Abstract
The nicotine metabolite cotinine (1-methyl-5-[3-pyridynl]-2-pyrrolidinone), like its precursor, has been found to exhibit procognitive and neuroprotective effects in some model systems; however, the mechanism of these effects is unknown. In this study, both the R-(+) and S-(−) isomers of cotinine were initially evaluated in an extensive profiling screen and found to be relatively inactive across a wide range of potential pharmacologic targets. Electrophysiological studies on human α4β2 and α7 nicotinic acetylcholine receptors (nAChRs) expressed in Xenopus oocytes confirmed the absence of agonistic activity of cotinine at α4β2 or α7 nAChRs. However, a significant increase in the current evoked by a low concentration of acetylcholine was observed at α7 nAChRs exposed to 1.0 μM R-(+)- or S-(−)-cotinine. Based on these results, we used a spontaneous novel object recognition (NOR) procedure for rodents to test the hypothesis that R-(+)- or S-(−)-cotinine might improve recognition memory when administered alone or in combination with the Alzheimer’s disease (AD) therapeutic agent donepezil. Although both isomers enhanced NOR performance when they were coadministered with donepezil, neither isomer was active alone. Moreover, the procognitive effects of the drug combinations were blocked by methyllycaconitine and dihydro-β-erythroidine, indicating that both α7 and α4β2 nAChRs contribute to the response. These results indicate that cotinine may sensitize α7 nAChRs to low levels of acetylcholine (a previously uncharacterized mechanism), and that cotinine could be used as an adjunctive agent to improve the effective dose range of cholinergic compounds (e.g., donepezil) in the treatment of AD and other memory disorders.
Introduction
Significant evidence from in vitro experiments as well as animal studies suggests that the nicotine metabolite cotinine (1-methyl-5-[3-pyridynl]-2-pyrrolidinone) might have potential as a therapeutic agent for some neurologic and psychiatric disorders, including Alzheimer’s disease (AD) and schizophrenia (see reviews in Terry et al., 2005, and Echeverria and Zeitlin, 2012). For example, in studies relevant to AD, cotinine has been shown to improve the survival of differentiated PC12 cells deprived of nerve growth factor (Buccafusco and Terry, 2003), as well as primary cortical neurons exposed to toxic concentrations of the amyloid-β peptide or glutamate (Burgess et al., 2011; Gao et al., 2014). Cotinine has also been shown to improve working/short-term memory performance in monkeys (Terry et al., 2005), prevent memory loss in transgenic 6799 Alzheimer’s disease mice, and stimulate the Akt/GSK3β pathway and reduce amyloid-β aggregation in mouse brains (Echeverria et al., 2011; Patel et al., 2014). In animal studies more closely related to schizophrenia, cotinine improved deficits in prepulse inhibition of the acoustic startle response in rats in three pharmacologic impairment models (Terry et al., 2005), attenuated the deficits of sustained attention in rats induced by the N-methyl-d-aspartate receptor antagonist MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine] (Terry et al., 2012), and improved deficits in working/short-term memory produced by the N-methyl-d-aspartate antagonist ketamine in monkeys (Buccafusco and Terry, 2009). In recent experiments in mice subjected to prolonged restraint (a chronic stress model), cotinine had antidepressant-like properties and reduced cognitive impairment and synaptic loss in the hippocampus and prefrontal cortex (Grizzell et al., 2014).
As a potential therapeutic agent for humans, cotinine has several advantages over nicotine, including a superior safety profile (Hatsukami et al., 1997), a much longer half-life (Benowitz, 1996), and a lower risk of abuse (Rosecrans, 1979). Although cotinine is (structurally) very closely related to nicotine, it is unclear if the previously described positive actions are related to pharmacologic effects at nicotinic acetylcholine receptors (nAChRs). The literature available on the effects of cotinine in nAChR binding experiments and in vitro functional assays suggests that it is a low-affinity ligand at heteromeric and homomeric nAChRs with weak agonist effects (Abood et al., 1981; Sloan et al., 1984; Anderson and Arneric, 1994; Briggs and McKenna, 1998; Dwoskin et al., 1999; Vainio and Tuominen, 2001; O’Leary et al., 2008). Recently, it has been speculated that cotinine might serve as a positive allosteric modulator of nAChRs (see Grizzell and Echeverria, 2014); however, we are unaware of any publications in which this hypothesis has been directly tested.
The purpose of the study described here was, therefore, to further elucidate the pharmacologic effects of cotinine. Both the R-(+) and S-(−) isomers of cotinine were first screened across more than 70 neurotransmitter receptors, transporters, ion channels, and enzymes. Subsequent experiments using a well described in vitro model system were conducted to further investigate the electrophysiological effects of both isomers of cotinine at α4β2 and α7 nAChRs. Based on the results of these experiments where both the R-(+) and S-(−) isomers of cotinine enhanced the response to acetylcholine (ACh) at α7 nAChRs (see Results), we designed a series of experiments in rodents to test the hypothesis that the isomers of cotinine might improve recognition memory when administered alone, or that they might amplify the procognitive effects of the commonly prescribed AD-cholinergic agent donepezil.
Materials and Methods
Pharmacological Activity of R-(+)- and S-(−)-Cotinine (In Vitro)
The R-(+) and S-(−) isomers of cotinine were screened at a single concentration (10 μM) across more than 70 neurotransmitter receptors, transporters, ion channels, and enzymes by Caliper Life Sciences (Hanover, MD). Binding or activity was determined according to standardized conditioned and validated protocols with reference standards included as an integral part of each assay. Details of each assay condition can be accessed through Caliper's website (www.caliperls.com).
Electrophysiological Recordings
Electrophysiological experiments were carried out with human α7 and α4β2 nAChRs expressed in Xenopus laevis oocytes. Oocytes were prepared, injected with cDNA encoding α7 nAChR subunits, and recorded using standard procedures (Hogg et al., 2008). In brief, ovaries were harvested from X. laevis females that were deeply anesthetized by cooling at 4°C and with tricaine mesylate (3-aminobenzoic acid ethyl ester, methane sulfonate salt, 150 mg/l). Small pieces of ovary were isolated in sterile Barth’s solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4·7H2O, 0.33 mM Ca(NO3)2·4H2O, and 0.41 mM CaCl2·6H2O, pH 7.4] and supplemented with 20 μg/ml kanamycin, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Injections of cDNAs encoding for the receptors were performed in at least 100 oocytes using an automated injection device (Roboinject; Multi Channel Systems, Reutlingen, Germany), and receptor expression was examined at least 2 days later. Oocytes were impaled with two electrodes filled with 3 M KCl, and their membrane potentials were maintained at −80 mV throughout the experiment. All recordings were performed at 18°C, and cells were superfused with oocyte Ringer's OR2 medium (82.5 mM NaCl, 2.5 mM KCl, 5 mM HEPES, 1.8 mM CaCl2·2H2O, and 1.8 mM MgCl2·6H2O, pH 7.4). Currents were recorded using an automated process equipped with standard two-electrode voltage-clamp configuration (HiClamp; Multi Channel Systems). The principle of this system differs from standard electrophysiology because, instead of applying the compound in the perfusion, the oocyte is moved into a well from a 96-well microtiter plate containing the desired solution. Data were captured and analyzed using Matlab (Mathworks, Inc., Natick, MA) or Excel (Microsoft, Redmond, WA) software. ACh and the isomers of cotinine were prepared as concentrated stock solutions in water and then diluted in the recording medium to obtain the desired test concentrations. All experiments were carried out using three or more cells.
Animal Care
All animal procedures used during this study were reviewed and approved by the Institutional Animal Care and Use Committee, and are consistent with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised 1996. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
Study Subjects.
Male albino Wistar rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) approximately 2 months old were housed in pairs in a temperature-controlled room (25°C), and were maintained on a standard 12-hour light/dark cycle with free access to food (Teklad Rodent Diet 8604 pellets; Harlan, Madison, WI) and water.
Behavioral Testing
Spontaneous Novel Object Recognition Task.
The novel object recognition (NOR) task was adapted from Ennaceur and Delacour (1988) as we have published previously (Callahan et al., 2014). In brief, test subjects were acclimated to laboratory conditions (i.e., tail marking, daily handling, and weighing) for at least 3 days prior to experimentation. During experimentation, the animals were transported to the laboratory and acclimated for 30 minutes prior to initiating the experimental phase; the animals remained in the laboratory for 15 minutes following study completion.
Habituation.
The animals were acclimated, weighed, and individually placed in a dimly lit (10 lux) training/testing environment (an opaque plastic chamber, 78.7 × 39.4 × 31.7 cm with bedding on the floor) for 10 minutes of chamber exploration. The NOR chamber was placed on a table positioned along the short wall of the laboratory. Heating, ventilating, and air conditioning ventilation provided masking noise to reduce any extraneous background noise, and there were no room-orienting cues or wall-mounted visual cues (except for the small black and white camera positioned above the NOR chamber). At the beginning of each series of NOR experiments, fresh bedding material was placed in the chamber prior to habituation and allowed to become saturated with animal odors. Animal droppings were removed between experimental sessions; however, the same bedding remained in the chamber for the remainder of each study (i.e., during training and testing), thus preventing any specific olfactory cues over the course of experimentation.
Training trial.
Twenty-four hours after the habituation session, the animals were acclimated, weighed, and injected with test compound (drug or vehicle), and after the appropriate pretreatment interval, they were placed in the chamber with their nose facing the center of a long wall and allowed to explore two identical objects for 10 minutes. The animals’ behavior was observed and recorded on videotape via a camera located 69 cm above the chamber; the investigator sat quietly 10–15 feet away from the NOR chamber.
Test trial.
