galili, 2003; this chimeric gene was originally described by Karchi et al., 1994). Twenty-four wild-type plants, transformed with the phas::dsLKR construct (termed hereafter phas::dsLKR genotypes), as well as 25 phas::DHPS plants retransformed with the phas::dsLKR construct (termed hereafter phas::dsLKR/DHPS genotypes), each containing a single phas::dsLKR insert, were selected based on progeny analysis for hygromycin resistance. Each line was selfed to obtain a series of T3 plants homozygous for the RNAi transgene.
Since the phas::dsLKR construct is controlled by the phaseolin promoter, which is active relatively late during seed development, we analyzed mature seeds of a number of independently transformed genotypes for the level of the LKR/SDH mRNA and polypeptide, using northern-blot, semiquantitative reverse transcription (RT)-PCR, and western-blot analyses with monoclonal anti LKR antibodies (Zhu et al., 2001). Unfortunately, the LKR/SDH RNA and protein were barely detectable in mature seeds of the wild-type plants, making it difficult to directly measure the extent of reduced expression of the AtLKR/SDH gene in the various phas::dsLKR lines (data not shown). This is probably because both the LKR/SDH mRNA and protein are present in very low levels in mature seeds and also because it is difficult to extract high quality RNA from mature seeds. Therefore, we tested the effect of phas::dsLKR expression on the profile of amino acids in the seeds of a large number of independently transformed phas::dsLKR and phas::dsLKR/DHPS lines, assuming that they both represented a wide range of gene expression levels for the AtLKR/SDH gene. The detailed amino acid analyses in these lines as well as in the wild-type and phas::DHPS lines are given in Supplemental Tables I to III (available at www.plantphysiol.org).
Effect of a Coordinated Seed-Specific Increase in Lys Synthesis and a Reduction of Its Catabolism on Lys Level in Mature Seeds
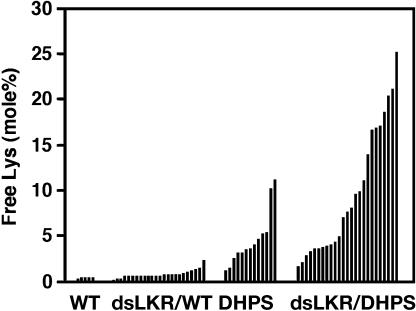
Next, we tested the effect of seed-specific expression of the phas::dsLKR construct on free Lys accumulation in mature seeds of the independently transformed phas::dsLKR and phas::dsLKR/DHPS genotypes and compared them to wild-type seeds. The proportion of free Lys in seeds of the wild-type, nontransformed plants ranged between 0.29 and 0.39 mol%, while seeds of the control plants expressing the phas::DHPS alone ranged from 1.15 to 10.99 mol% (Fig. 2). The free Lys level in seeds of the independently transformed phas::dsLKR genotypes ranged between 0.21 and 2.11 mol% (Fig. 2). The free Lys level varied significantly between the different independently transformed phas::dsLKR/DHPS plants, reaching 25.06 mol%. The highest value represented an increase of approximately 64- and 2.3-fold, compared to a wild-type plant and a plant expressing the phas::DHPS alone with the highest seed free Lys level, respectively (Fig. 2). The variation in free Lys levels in seeds of plants expressing the phas::DHPS alone was apparently due to environmental effects, while in the phas::dsLKR/DHPS plants it was apparently due to both environmental effects and the expression level of the phas::dsLKR construct. Hence, the varying degree of high free Lys in the different phas::dsLKR/DHPS transgenic lines versus a line expressing the phas::DHPS alone is likely due to differential suppression of AtLKR/SDH gene expression by the phas::dsLKR construct.
Figure 2.
Relative free Lys levels in mature seeds of different transgenic Arabidopsis plants. Relative levels of free Lys are given as mol% of total amino acids. Abbreviations: WT, wild-type plant; dsLKR/WT, independently transformed homozygous phas::dsLKR transgenic genotypes on a wild-type background; DHPS, transgenic plants expressing the phas::DHPS construct in a homozygous state; dsLKR/DHPS, independently transformed homozygous phas::dsLKR transgenic genotypes on a background containing the phas::DHPS construct in a homozygous state. Black histograms represent amino acid analyses of seeds derived from approximately 10 different plants of single lines.
