Abstract
The initial rate of Cu2+ movement across the thylakoid membrane of pea (Pisum sativum) chloroplasts was directly measured by stopped-flow spectrofluorometry using membranes loaded with the Cu2+-sensitive fluorophore Phen Green SK. Cu2+ transport was rapid, reaching completion within 0.5 s. The initial rate of uptake was dependent upon Cu2+ concentration and saturated at about 0.6 μm total Cu2+. Cu2+ uptake was maximal at a thylakoid lumen pH of 7.0. Cu2+ transport was inhibited by Zn2+ but was largely unaffected by Mn2+ and Cu+. Zn2+ inhibited Cu2+ transport to a maximum of 60%, indicating that there may be more than one transporter for copper in pea thylakoid membranes.
The transition metal, copper, an essential micronutrient in plants, is required as an enzyme cofactor for a number of physiological processes (Maksymiec, 1997; Rodriguez et al., 1999). Copper is required for mitochondrial electron transport, pathogen defense, cell wall lignification, vitamin C metabolism, ethylene perception, carbohydrate metabolism, nitrogen fixation, fatty acid desaturation/hydroxylation, and, in the chloroplast, for both electron transport (plastocyanin) and oxidative stress responses (Cu/Zn superoxide dismutase).
Copper can also be toxic at supraoptimal concentrations, leading to inhibition of leaf expansion, decrease in root mass, destruction of the thylakoid structure of chloroplasts, and considerable modification of the lipid and protein composition of thylakoid membranes (Maksymiec, 1997). The mechanisms of copper toxicity have not been fully elucidated but are thought to include the generation of oxygen and hydroxyl free radicals and the oxidation of dithiols in proteins to disulfides.
To maintain the concentration of essential metals within physiologically tolerable limits and to minimize their detrimental effects, all eukaryotes have evolved a number of mechanisms that control the uptake, accumulation, trafficking, and detoxification of metals. The main components of metal homeostasis are transport, chelation, and compartmentation.
Copper transport in plants likely involves members of the P-type ATPase and copper transporter (CTR) families and may involve members of the natural resistance-associated macrophage protein (NRAMP) and zinc IRT-like protein (ZIP)/iron-regulated transporter (IRT) transporter families. Copper transporting P-type ATPases were identified in the cytoplasmic and thylakoid membrane of the cyanobacterium Synechococcus (Kanamaru et al., 1994; Phung et al., 1994), and a related protein, PAA1, was shown to be involved in Cu2+ transport in chloroplasts of Pisum (Shikanai et al., 2003). The N terminus of PAA1 targets a fusion protein to the chloroplast, and deletion of PAA1 results in decreased chloroplast copper levels and reduced activity of Cu/Zn superoxide dismutase and plastocyanin. PAA1 is a member of the potential heavy metal transporting P-type ATPases found in Arabidopsis, for which a putative substrate has been identified (Baxter et al., 2003).
CTR family members are responsible for high-affinity copper transport in the plasma membrane and tonoplast of yeast (Saccharomyces cerevisiae; Lee et al., 2002; Puig and Thiele, 2002). Five CTR homologs (COPT1–COPT5) have been identified in Arabidopsis. All but COPT4 can complement the copper uptake deletion mutant of yeast (ctr1/ctr3) to some degree (Kampfenkel et al., 1995; Sancenon et al., 2003). The subcellular location of the plant CTR homologs has not been determined, but sequence analysis indicates that COPT1 may be targeted to the thylakoid membrane.
The NRAMP and ZIP/IRT families are thought to encode primarily iron and zinc transporters (Guerinot, 2000; Curie and Briat, 2003; Hell and Stephan, 2003), but the wide potential substrate range of family members indicates that they might also play a role in copper transport. Two Arabidopsis NRAMP genes complement both iron and manganese uptake mutants in yeast (Curie et al., 2000; Guerinot, 2000; Thomine et al., 2000), and expression of a mammalian NRAMP gene in frog oocytes confers iron, copper, zinc, cadmium, manganese, and cobalt transport equally (Gunshin et al., 1997). Similarly, expression in yeast of several ZIP/IRT genes rescues not only iron and zinc uptake mutants but also manganese uptake mutants, and the uptake can be inhibited by cadmium and copper, indicating these metals might also be transport substrates (Grotz et al., 1998; Korshunova et al., 1999; Ramesh et al., 2003).
