Abstract
The allosteric enzyme ADP-Glc pyrophosphorylase (AGPase) catalyzes the synthesis of ADP-Glc, a rate-limiting step in starch synthesis. Plant AGPases are heterotetramers, most of which are activated by 3-phosphoglyceric acid (3-PGA) and inhibited by phosphate. The objectives of these studies were to test a hypothesis concerning the relative roles of the two subunits and to identify regions in the subunits important in allosteric regulation. We exploited an Escherichia coli expression system and mosaic AGPases composed of potato (Solanum tuberosum) tuber and maize (Zea mays) endosperm subunit fragments to pursue this objective. Whereas potato and maize subunits have long been separated by speciation and evolution, they are sufficiently similar to form active mosaic enzymes. Potato tuber and maize endosperm AGPases exhibit radically different allosteric properties. Hence, comparing the kinetic properties of the mosaics to those of the maize endosperm and potato tuber AGPases has enabled us to identify regions important in regulation. The data herein conclusively show that both subunits are involved in the allosteric regulation of AGPase. Alterations in the small subunit condition drastically different allosteric properties. In addition, extent of 3-PGA activation and extent of 3-PGA affinity were found to be separate entities, mapping to different regions in both subunits.
Starch has many applications in industry as well as serving as an energy source in animal and human nutrition. ADP-Glc pyrophosphorylase (AGPase) catalyzes a rate-limiting step in starch synthesis. Modification of the regulatory properties of this enzyme increases starch yields in potato (Solanum tuberosum) tubers, and maize (Zea mays), wheat (Triticum aestivum), and rice (Oryza sativa) seeds (Stark et al., 1992; Giroux et al., 1996; Smidansky et al., 2002, 2003). Seed yield and plant biomass increases are conferred by deregulation of endosperm AGPase. Accordingly, AGPase has attracted wide interest for potential crop improvements.
Plant AGPases are tissue specific. They are heterotetramers of two small and two large subunits. Though forms of each subunit share significant sequence homology, the small subunit is generally more conserved than is the large subunit (Smith-White and Preiss, 1992). Sequence comparisons of the large subunits divide AGPases into four groups: stem/tuber, leaf, fruit/root, and endosperm AGPases. Further differences among AGPases are discernable in regulatory properties. While most plant AGPases are activated by 3-phosphoglyceric acid (3-PGA) and inhibited by phosphate (Ghosh and Preiss, 1966; Dickinson and Preiss, 1969; Sowokinos and Preiss, 1982; Kleczkowski et al., 1993a, 1993b; Sikka et al., 2001), extent of regulation varies from organ to organ. Leaf, potato tuber, and tomato (Lycopersicon esculentum) fruit AGPases are sensitive to 3-PGA and phosphate, while endosperm AGPases, except for that of rice (Sikka et al., 2001), are less sensitive to the regulators. AGPases also differ in subcellular localization. Leaf and potato tuber AGPases are found in the chloroplasts or amyloplasts (Okita et al., 1979; Kim et al., 1989). By contrast, the major activity of endosperm AGPases is localized in the cytosol, with a residual activity in the amyloplast (Denyer et al., 1996; Thorbjornsen et al., 1996; Sikka et al., 2001). The cytosolic and plastidial activities are encoded by different genes (Giroux and Hannah, 1994; Burton et al., 2002; Johnson et al., 2003). Surprisingly, the sequences of the proteins identified as barley (Hordeum vulgare) and wheat plastidial small subunits do not match any currently identified gene sequences (Burton et al., 2002; Johnson et al., 2003).
Mutagenesis experiments to pinpoint regions of the two subunits involved in catalysis and regulation have been greatly simplified by the expression of plant-derived AGPase genes in Escherichia coli (Iglesias et al., 1993; Ballicora et al., 1995; Giroux et al., 1996). These mutagenic studies (for example, Giroux et al., 1996; Greene et al., 1996a, 1996b, 1998; Laughlin et al., 1998; Kavakli et al., 2001; Salamone et al., 2002) as well as others reviewed recently elsewhere (Ballicora et al., 2003), coupled with binding assays of various substrate and effector analogs (for example, Morell et al., 1988; Ball and Preiss, 1994), have greatly aided the identification of important amino acids.
Here, we present a complementary approach to the mutagenic studies mentioned above. We used the E. coli expression system to map genuine regulatory regions in AGPase that distinguish maize endosperm and potato tuber AGPases. Whereas potato and maize subunits have long been separated by speciation and evolution, they are sufficiently similar to form active mosaic enzymes. Our approach then consisted of constructing mosaic subunits composed of regions from potato tuber and maize endosperm AGPases. These comparisons enabled us to identify regions important for regulation and that differ in potato tuber and maize endosperm AGPases. Our results conclusively show that the small subunit plays a vital role in allosteric regulation. These data, in conjunction with others recently published, are discussed in terms of the proposed delineation of the roles of the small and large subunit.
RESULTS
The Mosaics Display Variable Heterotetrameric AGPase Activity
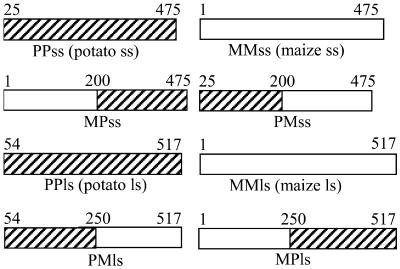
The mosaic subunits constructed for this study are shown in Figure 1. Each of the large and small subunits was first equally divided at conserved regions of the proteins, resulting in the mosaic subunits MPss, PMss, MPls, and PMls (where ss is small subunit and ls is large subunit). Heterotetramers involving all mosaics were then expressed in E. coli strain AC70R1-504. This strain is deficient in AGPase and consequently is unable to synthesize glycogen. If an active AGPase is expressed in AC70R1-504, the strain regains the ability to produce glycogen. Glycogen synthesis can be easily scored by growing the E. coli strain on Glc and staining resulting colonies with iodine. Colonies producing glycogen stain brown. Several mosaic AGPases did not stain (Table I). The reason for this lack of staining is under examination.
