Abstract
Apolipoprotein A-I (apoA-I) mimetic peptides are currently being developed as possible new agents for the treatment of cardiovascular disease based on their ability to promote cholesterol efflux and their other beneficial antiatherogenic properties. Many of these peptides, however, have been reported to cause transient hypertriglyceridemia due to inhibition of lipolysis by lipoprotein lipase (LPL). We describe a novel bihelical amphipathic peptide (C-II-a) that contains an amphipathic helix (18A) for binding to lipoproteins and stimulating cholesterol efflux as well as a motif based on the last helix of apolipoprotein C-II (apoC-II) that activates lipolysis by LPL. The C-II-a peptide promoted cholesterol efflux from ATP-binding cassette transporter ABCA1-transfected BHK cells similar to apoA-I mimetic peptides. Furthermore, it was shown in vitro to be comparable to the full-length apoC-II protein in activating lipolysis by LPL. When added to serum from a patient with apoC-II deficiency, it restored normal levels of LPL-induced lipolysis and also enhanced lipolysis in serum from patients with type IV and V hypertriglyceridemia. Intravenous injection of C-II-a (30 mg/kg) in apolipoprotein E–knockout mice resulted in a significant reduction of plasma cholesterol and triglycerides of 38 ± 6% and 85 ± 7%, respectively, at 4 hours. When coinjected with the 5A peptide (60 mg/kg), the C-II-a (30 mg/kg) peptide was found to completely block the hypertriglyceridemic effect of the 5A peptide in C57Bl/6 mice. In summary, C-II-a is a novel peptide based on apoC-II, which promotes cholesterol efflux and lipolysis and may therefore be useful for the treatment of apoC-II deficiency and other forms of hypertriglyceridemia.
Introduction
Apolipoprotein A-I (apoA-I) mimetic peptides are short amphipathic helical peptides that are designed to mimic the biologic properties of apoA-I (Sethi et al., 2007; Bielicki et al., 2010; Navab et al., 2010; Gordon and Davidson, 2012; Leman et al., 2014). In particular, these peptides have been designed to promote cholesterol efflux from cells by the ATP-binding cassette transporter ABCA1 as well as by other mechanisms. Several of these peptides are being developed as possible therapeutic agents for the rapid stabilization of acute coronary syndrome (ACS) patients based on promising clinical trials of full-length apoA-I associated with phospholipids, which can rapidly reduce plaque size and inflammation (Krause and Remaley, 2013). ApoA-I mimetic peptides may have advantages over full-length apoA-I as a therapy based on their low cost of production, safety, and route of administration (Sethi et al., 2007; Remaley et al., 2008).
One possible limitation of apoA-I mimetic peptides as well as full-length apoA-I is that they have been reported to transiently cause hypertriglyceridemia (Nanjee et al., 1996, 1999; Shaw et al., 2008; Carballo-Jane et al., 2010;). One proposed explanation for this phenomenon is their ability to displace apolipoprotein C-II (apoC-II), a known activator of lipoprotein lipase (LPL) (Kei et al., 2012), or they may, like apolipoprotein C-III (apoC-III), cause direct inhibition of LPL (Wang et al., 1985; Shachter et al., 1994; Jong et al., 1999; Ooi et al., 2008). Besides apoA-I, overexpression of several different apolipoproteins have been shown to cause hypertriglyceridemia (Nanjee et al., 1996; Melegh et al., 2012; Kersten, 2014). In contrast, apoC-II is known to be one of the main physiologic activators of LPL through the ability of its C-terminal helix to guide lipoproteins to the active site of LPL (Musliner et al., 1979; Zdunek et al., 2003). In particular, amino acid residues 63, 66, 69, and 70 in the last helix of apoC-II have been identified as being critical for LPL activation (Shen et al., 2002). Although peptides based on just the last helix of apoC-II can activate LPL when artificial lipid emulsion substrates are used, they are relatively inactive when very low density lipoprotein (VLDL) or chylomicrons are used as a substrate because of their poor lipoprotein binding affinity (Olivecrona and Beisiegel, 1997). Besides the last helix, apoC-II contains two other amphipathic helices, which facilitate its binding to lipoproteins (Zdunek et al., 2003).
Hypertriglyceridemia is typically defined as triglycerides (TGs) over 200 mg/dl (Berglund et al., 2012) and can be the result of several rare primary genetic defects but is more commonly due to secondary hypertriglyceridemia caused by obesity, diabetes mellitus, pregnancy, alcohol, and a wide variety of drugs (Dominguez-Munoz et al., 1991; Anderson et al., 2009). Triglycerides are enriched in chylomicrons and VLDL, and lipolysis by LPL is a critical step in the catabolism of these TG-rich lipoprotein particles. Rarely, hypertriglyceridemia can be due to genetic defects in LPL or apoC-II, and these patients are at risk for acute pancreatitis from their high triglycerides (Viljoen and Wierzbicki, 2012). Fibrates and fish oils are the main treatment of hypertriglyceridemia (Berglund et al., 2012), but treatment options for rapidly lowering triglycerides in patients with acute pancreatitis are limited and include plasmapheresis and intravenous infusion of heparin and/or insulin, which induces gene expression of LPL (Tsuang et al., 2009).
In the present study, we describe a novel bihelical mimetic peptide named C-II-a, which is based on the apoC-II protein. The first two helices of apoC-II are replaced with a synthetic amphipathic peptide called 18A (Anantharamaiah et al., 1985), which has a high affinity for binding to lipoproteins (Kei et al., 2012). The second helix is linked to the 18A helix by proline and contains 21 residues from the last helix of apoC-II, the LPL activating domain of apoC-II (Olivecrona and Beisiegel, 1997). We show that the CII-a peptide promotes lipolysis of triglycerides on native lipoproteins by LPL as well as cholesterol efflux by the ABCA1 transporter. This apoC-II mimetic peptide can thus possibly be used as a new therapeutic agent for the treatment of cardiovascular disease.
Materials and Methods
Peptide Synthesis.
C-II-a (DWLKAFYDKVAEKLKEAF-P-AMSTYTGIFTDQVLSVLKGEE), an inactive analog called C-II-i containing four alanine substitutions, (DWLKAFYDKVAEKLKEAF-P-AMSTATGAFTAAVLSVLKGEE), and an apoA-I mimetic peptide called 5A (DWLKAFYDKVAEKLKEAF-P- DWAKAAYDKAAEKAKEAA) (Sethi et al., 2008) were synthesized by a solid-phase procedure using N-(9-fluorenyl)methoxycarbonyl–protected amino acids on a Biosearch 9600 peptide synthesizer (Bioresearch, Tokyo, Japan). They were purified to greater than 95% purity by reverse-phase high-performance liquid chromatography on an Aquapore RP-300 column (PerkinElmer, Waltham, MA) (Sethi et al., 2008) and delivered intravenously into the retro-orbital sinus. Peptides were dissolved in saline, and the pH was adjusted to 7.4.
Peptide Modeling and Circular Dichroism Spectroscopy.