Copper is required for proteins located within the thylakoid lumen, such as polyphenol oxidase and plastocyanin (Heldt et al., 1973; Merchant and Dreyfuss, 1998). This requirement and the fact that copper is added to apoplastocyanin in the lumen (Merchant and Dreyfuss, 1998) indicate that there must be a mechanism by which copper crosses the thylakoid membrane. While substantial progress has been made recently in the identification of genes encoding for Cu2+ transporters, little biochemical characterization in plants has been reported. In this study, we have loaded Phen Green SK (PGSK) into isolated chloroplast thylakoid membranes and utilized stopped-flow spectrofluorometry to measure Cu2+ transport rates across the thylakoid membranes.
RESULTS
Cu2+ Transport across Pea Thylakoid Membranes
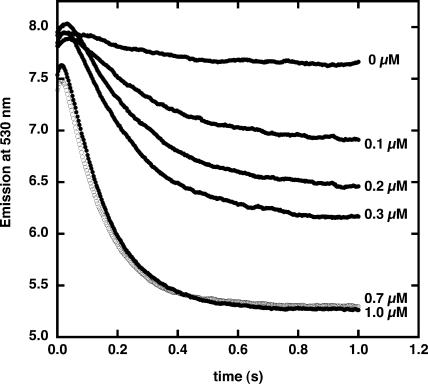
PGSK was entrapped within pea (Pisum sativum) thylakoid membranes by sonication. Sonication of thylakoid membranes produces membranes that are largely right-side-out in orientation, as evidenced by ATP synthesis (McCarty, 1968). Passing this preparation through a Sephadex G-50 column removed most of the external fluorophore, and addition of p-xylene bispyridinium dibromide (DPX) quenched the fluorescence of the fluorophore that was either bound to the outside of the membranes or free in solution. In a stopped-flow rapid mixing apparatus, the membranes were combined with buffer with no added Cu2+ and fluorescence emission at 530 nm monitored for 1.0 s with excitation at 506 nm (Fig. 1). There was a minimal drop in PGSK fluorescence over this time period. Addition of 0.1 μm Cu2+ resulted in an initial small increase in fluorescence, followed by a rapid quenching. The initial rise in fluorescence within the first 50 ms occurs in membrane-free solutions of PGSK mixed with Cu2+ and appears to be the result of an optical artifact after mixing of the solutions. At higher concentrations of Cu2+ added to PGSK-loaded thylakoid membranes, the rapid decrease was enhanced. The maximum decrease was achieved at about 0.7 μm. There was also a small offset in the starting fluorescence, presumably due to rapid Cu2+ quenching of PGSK fluorescence while mixing of the two solutions was occurring. The decrease in PGSK fluorescence is an indication that Cu2+ enters the thylakoid lumen and causes fluorescence quenching by binding to the fluorophore.
Figure 1.
Cu2+ quenching of Phen Green SK fluorescence in thylakoid membranes. Chloroplast thylakoid membranes were loaded with PGSK as described in “Materials and Methods” (pH 7.0). Membranes were mixed with varying concentrations of Cu2+ in external buffer (pH 7.0) as indicated. Fluorescence emission was monitored at 530 nm with excitation at 506 nm.
Phen Green SK Fluorescence Quenching by Cations
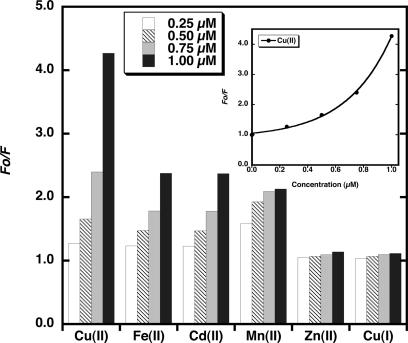
Additions of small aliquots of Cu2+, Cu+ Fe2+, Cd2+, Mn2+, and Zn2+ were made to PGSK in buffer A solution in a cuvette and the fluorescence emission measured. The peak of PGSK fluorescence emission depends on the buffer used (Shingles et al., 2001) and for buffer A was determined to be 530 nm. These data were used to construct a Stern-Volmer plot relating fluorescence changes to ion concentration (Fig. 2). The PGSK fluorescence was optimized relative to entrapped PGSK in thylakoid membranes so that data would be collected at a similar voltage. Stern-Volmer plots constructed at different PGSK concentrations all show a similar relationship for metal-dye interaction. The fluorescence data are shown as the ratio of the initial fluorescence, Fo (when no heavy metal ion is present), to the fluorescence measured after ion addition, F. The concentration dependence of quenching of PGSK fluorescence by Cu2+ is curvilinear (Fig. 2, inset). Approximation of the relation of PGSK fluorescence quenching to an exponential function allows for the calibration of fluorescence changes to the concentration of added ion. In this study, Cu2+ was a more effective quencher of PGSK fluorescence than previously reported (Shingles et al., 2001), indicating the importance of generating a standard curve for PGSK quenching for each lot of PGSK purchased.