Figure 1.
Summary of AGPase mosaics. White and hatched rectangles represent fragments originating from the maize endosperm (M) and potato tuber (P) small (ss) and large (ls) subunits, respectively. Numbers on top of the rectangles identify junctions. Numbers match amino acid positions in the maize endosperm subunit. Hence, potato tuber subunits start at residue 25 for the small subunit and 54 for the large subunit.
Table I.
Summary of iodine staining for detection of AGPase activity in the mosaic AGPases
| MMls (Maize ls) | PPls (Potato ls) | MP ls | PM ls | |
|---|---|---|---|---|
| MMss (Maize ss) | + | +−− | − | − |
| PPss (Potato ss) | + | + | + | + |
| MP ss | + | + | − | + |
| PM ss | + | − | − | − |
Each of the 16 mosaics examined contained the small subunit identified in the left column and the large subunit in the heading above the mosaic. AGPase staining is symbolized + for a dark brown stain, +−− for a very light stain, and − for no visible stain.
Active mosaics, as detected by staining, were assayed for 3-PGA activation and phosphate inhibition. To quantify any AGPase activity arising from homotetramers rather than heterotetramers, each of the wild-type potato and maize small and large subunits was expressed individually. Activity detected ranged from 2.5% to 10% of that obtained from expression of the recombinant wild-type maize or potato enzymes (Burger et al., 2003). In a similar manner, we expressed the mosaic small subunits of interest here alone or with a mutant large subunit (Table II). The mutant large subunit consisted of a maize large subunit protein with alterations at residues 512, 513, and 514. Expression of each small subunit alone led to low activity compared to activity arising from wild-type heterotetramers. Furthermore, activity decreased in the presence of the mutant large subunit. Hence, the vast majority of activity produced by coexpression of large and small subunits arises from heterotetramers.
Table II.
Activity arising from expression of each small subunit alone or with a mutant large subunit
| Small Subunit | Homotetrameric Activity | Activity with a Mutant SH2 |
|---|---|---|
| None | 2.1% | |
| PPss | 6.6% | 2.1% |
| MMss | 3.4% | 2.0% |
| MPss | 9.6% | 6.9% |
| PMss | 1.9% | 0.8% |
The mutant large subunit consisted of a virtually inactive MMls subunit altered at three residues. Assays were performed in the direction of ADP-Glc synthesis on crude extracts in the presence of 10 mm 3-PGA. Activity is expressed as the percentage of average activity detected in maize and potato heterotetramers.
Mosaics PPss/MMls, MPss/MMls, MPss/PMls, PPss/PMls, and PMss/MMls and the recombinant AGPases from potato tuber (PPss/PPls) and maize endosperm (MMss/MMls) were then partially purified for kinetic studies. Mosaic MMss/PPls could not be purified because of low activity and instability. Instability is inferred from the significant loss of activity during initial purification steps. The last purification step consisted of size separation by gel filtration chromatography. The active fraction eluted around 220 kD for all mosaics purified, a size compatible with a tetrameric structure. Purification varied from 15-fold for maize endosperm AGPase to 160-fold for potato AGPase. The variation in purification fold results mostly from differences in the stability of the various AGPases during purification. Indeed, activity lost during purification varied from enzyme to enzyme, with maize endosperm and potato tuber AGPase retaining 5% and 25% of their starting activity, respectively. Activity lost and final specific activities for the mosaic AGPases ranged between the values obtained for maize endosperm and potato tuber AGPases.
3-PGA Fold Activation and 3-PGA Ka Are Separate Entities
Mosaics were separated into different groups based on their Ka (concentration giving half-maximal activation) for 3-PGA, their fold activation by 3-PGA, or their cooperativity (Table III). Each parameter results in a different classification. For instance, PPss/MMls and PMss/MMls exhibit indistinguishable Ka values for3-PGA but vastly different extents of 3-PGA activation. Likewise, MMss/MMls and PMss/MMls belong to the same activation group but reside in different3-PGA affinity groups.
Table III.
Regulatory properties of the mosaic AGPases
|
|
Fold Activation
|
Gp
|
Ka
|
Gp
|
Cooperativity
|
Gp
|
|---|---|---|---|---|---|---|
| mM | ||||||
| PPss/PPls | 28 (12) | 1 | 0.02 (0.008) | 1 | H | 1 |
| MMss/MMls | 5 (1.7) | 2 | 0.40 (0.06) | 3 | H | 1 |
| PPss/PMls | 4.9 (1.2) | 2 | 0.66 (0.03) | 4 | S (n = 1.3) | 2 |
| MPss/PMls | 4.7 (0.02) | 2 | 0.07 (0.01) | 2 | H | 1 |
| PMss/MMls | 4.8 (1.6) | 2 | 0.05 (0.01) | 2 | H | 1 |
| PPss/MMls | 1.3 (0.15) | 3 | 0.11 (0.05) | 2 | H | 1 |
| MPss/MMls | 1.2 (0.07) | 3 | 3.72 (0.34) | 5 | S (n = 0.7) | 2 |
Proteins were partially purified through hydrophobic and gel filtration columns and assayed in the direction of ADP-Glc synthesis. Parameters of 3-PGA activation were calculated by nonlinear regression with the Prism program using a 1/y2 weighting and considering each replicated point as a separate datum. Each parameter was determined from two to three experiments with duplicate measurements. Numbers in parenthesis in the Ka and fold activation columns represent sd. Numbers in parentheses in the cooperativity column represent Hill coefficients. H, hyperbolic; S, sigmoidal; Gp, classification group based on the criterion of the left column.