The secondary structure of the peptides was predicted using PEPfold software (http://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD/). The interaction of peptides with phospholipids was determined by coarse grain modeling using the Orientations of Proteins in Membranes online database (http://opm.phar.umich.edu/). The depth/hydrophobic thickness (Å), ΔGtransfer (kcal/mol), and tilt angle (degrees) were also analyzed using the Orientations of Proteins in Membranes online software. The circular dichroism spectra of peptides were collected on a J-715 spectropolarimeter (JASCO, Easton, MD). The helicity of each peptide, which was monitored at 222 nm, was calculated by a previously reported formula (Lomize et al., 2006).
Mice and Diets.
C57Bl/6 and apolipoprotein E (apoE)–knockout mice (The Jackson Laboratory, Bar Harbor, ME) were fed a regular rodent chow diet (NIH-07 chow diet: 0.025% cholesterol, 4.5% fat; Ziegler Brothers, Inc., Gardner, PA). All animal studies were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee (protocols #H-0050 and H-0018).
Analysis of Lipids and Lipoproteins.
Lipids (total cholesterol, triglycerides, and phospholipids) were measured enzymatically (Wako Chemicals USA, Inc., Richmond, VA) on a ChemWell 2910 analyzer (Awareness Technology, Inc., Palm City, FL). Lipoproteins were separated by fast-protein liquid chromatography using two Superose 6 HR 10/30 columns (GE Healthcare, Piscataway, NJ) (Amar et al., 1998), followed by enzymatic colorimetric analysis of lipids, as described above.
LPL Activity Assays.
LPL activity was monitored with either intralipid (2.5 μg TGs per well) as the substrate or with serum (1:50 final dilution) in a reaction volume of 50 μl in phosphate-buffered saline (pH 7.4) containing 0.1% (w/v) fatty acid free bovine serum albumin. The activity was monitored by the release of free fatty acids (FFAs), as previously described (Carballo-Jane et al. 2010). A total of 0.2 units of LPL purified from bovine milk (Sigma-Aldrich, St. Louis, MO) was added per well. Recombinant full-length apoC-II protein was obtained from Meridian Life Science, Inc. (Memphis, TN). FFAs generated during the reaction were quantified in the same plate using commercially available reagents (Wako Chemicals USA). Samples were read at A550 in a SpectraMax 384 Plus plate reader (Molecular Devices, Sunnyvale, CA).
In Vitro Cholesterol Efflux Assay.
Cholesterol efflux studies were performed as previously described (Remaley et al., 2003), with the following modifications. BHK-mock (control) and BHK stably transfected cells with human ABCA1 cDNA (Vaughan and Oram, 2003; Sankaranarayanan et al., 2009) were labeled for 18 hours with 1 mCi/ml of [3H]cholesterol in Dulbecco’s modified Eagle’s medium (DMEM) media plus 10% fetal calf serum. Transporters were induced with 10 nM mifepristone in DMEM plus 0.1 mg/ml fatty acid–free bovine serum albumin for 18 hours. Cholesterol flux was measured after the addition of media containing an acceptor plus 10 nM mifepristone in DMEM with 0.1 mg/ml fatty acid–free bovine serum albumin. After 18 hours, media was collected and filtered (Whatman, 24 well 25-μm pore size; GE Healthcare) and cells were lysed in 0.5 ml of 0.1% SDS and 0.1 N NaOH. Radioactive counts in media and cell fractions were measured by liquid scintillation counting, and results are expressed as the percentage of total counts effluxed.
Statistical Analysis.
Unless otherwise indicated, all data are expressed as mean ± 1 S.D. P < 0.05 was considered to be statistically significant when using a two-tailed t-test.
Results
Design and Biophysical Characterization of C-II Peptides.
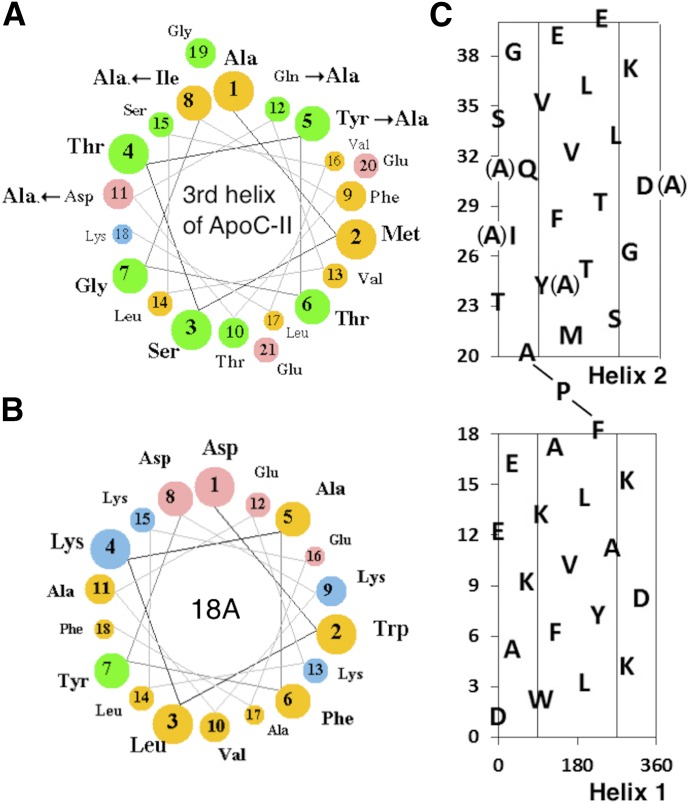
Figure 1A is a diagram of the active C-II-a peptide and an inactive analog called C-II-i. Both peptides contain two amphipathic helices linked by proline. The first or N-terminal helix, which is shared by both of these peptides, is a type A amphipathic helix based on the 18A peptide (Fig. 1B) (Anantharamaiah et al., 1985). It contains a relatively high hydrophobic moment of 9.68 μH/n and is known to have high affinity for lipids and lipoproteins, and thus was used to anchor the peptides to the lipoproteins. The second helix for both peptides is based on the third and last helix of apoC-II, which is known to activate LPL (Musliner et al., 1979; Connelly et al., 1987). This helix has a relatively low hydrophobic moment of 4.68 μH/n (Fig. 1A) and does not readily bind to lipoproteins. The inactive C-II-i peptide differs from the C-II-a active peptide in that four amino acid residues in the second helix, which are known to be critical in LPL activation (Shen et al., 2002), were substituted for Ala (Fig. 1C).
Fig. 1.
Helical wheel and net plots of apoC-II mimetic peptides. (A) Helical wheel plot of the third helix of apoC-II. Arrows show the site of Ala substitutions for the C-II-i peptide. The size of balls and color indicate the degree of charge and hydrophobicity (yellow, nonpolar; green, polar/uncharged; pink, acidic; blue, basic). (B) Helical wheel plot of 18A helix. (C) Helical net plot of C-II-a peptide. The central region indicates the position of the hydrophobic face. Arrows show sites of Ala substitutions for the C-II-i peptide. The first helix (1–18) is based on the 18A helix. The second helix (20–40) is based on the third helix of apoC-II.