Figure 2.
Quenching of Phen Green SK fluorescence by cations. The fluorescence emission was monitored at 530 nm with excitation at 506 nm of a solution of 3 μm PGSK in buffer A and was measured in the presence of varying concentrations of Cu2+, Fe2+, Cd2+, Mn2+, Zn2+, or Cu+. Inset, The line drawn through the data points for Cu2+ quenching of PGSK that fit the exponential equation described in “Materials and Methods” (R = 0.999).
Divalent Cation Transport across Chloroplast Thylakoid Membranes
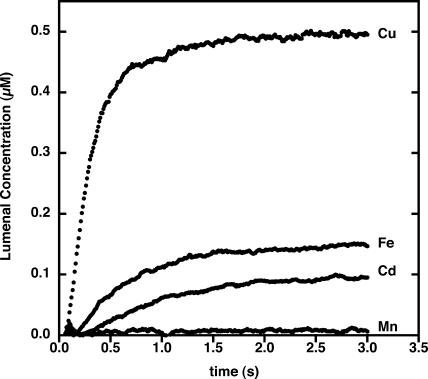
Several divalent cations at 1 μm concentration were tested for their ability to be taken up by thylakoid membranes loaded with PGSK. Utilizing the Stern-Volmer plots created for each cation, the lumenal concentration of each cation could be determined over time (Fig. 3). The final internal concentration of the cations was much lower than the concentration of cation added, indicating that the membranes and components of the incubation mixture buffer the ions. The product of the rate constant of the lumenal Cu2+ concentration change and the extent of the change can be used to calculate the initial rate of cation transport across these membranes. Cu2+ transport was the most rapid, with an initial rate of 1.4 μm/s. The internal volume of thylakoid membranes was determined to be about 3.3 μL/mg chlorophyll (Heldt et al., 1973). Assuming a protein to chlorophyll ratio of 4:1 for thylakoid membranes, the calculated initial rate of Cu2+ transport is approximately 70 pmol min−1 mg protein−1. By comparison, Fe2+, Cd2+, and Mn2+ were slowly transported across the thylakoid membranes, giving initial rates of transport of 5.0 and 2.0 pmol min−1 mg protein−1, respectively. Mn2+ transport was negligible.
Figure 3.
Transport of cations across thylakoid membranes loaded with PGSK. Thylakoid membranes were loaded with PGSK as described in “Materials and Methods.” Membranes were mixed with 1.0 μm cations in external buffer (pH 7.0) as indicated. Fluorescence emission was monitored at 530 nm with excitation at 506 nm.
Cu2+ Transport
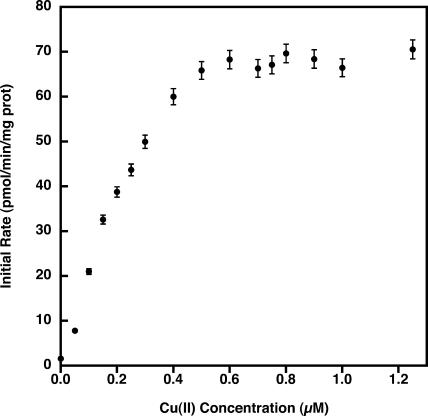
The determination of the initial rate of uptake as a function of Cu2+ concentration shows that Cu2+ uptake saturates at a concentration of approximately 0.6 μm added Cu2+ (Fig. 4). Cu2+ transport reached one-half of its maximal velocity at about 0.15 μm.
Figure 4.
Initial rates of Cu2+ transport across the thylakoid membrane. Lumenal Cu2+ concentration was determined as described in “Materials and Methods” at different concentrations of added Cu2+. Initial rates were determined from the equation describing a single exponential increase of lumenal Cu2+ concentration. Data shown are the mean ± sd of four pea thylakoid preparations with two to three replicates per experiment.
Low internal pH (5.0–6.0) resulted in the low transport rates, partly due to high background levels of PGSK quenching even in the absence of added Cu2+ (data not shown). The highest initial rates of Cu2+ transport were measured with an internal pH of 7.0. The effect of varying external pH on the initial rate of Cu2+ transport across thylakoid membranes showed that Cu2+ uptake was only slightly inhibited at an external pH of 5.0 or 8.0 (data not shown).