Initially, retention (delay) intervals ranging from 1 to 48 hours were evaluated for effects on the recognition of a novel object (see Fig. 4). Subsequently, a delay interval that reliably produced complete forgetting (48 hours) was used throughout the rest of the behavioral studies. In the NOR task, two objects, one object identical to training (familiar) and a novel object, were placed in the chamber, and the animal was allowed to explore the objects for 5 minutes. Experimental objects to be discriminated were a plastic multicolored Duplo-Lego block configured tower (12 cm in height, 6 cm in width) paired with a ceramic conical-shaped green Christmas tree salt/pepper shaker (12 cm in height, 5 cm in diameter); all objects existed in duplicate. The objects were placed 19.3 cm from the sides of the two short walls and 19.3 cm from the sides of the long walls of the chamber; distance between the two objects was approximately 40 cm. The role of familiar and novel object as well as chamber position of object was randomly assigned across subjects and treatments, and objects were cleaned between sessions with a dilute 50% EtOH solution to eliminate olfactory cues. Object exploration occurred when the animal directed its nose to the object at a distance of ≤2 cm and/or touched it with its nose, rearing up against the object to investigate if the object was also considered exploration, whereas physically climbing on the object, using the object to support itself while rearing to investigate the chamber arena or digging at the base of the object, was not considered appropriate object exploratory behavior. The primary behavioral measure was time (seconds) spent investigating each object. A discrimination index (d2) was calculated on each test trial and was defined as the difference in time spent exploring the novel and familiar objects divided by the total exploration time for both objects: d2 index = (novel – familiar)/(novel + familiar). This measure is considered as an index of recognition memory and takes into account individual differences in the total amount of exploration time. For data inclusion, the rat had to explore each individual object for at least 4 seconds and spend a minimum of 12 seconds of total object exploration. Experimental groups contained 6–9 rats per treatment (or testing) condition, which provided sufficient sample size to observe statistical significance. Animals were tested only once, and object exploration time was scored live under blind testing methods (i.e., the investigator was unaware of treatment assignment).
Fig. 4.
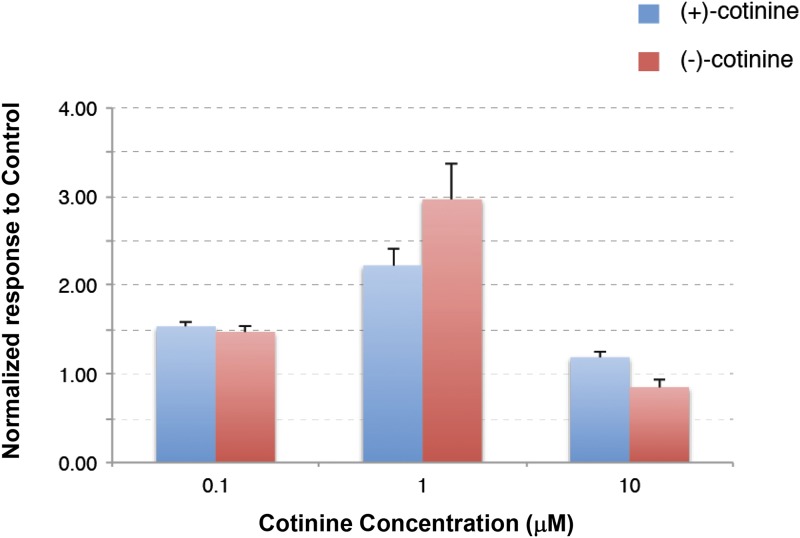
Concentration-dependent effects of sustained exposure to R-(+)- or S-(−)-cotinine at the human α7 nAChRs. Experiments were conducted using the same experimental protocol shown in Fig. 2. Currents were normalized to the average response recorded in control and represented in the form of histograms. Bars indicate the standard error for n = 5–6 for each isomer at α7 nAChRs.
Drug Administration
All compounds were prepared in physiologic saline (0.9% NaCl) and administered by intraperitoneal injection in a volume of 1 ml/kg. Doses refer to the weight of the salt, except where noted. The drugs used and suppliers were as follows: R-(+)-cotinine base (Toronto Research Chemicals Inc., North York, ON, Canada), S-(−)-cotinine (Toronto Research Chemicals Inc.), dihydro-β-erythroidine hydrobromide (Santa Cruz Biotechnology, Dallas, TX), donepezil HCl (Memory Pharmaceutical Corporation, Montvale, NJ), and methyllycaconitine citrate (Tocris Bioscience, Ellisville, MO). The pretreatment interval for donepezil and cotinine was 30 minutes prior to the NOR training trial. In the studies in which donepezil and cotinine were combined, donepezil was administered first followed immediately by the specific test dose of cotinine. For the antagonist studies in which methyllycaconitine (MLA) and dihydro-β-erythroidine (DHβE) were evaluated for their ability to reverse the behavioral effects of the combination of donepezil and cotinine, the antagonist was administered first (60 minutes before the NOR training trial) followed 30 minutes later (i.e., 30 minutes before the NOR training trial) with the combination of donepezil and cotinine.
Statistical Analyses
Statistical analysis was performed using SigmaPlot 11.2 (SPSS Inc., Chicago, IL), and statistical significance was assessed using an α level of 0.05. For one- and two-factor comparisons, analysis of variance (with repeated measures when indicated) was used followed by the Student-Newman-Keuls or Dunnett’s method (for comparisons with vehicle controls only) for post-hoc analysis. All results are expressed as the mean (± S.E.M.).
Results
R-(+) and S-(−) Isomers of Cotinine Lack Significant Activity across a Wide Range of Pharmacological Targets
Pharmacological Screen.
The results of the initial pharmacological screen for the R-(+) and S-(−) isomers of cotinine across more than 70 neurotransmitter receptors, transporters, ion channels, and enzymes are provided in Table 1. As per the standard protocols used by Caliper Life Sciences, significant activity at any of these sites was defined as the inhibition of ligand binding (or enzyme activity) by ≥50%. Using this criterion, neither the R-(+) nor the S-(−) isomer of cotinine demonstrated significant activity at any of the targets that were evaluated, although the R-(+) and S-(−) isomers were associated with ∼36 and ∼47% inhibition of ligand binding at D2 receptors, respectively.
TABLE 1.
In vitro binding/activity profile of R-(+)- and S-(−)-cotinine
Values are expressed as the percent inhibition of specific binding or activity and represent the average of replicate tubes at each of the concentrations tested.
| Target | % Inhibition at 10 μM |
|
|---|---|---|
| R-(+)-Cotinine | S-(−)-Cotinine | |
| Neurotransmitter related | ||
| Adenosine transporter (h) | −4.49 | 2.82 |
| Adenosine, A1 | −0.85 | 4.94 |
| Adenosine, A2A (h) | −0.04 | 2.53 |
| Adrenergic, α1A | 2.59 | −0.33 |
| Adrenergic, α1B | 14.72 | 5.99 |
| Adrenergic, α2A (h) | 22.43 | −7.00 |
| Adrenergic, α2B | 2.65 | −2.32 |
| Adrenergic, α2C (h) | −7.89 | −3.08 |
| Adrenergic, β1 (h) | 2.20 | −0.31 |
| Adrenergic, β2 (h) | −5.54 | −16.48 |
| Dopamine transporter | 7.69 | −17.26 |
| Dopamine, D1 (h) | −7.16 | −3.22 |
| Dopamine, D2s (h) | 36.01 | 46.99 |
| Dopamine, D3 | 7.78 | 23.27 |
| Dopamine, D4.4 (h) | 30.28 | 17.99 |
| GABAA, agonist site | −7.61 | −6.46 |
| GABAA, BDZ, α 1 site | 0.01 | 3.96 |
| GABAB | 19.30 | 20.92 |
| Glutamate, AMPA site (ionotropic) | −6.15 | 1.03 |
| Glutamate, kainate site (ionotropic) | −2.93 | −2.82 |
| Glutamate, MK-801 site (ionotropic) | 0.17 | 1.94 |
| Glutamate, NMDA agonist site (ionotropic) | −6.41 | 2.42 |
| Glutamate, NMDA phencyclidine site (ionotropic) | 2.48 | −5.90 |
| Glutamate, NMDA glycine (stry-insens site) (ionotropic) | 14.39 | 12.77 |
| Glycine, strychnine-sensitive | −2.19 | −17.48 |
| Histamine, H1 | −7.03 | −1.34 |
| Histamine, H2 | −9.70 | 13.70 |
| Histamine, H3 | 6.58 | 4.23 |
| Muscarinic, M1 (h) | 8.81 | 6.20 |
| Muscarinic, M2 (h) | 13.10 | 0.47 |
| Muscarinic, M3 (h) | 3.90 | −1.39 |
| Muscarinic, M4 (h) | −17.19 | −10.25 |
| Muscarinic, M5 (h) | 13.28 | 9.04 |
| Nicotinic, neuronal (a-BnTx insensitive) | −4.99 | 1.33 |
| Norepinephrine transporter | −12.99 | 0.98 |
| Opioid, δ2 (h) | −13.02 | −7.11 |
| Opioid, μ (h) | 4.82 | −3.74 |
| Serotonin transporter | 9.62 | 10.68 |
| Serotonin, 5HT1A (h) | 9.51 | 17.11 |
| Serotonin, 5HT1D | 28.35 | 14.73 |
| Serotonin, 5HT2A | 2.26 | 8.25 |
| Serotonin, 5HT2C | 6.61 | 3.89 |
| Serotonin, 5HT3 | 11.44 | 4.39 |
| Serotonin, 5HT4 | 7.47 | −1.65 |
| Serotonin, 5HT5A (h) | 8.82 | −13.11 |
| Serotonin, 5HT6 (h) | 8.39 | −4.09 |
| Serotonin, 5HT7 (h) | 29.64 | 17.10 |
| Sigma 1 | −8.50 | −8.91 |
| Sigma 2 | −12.49 | −2.54 |
| Ion channels | ||
| Calcium channel, type L (dihydropyridine site) | −10.72 | −11.01 |
| Calcium channel, type N | 4.35 | 5.77 |
| GABA, chloride, TBOB site | 5.62 | −11.07 |
| Potassium channel, ATP-sensitive | 4.37 | 3.42 |
| Potassium channel, Ca2+ Act., VI | 2.04 | 9.16 |
| Potassium channel, I [Kr] (hERG) (h) | 0.91 | −8.57 |
| Sodium, site 2 | 26.72 | 23.98 |
| Second messengers | ||
| Nitric oxide, NOS (neuronal-binding) | −7.02 | 3.35 |
| Prostaglandins | ||
| Leukotriene, LTB4 (BLT) | 6.08 | 6.06 |
| Leukotriene, LTD4 (CysLT1) | 1.87 | 5.42 |
| Thromboxane A2 (h) | −16.05 | −4.82 |
| Brain/gut peptides | ||
| Angiotensin II, AT1 (h) | 8.14 | 6.73 |
| Bradykinin, BK2 | −0.84 | −1.20 |
| Endothelin, ET-A (h) | −3.19 | 5.19 |
| Neurokinin, NK1 | 6.48 | 17.49 |
| Neuropeptide, NPY2 (h) | −14.58 | −2.44 |
| Enzymes | ||
| Esterase, acetylcholine | −5.12 | −1.22 |
| Phosphodiesterase, PDE3A1A (h) | 8.72 | 3.36 |
| Phosphodiesterase, PDE5A1 (h) | 5.21 | 3.41 |
| Enzymes, kinases | ||
| Kinase, protein, PKA (h) | 13.42 | 13.63 |
| Kinase, protein, PKCa (h) | −12.42 | −6.87 |
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDZ, benzodiazepine; BLT, high affinity receptor for the leukotriene LTB4; hERG, human ether-à-go-go–related gene; 5HT, 5-hydroxytryptamine; NMDA, N-methyl-d-aspartate; PKA, protein kinase A; PKCa, protein kinase C α; TBOB, t-butyl bicyclo-orthobenzoate.