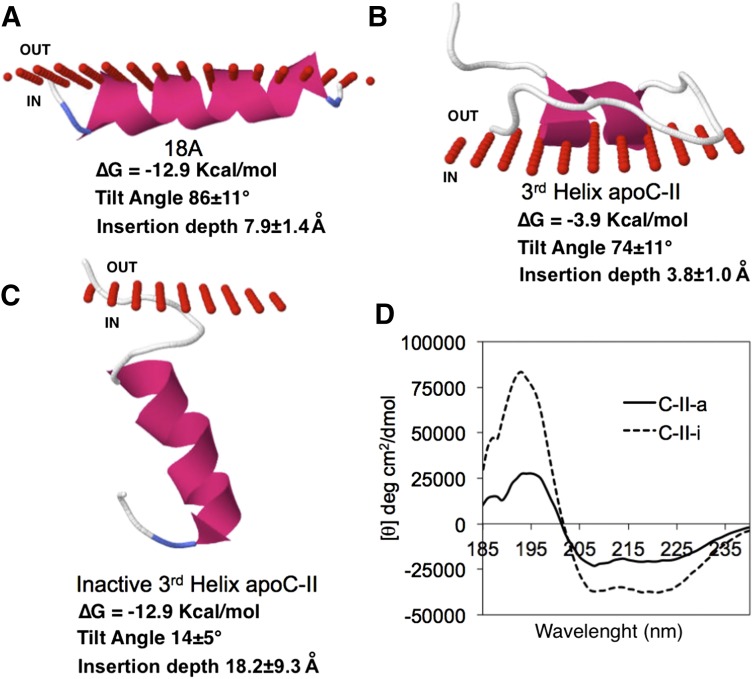
The interactions of these peptides with phospholipids were modeled in Fig. 2. As expected, based on its relatively large hydrophobic moment, the 18A helix was predicted to be buried relatively deeply in the acyl chains of phospholipids in a parallel orientation to the surface (Fig. 2A). In contrast, the second helix of C-II-a, which does not form a good amphipathic helix (Fig. 1A), was predicted to be only loosely associated with the outer phospholipid surface (Fig. 2B). Substitution of the four natural residues critical for LPL activation for Ala (Y23A, I26A, D29A, and Q30A) did not substantially alter its hydrophobic moment (4.50 μH/n) but increased its total hydrophobicity from 4.2 to 6.8 (Wimley-White scale), and it had a much greater predicted ∆G for lipid binding. Based on its increased ∆G and its now more circumferential arrangement of hydrophobic amino acids (Thevenet et al., 2012), it was predicted to deeply insert in phospholipid membranes in a parallel orientation to the acyl chains (Fig. 2C). These results are consistent with the analysis of the peptides by circular dichroism spectroscopy (Fig. 2D). The C-II-a and C-II-i peptides showed a similar helical content when dissolved in water (data not shown), but the C-II-i peptide was much more helical (C-II-i = 100.0%; CII-a = 59.3%) in the presence of 10% 2,2,2-trifluoroethanol (Fig. 2), which simulates a lipid environment.
Fig. 2.
Structure models of apoC-II mimetic peptides. Structural models for the binding of 18A (A), third helix apoC-II (B), and Ala-substituted third helix apoC-II (C) are shown. Red circles represent the boundary between phospholipid headgroups (out) and acyl chains below (in). Lipid binding parameters are listed below. (D) Circular dichroism spectrum of indicated peptides in 10% 2,2,2-trifluoroethanol.
Stimulation of In Vitro Lipolysis by C-II Peptides.
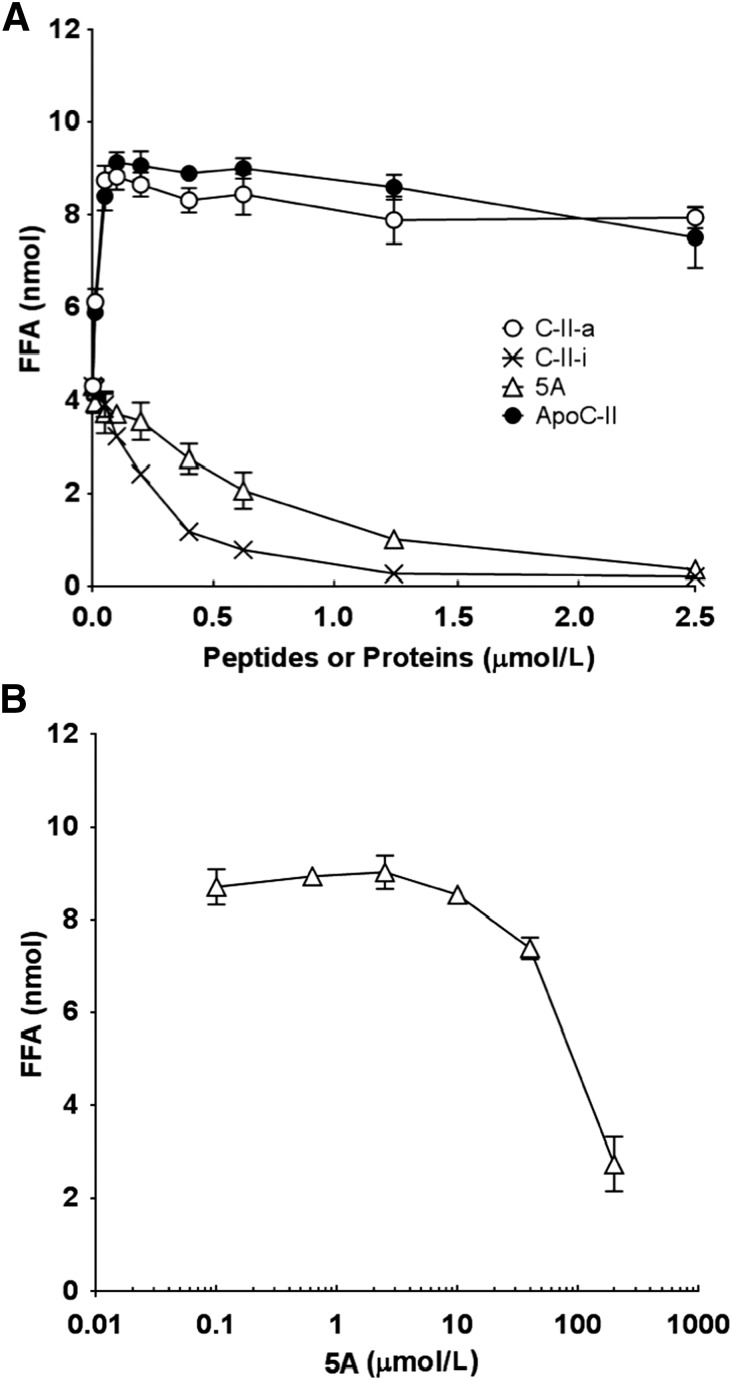
The ability of the C-II peptides to promote in vitro the lipolysis of intralipid, a phospholipid emulsion containing triglycerides, was tested and compared with full-length apoC-II and 5A, an apoA-I mimetic peptide that reduces atherosclerosis and inflammation in animal models (Amar et al., 2010; Tabet et al., 2010; Dai et al., 2012) but causes hypertriglyceridemia (Carballo-Jane et al., 2010). Increasing the concentration of C-II-a increased the generation of FFAs in the presence of LPL, with the maximum effect occurring at about 0.22 μmol/l, thus indicating that the peptide is a potent activator of LPL (Fig. 3). In fact, it had a very similar dose response relationship as the full-length apoC-II protein in LPL activation, with an apparent Km of 7.6 nmol/l and a Vmax of 8.51 nmol/h. In contrast, both the C-II-i and 5A peptide in the absence of any active apoC-II protein or peptide appeared to readily inhibit the production of FFAs by LPL (Fig. 3A), with an IC50 of less than 1 μmol/l. In Fig. 3B, the ability of 5A to inhibit LPL was tested in the presence of maximum activating levels of C-II-a. Inhibition of LPL by 5A in the presence of C-II-a was observed at doses of 5A greater than 2.5 μmol/l, and its IC50 for inhibiting LPL was approximately 100 μmol/l.