Both Subunits Are Equally Important in 3-PGA Activation
Mosaics were separated into three groups based on 3-PGA fold activation (Table III). Group 3 is insensitive to 3-PGA. Compared to the other mosaics, those of group 3 exhibit one common feature: a small subunit C terminus from potato tuber AGPase and a large subunit N terminus from maize endosperm AGPase. In all other mosaics, the small subunit C terminus and the large subunit N terminus originate from the same source. Interestingly, mosaics composed of the C terminus of the maize small subunit and the N terminus of the potato tuber large subunit exhibited very little activity as measured by staining (mosaics MMss/PPls, PMss/PMls, PMss/PPls, and MMss/PMls in Table I). These observations point to an interaction between the small subunit C terminus and the large subunit N terminus.
Group 1, composed of only the potato wild-type heterotetramer, is the most sensitive to 3-PGA activation. In contrast to the other mosaics, this enzyme contains the N terminus of the small subunit and the C terminus of the large subunit from potato tuber AGPase. Group 2 mosaics, which exhibit intermediate 3-PGA activation compared to other enzymes, are composed of either the N terminus of the maize endosperm small subunit or the C terminus of the maize endosperm large subunit. Since group 2 mosaics are less sensitive to 3-PGA activation compared to the potato tuber AGPase, we conclude that both the C terminus of the large subunit and the N terminus of the small subunit influence 3-PGA fold activation.
Nonadditive effects are also apparent. Replacement of the C terminus small subunit of the relative 3-PGA insensitive maize enzyme with its counterpart from the 3-PGA sensitive potato enzyme (compare PMss/MMls with PPss/MMls) reduces rather than increases 3-PGA activation.
Both Subunits Are Equally Important for 3-PGA Affinity
Mosaics were separated into five groups based on3-PGA affinity (Table III). Potato tuber and maize endosperm AGPase displayed hyperbolic curves as noted previously (Dickinson and Preiss, 1969; Sowokinos and Preiss, 1982). Mosaics MPss/MMls and PPss/PMls exhibited sigmoidal 3-PGA saturation kinetics in contrast to the potato tuber and maize endosperm AGPases. This implies modified subunit interactions compared with the potato tuber and maize endosperm AGPases. Hence, the protein conformation may be altered and may account for the high 3-PGA Ka value of these mosaics. Consequently, these mosaics were not used for mapping 3-PGA affinity.
Comparison of mosaics exhibiting hyperbolic3-PGA saturation kinetics led to the following conclusions. PMss/MMls has a lower Ka for 3-PGA than does MMss/MMls. Hence, the N terminus of the small subunit is important in 3-PGA affinity. PPss/MMls and PMss/MMls exhibit similar Ka values for 3-PGA. We conclude that the 3-PGA affinity difference(s) between potato tuber and maize endosperm does not map to the C terminus of the small subunit. MPss/PMls belongs to the same 3-PGA affinity group as do PPss/MMls and PMss/MMls. In addition, MPss/PMls displays a lower Ka than does MMss/MMls. Since we eliminated the C terminus of the small subunit as a determinant of 3-PGA affinity differences, we conclude that the N terminus of the large subunit is important for 3-PGA affinity. Furthermore, both subunits equally influence 3-PGA affinity differences. No mosaic exhibits a Ka as low as that of potato tuber AGPase. Hence, at least both N termini from potato tuber AGPase are required for a potato tuber-type Ka for 3-PGA.
Both Subunits Equally Influence Phosphate Inhibition
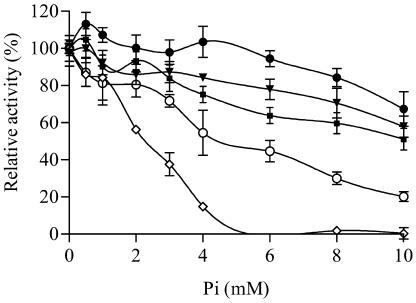
Phosphate inhibition studies were performed in the presence of 3 mm 3-PGA. All AGPases displayed significant activity in the absence of phosphate at this 3-PGA level. While this concentration is saturating for all mosaics except for MPss/MMls, this mosaic is the least sensitive to phosphate inhibition at 3 mm 3-PGA (Fig. 2) as well as at 10 mm (data not shown).
Figure 2.
Phosphate inhibition of potato tuber, maize endosperm, MPss/MMls, PPss/MMls, and PMss/MMls AGPases. Proteins were partially purified through hydrophobic and gel filtration columns. Inhibition studies were performed in the presence of 3 mm 3-PGA. AGPase activity was plotted as a percentage of activity in the absence of phosphate. Each point is the result of two experiments with duplicate measures. Error bars represent sd. •, MPss/MMls; ▾, maize endosperm; ▪, PPss/MMls; ○, PMss/MMls; ⋄, potato tuber.
Several mosaics as well as the maize endosperm AGPases are stimulated by low phosphate concentrations. This phenomenon has been well described in barley (Kleczkowski et al., 1993a; Kleczkowski, 1999) and can be seen in data published previously for the maize endosperm AGPase (Dickinson and Preiss, 1969).