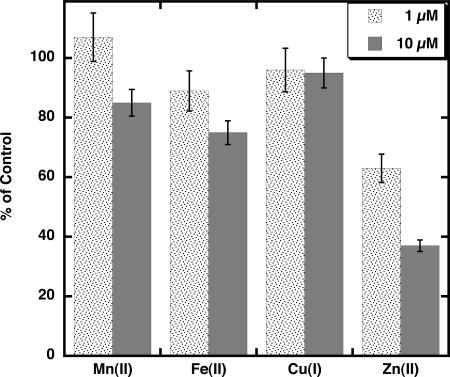
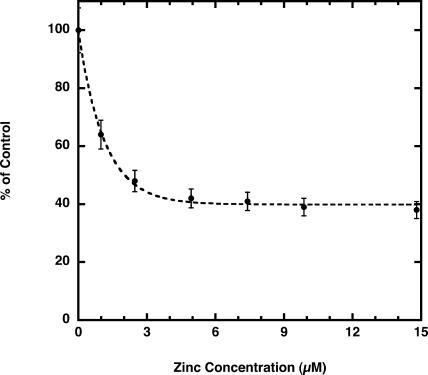
The effect of several metals on Cu2+ transport across thylakoid membranes was tested with Cu2+ at 1 μm. Equimolar Mn2+, which quenches PGSK fluorescence (Fig. 2) but shows negligible transport across thylakoid membranes in this study (Fig. 3), does not inhibit the initial rate of Cu2+ transport (Fig. 5). At 10 μm Mn2+, the initial rate of Cu2+ transport is inhibited by 10% to 20%. Fe2+, at 1 μm and 10 μm, inhibited the initial rate of Cu2+ transport by 15% and 25%, respectively. The inhibition is complicated by the fact that Fe2+ also quenches PGSK fluorescence (Fig. 2) and is itself transported across the thylakoid membranes (Fig. 3), although at a much slower rate than Cu2+. Cu+ does not quench PGSK fluorescence and does not inhibit Cu2+ transport across the thylakoid membranes (Fig. 5). Cu2+ transport is inhibited by Ag+ (35% at equimolar concentrations); however, Ag+ also quenches PGSK fluorescence. By contrast, Zn2+, which does not quench PGSK fluorescence (Fig. 2), strongly inhibits the initial rate of Cu2+ transport (Fig. 5). At 1 μm Zn2+, Cu2+ transport was inhibited by 35%, and at 10 μm Zn2+ transport was inhibited by 60%. Higher concentrations of Zn2+ resulted in little further inhibition of Cu2+ transport (Fig. 6), indicating a component of Cu2+ transport that may be insensitive to Zn2+. The Zn2+-insensitive component of Cu2+ transport is unaffected by the addition of 10 μm Mn2+ or Cd2+ (data not shown).
Figure 5.
Effect of cations on Cu2+ transport across thylakoid membranes. Membranes contained buffer at pH 7.0 inside and were mixed with external buffer at pH 7.0. The control rate of Cu2+ transport into PGSK-loaded membranes was established using 1 μm Cu2+ (74 pmol min−1 mg protein−1). Addition of all other cations was at 1, 5, and 10 μm as indicated. Lumenal Cu2+ concentration was determined as described in “Materials and Methods” at different concentrations of added Cu2+. Initial rates were determined from the equation describing a single exponential increase. Data shown are the mean ± sd (n = 3).
Figure 6.
Zinc inhibition of Cu2+ transport. Thylakoid membranes contained buffer A at pH 7.0 inside and were mixed with external buffer A at pH 7.0. Control rate of Cu2+ transport into PGSK-loaded membranes was established using 1 μm Cu2+ (76 pmol min−1 mg protein−1). Zn2+ was added at the concentrations indicated. Initial rates were determined from the equation describing a single exponential increase of lumenal Cu2+ concentration. Data shown are the mean ± sd (n = 3).
Chloroplast thylakoid membranes transport calcium (Ettinger et al., 1999). To determine if copper transport may use the calcium transporter, two inhibitors of calcium transport were utilized: lanthanum chloride and diltiazem (Roh et al., 1998). Neither of these inhibitors at 1 μm concentration affected Cu2+ transport across thylakoid membranes (data not shown).
