Abstract
Pain is associated with stimulation of some behaviors and depression of others, and μ-opioid receptor agonists are among the most widely used analgesics. This study used parallel assays of pain-stimulated and pain-depressed behavior in male Sprague-Dawley rats to compare antinociception profiles for six μ-agonists that varied in efficacy at μ-opioid receptors (from highest to lowest: methadone, fentanyl, morphine, hydrocodone, buprenorphine, and nalbuphine). Intraperitoneal injection of diluted lactic acid served as an acute noxious stimulus to either stimulate stretching or depress operant responding maintained by electrical stimulation in an intracranial self-stimulation (ICSS). All μ-agonists blocked both stimulation of stretching and depression of ICSS produced by 1.8% lactic acid. The high-efficacy agonists methadone and fentanyl were more potent at blocking acid-induced depression of ICSS than acid-stimulated stretching, whereas lower-efficacy agonists displayed similar potency across assays. All μ-agonists except morphine also facilitated ICSS in the absence of the noxious stimulus at doses similar to those that blocked acid-induced depression of ICSS. The potency of the low-efficacy μ-agonist nalbuphine, but not the high-efficacy μ-agonist methadone, to block acid-induced depression of ICSS was significantly reduced by increasing the intensity of the noxious stimulus to 5.6% acid. These results demonstrate sensitivity of acid-induced depression of ICSS to a range of clinically effective μ-opioid analgesics and reveal distinctions between opioids based on efficacy at the μ-receptor. These results also support the use of parallel assays of pain-stimulated and -depressed behaviors to evaluate analgesic efficacy of candidate drugs.
Introduction
Pain is a significant public health problem that is associated with sensory and affective components (Bair et al., 2003; Neugebauer et al., 2009). It has been estimated that 42% of US adults experience pain in their daily lives (Lethbridge-Cejku et al., 2004), and American Pain Society (2000) estimated the total cost to the US economy in pain-related healthcare and disability at over $100 billion per year. Efforts to improve pain medications include the search for new pharmacological targets as well as development of new strategies to minimize unwanted effects of existing medications. Development of improved preclinical assays may also contribute to analgesic development, because existing assays have been unreliable in predicting clinical drug effects (Negus et al., 2006; Whiteside et al., 2008; Mogil, 2009).
The imperfect predictive validity of conventional preclinical assays may result in part from the dependent variables they measure. Most preclinical assays of antinociception rely on measurements of “pain-stimulated behaviors,” which can be defined as behaviors that increase in rate, frequency, or intensity after noxious stimulus presentation (Negus et al., 2006; Stevenson et al., 2006; Negus, 2013). Common examples include withdrawal responses from stimuli that can be escaped (e.g., tail withdrawal from thermal stimuli) or pseudo-withdrawal responses from stimuli that cannot be escaped (e.g., stretching responses elicited by intraperitoneal injection of chemical stimuli). An exclusive reliance on these behaviors as dependent measures of nociception can be problematic for at least two reasons. First, drug-induced decreases in these behaviors can be produced by motor impairment (e.g., sedation, paralysis) rather than by a decrease in sensory sensitivity to the noxious stimulus. Second, assays of pain-stimulated behavior do not assess clinically relevant affective dimensions of pain. In particular, pain states that require clinical intervention are often associated with depression rather than stimulation of behavior, and in humans, pain-related depression of behavior is often accompanied by depression of mood (Bair et al., 2003; Lépine and Briley, 2004). Moreover, human and veterinary medicine often rely on diagnostic tools that measure pain-related depression of behavior and mood (Cleeland and Ryan, 1994; Brown et al., 2008).