R-(+) and S-(−) Isomers of Cotinine Display No Agonistic Activity at Human α4β2 or α7 nAChRs
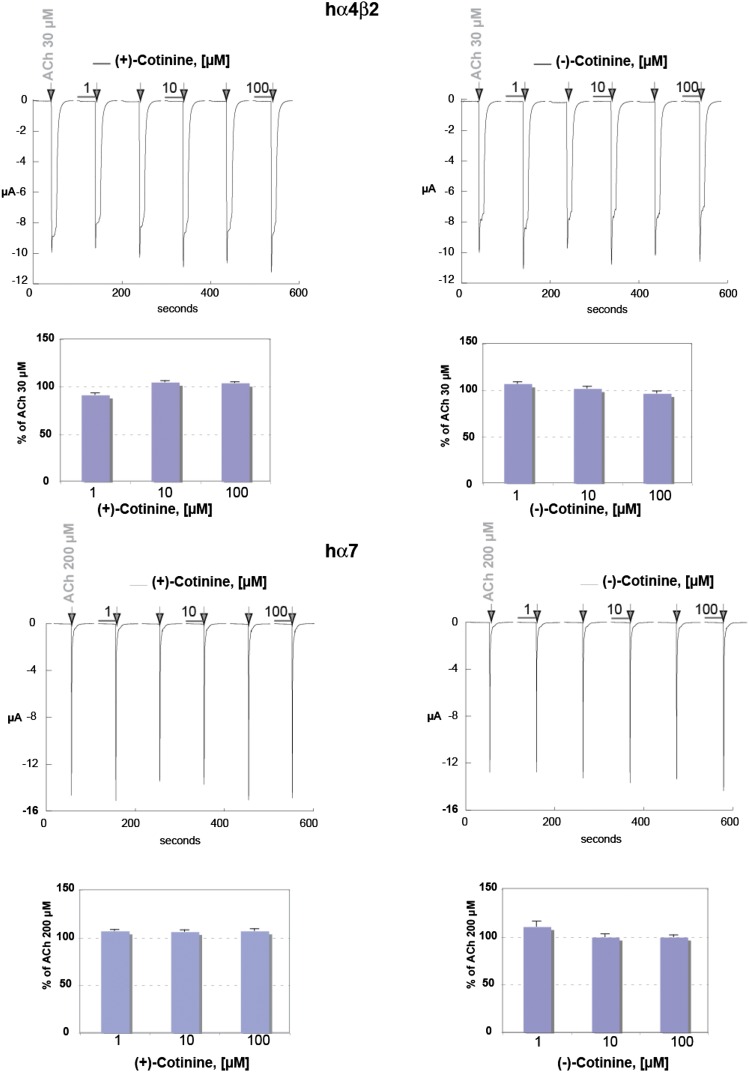
To evaluate the possible agonistic activity of R-(+)- or S-(−)-cotinine at human α4β2 and α7 receptors, a protocol of 30-second exposure to three concentrations of the compounds was designed. In addition, to test any putative antagonistic activity of cotinine on the nAChRs, compound exposure was immediately followed without wash by a brief ACh test pulse (see arrows in Fig. 1) at a concentration near the receptor EC50. Typical currents recorded at human α4β2 and average data obtained in seven cells are illustrated in the upper panels of Fig. 1. These data illustrate that exposure to cotinine at 1, 10, or 100 μM (see horizontal bars) evokes no detectable current and causes no significant inhibition of the subsequent ACh response. Results obtained using the same experimental protocol in cells expressing human α7 nAChRs are shown in the lower panel of Fig. 1. Typical currents recorded during drug exposure show that the isomers of cotinine cause no detectable activation of α7 receptors. Average amplitude of currents was obtained in nine cells and illustrates that R-(+)- or S-(−)-cotinine causes no inhibition of the ACh responses.
Fig. 1.
Putative agonistic activity of cotinine at human α4β2 and α7 nAChRs. To evaluate the possible agonistic activity of R-(+)- or S-(−)-cotinine, cells expressing either the human α4β2 or α7 receptor were exposed to brief pulses of the compounds (30 seconds) at three different concentrations (indicated by the horizontal bars). Current evoked by a brief ACh test pulse (30 μM for α4β2 and 200 μM for α7, indicated by the arrows) was applied immediately after cotinine exposure to evaluate a possible antagonistic activity of this compound. Typical currents obtained in a cell expressing α4β2 are shown in the upper panel. Average results, normalized versus the ACh response recorded in control conditions, are shown by the histogram (n = 7). Results obtained at α7 nAChRs are shown in the lower panel, and average responses were obtained for n = 9.
Sustained Exposure to the S-(−) Isomer of Cotinine Antagonizes the ACh-Evoked Current in Human α4β2, but Not α7 nAChRs
To probe a possible longer-term effect of cotinine, cells expressing α4β2 receptors were incubated for 48 hours in the presence of 10 μM S-(−)-cotinine, and the amplitude of the current evoked by 1 mM ACh was subsequently tested. The average amplitude of current cells exposed to 10 μM cotinine was 13.3 ± 2.4 μA (n = 18), whereas the average amplitude recorded in sibling oocytes recorded at the same time but not exposed to cotinine was 25.51 ± 4.2 μA (n = 13). These data suggest that sustained exposure to cotinine reduces the amplitude of the ACh-evoked response, and that cotinine might interact with the α4β2 receptors, although on a slow time course. Experiments conducted in cells expressing the α7 nAChR with the S-(−) isomer of cotinine using the 48-hour incubation protocol in the presence of 10 μM compound revealed no significant difference attributable to cotinine. Namely, cells incubated for 48 hours with 10 μM S-(−)-cotinine showed, on average, a current of 5.85 ± 0.28 μA (n = 9) when exposed to 1 mM ACh, whereas control cells responded with 6.28 ± 1.37 (n = 7). These data indicate that sustained exposure to S-(−)-cotinine causes no significant modification of the ACh-evoked currents in human α7 nAChRs.