DISCUSSION
Phen Green consists of a metal binding phenanthroline covalently attached to fluorescein. Phen Green SK was first used as a fluorescent probe to measure free iron levels in hepatocyte and human erythroleukemia K562 cells, which had been preloaded with the fluorophore (Petrat et al., 1999, 2000). We showed that PGSK entrapped in isolated vesicles could be used to measure the transport of ferrous iron across chloroplast inner envelope membranes (Shingles et al., 2001). Phen Green has also been used to measure copper transport in hepatopancreatic cells and mitochondria (Chavez-Crooker et al., 2001, 2002). The chloroplast thylakoid membrane is rich in chlorophyll and carotenoid. The wavelengths at which PGSK fluorescence is excited (506 nm) and emission measured (530 nm) avoid the problem of carotenoid and chlorophyll absorbance, making PGSK a suitable fluorophore to measure metal ion transport across this membrane. Using stopped-flow spectrofluorometry the kinetics of rapid ion transport can be followed on a millisecond timescale.
Cu2+ addition to chloroplast thylakoid membranes caused a rapid quenching of the fluorescence of entrapped PGSK, indicating transport of Cu2+ into the lumen. The rate of quenching of PGSK was dependent upon the concentration of added Cu2+ and saturated at approximately 0.6 μm (Fig. 4). This is within the same magnitude of the reported Km for Cu2+ uptake in Chlamydomonas reinhardtii (Hill et al., 1996). The reported ratio of PGSK to Fe2+ interaction is 3:1 (Petrat et al., 1999). If this is also the case for Cu2+, with 50 μm PGSK loaded inside the membranes, Cu2+ transport reaches completion before the probe is completely complexed.
Stern-Volmer plots of the quenching of PGSK fluorescence by Cu2+ are curvilinear at low concentrations (Fig. 2, inset). The fluorescence of PGSK is much more sensitive to quenching by Cu2+ than by Cu+, Cd2+, Mn2+, and Zn2+. The fluorescence of PGSK as it interacts with Cu2+ can be calibrated using a Stern-Volmer plot as described in “Materials and Methods.” The free Cu2+ concentration within the membranes can then be calculated. The final concentration of Cu2+ inside membranes, after equilibration, is less than the concentration of added Cu2+ (Fig. 3). This may be due to sequestration of Cu2+ by the membrane lipids and/or proteins as well as by components of the incubation mixture. Residual external PGSK may still bind Cu2+ through its phenanthroline component, even though its fluorescence is quenched by DPX (Shingles et al., 2002).
The presence of a Zn2+-insensitive component of Cu2+ transport (Fig. 6) seems to indicate two independent processes for Cu2+ transport across the thylakoid membranes. In addition, the fact that Cu2+ transport has saturation kinetics (Fig. 4) would suggest that transport proteins facilitate Cu2+ transport.
ZIP family members are high-affinity diffusion carriers that primarily transport zinc and iron. Cadmium and copper inhibit zinc uptake mediated by ZIP1, suggesting that these ions may also be substrates for this protein (Grotz et al., 1998). In fact, the ZIP/IRT family may have wide selectivity for cation transport, with Cu2+, Cd2+, Fe2+, Mn2+, and Zn2+ all acting as possible substrates (Grotz et al., 1998; Korshunova et al., 1999; Ramesh et al., 2003). The ZIP/IRT genes have not yet been expressed in a functional fashion to identify the ion specificity of the proteins, nor has cellular localization been confirmed for all members of this family.
The CTR, NRAMP, and HMA gene families may encode potential chloroplast thylakoid membrane copper transport proteins. Members of the CTR family act as homomultimers to transport copper in an energy-independent manner that is stimulated by acidic pH (Lee et al., 2002). Yeast cells have plasma membrane metal ion reductase activity (Hassett and Kosman, 1995), and expression of CTR transporters in yeast would indicate that copper uptake occurs as Cu+ (Sancenon et al., 2003). However, expression of a human CTR in Hek293 cells and addition of 64CuCl2 resulted in significant levels of Cu2+ transport in the absence of an external reductant to reduce Cu2+ to Cu+ (Lee et al., 2002), suggesting that Cu2+ and Cu+ are transported. Copper transport by CTR family members shows little inhibition by Fe2+, Zn2+, or Cd2+ (Lee et al., 2002; Sancenon et al., 2003). COPT1 from Arabidopsis was identified as being most likely involved in copper transport (Kampfenkel et al., 1995). While initially thought to be localized to the plasma membrane, sequence analysis using ARAMEMNON (Schwacke et al., 2003) indicates that COPT1 may be targeted to the chloroplast and quite possibly thylakoid targeted (score = 0.944), as predicted by PSORT (Nakai and Kanehisa, 1991).