In accordance with the clinical importance of pain-related behavioral depression, we have argued that analgesic drug development might benefit from preclinical assays of “pain-depressed behaviors,” which can be defined as behaviors that decrease in rate, frequency, or intensity after noxious stimulus presentation (Negus, 2013). We recently showed in rats that a commonly used and physiologically relevant noxious stimulus (intraperitoneal injection of diluted acid) could both stimulate a stretching response and depress intracranial self-stimulation (ICSS) (a positively reinforced operant behavior in which lever-press responding is maintained by delivery of electrical brain stimulation) (Do Carmo et al., 2009; Negus et al., 2010a; Altarifi et al., 2013; Negus, 2013; Negus and Altarifi, 2013). Both acid-stimulated stretching and acid-induced depression of ICSS could be blocked by clinically effective analgesics such as the μ-opioid receptor agonist morphine and the nonsteroidal anti-inflammatory drug ketoprofen. However, nonanalgesic drugs that produce motor impairment (e.g., the κ-opioid receptor agonists U69,593 [(+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide] and salvinorin A, the dopamine receptor antagonist flupenthixol) produced “false positive” antinociception in the assay of acid-stimulated stretching but “true negative” absence of antinociception in the assay of acid-depressed ICSS. These results provide one source of evidence to suggest that novel assays of pain-depressed behavior may be useful for dissociating analgesics from drugs that produce motor impairment.
The purpose of this study was to further compare effects of μ-agonist analgesics in the assays of acid-stimulated stretching and acid-depressed ICSS. Two sets of experiments were conducted. First, a relatively low-intensity noxious stimulus was used (intraperitoneal administration of 1.8% lactic acid in a volume of 1.0 ml/kg), and antinociception was assessed for a range of μ-agonists that vary in their relative efficacies at μ-receptors. Specifically, effects were examined for methadone, fentanyl, morphine, hydrocodone, buprenorphine, and nalbuphine [listed in order from highest to lowest efficacy as determined by in vitro functional assays of agonist-stimulated 5′-O-(3-thiotriphosphate) binding] (Selley et al., 1997, 1998; Thompson et al., 2004). We predicted that, consistent with their clinical effectiveness (Brunton et al., 2011), all μ-opioid receptor agonists would block acid effects in both assays. Second, a higher-intensity noxious stimulus was used (5.6% lactic acid), and the antinociceptive effects of the high-efficacy μ-agonist methadone and the low-efficacy μ-agonist nalbuphine were redetermined. Previous studies using assays of pain-stimulated behavior found that increased thermal noxious stimulus intensities resulted in decreased potency and/or efficacy of μ-agonists to produce thermal antinociception, with a greater impact of noxious stimulus intensity on lower-efficacy agonists (Walker et al., 1993; Morgan and Picker, 1996; Morgan et al., 1999; Negus and Mello, 1999). Accordingly, we predicted that a higher-intensity acid noxious stimulus would reduce potency and/or efficacy of nalbuphine more than of methadone in the assay of acid-depressed ICSS.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Harlan, Frederick, MD) with initial weights of 295–335 g were used for the studies of ICSS and acid-stimulated stretching. Male rats were selected for this study to facilitate comparison with our previous studies of μ-, κ-, and δ-opioid agonist effects on ICSS and pain-related depression of ICSS (Negus and Altarifi, 2013). Rats were individually housed and maintained on a 12-hour light/dark cycle with lights on from 6:00 AM to 6:00 PM. Food and water were continuously available except during experimental sessions. Animal maintenance and research were in compliance with National Institutes of Health guidelines on care and use of animal subjects in research (National Research Council, 2011), and all animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
All animals were anesthetized with isoflurane gas (2.5%–3% in oxygen; Webster Veterinary, Phoenix, AZ) for stereotaxic surgery to implant a stainless steel electrode (Plastics One, Roanoke, VA). The cathode of each electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip. The anode was 0.124 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior and 1.7 mm lateral from bregma, and 8.8 mm below the skull). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured with orthodontic resin. Rats received ketoprofen (5 mg/kg per day i.p. for 2 days) as the postoperative analgesic. ICSS training began after 7 days of recovery.
Assay of ICSS
Apparatus.
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever, colored stimulus lights above the lever, a house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via a swivel connector (Model SL2C; Plastics One). Control of experimental events and acquisition of data were accomplished with a computer operated by Med-PC IV software and connected to test chambers by an interface system (Med Associates).
Training Procedure.