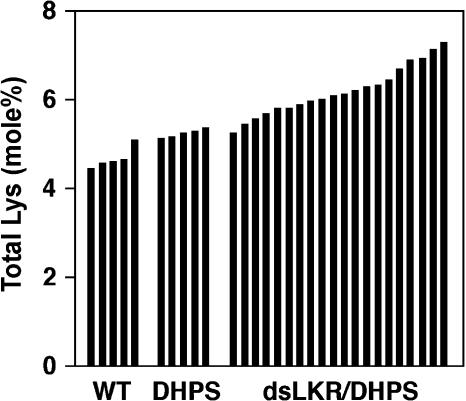
We also analyzed the effect of seed-specific expression of the phas::dsLKR construct on total (free plus protein-incorporated) Lys level in mature seeds of 20 representative, independently transformed phas::dsLKR/DHPS lines, as compared with wild-type plants, and the parental plants expressing the phas::DHPS alone. Total seed Lys levels were the lowest in wild-type plants, progressively increased in plants expressing the phas::DHPS alone, and further increased in the phas::dsLKR/DHPS plants (Fig. 3). The highest total Lys level in seeds of phas::dsLKR/DHPS plants was 7.28 mol%, which represents an approximately 42% increase compared to the wild-type plant with the highest total seed Lys level (5.12 mol%; Fig. 3).
Figure 3.
Relative total Lys levels in mature seeds of the different transgenic Arabidopsis plants. Relative levels of total Lys are given as mol% of total amino acids. Abbreviations refer to transgenic plant strains as described in Figure 2. Black histograms represent amino acid analyses of seeds derived from approximately 10 different plants of single lines.
Coordinated Seed-Specific Increase in Lys Synthesis and Reduction of Its Catabolism Alters Met Accumulation in Mature Seeds
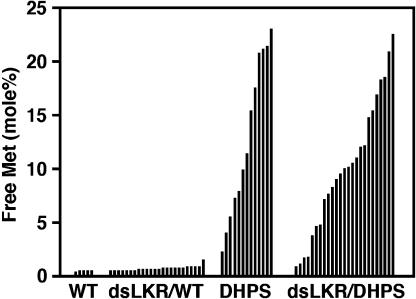
Met is synthesized by a branch that is competitive to the Lys branch in the Asp-family pathway (Fig. 1; Galili, 1995) and is therefore expected to be negatively associated with the Lys level. Yet, using an Arabidopsis AtLKR/SDH knockout mutant expressing the chimeric phas::DHPS gene, we previously found an unexpected positive association between free Lys and free Met levels in mature seeds (Zhu and Galili, 2003). Similar associations were also observed in the transgenic plants expressing the phas::dsLKR construct alone (Zhu and Galili, 2003). As shown in Figure 4, free Met level in mature seeds ranged between 0.36 and 0.43 mol% in the wild-type plants and between 0.45 and 2.11 mol% in the phas::dsLKR plants (Fig. 4). Notably, the range of free Met levels was similar in plants expressing the phas::DHPS alone and in the independently transformed genotypes of the phas::dsLKR/DHPS plants, reaching as high as approximately 22 mol%, an approximately 51-fold increase compared to the wild-type plant with the highest seed free Met level (Fig. 4). Assuming that environmental effects contributed similarly to Met level in all genotypes, these results suggest that the increase in free Met content is due to the bacterial DHPS, but not the phas::dsLKR construct.
Figure 4.
Relative free Met level in mature seeds of the different transgenic Arabidopsis plants. Relative levels of free Met are given as mol% of total amino acids. Abbreviations refer to transgenic plant strains as described in Figure 2. Black histograms represent amino acid analyses of seeds derived from approximately 10 different plants of single lines.
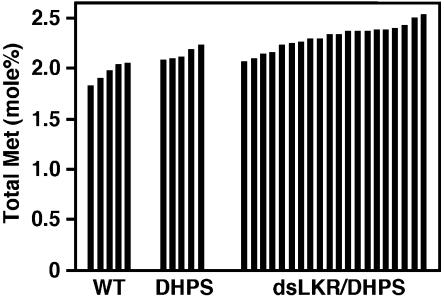
We also analyzed whether the seed-specific expression of the phas::dsLKR construct will increase the total Met level in mature seeds. Twenty representative, independently transformed phas::dsLKR/DHPS genotypes were analyzed in comparison with wild-type plants and parental plants expressing the phas::DHPS alone. Total seed Met ranged between 1.83 and 2.05 mol% in the wild-type plants; its level was increased in plants expressing the phas::DHPS alone and further increased in the phas::dsLKR/DHPS plants (Fig. 5). The highest total Met level in the phas::dsLKR/DHPS plants amounted to 2.53 mol%, which represented an approximately 23% increase compared to the wild-type plant with the highest total seed Met level (Fig. 5).