The Cu2+ transport measured in thylakoid membranes is generally consistent with the mechanism of action of ZIP/IRT and/or CTR transporters. Transport activity is concentration driven and energy independent. Both Cu2+ and Fe2+ are transported across thylakoid membranes (Fig. 3), consistent with the divalent cation transport activity of ZIP/IRT transporters. As generally expected of ZIP/IRT transporters, Cu2+ transport is inhibited by Zn2+ (Fig. 6). However, the initial rate of Cu2+ transport is inhibited to a maximum of 60%, even at high concentrations of Zn2+, suggesting the possibility of more than one type of Cu2+ transporter in the thylakoid membrane. Copper transport across the chloroplast thylakoid membrane is slightly inhibited by Fe2+ and Mn2+ (Fig. 5) but is relatively insensitive to Cd2+ (data not shown), consistent with the activity of the Arabidopsis (COPT1) CTR transporter. Furthermore, COPT1 Cu2+ transport activity is insensitive to high Zn2+ concentrations (Sancenon et al., 2003), as is at least 40% of the Cu2+ transport activity measured in thylakoid membranes (Fig. 6). However, acidic pH did not stimulate transport as it does for a human CTR transporter (Lee et al., 2002), and Cu+ does not inhibit Cu2+ transport (Fig. 5).
From primary sequence analysis, it was suggested that NRAMP1 from Arabidopsis may be plastid localized (Curie et al., 2000), and our sequence analysis of NRAMP6 using ARAMEMNON (Schwacke et al., 2003) indicates that this protein may be localized to the chloroplast, and PSORT (Nakai and Kanehisa, 1991) predicts thylakoid targeting (score = 0.964). However, members of the NRAMP family show pH and proton-coupled activity dependent upon membrane potential (Gunshin et al., 1997). Cu2+ transport activity across the thylakoid membranes appears to be unaffected by a pH gradient, indicating a lack of proton-linked activity. In addition, we would not expect there to be much of a potential gradient effect due to the presence of a chloride channel in this membrane (Schonknecht et al., 1988).
Copper chaperones appear to be involved in delivering copper to metal pumps. CCH copper chaperones have been identified in Arabidopsis (Himelblau et al., 1998), with four genes encoding proteins predicted to be localized to plastids (Wintz and Vulpe, 2002). The experiments performed in this study were conducted in the absence of any added ATP and copper chaperones, thus making it improbable that this protein is a candidate for the Cu2+ transport activity we see in these thylakoid membrane preparations.
Cu2+ transport in lobster hepatopancreatic mitochondria was shown to occur through both Ca2+ uniporters and H+/Ca2+ antiporters (Chavez-Crooker et al., 2002). Calcium was also reported to move across the pea thylakoid membranes by a proton antiporter (Ettinger et al., 1999). Cu2+ transport in thylakoids was not inhibited by addition of carbonyl cyanide m-chlorophenylhydrazone, a protonophore, confirming the lack of proton-linked effects. Inhibitors of Ca2+ uniport and antiport activity, diltiazem, LaCl3, and ruthenium red, had little effect on Cu2+ transport, indicating that transport proteins distinct from Ca2+ uniport or antiport mechanisms very likely mediate Cu2+ transport in thylakoids.
In cells, copper and other metal ions are needed to activate and stabilize enzymes such as superoxide dismutase, metalloproteases, cytochrome oxidase, protein kinases, and transcriptional factors. High metal concentrations, however, disrupt cellular processes and may lead to cell death. Homeostasis of copper levels in plant cells and subcellular compartments requires coordination between copper-binding proteins, copper chaperones, and copper transporters. Therefore, Cu2+ transporters may also play an important role in copper homeostasis by moving copper to subcellular compartments such as the thylakoid lumen, where copper will be incorporated into proteins or perhaps sequestered, preventing harmful effects at high concentrations. The measurement of free Cu2+ transport performed in this study reflects the capacity of copper transport across the thylakoid lumen and the possible presence of at least two copper transporters in this membrane.
MATERIALS AND METHODS
Reagents
Phen Green SK and DPX were purchased from Molecular Probes (Eugene, OR). Cuprous chloride dihydrate, copper (I) chloride, cadmium chloride anhydrous, manganese chloride tetrahydrate, and zinc chloride heptahydrate were purchased from Sigma (St. Louis). Pyrithione was purchased from Aldrich (Milwaukee, WI). Stock solutions of buffer components were made with reverse osmosis-treated and deionized water passed through a column containing Chelex-100 (Bio-Rad Laboratories, Hercules, CA) to reduce metal ion content.