Fig. 3.
Effects of peptides and apoC-II on lipolysis of intralipid by LPL. (A) Intralipid containing 2.5 μg of TG per well was mixed with phosphate buffer saline containing 0.1% (w/v) bovine serum albumin, 0.2 units of LPL from bovine milk, and the indicated amounts of C-II-a (○), C-II-I (×), 5A (∆), or apoC-II (●) into the well of 96-well microtiter plates. Free fatty acids were measured in the same plate using an enzymatic assay. (B) The inhibition of lipolysis by LPL was monitored using the above assay conditions, with 6 μmol/l of C-II-a in each well and the indicated concentration of 5A. Baseline FFA values in the absence of 5A peptide was 8.5 nmol. Results represent the mean of triplicates ± S.D.
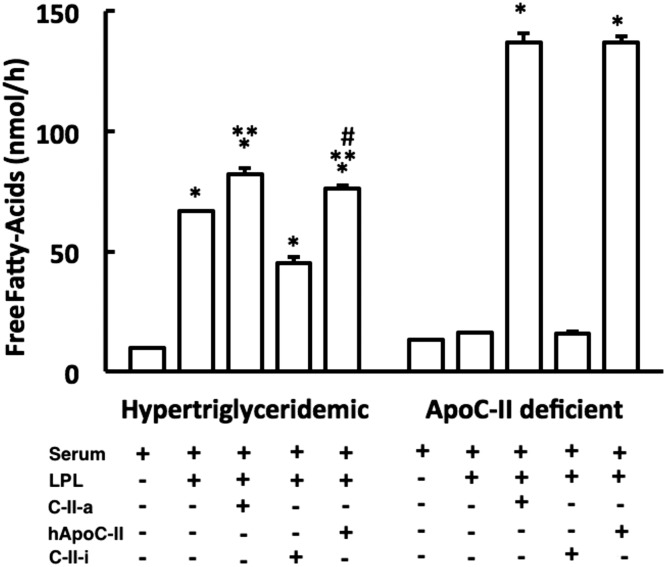
Next, we compared the ability of the two C-II peptides in promoting lipolysis in serum from a patient with type IV hypertriglyceridemia and a patient with type I hypertriglyceridemia from apoC-II deficiency with full-length apoC-II (Fig. 4) (Fojo and Brewer, 1992). The addition of just LPL to serum from a patient with type IV hypertriglyceridemia significantly increased the level of FFAs, and a small but statistically significant further increase in FFA generation was observed after also adding C-II-a. The addition of C-II-i along with LPL appeared to inhibit lipolysis, as there was a reduction in FFAs below what was observed after just LPL addition. The addition of C-II-a or C-II-i to serum or intralipid without LPL first did not generate any additional production of FFAs when compared with untreated samples (data not shown). Full-length apoC-II was almost as effective as the C-II-a peptide in stimulating lipolysis of LPL.
Fig. 4.
Effect of peptides on in vitro lipolysis of serum. Serum from a hypertriglyceridemic patient (TG = 302 mg/dl) or serum from an apoC-II deficient patient (TG = 638 mg/dl) was incubated in vitro with LPL alone or in conjunction with C-II-a (10 μmol/l), C-II-I (10 μmol/l), or full-length human apoC-II protein, and the appearance of FFAs was measured. Results represent the mean of triplicates ± S.D. *P < 0.05 compared with serum baseline; **P < 0.05 compared with serum plus LPL; #P < 0.05 compared with serum plus LPL plus C-II-a group.
When serum from an apoC-II deficient patient was used as the substrate, no additional FFA generation was observed after the addition of LPL, confirming the necessity for LPL activation by apoC-II. But when C-II-a was added along with LPL, it stimulated the production of FFAs to a similar level seen in the patient with type IV hypertriglyceridemia. In contrast, as before, the C-II-i peptide was inactive and did not result in any further production of FFAs in the presence of LPL. Full-length apoC-II behaved similar to the C-II-a peptide in restoring lipolysis to apoC-II deficient serum after LPL addition.
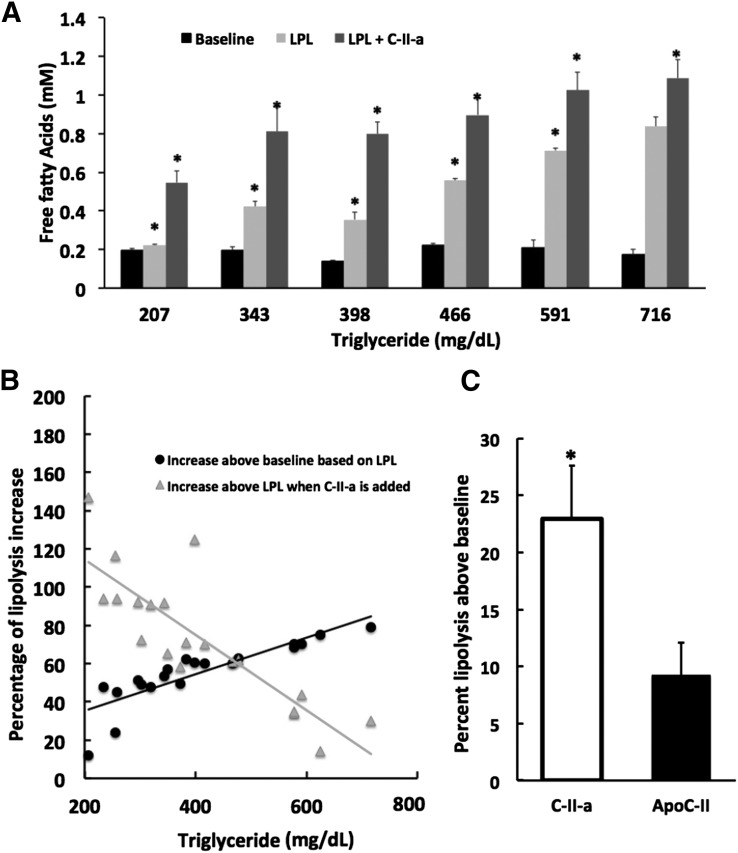
The effect of the C-II-a peptide was then tested in serum from patients with a wide range of hypertriglyceridemia due to type IV and V hyperlipidemia (Fig. 5A). The addition of LPL to serum caused a 2- to 4-fold increase in the production of FFAs over baseline, but the response was highly variable. Interestingly, several patients, particularly those with only a modest increase in triglycerides showed very little or no increase (see sample with TG = 207 mg/dl in Fig. 5A) in FFAs after the addition of LPL. The addition of C-II-a along with LPL, in all cases, however, showed an additional generation of FFAs above which was observed with just the LPL addition. As can be seen in Fig. 5B, the greatest percentage increase in lipolysis from the addition of C-II-a was from patients with only a moderate increase in triglycerides, which was less than approximately 500 mg/dl. Next, the ability of C-II-a to stimulate lipolysis in hypertriglyceridemic serum (TG < 500 mg/dl) above what was observed with just LPL addition was compared with the full-length apoC-II protein in Fig. 5C. Both increased lipolysis after LPL addition, but C-II-a treatment showed a higher rate of lipolysis for all samples than full-length apoC-II treatment and, on average, resulted in a more than 2-fold increase in FFA levels above baseline compared with the full-length apoC-II protein.