R-(+) and S-(−) Isomers of Cotinine Enhance the ACh-Evoked Current in Human α7 nAChRs
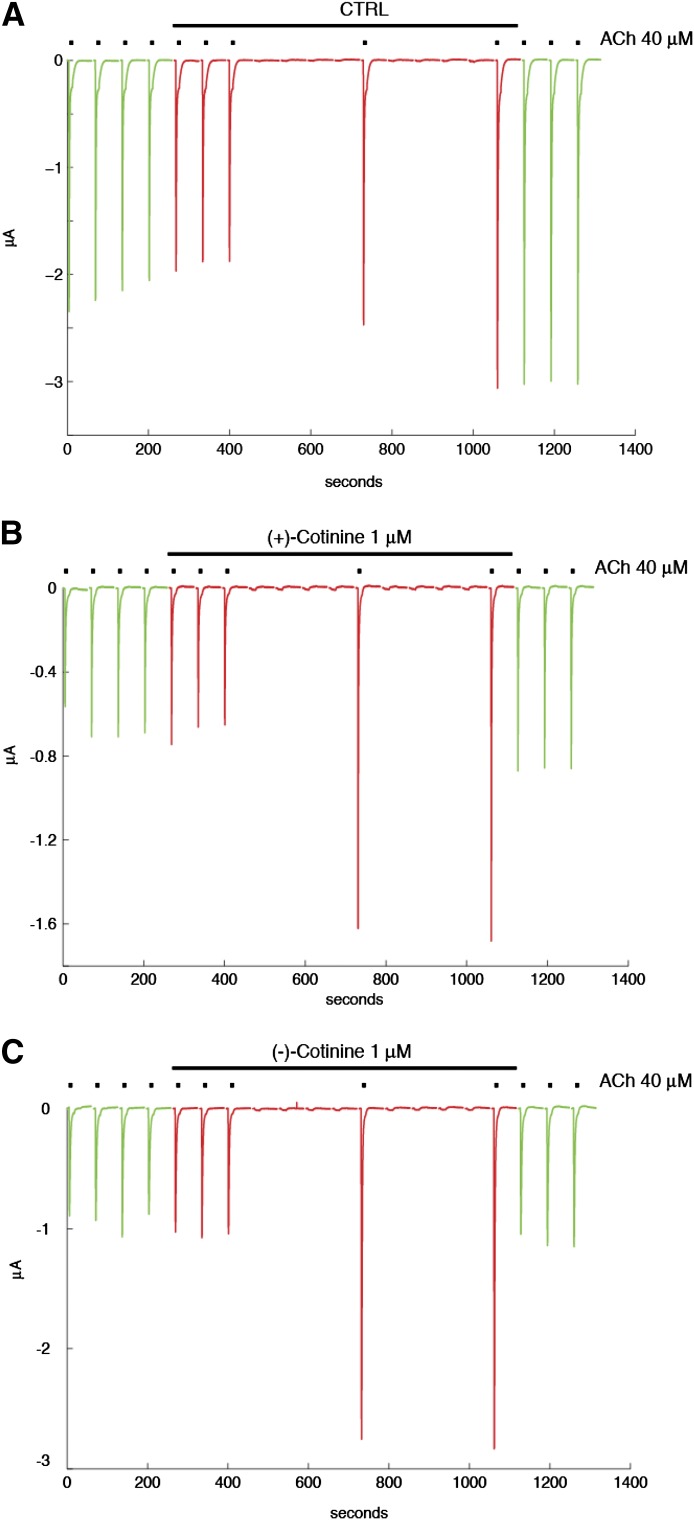
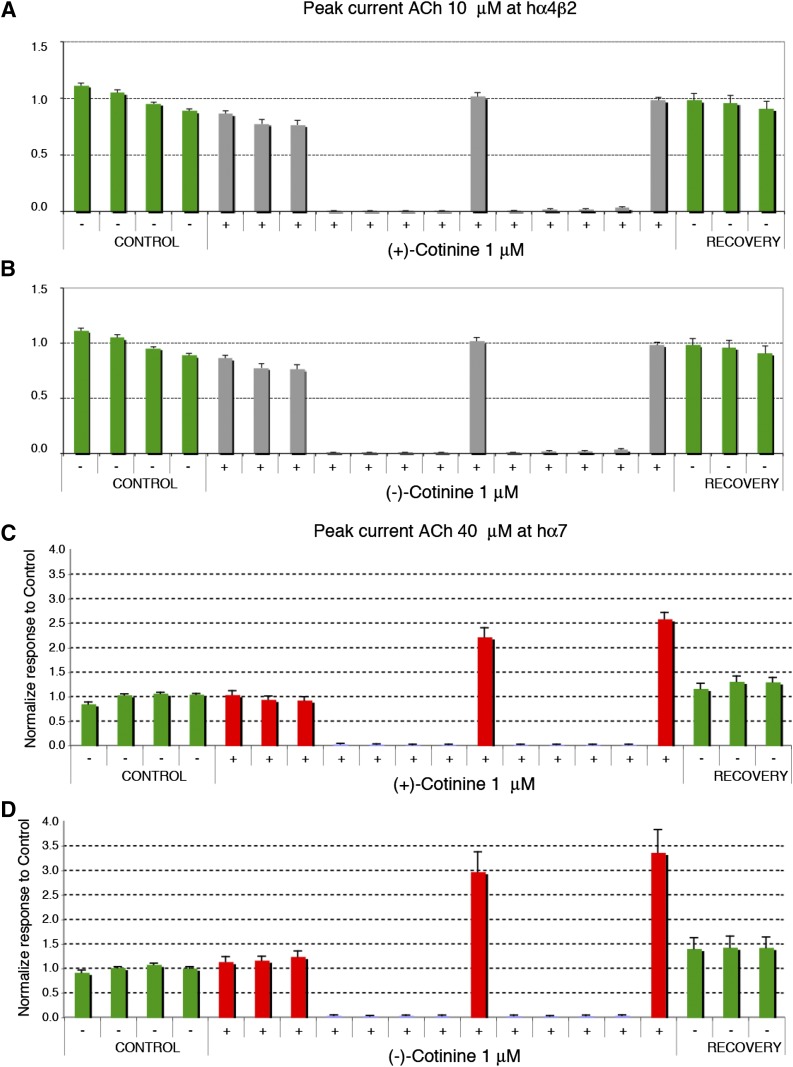
As has been shown previously, in some conditions, low concentrations of agonist can yield an unusual potentiation of ACh-evoked currents at α7 nAChRs (Wallace et al., 2010; Prickaerts et al., 2012). Thus, we tested if this protocol of irregular stimulation of the receptor would allow us to detect a possible interaction of the cotinine isomers with nAChRs. This protocol is designed to mimic the fact that neurons do not discharge in a sustained and continuous manner, but rather in bursts of different durations. Cells are exposed first at regular intervals to brief ACh test pulses to assess the stability of their responses to the ACh test pulse. After this first phase, cells are exposed to a sustained concentration of cotinine, and the ACh-evoked currents are measured for 6 minutes. Stimulation by ACh is then suspended for 8 minutes to mimic a period of silence in neuronal activity. The effect of cotinine on the ACh-evoked current is then examined by a brief ACh test pulse. The process is repeated a second time before returning to control conditions. As shown in Fig. 2A, only minor variations in the amplitude of the ACh-evoked current were observed in cells exposed to control conditions, which indicates that brief exposure to 40 μM ACh causes no major desensitization of the receptors that could be revealed by a longer time interval between applications. Strikingly different results were observed when testing the effects of R-(+)- or S-(−)-cotinine with a significant increase in the ACh-evoked current (Fig. 2, B and C). Typical currents evoked by 40 μM ACh at human α7 receptors using this protocol are illustrated in Fig. 2. Average results obtained using this same experimental protocol obtained for R-(+)- or S-(−)-cotinine at α4β2 and α7 nAChRs are shown in Fig. 3. Surprisingly, exposure to 1 μM R-(+)- or S-(−)-cotinine caused a significant enhancement of the ACh-evoked current at the α7 receptor, whereas the same experimental condition yielded no significant modification of the α4β2 response. To examine the concentration dependency of the α7 potentiation, cells were exposed either to 0.1 or 10 μM R-(+)- or S-(−)-cotinine using the same experimental paradigm and are summarized (with the response to 1.0 μM concentrations of the isomers) in the histogram in Fig. 4. Collectively, these data suggest that both the R-(+) and S-(−) isomers of cotinine can modulate the ACh-evoked current at the α7 nAChRs with a maximal efficacy at about 1 μM, and should yield functional differences in vivo.
Fig. 2.
Sustained exposure to R-(+)- and S-(−)-cotinine at human α7 nAChRs. Probing the effects of sustained exposure to 1 μM cotinine on currents evoked by 40 μM ACh was conducted using the irregular stimulation paradigm described in Prickaerts et al. (2012). ACh-evoked currents were recorded first in control using a 2-minute interval between brief ACh test pulse (40 μM, 5 seconds, green traces). Exposure to 1 μM cotinine was then applied (indicated by the horizontal bar above the traces), and currents evoked by 40 μM were tested at irregular intervals (red traces). Note the enhancement of the response observed after the 8-minute time period (middle trace) in the α7 nAChRs treated with both the R-(+) isomer (B) and S-(−) isomer (C) of cotinine. Recovery from cotinine exposure was determined by applying the same ACh test pulses at regular intervals upon return in control (green traces). A control trace obtained without cotinine is presented in (A). CTRL, control.
Fig. 3.
Average effects of sustained exposure to 1 μM R-(+)- or S-(−)-cotinine at the human α4β2 (A and B, respectively) and α7 nAChRs (C and D, respectively). Experiments were conducted using the same experimental protocol shown in Fig. 2. Currents were normalized to the average response recorded in control and represented in the form of histograms. Bars indicate the standard error for n = 9 for S-(−)-cotinine at α4β2, and n = 5 for α7 and n = 6 for R-(+)-cotinine at α4β2 and n = 5 for α7. Green bars indicate the ACh-evoked responses recorded in control, and gray or red bars indicate the response recorded during cotinine exposure. Note the difference in scale used for the α4β2 and α7 graphs.
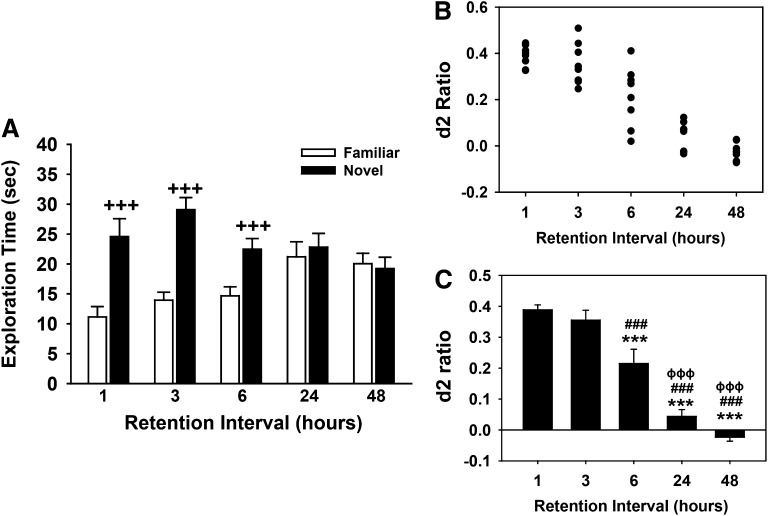
Performance of a Rodent Task of Recognition Memory Is Delay Dependent
The effects of different delay intervals in the NOR task (A/B retention sessions) are provided in Fig. 5. The individual exploration times of the novel and familiar objects are illustrated in Fig. 5A, with the calculated discrimination (d2) ratios illustrated as scatter plots and histograms in Fig. 5, B and C, respectively. As shown, there was a delay-dependent decrease in preference for the novel object with subjects displaying complete forgetting of the familiar object at the 24- and 48-hour time points, with discrimination (d2) ratios near zero. Statistical analysis of exploration times revealed the following: main effect of delay [F(4,35) = 0.86, P = 0.50], object type [F(1,35) = 171.57, P < 0.001], delay by object type interaction [F(4,35) = 30.59, P < 0.001]. Post-hoc analysis indicated that there was a significant preference for the novel object (i.e., versus the familiar object; P < 0.001) at the 1-, 3-, and 6-hour delays, but that this preference was lost at the longer delays. This (delay-related) effect on preference for the novel object was also evident when d2 ratios were analyzed [F(4,35) = 41.40, P < 0.001; see Fig. 5 for the significant differences between individual delays].
Fig. 5.
Delay-dependent decrease in performance (recognition memory) by young Wistar rats in a spontaneous NOR procedure. (A) Mean (± S.E.M.) exploration times of the familiar and novel objects (A/B retention sessions). (B) Scatter plots of discrimination (d2) ratios by individual rats. (C) Mean (± S.E.M.) discrimination (d2) ratios illustrated as histograms. d2 ratio = (novel − familiar)/(novel + familiar). Bars represent the mean (± S.E.M.), N = 8 rats per delay condition. +++P < 0.001 novel versus familiar object; ***P < 0.001, significantly different from the 1-hour time point; ###P < 0.001 significantly, different from the 3-hour time point; φφφP < 0.001, significantly different from the 6-hour time point.