Plant Material
Pea plants (Pisum sativum L. cv Laxton's Progress No. 9) were grown from seed for 16 to 18 d in vermiculite in a controlled environment growth cabinet (Revco, Asheville, NC) set for 16-h-day (24°C)/8-h-night (20°C) periods using standard fluorescent lighting.
Thylakoid Membrane Isolation
Ten grams of leaf tissue were homogenized in 70 mL of STN (0.4 m Suc, 10 mm Tris-HCl, pH 8.0, and 10 mm NaCl), the homogenate filtered through 100 μm Nitex cloth, and the filtrate centrifuged at 6,000g for 5 min at 4°C. The pellet was resuspended in 35 mL of STN and centrifuged at 6,000g for 5 min at 4°C. The resulting pellet was resuspended in 5 mL of thylakoid resuspension buffer (0.2 m Suc, 10 mm HEPES-KOH, pH 7.0, 50 mm KCl, and 5 mm MgCl2). Chlorophyll concentration was determined and the resuspension adjusted to 0.1 mg/mL. One milliliter of the thylakoid suspension was added to 1.0 mL of thylakoid resuspension buffer and 1.0 mL of 150 μm PGSK and 0.1 mL of 150 mm 2,2′-dipyridyl. The suspension was sonicated four times for 5 s for 20 s total in a Fisher Scientific 60 sonic dismembrator (8-W power output; Springfield, NJ). Disruption of the thylakoid membranes and subsequent resealing entrapped some of the PGSK within thylakoid membranes. The membrane preparation was then passed through a 1.6 × 10-cm Sephadex G-50 column equilibrated with buffer A (0.1 m Suc, 10 mm HEPES-KOH, pH 7.0, 50 mm KCl, and 5 mm MgCl2) at 4°C to remove external PGSK, and the eluant diluted to 25 mL with the same buffer.
Stopped-Flow Spectrofluorometric Assay of Copper Transport in Membranes
Fluorescence measurements were collected with an OLIS-modified SLM-SPF-500C spectrofluorometer equipped with an OLIS USA-SF stopped-flow apparatus (Bogart, GA). For thylakoid membrane preparations, chamber A contained 2.0 mL of membrane suspension in buffer A at pH 7.0 plus 5 mm DPX to quench the fluorescence of residual external PGSK. Chamber B contained various concentrations of copper and/or other divalent cations in 2.0 mL of buffer A. A nitrogen-driven piston at 80 psi achieved mixing of 0.35-mL solutions from chambers A and B. Fluorescence was followed at an emission wavelength of 530 nm with excitation at 506 nm. Slit widths were set at 10 nm with a cutoff filter (LP510; Oriel, Stamford, CT) placed over the entrance to the emission monochromator. All measurements were taken at 25°C.
Standard Curve for Quenching of Phen Green
To a stirred PGSK solution (3 μm) in buffer A, small aliquots of metal ions were added to the cuvette and fluorescence emission measured between 510 nm to 540 nm with excitation at 506 nm. The fluorescence at the peak emission (530 nm) was used to determine the Fo/F ratio and plotted against copper concentration, where Fo is the fluorescence measured in the absence of metal, and F is the fluorescence measured in the presence of metal.
Data Reduction and Handling
Curve fitting was carried out using the graphing program Kaleidagraph (Synergy Software, Reading, PA). Stern-Volmer data were fit to the exponential equation y = a + b × exp(c × x); a = offset; b = extent; c = constant. The lumenal concentration of added ion was determined using the exponential equation as a standard curve. The rates of Cu2+ flux were calculated from the lumenal ion concentration change over the first 1 s of data collected and fit to the equation describing a single exponential rise y = p × (1 − (exp(−q × x))); P = extent; q = rate constant. All fits used R values greater than 0.98.
Assays
Chlorophyll was determined as described in Arnon (1949).
Acknowledgments
We would like to thank Lynn (Hoji) Scott for her technical assistance in planting and nurturing of pea plants and Dr. Douglas Fambrough for use of the probe sonicator.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.037895.