Fig. 5.
Effect of peptides on lipolysis in serum. (A) A wide range of serum samples from 20 hypertriglyceridemic patients (TG = 207–716 mg/dl) were incubated in vitro with LPL alone (0.2 U) or LPL plus C-II-a peptide (10 μmol/l), and the appearance of FFAs was measured. Representative results from six patients are shown. (B) Mean percentage change in FFAs after addition of LPL (●) compared with baseline (no LPL) or LPL plus C-II-a peptide (▪) compared with LPL only baseline in hypertriglyceridemic serum. (C) Mean percentage change in FFAs after addition of LPL and C-II-a peptide (50 μmol/l) or full-length apoC-II protein (10 μmol/l) compared with LPL only baseline for hypertriglyceridemic serum samples (n = 9), with a mean TG level of 314 mg/dl (range, 224–436 mg/dl). Results represent the mean of triplicates ± S.D. *P < 0.05 compared with baseline.
Stimulation of In Vitro Cholesterol Efflux by C-II Peptides.
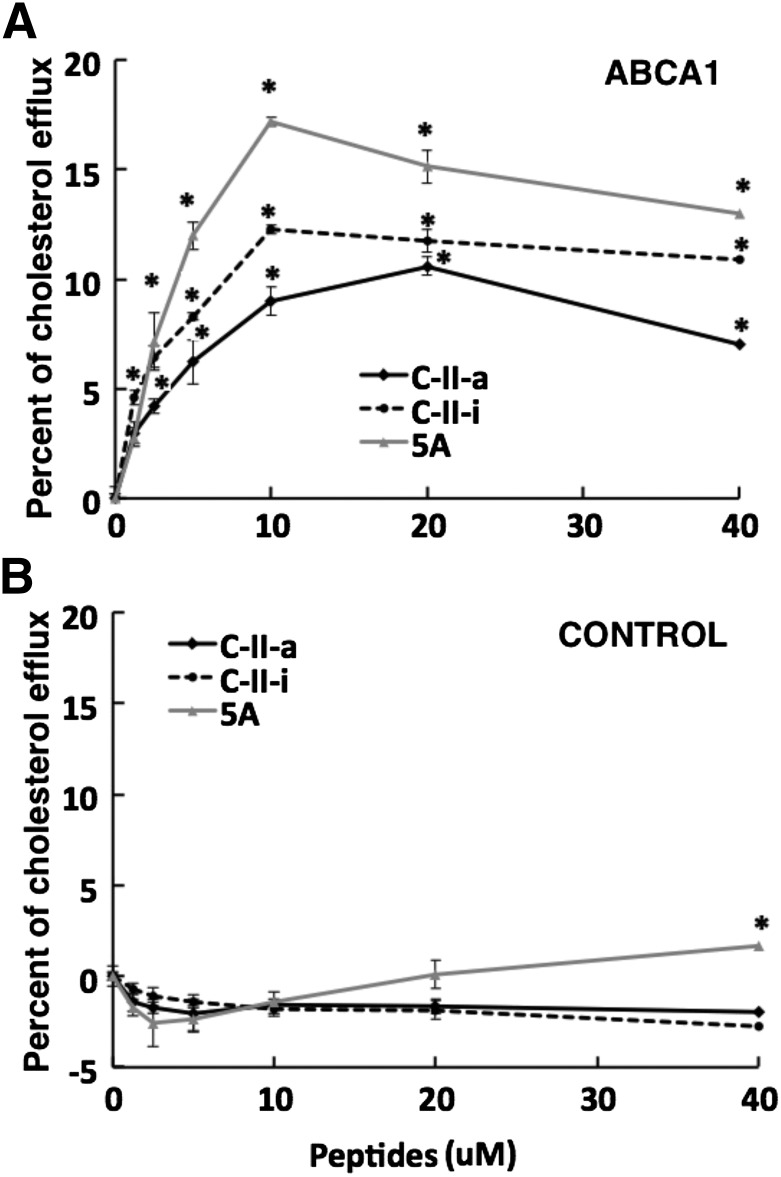
The C-II peptides and 5A peptide, which has been shown to mediate cholesterol efflux by the ABCA1 transporter (Sethi et al., 2007), both share the first 18A helix. Like the C-II-a peptide, the second helix of the 5A peptide has a relatively low lipid binding affinity. Its second helix is a modified 18A helix containing five Ala substitutions for hydrophobic residues, which reduces its lipid affinity but has been shown to improve its specificity for cholesterol efflux by the ABCA1 transporter and reduce cytotoxicity (Sethi et al., 2007). We, therefore, compared the ability of the C-II peptides and 5A peptide in promoting cholesterol efflux from ABCA1-transfected cells (Fig. 6A). The 5A peptide showed the most cholesterol efflux from ABCA1-transfected cells, but both the C-II-a and C-II-i peptides also showed considerable specific efflux from ABCA1-transfected cells above that observed with the mock transfected cells (Fig. 6B).
Fig. 6.
Effect of peptides on cholesterol efflux. Cholesterol efflux was measured on BHK-ABCA1 (A) and the control BHK cell line (B) after 18 hours at 37°C with the following peptides at the indicated doses: 5A (gray lines), C-II-i (dotted lines), or C-II-a peptide (black lines). Results represent the mean of triplicates ± S.D. *P < 0.05 compared with baseline.
Stimulation of In Vivo Lipolysis C-II Peptides.