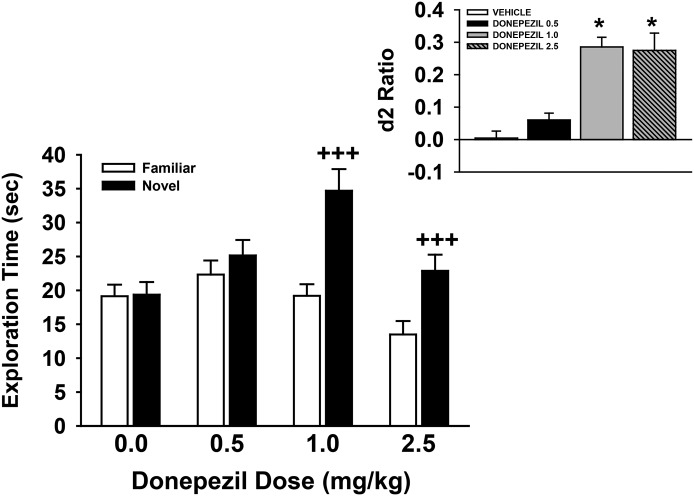
Donepezil Is Associated with Dose-Dependent Improvements in Recognition Memory
The effects of the AD treatment, donepezil, in the NOR task (A/B retention sessions) after a 48-hour retention interval are provided in Fig. 6. The individual exploration times of the novel and familiar objects are illustrated in the main figure, with the calculated discrimination (d2) ratios illustrated in the inset. As shown, donepezil was associated with a dose-dependent increase in preference for the novel object [main effect of dose: F(3,29) = 4.0, P = 0.017; object type: F(1,29) = 74.9, P < 0.001; dose by object type interaction: F(3,29) = 18.2, P < 0.001]. Post-hoc analysis indicated that the 1.0- and 2.5-mg/kg doses of donepezil were associated with a significant preference for the novel object (P < 0.001 versus familiar). This (dose-related) effect of donepezil was also observed when the d2 ratios were analyzed [F(3,29) = 19.0, P < 0.001; see Fig. 6 for the significant dose-related difference in d2 ratios].
Fig. 6.
Dose-related effects of donepezil on performance of a spontaneous novel object recognition task by young Wistar rats. In these experiments, donepezil (or vehicle) was administered by intraperitoneal injection 30 minutes before the training trial. Mean (± S.E.M.) exploration times of the familiar and novel objects after 48-hour delays (A/B retention sessions) are illustrated in the main figure. The inset illustrates the mean (± S.E.M.) discrimination (d2) ratios. d2 ratio = (novel − familiar)/(novel + familiar). +++P < 0.001, novel versus familiar object; *P < 0.05 versus vehicle. N = 8–9 for each group.
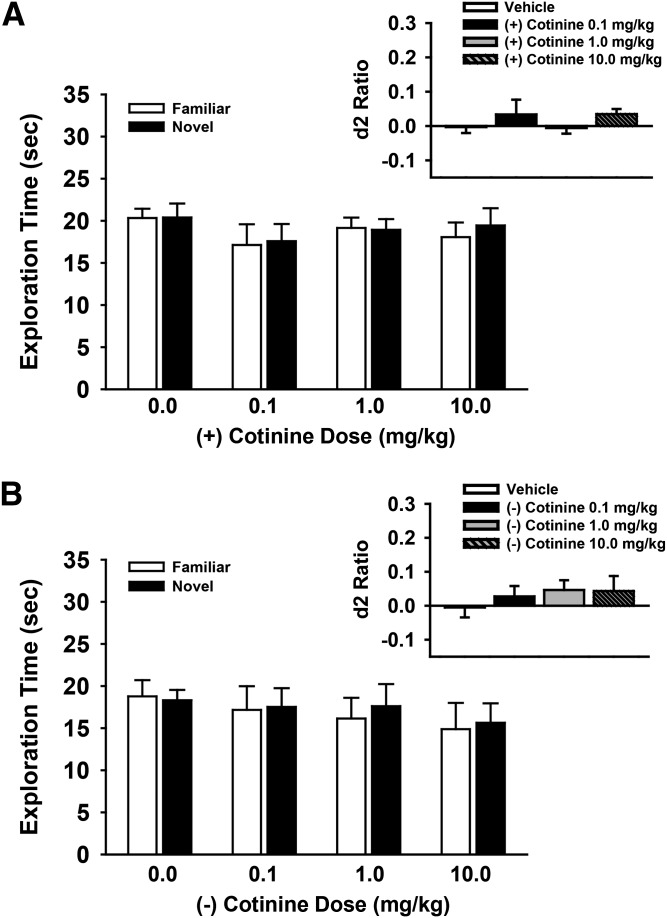
R-(+) and S-(−) Isomers of Cotinine (Administered Alone) Do Not Affect Performance of the NOR Task
The effects of different doses (0.1–10.0 mg/kg) of R-(+)- and S-(−)-cotinine in the NOR task (A/B retention sessions) after a 48-hour retention interval are provided in Fig. 7, A and B, respectively. The individual exploration times of the novel and familiar objects are illustrated in the main figure, with the calculated discrimination (d2) ratios illustrated in the figure insets. There were no significant effects of either isomer of cotinine at any of the doses that were evaluated (main effects of dose and the dose by object type interactions, P > 0.05).
Fig. 7.
Dose-related effects of R-(+)-cotinine (A) and S-(−)-cotinine (B) in the NOR task on performance of a spontaneous novel object recognition task by young Wistar rats. In these experiments, cotinine (or vehicle) was administered by intraperitoneal injection 30 minutes before the training trial. Mean (± S.E.M.) exploration times of the familiar and novel objects after 48-hour delays (A/B retention sessions) are illustrated in the main figures. Insets illustrate the mean (± S.E.M.) discrimination (d2) ratios. d2 ratio = (novel − familiar)/(novel + familiar). N = 6 for each group.
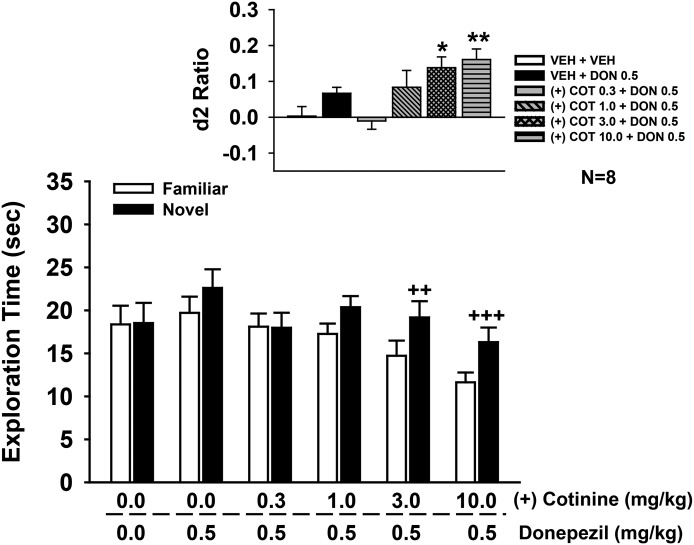
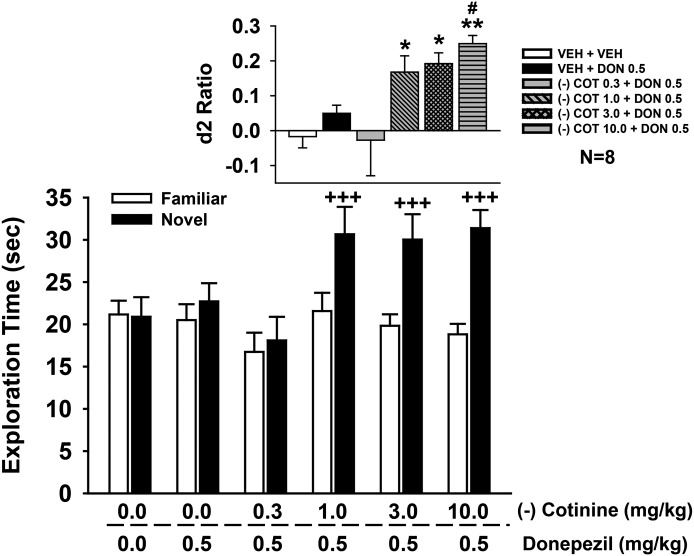
R-(+) Isomer of Cotinine Enhances the Effects of a Subthreshold Dose of Donepezil on NOR Performance
In these experiments, several doses of R-(+)-cotinine were combined with a subthreshold dose of donepezil (i.e., 0.5 mg/kg) operationally defined as a dose that did not significantly affect NOR performance in prior studies. The combined drug effects on the individual exploration times of the novel and familiar objects are illustrated in the main part of Fig. 8, with the calculated discrimination (d2) ratios illustrated in the inset. Statistical analysis of object exploration revealed the following: main effect of treatment [F(5,42) = 2.0, P = 0.11], object type [F(1,42) = 33.6, P < 0.001], treatment by object type interaction [F(5,42) = 3.76, P = 0.007]. Post-hoc analysis indicated that the combinations of 3.0 and 10.0 mg/kg R-(+)-cotinine with 0.5 mg/kg donepezil were associated a significant preference for the novel object (P < 0.01 versus familiar). This effect was also observed in the statistical analysis of d2 ratios [main effect of treatment: F(5,42) = 5.25, P < 0.001; see Fig. 8 for the significant treatment-related difference in d2 ratios].
Fig. 8.
Effects of coadministration of a subthreshold dose of donepezil (0.5 mg/kg i.p.) plus R-(+)-cotinine (1.0–10 mg/kg i.p.) on performance of a spontaneous novel object recognition task. In these experiments, donepezil (or vehicle) was administered 30 minutes before the training trial followed immediately by the specific test dose of cotinine. Mean (± S.E.M.) exploration times of the familiar and novel objects after 48-hour delays (A/B retention sessions) are illustrated in the main figure. Inset illustrates the mean (± S.E.M.) discrimination (d2) ratios. d2 ratio = (novel − familiar)/(novel + familiar). ++P < 0.01; +++P < 0.001 novel versus familiar object; *P < 0.05; **P < 0.01 significantly different from vehicle response. N = 8 for each group. COT, cotinine; DON, donepezil; VEH, vehicle.