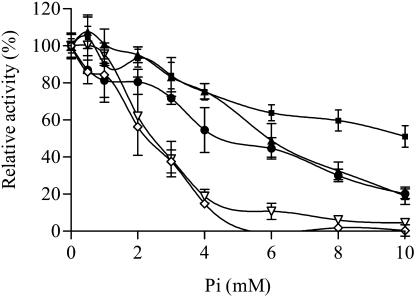
Phosphate inhibition divided the AGPases into three groups. MPss/MMls and PPss/MMls were relatively insensitive to phosphate (Fig. 2). MPss/PMls and PMss/MMls displayed phosphate inhibition curves intermediate between those of maize endosperm and potato tuber AGPases (Fig. 3), while PPss/PMls was inhibited comparatively to potato tuber AGPase (Fig. 3). Since PPss/PMls belongs to the same inhibition group as potato tuber AGPase (Fig. 3), phosphate inhibition differences between potato tuber and maize endosperm do not map to the C terminus of the large subunit. PMss/MMls is more sensitive to phosphate than is MMss/MMls (Fig. 2). Hence, phosphate inhibition differences map to the N terminus of the small subunit. PPss/MMls is less sensitive to phosphate inhibition than is PMss/MMls (Fig. 3). This may result from PPss/MMls containing a C terminus of the small subunit and an N terminus of the large subunit from different origins. Indeed, MPss/MMls is also insensitive to phosphate inhibition, while MPss/PMls is relatively sensitive to phosphate inhibition (Figs. 2 and 3). In fact, MPss/PMls and PMss/MMls belong to the same group of intermediate phosphate inhibition. MPss/PMls and PMss/MMls both contain a C terminus of the small subunit and an N terminus of the large subunit from identical origins. However, in MPss/PMls, those two fragments originate from potato tuber, while in PMss/MMls they come from maize endosperm. Hence, sensitivity to phosphate inhibition is possible when the C terminus of the small subunit and the N terminus of the large subunit originate from the same parental AGPase. Whether both fragments come from potato tuber or maize endosperm does not seem important. Finally, MPss/PMls is more sensitive to phosphate inhibition than is MMss/MMls. Hence, phosphate inhibition differences between potato tuber and maize endosperm AGPases map to the C terminus of the small subunit or the N terminus of the large subunit. Since the C terminus of the small subunit and the N terminus of the large subunit must originate from the same AGPase for phosphate inhibition to occur, one cannot distinguish which of these fragments influences the phosphate inhibition differences distinguishing potato tuber and maize endosperm AGPases.
Figure 3.
Phosphate inhibition of PPss/MMls, PMss/MMls, MPss/PMls, PPss/PMls, and potato tuber AGPases. Experimental approach was identical to that of Figure 2. ▪, PPss/MMls; ▴, MPss/PMls; •, PMss/MMls; ∇, PPss/PMls; and ⋄, potato tuber.
Extent of 3-PGA Activation, Ka for 3-PGA, and Phosphate Inhibition Map to Different Motifs
While the maize endosperm AGPase is less sensitive to 3-PGA activation and phosphate inhibition compared to the potato tuber AGPase, the data presented here show that these parameters map to different motifs in the enzyme. A particularly striking example of this is the mosaic PP/PM. This enzyme exhibits the phosphate sensitivity of potato, is activated by 3-PGA to the extent noted for maize, but has a Ka for 3-PGA outside of the range defined by potato and maize.
DISCUSSION
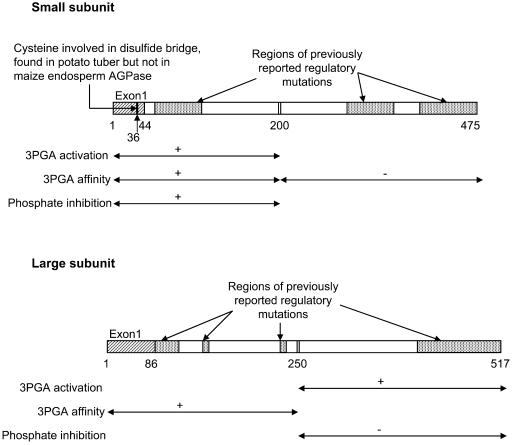
Experiments were done to map sites in the small and large subunits of AGPase that are important in conditioning the allosteric properties of the enzyme. Sites mapped are ones that differ in the allosterically distinguishable forms of the maize endosperm and the potato tuber. Here, we switched regions of maize endosperm and the potato tuber AGPase and monitored alterations in allosteric properties. Changes in both subunits have substantial effects on allosteric properties. For example, replacement of the potato large subunit with that from maize effectively abolishes 3-PGA activation of the potato tuber AGPase and increases the Ka for 3-PGA by a factor of 5. Substitution of the C terminus of the potato small subunit for the maize counterpart decreases 3-PGA activation of the maize endosperm AGPase by 70% and increases the Ka for 3-PGA by an order of magnitude. Because of the conserved nature of the small subunit, swapping of the small subunit C termini involves substitutions of only 27 amino acids. These data are summarized in Figure 4.
Figure 4.
Regulatory sites within each subunit. Exon 1 and junction regions (double vertical bars) have been placed on the rectangles representing each subunit. Numbers underneath the rectangles identify the beginning and end of exon 1 as well as the junction regions. Numbers correspond to amino acid positions in the maize endosperm AGPase. The regions of previously reported mutations that modify regulatory properties were derived from the data of Giroux et al. (1996), Greene et al. (1996a, 1996b, 1998), Laughlin et al. (1998), Kavakli et al. (2001), and Salamone et al. (2002). Information from this study has been placed beneath the rectangles representing each subunit. For each parameter, i.e. phosphate inhibition, 3-PGA activation fold, and 3-PGA affinity, the symbols are the following: ↔, region of the subunit delineated; +, region conditioning differences between potato tuber and maize endosperm AGPase; −, region not conditioning differences between potato tuber and maize endosperm AGPase.