Rats were trained under a fixed-ratio 1 schedule of brain stimulation using procedures identical to those previously described (Altarifi and Negus, 2011; Altarifi et al., 2012; Negus et al., 2012b). During initial training, sessions lasted from 30 to 60 minutes, the house light was illuminated, and each lever press resulted in delivery of a 500-millisecond train of square-wave cathodal pulses (0.1-millisecond pulse duration) and illumination for 500 milliseconds of the stimulus lights. Responses during the stimulation did not earn additional stimulation. Initially, the frequency of stimulation was held constant at 158 Hz, and stimulation intensity for each rat was adjusted to the lowest value that would sustain a high rate of ICSS (≥30 stimulations per minute). Frequency manipulations were then introduced, and the terminal schedule consisted of sequential 10-minute components. During each component, a descending series of 10 current frequencies (from 158 to 56 Hz in 0.05-log increments) was presented, with a 60-second trial at each frequency. Each frequency trial began with a 10-second time out, during which the house light was off, and responding had no scheduled consequences. During the last 5 seconds of this time out, five noncontingent stimulations were delivered once per second at the frequency available during that trial, and the stimulus lights were illuminated during each stimulation. This noncontingent stimulation was followed by a 50-second response phase, during which the house light was illuminated, and responding produced electrical stimulation under the fixed-ratio 1 schedule. Training continued with presentation of three consecutive components per day, and intensity was again adjusted as necessary until the following criteria were met for the last two components for at least 2 consecutive days: 1) ICSS rate increased as a function of brain stimulation frequency; 2) ICSS rates were ≥ 50% maximum control rates (MCRs) for at least three and no more than six of the highest brain stimulation frequencies (see the data analysis section for a definition of MCR); and 3) the lowest frequency to maintain ≥ 50% MCR varied by no more than 1 frequency increment from the median. In general, rats were trained in groups of 10–14. The first six rats to meet training criteria were advanced to ICSS testing. The remaining rats from each group were assigned to studies of acid-stimulated stretching using methods described below.
Testing Procedure.
ICSS testing was conducted in two phases. First, the effects of methadone (0.032–1.0 mg/kg), fentanyl (0.0032–0.032 mg/kg), morphine (0.1–3.2 mg/kg), hydrocodone (0.1–3.2 mg/kg), buprenorphine (0.001–0.032 mg/kg), and nalbuphine (0.1–1.0 mg/kg) were examined as pretreatments to 1.8% lactic acid or acid vehicle (sterile water). Each drug was tested in a separate group of five to six rats that were opioid naïve at the start of the study. ICSS test sessions consisted of three consecutive “baseline” components followed first by a 30-minute time out when opioid/vehicle and acid/vehicle were administered and then by two consecutive test components. The first baseline component of each test session was considered to be an acclimation component, and data were discarded. Data from the second and third ‘‘baseline’’ components were used to calculate baseline parameters of frequency-rate curves for that session (see “Data Analysis”). The opioid or its vehicle was administered subcutaneously at the beginning of the time out as a 30-minute pretreatment to 1.8% lactic acid or its vehicle (intraperitoneally in a volume of 1.0 ml/kg), which was administered at the end of the time out and immediately before initiation of the test components. Test sessions were conducted on Tuesdays and Fridays, with one test day each week devoted to evaluation of an opioid dose plus acid and the other day devoted to evaluation of the same opioid dose plus acid vehicle. For all drugs, doses were delivered in a mixed order across rats. Three-component training sessions were conducted during other weekdays.
The second phase of the study compared effects of methadone and nalbuphine on depression of ICSS by a higher-intensity noxious stimulus. First, 13 experimentally naïve rats were treated at weekly intervals with subcutaneous saline as a 30-minute pretreatment to vehicle, 0.56%, 1.8%, or 5.6% lactic acid (in order of testing; intraperitoneally in a volume of 1.0 ml/kg). Test sessions began with three baseline components followed first by delivery of injections during a 30-minute time out and then by two consecutive test components. Subsequently, the rats were divided into two groups, and effects of 5.6% lactic acid on ICSS were redetermined after pretreatment with methadone (0.1 and 1.0 mg/kg; n = 6) or nalbuphine (1.0 or 10 mg/kg; n = 7). For each drug, the lowest dose was the dose that produced peak antinociception against 1.8% lactic acid in the first phase of the study, and this was the first dose tested. Rats were then tested a week later with a 10-fold higher dose of each drug.
Data Analysis.