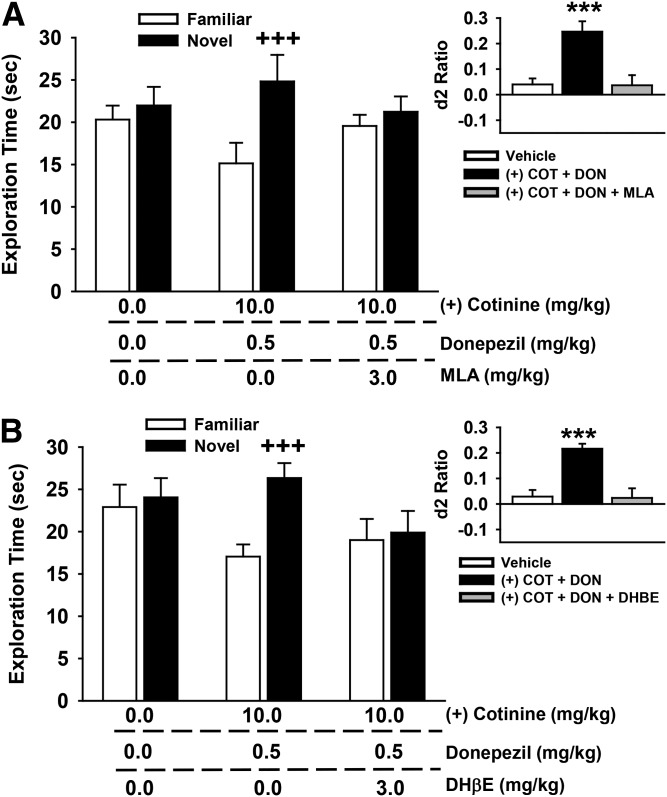
Selective Nicotinic Antagonists Block the Positive Effects of R-(+)-Cotinine Plus Donepezil on NOR Performance
In these experiments (Fig. 9), test subjects were pretreated with either the α7-selective nAChR antagonist MLA (Fig. 9A) or the α4β2-selective nAChR antagonist DHβE (Fig. 9B) before administration of an active dose combination of R-(+)-cotinine and donepezil (identified in the previous set of studies). The individual exploration times of the novel and familiar objects are illustrated in the main portion of Fig. 9, with the calculated discrimination (d2) ratios illustrated in the insets. For the MLA experiments, the following statistical results were obtained: main effect of treatment [F(2,21) = 0.08, P = 0.92], object type [F(1,21) = 18.7, P < 0.001], treatment by object type interaction [F(2,21) = 7.10, P = 0.004]. Post-hoc analysis indicated that the combination of 10.0 mg/kg R-(+)-cotinine with 0.5 mg/kg donepezil was associated with a significant preference for the novel object (P < 0.001 versus familiar), and that 3.0 mg/kg MLA blocked this effect. The same effect was observed when the d2 ratios were analyzed [main effect of treatment: F(2,21) = 9.21, P = 0.001; see Fig. 9 for the significant treatment-related difference in d2 ratios]. For the DHβE experiments, the following statistical results were obtained: main effect of treatment [F(2,21) = 0.91, P = 0.42], object type [F(1,21) = 45.17, P < 0.001], treatment by object type interaction [F(2,21) = 24.15, P < 0.001]. Post-hoc analysis indicated (again) that the combination of 10.0 mg/kg R-(+)-cotinine with 0.5 mg/kg donepezil was associated with a significant preference for the novel object (P < 0.001 versus familiar), and that 3.0 mg/kg DHβE blocked this effect. The same effect was observed when the d2 ratios were analyzed [main effect of treatment: F(2,21) = 16.46, P < 0.001; see Fig. 9 for the significant treatment-related difference in d2 ratios].
Fig. 9.
Effects of coadministration of a subthreshold dose of donepezil (0.5 mg/kg), an active dose of R-(+)-cotinine (10 mg/kg), and the nicotinic antagonists methyllycaconitine (A) and dihydro-β-erythroidine (B) on performance of a spontaneous novel object recognition task. In these experiments, the antagonist was administered first (60 minutes before the NOR training trial) followed 30 minutes later (i.e., 30 minutes before the NOR training trial) with the combination of donepezil and cotinine. All compounds were administered by intraperitoneal injection. Mean (± S.E.M.) exploration times of the familiar and novel objects after 48-hour delays (A/B retention sessions) are illustrated in the main figures. Insets illustrate the mean (± S.E.M.) discrimination (d2) ratios. d2 ratio = (novel − familiar)/(novel + familiar). +++P < 0.001, novel versus familiar object; ***P < 0.001 significantly different from vehicle response. N = 8 for each group. COT, cotinine; DON, donepezil.
S-(−) Isomer of Cotinine Enhances the Effects of a Subthreshold Dose of Donepezil on NOR Performance
In the next set of experiments, several doses of S-(−)-cotinine were combined with a subthreshold dose of donepezil. The combined drug effects on the individual exploration times of the novel and familiar objects are illustrated in the main part of Fig. 10, with the calculated discrimination (d2) ratios illustrated in the inset. Statistical analysis of object exploration revealed the following: main effect of treatment [F(5,42) = 2.5, P = 0.045], object type [F(1,42) = 66.81, P < 0.001], treatment by object type interaction [F(5,42) = 9.44, P < 0.001]. Post-hoc analysis indicated that the combinations of 1.0, 3.0, and 10.0 mg/kg S-(−)-cotinine with 0.5 mg/kg donepezil were associated with a significant preference for the novel object (P < 0.001 versus familiar). This effect was also observed in the statistical analysis of d2 ratios [main effect of treatment: F(5,42) = 5.20, P < 0.001; see Fig. 10 for the significant treatment-related difference in d2 ratios].
Fig. 10.
Effects of coadministration of a subthreshold dose of donepezil (0.5 mg/kg i.p.) plus S-(−)-cotinine (1.0–10 mg/kg i.p.) on performance of a spontaneous novel object recognition task. In these experiments, donepezil (or vehicle) was administered 30 minutes before the training trial followed immediately by the specific test dose of cotinine. Mean (± S.E.M.) exploration times of the familiar and novel objects after 48-hour delays (A/B retention sessions) are illustrated in the main figure. The inset illustrates the mean (± S.E.M.) discrimination (d2) ratios. d2 ratio = (novel − familiar)/(novel + familiar). +++P < 0.001, novel versus familiar object; *P < 0.05; **P < 0.01, significantly different from vehicle response. #P < 0.05 significantly different versus DON alone response. N = 8 for each group. COT, cotinine; DON, donepezil; VEH, vehicle.
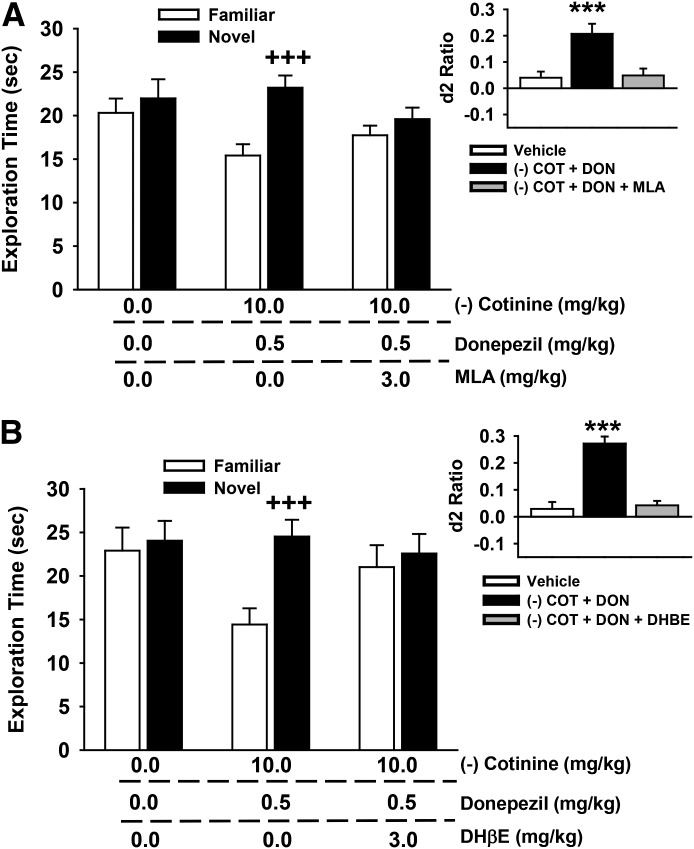
Selective Nicotinic Antagonists Block the Positive Effects of S-(−)-Cotinine and Donepezil on NOR Performance
As in the case of the R-(+) isomer of cotinine, in subsequent experiments (Fig. 11), we pretreated test subjects with either MLA (Fig. 11A) or DHβE (Fig. 11B) before administering an active dose combination of R-(−)-cotinine and donepezil. The individual exploration times of the novel and familiar objects are illustrated in the main portion of the figure, with the calculated discrimination (d2) ratios illustrated in the inset. For the MLA experiments, the following statistical results were obtained: main effect of treatment [F(2,21) = 0.74, P = 0.49], object type [F(1,21) = 24.0, P < 0.001], treatment by object type interaction [F(2,21) = 6.84, P = 0.005]. Post-hoc analysis indicated that the combination of 10.0 mg/kg S-(−)-cotinine with 0.5 mg/kg donepezil was associated with a significant preference for the novel object (P < 0.001 versus vehicle), and that 3.0 mg/kg MLA blocked this effect. The same effect was observed when the d2 ratios were analyzed [main effect of treatment: F(2,21) = 7.27, P = 0.004; see Fig. 11 for the significant treatment-related difference in d2 ratios]. For the DHβE experiments, the following statistical results were obtained: main effect of treatment [F(2,21) = 0.86, P = 0.44], object type [F(1,21) = 89.76, P < 0.001], treatment by object type interaction [F(2,21) = 41.90, P < 0.001]. Post-hoc analysis indicated that the combination of 10.0 mg/kg S-(−)-cotinine with 0.5 mg/kg donepezil was associated with a significant preference for the novel object (P < 0.001 versus vehicle), and that 3.0 mg/kg DHβE blocked this effect. The same effect was observed when the d2 ratios were analyzed [main effect of treatment: F(2,21) = 41.15, P < 0.001; see Fig. 11 for the significant treatment-related difference in d2 ratios].