The data presented show that there are multiple important sites as well as interactions occurring among the various motifs for each of the three traits studied (Ka for 3-PGA, degree of activation by 3-PGA, and degree of phosphate inhibition). The large differences seen in 3-PGA activation and phosphate inhibition are controlled to a great extent by the C terminus of the small subunit apparently interacting with the N terminus of the large subunit. The N terminus of the small subunit, in conjunction with the C terminus of the large subunit, greatly modulates3-PGA activation. Also, differences distinguishing maize endosperm and potato tuber in the N termini of both subunits are important for 3-PGA affinity but not those in the C terminus of the small subunit.
It is also interesting to note that some of the mosaic AGPases described above exhibit a Ka for 3-PGA, degree of 3-PGA activation, or extent of phosphate inhibition that falls outside the range defined by the two parental AGPases. These variants likely may have agricultural importance in light of the role played by the enzyme's allosteric properties in controlling the amount of starch (Stark et al., 1992; Giroux et al., 1996) in starch-rich plant organs. These are under test.
The experiments presented here were designed and initiated in part to test a hypothesis arising from two important but unrelated facts concerning plant AGPases: (1) the small subunits of plant AGPases are, in general, much more conserved compared to the large subunits and (2) the enzymes from different plant organs exhibit dramatic differences in allosteric properties. One reasonable explanation for these two facts is the idea that the small subunit is catalytic, whereas the large subunit is allosteric. This hypothesis and its rationale have been reviewed in detail elsewhere (Smith-White and Preiss, 1992; Hannah, 1997; Ballicora et al., 2003).
In contrast to the prediction of the hypothesis above, resultant allosteric data from mosaic subunits derived from these two isoforms of AGPase show that both the small and the large subunits participate equally in controlling the regulatory properties of AGPase.
Of relevance to the hypothesis above, we note the recent data from expression of the Arabidopsis small and large subunits in E. coli. Crevillen et al. (2003) expressed the lone Arabidopsis small subunit with the various Arabidopsis large subunits. Resultant constructs exhibited different substrate affinities, implying a catalytic role for the large subunit.
We also note expression of either small or large AGPase subunits from potato tuber or maize endosperm in E. coli gave rise to detectable AGPase activity (Burger et al., 2003). Comparable activity levels, equaling only a small percentage of wild-type heterotetrameric activity, were detected when each of the four subunits was expressed separately in E. coli. Taken together, the combined data clearly show that both subunits are important for catalysis and for regulation.
Finally, we note that the structure of the tetrameric TDP-Glc pyrophosphorylase, an enzyme with significant sequence similarity to AGPase, was solved recently (Blankenfeldt et al., 2000). This enzyme contains four catalytic sites.
Since there is not a simple delineation of function distinguishing the small and large subunits of AGPase, the discrepancy in the rate of sequence divergence exhibited by the two plant AGPase genes deserves attention. Two facts are relevant.
First, regions of the small subunit are as variable as the large subunit. Amino acids encoded by exon 1 of the small subunit are more variable than those of the large subunit (Hannah et al., 2001). Interestingly, none of the bacterial AGPases contain sequences corresponding to the plant exon 1 sequences, and of the several structurally solved pyrophosphorylase domains (Brown et al., 1999; Blankenfeldt et al., 2000; Sulzenbacher et al., 2001; Zuccotti et al., 2001), sequence alignments show that the first residues of the pyrophosphorylase domains align with exon 2 of AGPase. Furthermore, a Cys residue involved in redox regulation of AGPase activity is encoded by exon 1 (Fu et al., 1998; Tiessen et al., 2002). This Cys is present in the potato tuber small subunit but is not found in the maize endosperm small subunit or in some other small subunits. Hence, exon 1 represents a viable candidate for determining the allosteric properties unique to plant AGPases. In view of this, attempts were made to examine mosaics containing exchanged exon 1 sequences. Resulting small subunit mosaics exhibited high levels of activity when expressed alone, negating analysis of heterotetrameric activities in these small subunit/large subunit combinations. These subunits are currently under further investigation.
Secondly, inherent in the argument of different roles for the two subunits is the assumption that both genes are equally subject to mutation, and the differences in sequence variation exhibited by the two subunits are caused by different evolutionary selection pressures on the proteins. An examination of the data shows that this assumption may not be correct. Sequences of 33 Sh2 alleles and 33 Bt2 alleles isolated from diverse maize inbreds and landraces and recently deposited in GenBank were examined. Consistent with the more conserved nature of the small subunit, three amino acid changes were found in the 1,693 sequenced coding bases of the 33 small subunit Bt2 alleles, while eight amino acid changes were found in the 1,551 bases of coding information in the 33 Sh2 alleles.
Significantly, an even larger bias was found in intron changes: 137 mutations were found in the 4,356 bases of sequenced Sh2 introns, but only 30 alterations occurred in the 4,319 bases of sequenced Bt2 introns. Intron sequences obviously have no bearing on the structure of the protein encoded by the gene. That intron sequences exhibit the same pattern of differential sequence divergence as noted in the coding regions has a number of explanations. Since there are now ample examples of genes with differing intrinsic mutation rates, we cannot exclude the possibility that the differential rate of divergence exhibited by genes for the large and small subunits simply reflects differing mutation rates of the two genes.