Figure 5.
Relative total Met levels in mature seeds of the different transgenic Arabidopsis plants. Relative levels of total Met are given as mol% of total amino acids. Abbreviations refer to transgenic plant strains as described in Figure 2. Black histograms represent amino acid analyses of seeds derived from approximately 10 different plants of single lines.
Correlation between Lys Level and the Content of Other Metabolically Related Amino Acids
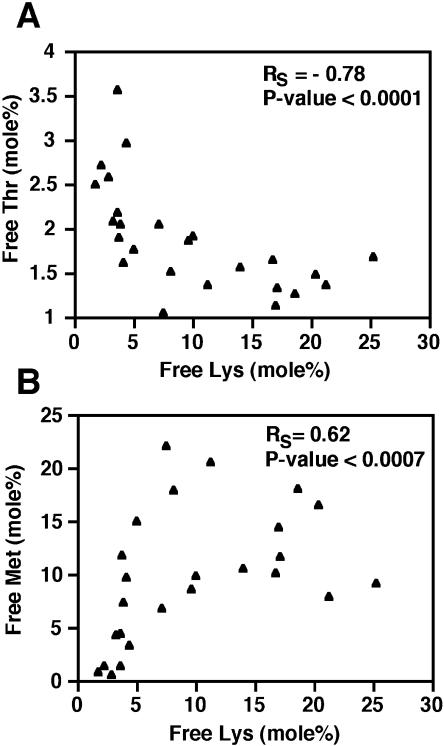
The correlation between free Lys and its other metabolically related amino acids was analyzed in the population of independently transformed phas::dsLKR/DHPS genotypes, which possess the highest variation in free Lys content. In all cases, the correlation did not fit a linear model, so we chose to represent the correlation by a Spearman correlation coefficient, which measures the strength of monotonic relationships between two variables. First, we first tested the correlations of Lys with free Thr and Met, which are synthesized by the other branch of the Asp-family pathway (Fig. 1). As shown in Figure 6, section A, the free Thr level was negatively correlated with the free Lys level, fitting a model in which Thr levels were strongly reduced at a low level of increased Lys and then reached a plateau. The correlation between free Met and free Lys levels was less pronounced than that between free Thr and Lys. However, the free Met level was still positively correlated with the free Lys level, fitting a second degree polynomial in which the Met level was strongly increased with increasing Lys content at the lower range of Lys levels, and then decreased at the high range of Lys levels (Fig. 6, section B).
Figure 6.
Correlation between free Lys and free Thr or free Met levels in mature seeds of the different phas::dsLKR/DHPS Arabidopsis lines. Spearman correlation between the mol% of free Lys and free Thr (section A) or free Met (section B).
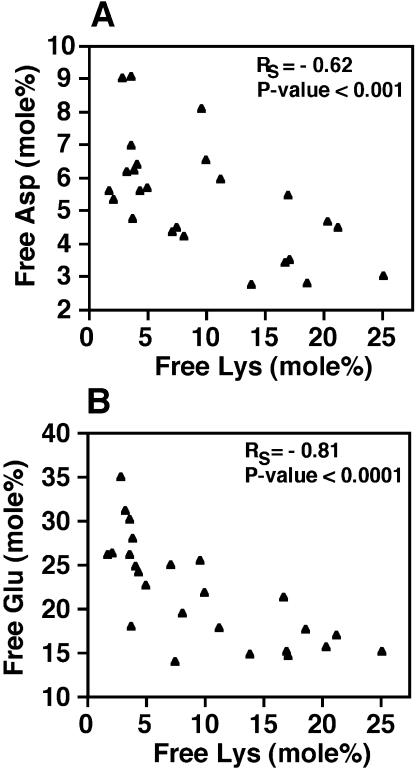
Lys is synthesized from Asp, which together with Glu, Gln, and Asn constitute a central regulatory metabolic network in plants (Lam et al., 1995). Moreover, Glu is also a major product of the Lys catabolism pathway (Galili et al., 2001). We therefore also determined how specific changes in Lys metabolism correlate with changes in the levels of these other four amino acids in the independently transformed genotypes of the phas::dsLKR/DHPS plants. As shown in Figure 7, sections A and B, Spearman correlation coefficients showed that free Asp and Glu levels were negatively correlated with the free Lys level in mature seeds (section D). This correlation fit a model similar to that found between free Thr and Lys (see Fig. 6A), namely that free Asp and Glu levels were relatively strongly reduced with increased Lys content at the low range of Lys levels and then their rates of reduction tended to diminish at the high Lys levels. No significant correlation was found between the level of free Lys and the levels of free Gln and Asn (data not shown).