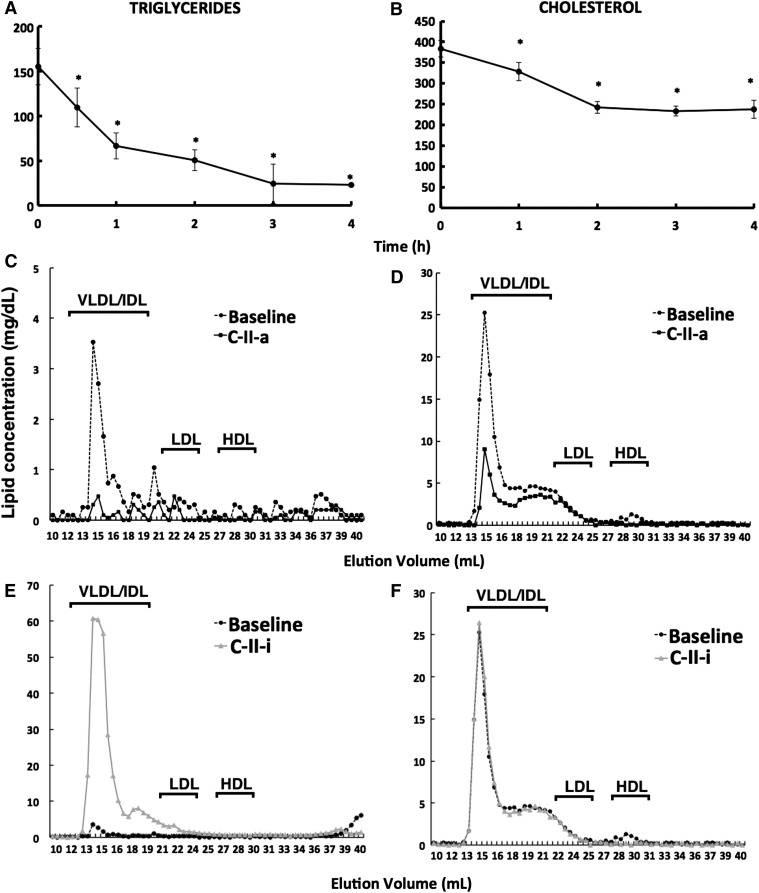
Next, we examined the ability of the C-II-a peptide to promote lipolysis in apoE-knockout mice in Fig. 7. Intravenous injection of C-II-a caused a rapid reduction in serum triglycerides. By 4 hours, C-II-a reduced serum triglycerides by approximately 85% compared with baseline (Fig. 7A). Total cholesterol also showed a similar trend as triglycerides but showed less of a change, with a maximum decrease of 38% at 4 hours (Fig. 7B). Four hours after injection of the peptide, plasma lipids were also analyzed by fast-protein liquid chromatography analysis (Fig. 7, C and D). Based on this analysis, the majority of the decrease in triglycerides and cholesterol occurred on VLDL and, to a lesser degree, low-density lipoprotein. In contrast, the C-II-i peptide caused hypertriglyceridemia, with most of the changes due to an increase in triglycerides on VLDL (Fig. 7E). Total cholesterol did not show a major change following C-II-i treatment (Fig. 7F).
Fig. 7.
Effect of apoC-II mimetic peptides on lipids in apoE-knockout mice. ApoE-knockout mice (female; n = 3 per group) received an intravenous bolus injection of C-II-a or C-II-i peptide (30 mg/kg). Retro-orbital bleedings were performed up to 4 hours postinjection. Fast-protein liquid chromatography separation and lipid analysis were performed on pooled samples for triglycerides (A, C, and E) and cholesterol (B, D, and F). Results represent the mean of triplicates ± S.D. *P < 0.05 compared with baseline. IDL, intermediate-density lipoprotein.
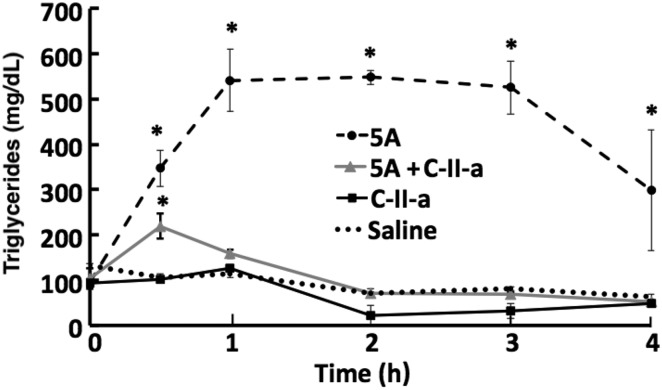
In Fig. 8, we examined the effect of intravenously injecting C57Bl/6 mice with 5A with and without the C-II-a peptide on serum triglycerides. The 5A peptide caused a marked but transient increase in triglycerides, whereas the C-II-a peptide by itself had no significant effect on triglycerides compared with baseline or saline control. When both the 5A and C-II-a peptides were coinjected into mice, the C-II-a peptide blocked the increase in triglycerides observed when the 5A peptide was injected alone. This suggests that the C-II-a peptide can prevent hypertriglyceridemia from the 5A apoA-I mimetic peptide treatment by promoting lipolysis.
Fig. 8.
Effect of coadministration of C-II-a along with 5A on hypertriglyceridemia in mice. C57Bl/6 mice (female; n = 3 per group) received either a 2-mg (60 mg/kg) bolus of intravenous injection of 5A (dotted line), 1 mg (30 mg/kg) of C-II-a peptide coinjected with 2 mg of 5A peptide (gray line), or just 1 mg (30 mg/kg) of C-II-a (black line). Results from saline-injected mice are shown as a black dotted line. Plasma was collected from the retro-orbital sinus and analyzed for triglycerides. Results represent the mean of triplicates ± S.D. *P < 0.05 compared with saline baseline.
Discussion
Recently, there has been great interest in the development of apolipoprotein mimetic peptides for cardiovascular disease and also a wide variety of other disorders related to inflammation and Alzheimer’s disease (Sethi et al., 2007; Bielicki et al., 2010; Navab et al., 2010), but most of these peptides are based on sequences derived from apoA-I and apoE. In this report, we describe a novel apolipoprotein mimetic peptide based on apoC-II, which is an important physiologic activator of lipolysis by LPL. It was already known that the last helix of apoC-II was critical in the activation of LPL, and four amino acid residues critical in this process have been mapped to the last helix of apoC-II (Shen et al., 2002). Most of these amino acid residues are near the polar face of the helix and are thought to possibly interact with LPL and tether it to the surface of lipoproteins, where it can interact with its triglyceride substrate. Although the last helix of apoC-II by itself can activate LPL when using artificial lipid emulsions as a substrate, it is inactive with natural lipoprotein substrates because of its poor affinity for lipoproteins (Olivecrona and Beisiegel, 1997). To address this issue, we attached the last helix of apoC-II to the 18A helix, which has been previously used in the design of other apolipoprotein mimetic peptides, because of its high lipid binding affinity (Sethi et al., 2008). The resulting bihelical peptide, called C-II-a, was considerably shorter than full-length apoC-II protein but similar in its ability to activate LPL with both intralipid emulsions (Fig. 3) and natural lipoprotein substrates (Fig. 5). C-II-i, the inactive version of the peptide, was not only ineffective in activating LPL, but, in fact, inhibited LPL activity (Fig. 3A). It did so even in the absence of any active C-II-a peptide or full-length apoC-II protein (Fig. 3), so it may perhaps directly inhibit LPL by changing the lipid interface and/or possibly by competing with LPL for lipid binding. The predicted orientation of C-II-i in the lipid surface was much different than C-II-a (Fig. 2), which could possibly account for its ability to act as a LPL inhibitor.