Finally, we note that while the approach used in these studies is powerful in identifying and mapping extant variation among different AGPases, conserved regions important in regulatory properties are not resolved by this methodology. For instance, whereas the C terminus of the large subunit was shown by mutagenic studies to be involved in phosphate inhibition, our data did not identify this region as important for this parameter. The approach used here and resultant data complement more conventional mutagenic studies reported previously. Taken together, data from mutagenic approaches and this approach can identify regions important in various parameters but that are evolutionarily conserved across the various AGPases. This is especially important in regions exhibiting significant sequence dissimilarity.
MATERIAL AND METHODS
Escherichia coli Strains and Plasmids
Mosaics were constructed from the plasmids pMONcSh2-377.1, pMONcBt2-375.6 (Giroux et al., 1996), pML10 (Ballicora et al., 1995), and pMON17336 (Iglesias et al., 1993) containing the genes for Sh2, Bt2, the potato (Solanum tuberosum) tuber complete small subunit, and the potato tuber large subunit, respectively. Cloning was performed in Escherichia coli strain DH5α, while enzyme expression was carried out in the AGPase-deficient E. coli strain AC70R1-504.
Construction of Mosaics
The mosaics are described in Figure 1. Two PCR reactions were performed on each small subunit plasmid, pMONcBt2-375.6 and pML10, to create mosaics MPss and PMss. One reaction amplified the vector and half of the AGPase gene, while the other reaction amplified the second half of the gene. The resulting products were then blunt-end ligated to form the mosaic plasmids. Primers used were CAG TTC GAT GTA ACC CAC TCG, P GAT CTC AAC AGC GGT AAG ATC C in addition to P AAA CGT GCA ACT GCA TTT GGC CTC, and CTC GTC CAT TGG CAG TGC GGC AAC for PMss, and P AAG CGT GCC ACT GCA TTT GGT CTC and CTC ATC CAT CGG TAG GGC AGC AAC for MPss. The same method was used to synthesize PMls with the primers CCA GCC CAA AAT CTG ATG CT, P TGA AGA TTG ATC ATA CTG GA, P ATC CCC GGG TAC CGA GCT, and TCT CCC TAT AGT GAG TCG TA, and MPls with the primers CTA GCC CAT TTT TAG AAG CT, P TCA AGA TTG ACA GCA GAG GC, P ATC CCC GGG TAC CGA GCT, and TCT CCC TAT AGT GAG TCG TA. Reaction conditions were the following: a heating step of 4 min at 94°C, followed by 25 cycles of 1 min denaturing at 94°C, 1 min annealing at 55°C, and 1 min/kb extension at 72°C. Amplification was performed with Vent DNA polymerase (New England Biolabs, Beverly, MA). Resulting clones were sequenced to verify proper junctions.
Enzyme Expression and Purification
E. coli AC70R1-504 with the constructs of interest was inoculated into 10 mL of Luria-Bertani broth (LB) plus spectinomycin (75 μg/mL) and kanamycin (50 μg/mL) and grown overnight at 37°C. The culture was then transferred to 1 L of LB plus spectinomycin (75 μg/mL) and kanamycin (50 μg/mL) and grown at 37°C until an optical density at 600 nm of 0.5 to 0.6 was reached. Induction was then performed for 6.5 h at room temperature with the addition of 0.2 mm isopropyl β-d-thiogalactoside and 0.02 mg/mL of nalixidic acid. After induction, cells were pelleted and resuspended in extraction buffer (200 mm KCl, 50 mm HEPES, pH 7.5, 10 mm MgCl2, 5 mm EDTA, 30% ammonium sulfate, 20% Suc, and freshly added 1 mm dithiothreitol, 50 μg/mL of lysozyme, 1 μg/mL of pepstatin, 1 μg/mL of leupeptin,10 μg/mL of chymostatin, 1 mm phenylmethylsulfonyl fluoride, and 1 mm benzamidine). Cells were then sonicated to extract protein. The extract was filtered to 0.45 μm. For assaying crude extracts, the same induction and extraction procedures were used on a final LB volume of 100 mL.
After filtration, the extract was loaded onto a Pharmacia HR 10/10 column packed with a Pharmacia Fractogel EMD C3 aminopropyl medium (Piscataway, NJ). Step gradients of 1 m, 0.8 m, 0.6 m, 0.4 m, 0.2 m, and 0 m ammonium sulfate were performed at 3 mL/min by combining different proportions of buffer A (50 mm HEPES, pH 7.5, 5 mm EDTA, 10 mm magnesium chloride, 20% Suc, 1 m ammonium sulfate, and freshly added 1 mm dithiothreitol) and buffer B (buffer A without ammonium sulfate). One fraction per gradient was collected. Each fraction was tested for activity in the direction of ATP synthesis as described previously (Greene et al., 1996b). The active fraction was concentrated before gel filtration chromatography. Gel filtration chromatography was performed at 0.5 mL/min on a prepacked HR 10/30 Pharmacia Superdex 200 column with equilibration buffer (50 mm HEPES, pH 7.5, 5 mm EDTA, 10 mm magnesium chloride, 200 mm potassium chloride, 5% Suc, and freshly added 1 mm dithiothreitol). Fractions of 250 μL were collected after elution of the void volume. Active fractions, as determined by the ATP synthesis assay, were pooled, supplemented with 2 mg of bovine serum albumin, concentrated to 0.6 mL, mixed with 0.4 mL of 100% glycerol, and stored in aliquots at −20°C. A subset of mosaic AGPases monitored on western blots did not show any signs of degradation (data not shown).