Figure 7.
Correlation between free Lys and free Asp and Glu levels in mature seeds of different phas::dsLKR transgenic Arabidopsis lines. Spearman correlation between the mol% of free Lys and Asp and Glu are given in sections A and B, respectively.
In our previous report (Zhu and Galili, 2003) using Arabidopsis plants expressing the phas::DHPS in an AtLKR/SDH knockout background, we found a negative correlation between free Lys and free Gln in mature seeds of plants accumulating extra high Lys levels (20–35 mol%). This suggests that very high fluxes of Lys biosynthesis might trigger the conversion of Gln, via Glu, to Asp en route to Lys. Unfortunately, we could not substantiate this correlation with the phas::dsLKR construct because we were unable to obtain lines with free Lys above 25 mol% (Fig. 2).
Effect of a Coordinated Seed-Specific Increase in Lys Synthesis and a Reduction of Its Catabolism on Seed Germination
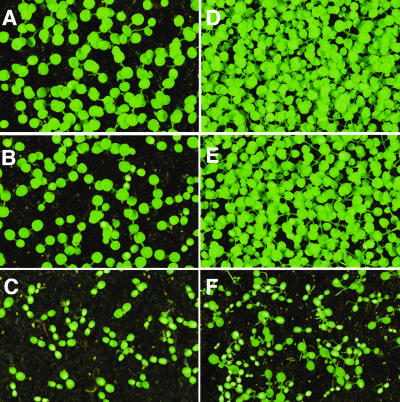
We previously showed that Lys overaccumulation in seeds of transgenic Arabidopsis plants expressing the phas::DHPS construct in an AtLKR/SDH knockout background interfered with seed germination (Zhu and Galili, 2003). Two types of interference were noted: (1) arrest of seed germination (reduced germination frequency), which was generally restricted to seeds derived from lines accumulating the highest levels of free Lys; and (2) defective postgermination seedling establishment, which was manifested by slower seedling growth and the appearance of white spots on the emerging cotyledons; this was observed in seeds derived from lines accumulating moderate to high free Lys levels. The arrest of seed germination is likely due to negative effects of Lys on processes occurring during seed maturation, but whether imperfect seedling growth was also due to impaired Lys catabolism during seed maturation or a postgermination deficiency in Lys catabolism could not be determined, because Lys catabolism was eliminated in all plant tissues as a result in the AtLKR/SDH knockout mutation (Zhu and Galili, 2003). It was therefore interesting to compare the effects of seed Lys levels on seed germination and seedling growth in the phas::dsLKR/DHPS line in comparison to seeds expressing the phas::DHPS construct in an AtLKR/SDH knockout background. Seeds from all the independently transformed phas::dsLKR/DHPS plants germinated at relatively high frequencies. However, none of them contained the extremely high Lys level that was found to retard the germination of seeds expressing the phas::DHPS construct in an AtLKR/SDH knockout background (Zhu and Galili, 2003). This was perhaps because of the incomplete suppression of the AtLKR/SDH gene expression by the phas::dsLKR construct. Yet, as shown in Figure 8, when seeds accumulating comparable moderately high Lys levels were tested, those expressing the phas::DHPS construct in an AtLKR/SDH knockout background had a much slower rate of seedling growth and many more white spots on the cotyledons (Fig. 8, sections C and F) than the phas::dsLKR/DHPS seeds (Fig. 8, sections B and E). In fact, the rate of seedling growth was comparable between the high-Lys phas::dsLKR/DHPS seeds and those of the wild-type seeds (Fig. 8, compare sections B and E with A and D, respectively).
Figure 8.