Fig. 11.
Effects of coadministration of a subthreshold dose of donepezil (0.5 mg/kg), an active dose of S-(−)-cotinine (10 mg/kg), and the nicotinic antagonists methyllycaconitine (A) and dihydro-β-erythroidine (B) on performance of a spontaneous novel object recognition task. In these experiments, the antagonist was administered first (60 minutes before the NOR training trial) followed 30 minutes later (i.e., 30 minutes before the NOR training trial) with the combination of donepezil and cotinine. All compounds were administered by intraperitoneal injection. Mean (± S.E.M.) exploration times of the familiar and novel objects after 48-hour delays (A/B retention sessions) are illustrated in the main figures. Insets illustrate the mean (± S.E.M) discrimination (d2) ratios. d2 ratio = (novel × familiar)/(novel + familiar). +++P < 0.001, novel versus familiar object; ***P < 0.001, significantly different from vehicle response. COT, cotinine; DON, donepezil. N = 8 for each group.
Discussion
The most notable results of this study can be summarized as follows: 1) the R-(+) and the S-(−) isomers of cotinine appear to be relatively inactive across a wide range of potential pharmacologic targets, including those that might have relevance to neuropsychiatric disorders or be associated with adverse drug reactions; 2) however, in electrophysiological studies, both isomers of cotinine significantly increased responses evoked by low concentrations of acetylcholine in oocytes expressing the human α7 nAChR; 3) in the behavioral (NOR) studies, both isomers of cotinine enhanced NOR performance when coadministered with a subthreshold dose of donepezil, whereas neither isomer was active alone; and 4) the positive effects of the combinations of the isomers of cotinine and donepezil on NOR performance were blocked by MLA and DHβE, indicating that both α7 and α4β2 nAChRs contribute to this specific behavioral response.
The electrophysiological studies conducted in human receptors expressed in Xenopus oocytes indicate without ambiguity that the R-(+) and the S-(−) isomers of cotinine (when administered in brief pulses of 30 seconds) do not evoke inward currents at either the α4β2 or α7 receptor, nor do they cause any significant inhibition of these receptors. These data are in agreement with previously reported studies (Briggs and McKenna, 1998). This suggests that in vivo, at concentrations relevant to those commonly observed in smokers’ blood (e.g., 250–300 ng/ml or 1.4–1.7 μM; see Hukkanen et al., 2005), cotinine does not act as an agonist or antagonist at α4β2 or α7 receptors. We did, however, observe that sustained (48-hour) exposure to S-(−)-cotinine reduced the amplitude of the ACh-evoked response in α4β2, but not α7 receptors, thus indicating that cotinine might interact with α4β2 receptors, although on a slow time course. As the reduction of the amplitude of current can occur through different mechanisms, it would be too speculative to interpret these data further at this point.
Additional electrophysiological experiments were conducted with a low concentration of ACh applied at irregular intervals, using a protocol that unveiled the coagonist activity of RG 3487 (N-[(3S)-1-azabicyclo[2.2.2]oct-3-yl]-1H-indazole-3-carboxamide hydrochloride) (Wallace et al., 2010) and EVP-6124 [(R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide] at α7 nAChRs (Prickaerts et al., 2012). Experiments conducted with this protocol at human α4β2 receptors showed no significant effect of cotinine (see Fig. 2), whereas under the same experimental conditions, a major effect was observed at α7 nAChRs. These data clearly indicate that exposure to 1 μM R-(+)- or S-(−)-cotinine enhances the response of α7 receptors to 40 μM ACh, an effect that could explain the procognitive effects observed with cotinine treatment in NOR experiments. These results are suggestive of an interaction of cotinine with the α7 binding site, which is supported by the observation that cotinine acts, albeit at a high concentration, as an agonist at the L9′T mutant of α7 (Briggs et al., 1999).
Although there was some evidence in both the electrophysiological experiments with α7 nAChRs (at the 1 μM concentration; see Fig. 4) and the NOR experiments, where cotinine was combined with donepezil (see Figs. 8 and 10, respectively), that the responses to the R-(+) isomer might be somewhat lower in magnitude when compared with the S-(−) isomer, there was little evidence of clear (i.e., logarithmic) differences in stereospecificity detected in this study. Although these observations are bit difficult to interpret, the behavioral effects might imply that the compounds have subtle effects across multiple targets (i.e., at different nAChR subtypes or at other receptors not yet identified). The fact that both an α7 nAChR antagonist (MLA) and an α4β2 antagonist (DHβE) blocked the effects of the drug combination does imply that both of these nAChR subtypes contribute to the capacity of the cotinine isomers to amplify the effects of donepezil on synaptic acetylcholine levels. Interestingly, in a sensory inhibition paradigm in DBA/2 mice, blockade of α4β2 nAChRs with DHβE or α7 nAChRs with α-bungarotoxin blocked the increase in the conditioning amplitude and sensory gating improvements (respectively) induced by cotinine (Robb et al., 2013; Wildeboer-Andrud et al., 2014), indicating that both nAChR subtypes contribute to these in vivo responses as well.
Although it is always a challenge to reconcile data obtained in vitro with results obtained in vivo using very different approaches, results obtained from brain slice experiments are shining a new light on our understanding of the role of nAChRs in brain circuits (Arroyo et al., 2014; Bloem et al., 2014). These studies clearly show that α7 and α4β2 nAChRs are expressed in different layers of the cortex, but that some interneurons express both types of receptors as shown by MLA and DHβE inhibition (Bennett et al., 2012). Similar observations were also reported for recordings in the hippocampus (Alkondon and Albuquerque, 1993, 2001; Christophe et al., 2002). Moreover, in this work, it was shown that treatment with an acetylcholinesterase inhibitor (ambenonium dichloride) caused a marked slowing down of the slow phase of the response time course of synaptic potentials evoked by basal forebrain stimulations. These results were interpreted as reflecting the slowing down of degradation of ACh, which caused a more prolonged response at α4β2 receptors that was sensitive to DHβE (Bennett et al., 2012). These dual phases in the synaptic-evoked currents with α7 and α4β2 receptors suggest that both the transient and phasic responses might contribute to the regulation of brain function, and it was shown that α4β2 responses of interneurons can cause a prolonged disynaptic inhibition of cortical neurons in the mouse forebrain (Arroyo et al., 2012). The complexity of receptor expression with the phasic and tonic responses evoked in the same cell might reconcile the puzzling observation (noted earlier) that the procognitive effects of cotinine were inhibited by both MLA and DHβE. Namely, as procognitive effects of cotinine are observed in the presence of a low concentration of acetylcholinesterase inhibitor, the subsequent exposure to DHβE might reduce the α4β2 component of the response that is otherwise indispensable to unveil the enhancement of α7 activity caused by cotinine.
The ability of the isomers of cotinine to effectively increase the response to ACh at α7 nAChRs may have several implications. The α7 nAChR has long been considered a therapeutic target in disorders such as AD and schizophrenia given the deficits in α7 nAChR protein that have been observed in the brains of patients who suffered from these disorders (Freedman et al., 1995; Burghaus et al., 2000; Guan et al., 2000). Moreover, α7 nAChRs are abundant in the hippocampus and prefrontal cortex (important structures for cognition and AD; reviewed in Gotti et al., 2007), and they modulate several calcium-dependent events in neurons, including neurotransmitter release (McGehee et al., 1995; Gray et al., 1996), postsynaptic signaling (Chang and Berg, 1999; Hefft et al., 1999), and neuronal survival (Messi et al., 1997; Berger et al., 1998). In addition, agonists of α7 nAChRs have been shown to increase the phosphorylation of extracellular signal-regulated kinase and cAMP response element-binding protein (signaling pathways linked long-term potentiation and memory formation) in the rodent brain (Bitner et al., 2007, 2010) and to improve performance in a variety of learning and memory-related tasks in animals (for review, see Kem, 2000).
The ability of the isomers of cotinine to improve the procognitive dose range of donepezil could also have important clinical implications, and there is significant interest in the AD field in any strategy that might enhance the efficacy of the currently available treatments (see Riordan et al., 2011). One limitation to donepezil [and other acetylcholinesterase inhibitor (AChEIs)] is the variety of dose-limiting side effects that may prevent the administration of doses that are high enough for optimal effects on cognition. Although both muscarinic acetylcholine receptors and nAChRs are considered important therapeutic targets in AD, doses of AChEIs high enough to significantly improve nAChR signaling (via the increase in synaptic acetylcholine) are often accompanied by adverse reactions (e.g., nausea, vomiting, and diarrhea) that likely result from muscarinic overstimulation (see Maelicke and Albuquerque, 2000). Accordingly, an alternative (nAChR-based) treatment strategy that would theoretically be less susceptible to adverse reactions would be to selectively activate or “sensitize” nAChRs to acetylcholine, thus allowing for lower doses of the AChEI to be used. Recently, we tested an adjunctive treatment strategy which included the nicotinic positive allosteric modulator, PNU-120596 [N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea], and subthreshold doses of donepezil and found it to be effective in both aged rats and aged monkeys (Callahan et al., 2013). The results of the current study indicate that the isomers of cotinine might also serve as an important part of a similar adjunctive strategy.
The translational significance of the rodent behavioral experiments described in this report is also an important subject of discussion. Object recognition memory is one of the domains of cognition that is often impaired in aged (nondemented) individuals as well as in patients with AD (Flicker et al., 1987; Purdy et al., 2002; Schiavetto et al., 2002). The rodent NOR task has been described as a model of (nonspatial) recognition memory (Ennaceur and Delacour, 1988). This form of memory is believed to consist of a recollective (episodic) and a familiarity component (Squire et al., 2004), i.e., behaviors that are demonstrated in the NOR task when subjects explore a novel object more than a familiar one. Moreover, there is considerable (albeit debated) evidence that the hippocampus (an important structure in the neuropathology of AD) is actively involved in object recognition memory in both rodents (Myhrer, 1988; Rampon et al., 2000; Broadbent et al., 2004) and humans (Squire, 1992; Reed and Squire, 1997).