Kinetic Studies
Kinetic studies were performed in the direction of ADP-Glc synthesis as described previously (Dickinson and Preiss, 1969). Incorporation of [14C]Glc-1-P into ADP-Glc was measured. The reaction mixture contained 80 mm HEPES, pH 7.5, 1 mm dithiothreitol, 1 mm Glc-1-P, 4 mm MgCl2, 0.5 mg/mL of bovine serum albumin, 15,000 cpm of [14C]Glc-1-P and, where appropriate,3-PGA and/or inorganic phosphate. Reaction volume was 50 μL. Assays were initiated by addition of 1.5 mm ATP. Reaction mixtures were incubated for 30 min at 37°C and terminated by boiling for 2 min. Unincorporated Glc-1-P was cleaved by addition of 0.6 units of bacterial alkaline phosphatase (Worthington Biochemical, Lakewood, NJ) and incubation for 1 h at 37°C. A 30-μL aliquot of the reaction mixture was spotted on DEAE paper, washed with distilled water, dried, and quantified in a liquid scintillation counter. The Ka (concentration giving half-maximal activation) for 3-PGA was determined by nonlinear regression analysis with the Prism program version 3.00 for Windows 95 and NT (GraphPad Software, San Diego). The Michaelis and Menten equation [v = Vm × S/(Km + S)] in the Prism program was modified to v = Vmin + Vm × S/(Km + S) to account for significant activity levels in the absence of 3-PGA. The parameters for sigmoidal curves were determined by nonlinear regression analysis by use of the Prism program curve for a sigmoidal response with a variable slope. The equation was: y = Vmin + (Vmax − Vmin)/(1 + 10(logKa−logx) × Hillcoeff), where Vmin is the minimal velocity, Vmax is the maximal velocity, and Hillcoeff the Hill coefficient. The Hill coefficients determined by the Prism equation were checked for accuracy by Hill plots.
Acknowledgments
We thank Drs. William Gurley and Andrew Hanson for use of lab equipment, Drs. Paul Sehnke, Carla Linebarger, Sanja Roje, Philippe Ranocha, and Gilles Basset for technical help, Dr. Edward Buckler for interesting evolutionary discussions as well as placement of the Sh2 and Bt2 alleles in GenBank, and the Interdisciplinary Center for Biotechnology Research at the University of Florida for DNA sequencing.
This work was supported by the National Science Foundation (grant nos. IBN–9316887, IBN–960416, IBN–9982626, and MCB–9420422 to L.C.H.), the U.S. Department of Agriculture Competitive Grants Program (grant nos. 94–37300–453, 9500836, 95–37301–2080, 9701964, 97–36306–4461, 98–01006, and 2000–01488 to L.C.H.), the Florida Agricultural Experiment Station (Journal Series no.R–08819), and the U.S. Department of Energy (grant no. DEFG03–96ER20216 to T.W.O.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036699.
References
- Ball K, Preiss J (1994) Allosteric sites of the large subunit of the spinach leaf ADPglucose pyrophosphorylase. J Biol Chem 269: 24706–24711 [PubMed] [Google Scholar]
- Ballicora MA, Iglesias AA, Preiss J (2003) ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol Mol Biol Rev 67: 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballicora MA, Laughlin MJ, Fu Y, Okita TW, Barry GF, Preiss J (1995) Adenosine 5′-diphosphate-glucose pyrophosphorylase from potato tuber. Significance of the N terminus of the small subunit for catalytic properties and heat stability. Plant Physiol 109: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenfeldt W, Asuncion M, Lam JS, Naismith JH (2000) The structural basis of the catalytic mechanism and regulation of glucose-1-phosphate thymidylyltransferase (Rml). EMBO J 19: 6652–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Pompeo F, Dixon S, Mengin-Lecreulx D, Cambillau C, Bourne Y (1999) Crystal structure of the bifunctional N-acetylglucosamine1-phosphate uridyltransferase from Escherichia coli: a paradigm for the related pyrophosphorylase superfamily. EMBO J 18: 4096–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Johnson PE, Beckles DM, Fincher GB, Jenner HL, Naldrett MJ, Denyer K (2002) Characterization of the genes encoding the cytosolic and plastidial forms of ADP-glucose pyrophosphorylase in wheat endosperm. Plant Physiol 130: 1464–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger BT, Cross JM, Shaw JR, Caren JR, Greene TW, Okita TW, Hannah LC (2003) Relative turnover numbers of maize endosperm and potato tuber ADP-glucose pyrophosphorylases in the absence and presence of 3-PGA. Planta 217: 449–456 [DOI] [PubMed] [Google Scholar]
- Crevillen P, Ballicora MA, Merida A, Preiss J, Romero JM (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278: 28508–28515 [DOI] [PubMed] [Google Scholar]
- Denyer K, Dunlap F, Thorbjornsen T, Keeling P, Smith AM (1996) The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiol 112: 779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DB, Preiss J (1969) ADP glucose pyrophosphorylase from maize endosperm. Arch Biochem Biophys 130: 119–128 [DOI] [PubMed] [Google Scholar]
- Fu Y, Ballicora MA, Leykam JF, Preiss J (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273: 25045–25052 [DOI] [PubMed] [Google Scholar]
- Ghosh HP, Preiss J (1966) Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem 241: 4491–4504 [PubMed] [Google Scholar]
- Giroux MJ, Hannah LC (1994) ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol Gen Genet 243: 400–408 [DOI] [PubMed] [Google Scholar]