Morphological phenotypes of seedlings derived from phas::dsLKR/DHPS and AtLKR/SDH knockout plants. The morphological phenotypes of 1-week-old (sections A–C) and 2-week-old (sections D–F) seedlings derived from seeds of wild-type plants (sections A and D) as well as from seeds of phas::dsLKR/DHPS (sections B and E) and plants expressing the phas::DHPS in a AtLKR/SDH knockout background (sections C and F) containing comparable average free seed Lys levels.
Lys Catabolism Plays an Important Regulatory Role in Balancing Lys Levels during Vegetative Growth
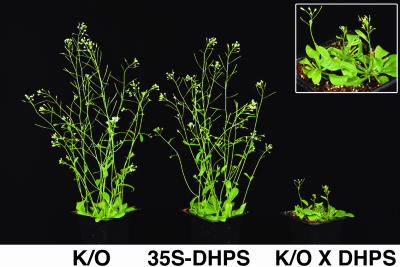
The faster growth rate of seedlings derived from high Lys plants in which Lys catabolism was specifically reduced during seed maturation versus those in which Lys catabolism was entirely knocked out (Fig. 8) suggested that Lys catabolism balances Lys levels during vegetative growth. Since evidence of a functional role for Lys catabolism was only observed in developing seeds, we wished to demonstrate further that this pathway is also operative in vegetative tissues. To do so we transformed Arabidopsis plants with a construct expressing the bacterial DHPS gene under control of the constitutive 35S promoter (Ben Tzvi-Tzchori et al., 1996). A number of transgenic plants were produced and confirmed by western blot to express the bacterial DHPS analysis (data not shown). One of these plants, which expressed a relatively low level of the bacterial DHPS, accumulated only a slightly higher Lys level than the wild-type plants (average 2.84 mol%, compared to 2.30 mol% in wild-type plants), and had a phenotype comparable to the wild-type plants (Fig. 9, left and center plants, respectively), was crossed with the homozygous AtLKR/SDH knockout mutant. Notably, this cross yielded F1 plants with a severe abnormal phenotype (Fig. 9, right; see also enlargement), which is a characteristic of plants that significantly overaccumulate Lys in vegetative tissues (Ben Tzvi-Tzchori et al., 1996). Free amino acid analysis further showed that leaves of the F1 plants had nearly 8-fold higher Lys (average 20.19 mol%) than leaves of both of the parents (average 2.61 and 2.84 mol%; see above).
Figure 9.
Morphological phenotypes of transgenic plants with modified Lys metabolism in vegetative tissues. The morphological phenotypes of a homozygous AtLKR/SDH knockout mutant (K/O; left), a transgenic line constitutively expressing relatively low levels of the bacterial DHPS via the 35S promoter (35S-DHPS; center), and their F1 hybrid (K/O × DHPS; right) are shown. An enlargement of the F1 hybrid is shown on the top right corner.
DISCUSSION
Lys Metabolism in Developing Arabidopsis Seeds Is Coordinately Regulated by Anabolic and Catabolic Processes
In previous studies, we expressed a bacterial feedback-insensitive DHPS under the control of the seed-specific phaseolin promoter in a homozygous AtLKR/SDH knockout mutant. We used this transgenic mutant to show that Lys accumulation in developing Arabidopsis seeds is regulated both by synthesis and catabolism (Zhu and Galili, 2003). However, since Lys catabolism was eliminated through the entire life cycle of these plants, including the entire period of seed maturation, we could not determine whether Lys synthesis and catabolism operate in a concerted spatial and temporal manner to regulate Lys accumulation during seed development. In this report, by expressing both the bacterial DHPS and an AtLKR/SDH RNAi construct under control of the phaseolin promoter, we showed that spatially and temporally coordinated processes of synthesis and catabolism operate in concert to regulate Lys metabolism during seed development.