References
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I, Tchieu J, Sussman MR, Boutry M, Palmgren MG, Gribskov M, Harper JF, Axelsen KB (2003) Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol 132: 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Crooker P, Garrido N, Ahearn GA (2001) Copper transport by lobster hepatopancreatic epithelial cells separated by centrifugal elutriation: measurements with the fluorescent dye Phen Green. J Exp Biol 204: 1433–1444 [DOI] [PubMed] [Google Scholar]
- Chavez-Crooker P, Garrido N, Ahearn GA (2002) Copper transport by lobster (Homarus americanus) hepatopancreatic mitochondria. J Exp Biol 205: 405–413 [DOI] [PubMed] [Google Scholar]
- Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347: 749–755 [PMC free article] [PubMed] [Google Scholar]
- Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119: 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA 95: 7220–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465: 190–198 [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488 [DOI] [PubMed] [Google Scholar]
- Hassett R, Kosman DJ (1995) Evidence for Cu (II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem 270: 128–134 [DOI] [PubMed] [Google Scholar]
- Heldt HW, Werdan K, Milovanc M, Geller G (1973) Alkalization of chloroplast stroma caused by light-dependent proton flux into thylakoid space. Biochim Biophys Acta 314: 224–241 [DOI] [PubMed] [Google Scholar]
- Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216: 541–551 [DOI] [PubMed] [Google Scholar]
- Hill KL, Hassett R, Kosman D, Merchant S (1996) Regulated copper uptake in Chlamydomonas reinhardtii in response to copper availability. Plant Physiol 112: 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau E, Mira H, Lin SJ, Culotta VC, Penarrubia L, Amasino RM (1998) Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol 117: 1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Vanmontagu M (1995) Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homolog. J Biol Chem 270: 28479–28486 [DOI] [PubMed] [Google Scholar]
- Kanamaru K, Kashiwagi S, Mizuno T (1994) A copper-transporting P-type ATPase found in the thylakoid membrane of the cyanobacterium Synechococcus species Pcc7942. Mol Microbiol 13: 369–377 [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40: 37–44 [DOI] [PubMed] [Google Scholar]
- Lee J, Pena MMO, Nose Y, Thiele DJ (2002) Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277: 4380–4387 [DOI] [PubMed] [Google Scholar]
- Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34: 321–342 [Google Scholar]
- McCarty RE (1968) Relation of photophosphorylation to hydrogen ion transport. Biochim Biophys Acta 32: 37–43 [DOI] [PubMed] [Google Scholar]
- Merchant S, Dreyfuss BW (1998) Posttranslational assembly of photosynthetic metalloproteins. Annu Rev Plant Physiol Plant Mol Biol 49: 25–51 [DOI] [PubMed] [Google Scholar]
- Nakai K, Kanehisa M (1991) Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 11: 95–110 [DOI] [PubMed] [Google Scholar]
- Petrat F, de Groot H, Rauen U (2000) Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch Biochem Biophys 376: 74–81 [DOI] [PubMed] [Google Scholar]
- Petrat F, Rauen U, de Groot H (1999) Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology 29: 1171–1179 [DOI] [PubMed] [Google Scholar]
- Phung LT, Ajlani G, Haselkorn R (1994) P-type ATPase from the cyanobacterium Synechococcus-7942 related to the human Menkes and Wilson disease gene products. Proc Natl Acad Sci USA 91: 9651–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6: 171–180 [DOI] [PubMed] [Google Scholar]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP (2003) Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol 133: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283: 996–998 [DOI] [PubMed] [Google Scholar]
- Roh MH, Shingles R, Cleveland MJ, McCarty RE (1998) Direct measurement of calcium transport across chloroplast inner envelope vesicles. Plant Physiol 118: 1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancenon V, Puig S, Mira H, Thiele DJ, Penarrubia L (2003) Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol 51: 577–587 [DOI] [PubMed] [Google Scholar]
- Schonknecht G, Hedrich R, Junge W, Raschke K (1988) A voltage-dependent chloride channel in the photosynthetic membrane of a higher plant. Nature 336: 589–592 [Google Scholar]
- Schwacke R, Schneider A, van der Graff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flugge UI, Kunze R (2003) ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol 131: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Muller-Moule P, Munekage Y, Niyogi KK, Pilon M (2003) PAA1, a P-type ATPase of Arabidopsis, functions in copper transport in chloroplasts. Plant Cell 15: 1333–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingles R, North M, McCarty RE (2001) Direct measurement of ferrous ion transport across membranes using a sensitive fluorometric assay. Anal Biochem 296: 106–113 [DOI] [PubMed] [Google Scholar]
- Shingles R, North M, McCarty RE (2002) Ferrous ion transport across chloroplast inner envelope membranes. Plant Physiol 128: 1022–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomine S, Wang RC, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA 97: 4991–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintz H, Vulpe C (2002) Plant copper chaperones. Biochem Soc Trans 30: 732–735 [DOI] [PubMed] [Google Scholar]