- Giroux MJ, Shaw J, Barry G, Cobb BG, Greene T, Okita T, Hannah LC (1996) A single mutation that increases maize seed weight. Proc Natl Acad Sci USA 93: 5824–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene TW, Chantler SE, Kahn ML, Barry GF, Preiss J, Okita TW (1996. b) Mutagenesis of the potato ADPglucose pyrophosphorylase and characterization of an allosteric mutant defective in 3-phosphoglycerate activation. Proc Natl Acad Sci USA 93: 1509–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene TW, Kavakli IH, Kahn ML, Okita TW (1998) Generation of up-regulated allosteric variants of potato ADP-glucose pyrophosphorylase by reversion genetics. Proc Natl Acad Sci USA 95: 10322–10327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene TW, Woodbury RL, Okita TW (1996. a) Aspartic acid 413 is important for the normal allosteric functioning of ADP-glucose pyrophosphorylase. Plant Physiol 112: 1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah LC (1997) Starch synthesis in the maize endosperm. In BA Larkins, IK Vasil, eds, Advances in Cellular and Molecular Biology of Plants. Cellular and Molecular Biology of Plant Seed Development, Vol 4. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 375–405
- Hannah LC, Shaw JR, Giroux MJ, Reyss A, Prioul JL, Bae JM, Lee JY (2001) Maize genes encoding the small subunit of ADP-glucose pyrophosphorylase. Plant Physiol 127: 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias AA, Barry GF, Meyer C, Bloksberg L, Nakata PA, Greene T, Laughlin MJ, Okita TW, Kishore GM, Preiss J (1993) Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem 268: 1081–1086 [PubMed] [Google Scholar]
- Johnson PE, Patron NJ, Bottrill AR, Dinges JR, Fahy BF, Parker ML, Waite DN, Denyer KA (2003) Low-starch barley mutant, riso 16, lacking the cytosolic small subunit of ADP-glucose pyrophosphorylase, reveals the importance of the cytosolic isoform and the identity of the plastidial small subunit. Plant Physiol 131: 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavakli IH, Park JS, Slattery CJ, Salamone PR, Frohlick J, Okita TW (2001) Analysis of allosteric effector binding sites of potato ADP-glucose pyrophosphorylase through reverse genetics. J Biol Chem 276: 40834–40840 [DOI] [PubMed] [Google Scholar]
- Kim W, Franceschi V, Okita T, Robinson N, Morell M, Preiss J (1989) Immunocytochemical localization of ADPglucose pyrophosphorylase in developing potato tuber cells. Plant Physiol 91: 217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski LA (1999) A phosphoglycerate to inorganic phosphate ratio is the major factor in controlling starch levels in chloroplasts via ADP-glucose pyrophosphorylase regulation. FEBS Lett 448: 153–156 [DOI] [PubMed] [Google Scholar]
- Kleczkowski LA, Villand P, Luthi E, Olsen OA, Preiss J (1993. b) Insensitivity of barley endosperm ADP-glucose pyrophosphorylase to 3- phosphoglycerate and orthophosphate regulation. Plant Physiol 101: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski LA, Villand P, Preiss J, Olsen OA (1993. a) Kinetic mechanism and regulation of ADP-glucose pyrophosphorylase from barley (Hordeum vulgare) leaves. J Biol Chem 268: 6228–6233 [PubMed] [Google Scholar]
- Laughlin MJ, Payne JW, Okita TW (1998) Substrate binding mutants of the higher plant ADP-glucose pyrophosphorylase. Phytochemistry 47: 621–629 [DOI] [PubMed] [Google Scholar]
- Morell M, Bloom M, Preiss J (1988) Affinity labeling of the allosteric activator site(s) of spinach leaf ADP-glucose pyrophosphorylase. J Biol Chem 263: 633–637 [PubMed] [Google Scholar]
- Okita TW, Greenberg E, Kuhn DN, Preiss J (1979) Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol 64: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone PR, Kavakli IH, Slattery CJ, Okita TW (2002) Directed molecular evolution of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 99: 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikka VK, Choi S, Kavakli IH, Sakulsingharoj C, Gupta S, Ito H, Okita TW (2001) Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase. Plant Sci 161: 461–468 [Google Scholar]
- Smidansky ED, Clancy M, Meyer FD, Lanning SP, Blake NK, Talbert LE, Giroux MJ (2002) Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc Natl Acad Sci USA 99: 1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidansky ED, Martin JM, Hannah LC, Fischer AM, Giroux MJ (2003) Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 6: 656–664 [DOI] [PubMed] [Google Scholar]
- Smith-White BJ, Preiss J (1992) Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources. J Mol Evol 34: 449–464 [DOI] [PubMed] [Google Scholar]
- Sowokinos J, Preiss J (1982) Pyrophosphorylases in Solanum tuberosum. III. Purification, physical, and catalytic properties of ADPglucose pyrophosphorylase in potatoes. Plant Physiol 69: 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D, Timmerman K, Barry G, Preiss J, Kishore G (1992) Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science 258: 287–291 [DOI] [PubMed] [Google Scholar]
- Sulzenbacher G, Gal L, Peneff C, Fassy F, Bourne Y (2001) Crystal structure of Streptococcus pneumoniae N-acetylglucosamine-1-phosphate uridyltransferase bound to acetyl-coenzyme A reveals a novel active site architecture. J Biol Chem 276: 11844–11851 [DOI] [PubMed] [Google Scholar]
- Thorbjornsen T, Villand P, Denyer K, Olsen O, Smith AM (1996) Distinct isoforms of ADP-glucose pyrophosphorylase occur inside and outside the amyloplast in barley endosperm. Plant J 10: 243–250 [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti S, Zanardi D, Rosano C, Sturla L, Tonetti M, Bolognesi M (2001) Kinetic and crystallographic analyses support a sequential-ordered bi bi catalytic mechanism for Escherichia coli glucose-1-phosphate thymidylyltransferase. J Mol Biol 313: 831–843 [DOI] [PubMed] [Google Scholar]