Association of Lys with Regulatory Metabolic Networks of the Asp-Family Pathway and Nitrogen Metabolism in Arabidopsis Seeds
By expressing the phas::DHPS in the AtLKR/SDH knockout mutant, we previously showed that increased Lys levels in the seeds were associated with significant alterations in the levels of several amino acids (Zhu and Galili, 2003). The most pronounced changes were an inverse relationship between free Lys and Thr levels, a metabolically unexpected positive relationship between free Lys and Met levels and inverse relationships between free Lys and the levels of Glu and Asp, but not Gln and Asn (Zhu and Galili, 2003). These metabolic associations were essentially supported by the results of the experiments in this study. However, our ability to obtain transgenic lines with a gradual range of free Lys levels from 1.08 mol% to 25.06 mol%, apparently as a result of various levels of AtLKR/SDH gene expression, allowed us to further explore the correlations between levels of different amino acids. Thus, assuming that free amino acid levels in mature seeds reflect their metabolism during earlier stages of seed maturation, the following conclusions can be drawn: (1) The flux of Lys biosynthesis, which is strongly regulated by the sensitivity of DHPS to feedback inhibition by Lys, competes with the flux of Thr synthesis and uses mostly Glu and Asp and only to a lesser extent Gln and Asn as donor metabolites, and (2) The unexpected increase in free Met when free Lys synthesis is enhanced is not directly related to Lys catabolism or to metabolic interactions between the Lys and Met fluxes. This is because very high levels of Met could also be obtained in plants expressing the phas::DHPS alone (Fig. 4), and the Met level regressed upon massive accumulation of free Lys (Fig. 6B). The increase in Met level may be explained by a plausible competition between the biosynthesis branches of Met and Thr whose level was reduced in our transgenic plants. However, the increased Met level cannot be solely explained by anabolic interactions because the entire flux of the Thr and Met branches is likely reduced in the transgenic plants because excess Lys level feedback inhibits the Lys-sensitive AK isozymes (Fig. 1). Thus, we hypothesize that the reduced Met catabolism also contributes to the increased free Met levels and may in fact be an important regulatory factor in this unexpected metabolic interaction.
Postgermination Lys Catabolism Plays an Important Regulatory Role in Balancing the Lys Level during Seedling Growth
When germination of seeds containing comparable high Lys levels was tested, those expressing the phas::DHPS construct in an AtLKR/SDH knockout background showed much slower seedling growth and much more severe appearance of white spots on the cotyledons than germinated seeds of phas::dsLKR/DHPS plants (Fig. 8). Since the AtLKR/SDH knockout mutant lacks Lys catabolism both in maturing seeds and germinating seedlings, while in the phas::dsLKR expressing plants Lys catabolism is reduced particularly in maturing seeds, this implies that the defective seedling growth is due to impaired postgermination Lys catabolism rather than to defective Lys catabolism during seed maturation. This observation is of particular interest because it suggests that the seed Lys overaccumulation and its inhibitory effect on seedling growth are two spatially distinct processes that can be genetically dissected. This issue is of particular significance not only for our understanding of Lys metabolism, but also from a biotechnological standpoint, because negative germination-associated traits must be eliminated in the production of nutritionally balanced high-Lys crop plants.
Finally, the fact that seedlings derived from the phas::dsLKR/DHPS seeds developed much faster than those of seeds expressing the phas::DHPS construct in an AtLKR/SDH knockout background also suggests that the function of Lys catabolism in balancing Lys levels is not restricted to seeds, but also operates in vegetative tissues. This assumption was also supported by our observation that F1 plants, obtained by crossing of transgenic plants constitutively expressing the 35S-DHPS with an AtLKR/SDH knockout mutant, had abnormal vegetative phenotypes compared to their parents (Fig. 9) and also accumulated significantly higher Lys levels in vegetative tissues. Since these F1 plants were heterozygous for the AtLKR/SDH knockout mutation, it can also be concluded that a quantitative reduction, rather than complete elimination of the Lys catabolism pathway, is sufficient to exert a regulatory effect of the steady state Lys levels in vegetative tissues.
MATERIALS AND METHODS
Materials
Arabidopsis plants were grown in pots with greenhouse conditions (16-h photoperiod at 23°C ± 5°C). For screening transgenic plants, sterile seeds were germinated on Murashige and Skoog medium (Duchefa, Israel) containing 10 μg/mL hygromycin. To test germination and observe seedling establishment, mature seeds were spread directly on the soil surface in a petri dish.
Gene Construction and Generation of Transgenic Plants Expressing the phas::dsLKR RNAi Construct
The phas::dsLKR RNAi construct contains sense and antisense LKR cDNA sequences flanking a 1,300-bp spacer fragment derived from the first intron of a potassium transporter gene (ATU06745). The original dsLKR created in SK was subcloned into the plant expression vector pCAMBIA 1300 (AF234296) containing a seed-specific phaseolin promoter, a nopaline synthase terminator, and a hygromycin resistance gene, to produce the plasmid BIA-dsLKR.