It is also possible that other mechanisms independent of LPL lipolysis may also play a role, at least partially, on the observed in vivo reduction of triglycerides in mice by the C-II-a peptide. It was recently reported that apoC-III inhibition by antisense oligonucleotides in type I patients with defective LPL unexpectedly lowered serum triglycerides (Graham et al., 2013). This may be possibly related to the role of apoC-III in inhibiting other lipases, such as hepatic lipase or endothelial lipase, whereas apoC-II and possibly the C-II-a peptide could, in contrast, stimulate lipolysis by activating other lipases. Alternatively, apoC-III may possibly be interfering with hepatic uptake of remnant lipoproteins (Graham et al., 2013). It has been shown that catalytically inactive LPL can, in fact, promote the hepatic uptake of remnant lipoproteins by acting as an alternative ligand for binding to hepatocytes (Gonzalez-Navarro et al., 2004). ApoC-III could possibly interfere with this process, whereas apoC-II and the C-II-a peptides could facilitate this process and hence accelerate hepatic clearance of remnant lipoproteins independent of lipolysis by promoting the interaction of LPL with remnant lipoproteins. These other possible mechanisms of action of the C-II-a peptide on triglyceride metabolism will be investigated in future studies.
Our initial objective for developing apoC-II mimetic peptides was to investigate and possibly address hypertriglyceridemia caused by apoA-I mimetic peptides (Carballo-Jane et al., 2010). ApoA-I mimetic peptides were first developed as structural probes of apolipoproteins (Anantharamaiah et al., 1985) but were then discovered to share many of the beneficial antiatherogenic properties of apoA-I. Recently, some of these peptides have been tested in early stage clinical trials as alternatives to full-length apoA-I for the rapid stabilization of patients with ACS (Krause and Remaley, 2013). The 5A peptide, like other apoA-I mimetic peptides, can acutely raise triglycerides quite significantly (Fig. 8), which appears to be due to inhibition of LPL (Fig. 3). In contrast to 5A, we observed that the C-II-a peptide activated LPL with a similar efficiency as the full-length apoC-II protein using both artificial lipid emulsion substrates (Fig. 3) as well as with lipoprotein substrates (Figs. 4 and 5). The C-II-a peptide was also quite effective in promoting cholesterol efflux by ABCA1 (Fig. 6), and thus possibly could be used like 5A and other apoA-I mimetic peptides as a treatment of ACS without the perhaps dose-limiting effects from hypertriglyceridemia. Alternatively, as shown in Fig. 8, C-II-a could possibly be used in conjunction with 5A and other apoA-I mimetic peptides for blunting their hypertriglyceridemic effect.
The newly described C-II-a peptide has some other potential therapeutic indications. Genetic deficiency of apoC-II is a relatively rare cause of type I hyperlipidemia, but, like genetic defects due to mutations in LPL, these patients can develop remarkably elevated levels of plasma triglycerides, which can lead to acute pancreatitis (Fojo and Brewer, 1992; Viljoen and Wierzbicki, 2012). Currently, there is no specific therapy for such patients, but given the normalization of lipolysis that occurred after adding the peptide to plasma from a patient with apoC-II deficiency, the C-II-a peptide may be useful as a therapy for this disorder. Based on the results shown, it would be predicted to rapidly lower plasma triglycerides in apoC-II deficient patients if given during acute pancreatitis, which would be expected to help in its resolution (Tsuang et al., 2009; Viljoen and Wierzbicki, 2012). If a convenient formulation could be developed so that it could be delivered subcutaneously or orally, it could perhaps also be used prophylactically in these patients for preventing the initial development of hypertriglyceridemia and the triggering of pancreatitis.
Interestingly, we observed that the addition of the C-II-a peptide to patients with more common forms of hypertriglyceridemia also showed increased lipolysis after the addition of LPL (Fig. 5). Besides apoC-II and apoA-V (Fruchart-Najib et al., 2004), the other known protein activator of LPL, many other apolipoproteins are known to inhibit LPL (Kersten, 2014), particularly apoC-III. It may be that the ratio of activator to inhibitor proteins on lipoproteins determines their overall rate of lipolysis by LPL, and the presence of additional activator proteins in the form of the C-II-a peptide may help promote lipolysis. Interestingly, we observed that many patients with only modest increases in triglycerides appeared to be relatively resistant to lipolysis after adding LPL, but this was relieved after adding the C-II-a peptide (Fig. 5). This possibly suggests that intermediate or small remnant-like particles may be relatively depleted in apoC-II or perhaps enriched in apoC-III and that the C-II-a peptide could also be potentially useful for more common forms of hypertriglyceridemia. It is a rare complication, but some patients with type IV and V hypertriglyceridemia can also develop pancreatitis and thus could perhaps also benefit from apoC-II mimetic peptide therapy. Recently, hypertriglyceridemia has also been recognized as an important independent risk factor for cardiovascular disease. From genome-wide association studies, many of the genes related to triglyceride metabolism have been associated with myocardial disease (Musunuru et al., 2010). For example, mutations in the apoC-III gene leading to low plasma levels of triglycerides have been associated with a markedly lower cardiovascular disease risk (Pollin et al., 2008). Polymorphisms in the apoC-III gene have also recently been found to be closely linked to cardiovascular disease (Crosby et al., 2014). These findings suggest that strategies for lowering serum triglycerides, perhaps by apoC-II mimetic peptides, could also be a useful approach for decreasing cardiovascular disease risk in the general population with hypertriglyceridemia.
In summary, we describe a new class of apolipoprotein mimetic peptides based on apoC-II. The novel apoC-II mimetic peptide, called C-II-a, is a potent activator of LPL and lowers serum triglycerides. Additional studies are needed, but the current results suggest that the C-II-a peptide and/or related apoC-II mimetic peptides may have several clinical indications for the treatment and prevention of cardiovascular disease.
Abbreviations
- ACS
acute coronary syndrome
- apoA-I
apolipoprotein A-I
- apoC-II
apolipoprotein C-II
- apoC-III
apolipoprotein C-III
- apoE
apolipoprotein E
- DMEM
Dulbecco’s modified Eagle’s medium
- FFAs
free fatty acids
- LPL
lipoprotein lipase
- TGs
triglycerides
- VLDL
very low density lipoprotein
Authorship Contributions
Participated in research design: Amar, Remaley.
Conducted experiments: Amar, Sakurai, Sviridov, Freeman, Ahsan, Sakurai-Ikuta.
Performed data analysis: Amar, Sakurai, Sviridov, Freeman, Ahsan, Sakurai-Ikuta.
Wrote or contributed to the writing of the manuscript: Amar, Remaley.