In conclusion, the results of this study indicate that cotinine may sensitize α7 nAChRs to low levels of acetylcholine (a previously uncharacterized mechanism), and that both the R-(+) and S-(−) isomers of cotinine could be used as part of an adjunctive treatment strategy to improve the effective dose range of cholinergic compounds (e.g., donepezil) used in AD. Our behavioral data in young rats also support the premise that neither the procognitive effects of donepezil nor the donepezil-cotinine combination requires innate cholinergic deficits (as are normally associated with old age or AD), suggesting potential applications across a wide range of cognitive disorders.
Acknowledgments
The authors thank Ashley Davis for administrative assistance in preparing this article. The authors also thank Sonia Bertrand for help in performing the electrophysiological experiments.
Abbreviations
- ACh
acetylcholine
- AChEI
acetylcholinesterase inhibitor
- AD
Alzheimer’s disease
- DHβE
dihydro-β-erythroidine
- EVP-6124
(R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide
- MK-801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- NOR
novel object recognition
- PNU-120596
N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea
- RG 3487
N-[(3S)-1-azabicyclo[2.2.2]oct-3-yl]-1H-indazole-3-carboxamide hydrochloride
Authorship Contributions
Participated in research design: Terry, Callahan, Bertrand.
Conducted experiments: Callahan, Bertrand.
Performed data analysis: Terry, Callahan, Bertrand.
Wrote or contributed to the writing of the manuscript: Terry, Callahan, Bertrand.
Footnotes
The work described in this manuscript was supported by the National Institutes of Health National Institute on Aging [Grant R01-AG029617]; and the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA029127].
References
- Abood LG, Reynolds DT, Booth H, Bidlack JM. (1981) Sites and mechanisms for nicotine’s action in the brain. Neurosci Biobehav Rev 5:479–486. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. (1993) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther 265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. (2001) Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol 86:3043–3055. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Arneric SP. (1994) Nicotinic receptor binding of [3H]cytisine, [3H]nicotine and [3H]methylcarbamylcholine in rat brain. Eur J Pharmacol 253:261–267. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. (2012) Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci 32:3859–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo S, Bennett C, Hestrin S. (2014) Nicotinic modulation of cortical circuits. Front Neural Circuits 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HM, Lees K, Harper KM, Jones AK, Sattelle DB, Wonnacott S, Wolstenholme AJ. (2012) Xenopus laevis RIC-3 enhances the functional expression of the C. elegans homomeric nicotinic receptor, ACR-16, in Xenopus oocytes. J Neurochem 123:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. (1996) Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 18:188–204. [DOI] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. (1998) Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci 18:6871–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, et al. (2007) Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci 27:10578–10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, Marsh KC, Nikkel AL, Browman K, Radek R, et al. (2010) In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer's disease. J Pharmacol Exp Ther 334:875–886. [DOI] [PubMed] [Google Scholar]
- Bloem B, Poorthuis RB, Mansvelder HD. (2014) Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity. Front Neural Circuits 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG. (1998) Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology 37:1095–1102. [DOI] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG, Monteggia LM, Touma E, Roch JM, Arneric SP, Gopalakrishnan M, Sullivan JP. (1999) Gain of function mutation of the alpha7 nicotinic receptor: distinct pharmacology of the human alpha7V274T variant. Eur J Pharmacol 366:301–308. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101:14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV., Jr (2003) The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sci 72:2931–2942. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV., Jr (2009) A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochem Pharmacol 78:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Zeitlin R, Echeverria V. (2011) Cotinine inhibits amyloid-β peptide neurotoxicity and oligomerization. J Clinic Toxicol S6:003. [Google Scholar]
- Burghaus L, Schütz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, Lindstrom J, Schröder H. (2000) Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res Mol Brain Res 76:385–388. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Hutchings EJ, Kille NJ, Chapman JM, Terry AV., Jr (2013) Positive allosteric modulator of α7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology 67:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Terry AV, Jr, Tehim A. (2014) Effects of the nicotinic α7 receptor partial agonist GTS-21 on NMDA-glutamatergic receptor related deficits in sensorimotor gating and recognition memory in rats. Psychopharmacology (Berl) 231:3695–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KT, Berg DK. (1999) Nicotinic acetylcholine receptors containing alpha7 subunits are required for reliable synaptic transmission in situ. J Neurosci 19:3701–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, Audinat E. (2002) Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol 88:1318–1327. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Crooks PA. (1999) (S)-(-)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther 288:905–911. [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R. (2012) Cotinine: a potential new therapeutic agent against Alzheimer’s disease. CNS Neurosci Ther 18:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirschner DA, et al. (2011) Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J Alzheimers Dis 24:817–835. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31:47–59. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Crook T, Bartus RT. (1987) A visual recognition memory test for the assessment of cognitive function in aging and dementia. Exp Aging Res 13:127–132. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. (1995) Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33. [DOI] [PubMed] [Google Scholar]
- Gao J, Adam BL, Terry AV., Jr (2014) Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer's disease. Bioorg Med Chem Lett 24:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. (2007) Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74:1102–1111. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. (1996) Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383:713–716. [DOI] [PubMed] [Google Scholar]
- Grizzell JA, Echeverria V. (2014) New Insights into the Mechanisms of Action of Cotinine and its Distinctive Effects from Nicotine. Neurochem Res [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Grizzell JA, Iarkov A, Holmes R, Mori T, Echeverria V. (2014) Cotinine reduces depressive-like behavior, working memory deficits, and synaptic loss associated with chronic stress in mice. Behav Brain Res 268:55–65. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. (2000) Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J Neurochem 74:237–243. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Grillo M, Pentel PR, Oncken C, Bliss R. (1997) Safety of cotinine in humans: physiologic, subjective, and cognitive effects. Pharmacol Biochem Behav 57:643–650. [DOI] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. (1999) Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol 515:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Bandelier F, Benoit A, Dosch R, Bertrand D. (2008) An automated system for intracellular and intranuclear injection. J Neurosci Methods 169:65–75. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57:79–115. [DOI] [PubMed] [Google Scholar]
- Kem WR. (2000) The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21). Behav Brain Res 113:169–181. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Albuquerque EX. (2000) Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer's disease. Eur J Pharmacol 393:165–170. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. (1995) Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269:1692–1696. [DOI] [PubMed] [Google Scholar]
- Messi ML, Renganathan M, Grigorenko E, Delbono O. (1997) Activation of alpha7 nicotinic acetylcholine receptor promotes survival of spinal cord motoneurons. FEBS Lett 411:32–38. [DOI] [PubMed] [Google Scholar]
- Myhrer T. (1988) The role of medial and lateral hippocampal perforant path lesions and object distinctiveness in rats’ reaction to novelty. Physiol Behav 42:371–377. [DOI] [PubMed] [Google Scholar]
- O’Leary K, Parameswaran N, McIntosh JM, Quik M. (2008) Cotinine selectively activates a subpopulation of alpha3/alpha6beta2 nicotinic receptors in monkey striatum. J Pharmacol Exp Ther 325:646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Grizzell JA, Holmes R, Zeitlin R, Solomon R, Sutton TL, Rohani A, Charry LC, Iarkov A, Mori T, et al. (2014) Cotinine halts the advance of Alzheimer's disease-like pathology and associated depressive-like behavior in Tg6799 mice. Front Aging Neurosci 6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, Reneerkens OA, Flood DG, Hilt D, Gawryl M, et al. (2012) EVP-6124, a novel and selective α7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of α7 nicotinic acetylcholine receptors. Neuropharmacology 62:1099–1110. [DOI] [PubMed] [Google Scholar]
- Purdy KS, McMullen PA, Freedman M. (2002) Changes to the object recognition system in patients with dementia of the Alzheimer’s type. Brain Cogn 49:213–216. [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. (2000) Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 3:238–244. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. (1997) Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci 111:667–675. [DOI] [PubMed] [Google Scholar]
- Riordan KC, Hoffman Snyder CR, Wellik KE, Caselli RJ, Wingerchuk DM, Demaerschalk BM. (2011) Effectiveness of adding memantine to an Alzheimer dementia treatment regimen which already includes stable donepezil therapy: a critically appraised topic. Neurologist 17:121–123. [DOI] [PubMed] [Google Scholar]
- Robb H, McGaraughty S, Lee C-H, Radek R (2013) Effects of the nicotinic metabolite cotinine on EGG evoked potential sensory gating in DBA/2 mice. Society for Neuroscience, p702.16.
- Rosecrans JA. (1979) Nicotine as a discriminative stimulus to behavior: its characterization and relevance to smoking behavior. NIDA Res Monogr (23):58–69. [PubMed] [Google Scholar]
- Schiavetto A, Köhler S, Grady CL, Winocur G, Moscovitch M. (2002) Neural correlates of memory for object identity and object location: effects of aging. Neuropsychologia 40:1428–1442. [DOI] [PubMed] [Google Scholar]
- Sloan JW, Todd GD, Martin WR. (1984) Nature of nicotine binding to rat brain P2 fraction. Pharmacol Biochem Behav 20:899–909. [DOI] [PubMed] [Google Scholar]
- Squire LR. (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. (2004) The medial temporal lobe. Annu Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. (2012) The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol 83:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. (2005) Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev 11:229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio PJ, Tuominen RK. (2001) Cotinine binding to nicotinic acetylcholine receptors in bovine chromaffin cell and rat brain membranes. Nicotine Tob Res 3:177–182. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Chiu G, Dao H, Santarelli L, Porter R, Bertrand S, Bertrand D (2010) Modulation of cholinergic tone impacts the efficacy of RG3487/MEM3454, a novel, nicotinic alpha 7 receptor agonist. Society for Neuroscience, p236.1.
- Wildeboer-Andrud KM, Zheng L, Choo KS, Stevens KE. (2014) Cotinine impacts sensory processing in DBA/2 mice through changes in the conditioning amplitude. Pharmacol Biochem Behav 117:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]