Transgenic plants expressing a bacterial feedback-insensitive DHPS under the control of a phaseolin promoter were previously described (Karchi et al., 1994). The BIA-dsLKR construct was transformed into either the homozygous DHPS transgenic plants or wild-type plants (see “Results”) to generate phas::dsLKR/DHPS and phas::dsLKR transgenic plants, respectively. Arabidopsis plant transformation was performed as described previously (Clough and Bent, 1998).
Amino Acid Analysis
Free amino acids were extracted from mature Arabidopsis seeds or from vegetative tissues as previously described (Shaul and Galili, 1992). To measure the total amino acid composition of mature seeds, 100 mg of mature seeds was ground to a powder, in liquid nitrogen followed by hydrolysis in 6 m HCl for 22 h at 115°C. Amino acid analysis was performed by reverse-phase HPLC on a C-18 column (Waters, Milford, MA) using precolumn phenylisothiocyanate derivatization.
Statistical Analysis
Statistical analysis was performed in order to evaluate the correlation between Lys and the variables Thr, Met, Asp, Glu, Gln, and Asn. Since the data are not normally distributed and the relationships are not necessarily linear, Spearman rank correlation coefficient was used. This correlation coefficient detects any monotonic relationship, not necessarily linear (Sokal and Rohlf, 1981). Analysis was using the SAS program (SAS Institute, Cory, NC).
Supplementary Material
Acknowledgments
We thank Tamar Golan for assisting with the selection of different Arabidopsis genotypes. GG is an incumbent of the Bronfman Chair of Plant Sciences.
This work was supported by grants from the FrameWork Program of the Commission of the European Communities and by the Israel Academy of Sciences and Humanities, National Council for Research and Development.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.037168.
References
- Arruda P, Kemper EL, Papes F, Leite A (2000) Regulation of lysine catabolism in higher plants. Trends Plant Sci 5: 324–330 [DOI] [PubMed] [Google Scholar]
- Ben Tzvi-Tzchori I, Perl A, Galili G (1996) Lysine and threonine metabolism are subject to complex patterns of regulation in Arabidopsis. Plant Mol Biol 32: 727–734 [DOI] [PubMed] [Google Scholar]
- Bright SWJ, Shewry PR (1983) Improvement of protein quality in cereals. CRC Crit Rev Plant Sci 1: 49–93 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Falco SC, Guida T, Locke M, Mauvais J, Sandres C, Ward RT, Webber P (1995) Transgenic canola and soybean seeds with increased lysine. Biotechnology 13: 577–582 [DOI] [PubMed] [Google Scholar]
- Galili G (1995) Regulation of lysine and threonine synthesis. Plant Cell 7: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G, Shaul O, Perl A, Karchi H (1994) Synthesis and accumulation of the essential amino acids lysine and threonine in seeds. In H Kigel, G Galili, eds, Seed Development and Germination. Marcel Dekker, New York, pp 811–831
- Galili G, Tang G, Zhu X, Gakiere B (2001) Lysine catabolism: a stress and development super-regulated metabolic pathway. Curr Opin Plant Biol 4: 261–266 [DOI] [PubMed] [Google Scholar]
- Karchi H, Shaul O, Galili G (1994) Lysine synthesis and catabolism are coordinately regulated during tobacco seed development. Proc Natl Acad Sci USA 91: 2577–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H-M, Coschigano K, Schultz C, Melo-Oliveira R, Tjagen G, Oliveira I, Ngai N, Hsieh M-H, Coruzzi GM (1995) Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell 7: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B, Krebbers E, Tingey S (1999) Gene discovery and product development for grain quality traits. Science 285: 372–375 [DOI] [PubMed] [Google Scholar]
- Shaul O, Galili G (1992) Increased lysine synthesis in transgenic tobacco plants expressing a bacterial dihydrodipicolinate synthase in their chloroplasts. Plant J 2: 203–209 [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ (1981) Biometry, Ed 2. Freeman and Company, New York
- Zhu X, Galili G (2003) Increased lysine synthesis coupled with a knockout of its catabolism synergistically boosts lysine content and also trans regulates the metabolism of other amino acids in arabidopsis seeds. Plant Cell 15: 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Tang G, Granier F, Bouchez D, Galili G (2001) A T-DNA insertion knockout of the bifunctional lysine-ketoglutarate reductase/saccharopine dehydrogenase gene elevates lysine levels in Arabidopsis seeds. Plant Physiol 126: 1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.