Footnotes
This research was supported by Intramural Research Program of the National Institutes of Health [National Heart, Lung, and Blood Institute]
References
- Amar MJ, D’Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, et al. (2010) 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther 334:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar MJ, Dugi KA, Haudenschild CC, Shamburek RD, Foger B, Chase M, Bensadoun A, Hoyt RF, Jr, Brewer HB, Jr, Santamarina-Fojo S. (1998) Hepatic lipase facilitates the selective uptake of cholesteryl esters from remnant lipoproteins in apoE-deficient mice. J Lipid Res 39:2436–2442. [PubMed] [Google Scholar]
- Anantharamaiah GM, Jones JL, Brouillette CG, Schmidt CF, Chung BH, Hughes TA, Bhown AS, Segrest JP. (1985) Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem 260:10248–10255. [PubMed] [Google Scholar]
- Anderson F, Thomson SR, Clarke DL, Buccimazza I. (2009) Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes. Pancreatology 9:252–257. [DOI] [PubMed] [Google Scholar]
- Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AF. (2012) Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:2969–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S. (2010) A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res 51:1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Jane E, Chen Z, O’Neill E, Wang J, Burton C, Chang CH, Chen X, Eveland S, Frantz-Wattley B, Gagen K, et al. (2010) ApoA-I mimetic peptides promote pre-β HDL formation in vivo causing remodeling of HDL and triglyceride accumulation at higher dose. Bioorg Med Chem 18:8669–8678. [DOI] [PubMed] [Google Scholar]
- Connelly PW, Maguire GF, Hofmann T, Little JA. (1987) Structure of apolipoprotein C-IIToronto, a nonfunctional human apolipoprotein. Proc Natl Acad Sci USA 84:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, et al. TG and HDL Working Group of the Exome Sequencing Project. National Heart, Lung, and Blood Institute (2014) Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Yao X, Keeran KJ, Zywicke GJ, Qu X, Yu ZX, Dagur PK, McCoy JP, Remaley AT, Levine SJ. (2012) Apolipoprotein A-I attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. Am J Respir Cell Mol Biol 47:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Munoz JE, Malfertheiner P, Ditschuneit HH, Blanco-Chavez J, Uhl W, Buchler M, Ditschuneit H. (1991) Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int J Pancreatol 10:261–267. [PubMed] [Google Scholar]
- Fojo SS, Brewer HB. (1992) Hypertriglyceridaemia due to genetic defects in lipoprotein lipase and apolipoprotein C-II. J Intern Med 231:669–677. [DOI] [PubMed] [Google Scholar]
- Fruchart-Najib J, Baugé E, Niculescu LS, Pham T, Thomas B, Rommens C, Majd Z, Brewer B, Pennacchio LA, Fruchart JC. (2004) Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun 319:397–404. [DOI] [PubMed] [Google Scholar]
- González-Navarro H, Nong Z, Amar MJ, Shamburek RD, Najib-Fruchart J, Paigen BJ, Brewer HB, Jr, Santamarina-Fojo S. (2004) The ligand-binding function of hepatic lipase modulates the development of atherosclerosis in transgenic mice. J Biol Chem 279:45312–45321. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Davidson WS. (2012) Apolipoprotein A-I mimetics and high-density lipoprotein function. Curr Opin Endocrinol Diabetes Obes 19:109–114. [DOI] [PubMed] [Google Scholar]
- Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, et al. (2013) Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 112:1479–1490. [DOI] [PubMed] [Google Scholar]
- Jong MC, Hofker MH, Havekes LM. (1999) Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol 19:472–484. [DOI] [PubMed] [Google Scholar]
- Kei AA, Filippatos TD, Tsimihodimos V, Elisaf MS. (2012) A review of the role of apolipoprotein C-II in lipoprotein metabolism and cardiovascular disease. Metabolism 61:906–921. [DOI] [PubMed] [Google Scholar]
- Kersten S. (2014) Physiological regulation of lipoprotein lipase. Biochim Biophys Acta 1841:919–933. [DOI] [PubMed] [Google Scholar]
- Krause BR, Remaley AT. (2013) Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol 24:480–486. [DOI] [PubMed] [Google Scholar]
- Leman LJ, Maryanoff BE, Ghadiri MR. (2014) Molecules that mimic apolipoprotein A-I: potential agents for treating atherosclerosis. J Med Chem 57:2169–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomize AL, Pogozheva ID, Lomize MA, Mosberg HI. (2006) Positioning of proteins in membranes: a computational approach. Protein Sci 15:1318–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegh BI, Duga B, Sümegi K, Kisfali P, Maász A, Komlósi K, Hadzsiev K, Komoly S, Kosztolányi G, Melegh B. (2012) Mutations of the apolipoprotein A5 gene with inherited hypertriglyceridaemia: review of the current literature. Curr Med Chem 19:6163–6170. [DOI] [PubMed] [Google Scholar]
- Musliner TA, Herbert PN, Church EC. (1979) Activation of lipoprotein lipase by native and acylated peptides of apolipoprotein C-II. Biochim Biophys Acta 573:501–509. [DOI] [PubMed] [Google Scholar]
- Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, et al. (2010) From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjee MN, Crouse JR, King JM, Hovorka R, Rees SE, Carson ER, Morgenthaler JJ, Lerch P, Miller NE. (1996) Effects of intravenous infusion of lipid-free apo A-I in humans. Arterioscler Thromb Vasc Biol 16:1203–1214. [DOI] [PubMed] [Google Scholar]
- Nanjee MN, Doran JE, Lerch PG, Miller NE. (1999) Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans. Arterioscler Thromb Vasc Biol 19:979–989. [DOI] [PubMed] [Google Scholar]
- Navab M, Shechter I, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. (2010) Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol 30:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivecrona G, Beisiegel U. (1997) Lipid binding of apolipoprotein CII is required for stimulation of lipoprotein lipase activity against apolipoprotein CII-deficient chylomicrons. Arterioscler Thromb Vasc Biol 17:1545–1549. [DOI] [PubMed] [Google Scholar]
- Ooi EM, Barrett PH, Chan DC, Watts GF. (2008) Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci (Lond) 114:611–624. [DOI] [PubMed] [Google Scholar]
- Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, et al. (2008) A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 322:1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaley AT, Amar M, Sviridov D. (2008) HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther 6:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, et al. (2003) Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res 44:828–836. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH. (2009) Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res 50:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi AA, Amar M, Shamburek RD, Remaley AT. (2007) Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis. Curr Opin Investig Drugs 8:201–212. [PubMed] [Google Scholar]
- Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D’Souza W, Sviridov D, et al. (2008) Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem 283:32273–32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachter NS, Hayek T, Leff T, Smith JD, Rosenberg DW, Walsh A, Ramakrishnan R, Goldberg IJ, Ginsberg HN, Breslow JL. (1994) Overexpression of apolipoprotein CII causes hypertriglyceridemia in transgenic mice. J Clin Invest 93:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. (2008) Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res 103:1084–1091. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lookene A, Nilsson S, Olivecrona G. (2002) Functional analyses of human apolipoprotein CII by site-directed mutagenesis: identification of residues important for activation of lipoprotein lipase. J Biol Chem 277:4334–4342. [DOI] [PubMed] [Google Scholar]
- Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G. (2010) The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol 30:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tufféry P. (2012) PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res 40:W288–W293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. (2009) Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol 104:984–991. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Oram JF. (2003) ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J Lipid Res 44:1373–1380. [DOI] [PubMed] [Google Scholar]
- Viljoen A, Wierzbicki AS. (2012) Diagnosis and treatment of severe hypertriglyceridemia. Expert Rev Cardiovasc Ther 10:505–514. [DOI] [PubMed] [Google Scholar]
- Wang CS, McConathy WJ, Kloer HU, Alaupovic P. (1985) Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest 75:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdunek J, Martinez GV, Schleucher J, Lycksell PO, Yin Y, Nilsson S, Shen Y, Olivecrona G, Wijmenga S. (2003) Global structure and dynamics of human apolipoprotein CII in complex with micelles: evidence for increased mobility of the helix involved in the activation of lipoprotein lipase. Biochemistry 42:1872–1889. [DOI] [PubMed] [Google Scholar]