Data were analyzed as previously described (Rosenberg et al., 2013; Negus and Miller, 2014). The primary dependent variable was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial were converted to the %MCR for that rat on that day. The MCR was determined during the baseline components of each test session and was defined as the mean of the maximal rates observed in any frequency trial during the second and third baseline components. Thus, %MCR for each trial was calculated as follows: (reinforcement rate during a frequency trial ÷ MCR) × 100. Normalized data from the frequency trials of each pair of consecutive test components were then averaged within each rat and then across rats for display and for statistical analysis using two-way analysis of variance (ANOVA), with drug dose as one factor and ICSS frequency as the other factor. A significant ANOVA was followed by a Holm–Sidak post hoc test, and the criterion for significance was set at P < 0.05.
To provide an additional summary measure of ICSS performance, the total number of stimulations obtained at all frequencies was summed for each component. The average test number of stimulations per component was expressed as a percentage of the average baseline number of stimulations per component in each rat and averaged across rats. These data were also used to quantify blockade of acid-induced depression of ICSS. Specifically, “percent acid blockade” was quantified using the following equation: [(test − acid)/(100 − acid)] × 100, where “test” was the percent baseline number of stimulations per component after treatment with drug plus acid, and “acid” was the percent baseline number of stimulations per component after vehicle plus acid. For all drugs producing greater than 50% acid blockade, linear regression was used to calculate an ED50 and 95% confidence limits, with ED50 defined as the effective dose producing 50% acid blockade. For drugs that produced inverted U-shaped dose-effect curves (methadone, fentanyl, and morphine), the ED50 value was determined from the ascending limb of the dose-effect curve. ED50 values were considered to be significantly different if 95% confidence limits did not overlap.
Assay of Lactic Acid–Stimulated Stretching
Behavioral Procedure.
Test sessions were conducted once per week. Test drugs were administered subcutaneously 30 minutes prior to treatment with 1.8% lactic acid (intraperitoneally in a volume of 1.0 ml/kg). Immediately after acid injection, rats were placed into acrylic test chambers (31.0 × 20.1 × 20.0 cm) for 30-minute observation periods. A stretch was operationally defined as a contraction of the abdomen followed by extension of the hind limbs, and the number of stretches during the observation period was counted. Effects of methadone (0.1–1.0 mg/kg), fentanyl (0.0032–0.032 mg/kg), morphine (0.1–1.0 mg/kg), hydrocodone (0.1–1.0 mg/kg), buprenorphine (0.00032–0.01 mg/kg), and nalbuphine (0.1–1.0 mg/kg) were examined in separate groups of 5–8 rats. Drug doses were tested in a mixed order across rats.
Similar to ICSS experiments, a separate group of naïve rats (n = 7) was treated with subcutaneous saline as a 30-minute pretreatment to vehicle (water), 0.56%, 1.8%, or 5.6% lactic acid (in order of testing; intraperitoneally in a volume of 1.0 ml/kg). After acid administration, rats were observed for 30 minutes, and the total number of stretches was counted as previously described. Opioids were not tested as pretreatments to 5.6% acid, because this high-intensity acid concentration failed to elicit a significant number of stretches.
Data Analysis.
Data were analyzed as previously described (Rosenberg et al., 2013). The primary dependent variable was the number of stretches counted during each observation period in each rat. To normalize these data, raw counts were converted to percent vehicle control using the following: equation (test/acid) × 100, where “test” was the number of stretches observed after drug plus acid, and “acid” was the number of stretches after drug vehicle plus acid. These data were then averaged across rats. For all drugs producing greater than 50% reduction in stretching, linear regression was used to calculate an ED50 and 95% confidence limits, with ED50 defined as the effective dose producing 50% control writhing. ED50 values were considered to be significantly different if 95% confidence limits did not overlap.
To evaluate significance during tests with different acid concentrations, data were averaged across rats and submitted to one-way ANOVA with acid concentration as the single factor. A significant ANOVA was followed by a Dunnett post hoc test with P < 0.05.
Drugs
Methadone HCl, fentanyl HCl, morphine sulfate, hydrocodone bitartrate, and buprenorphine HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Nalbuphine HCl was provided by K.C.R. (Chemical Biology Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD). All opioids were dissolved in saline and delivered subcutaneously in a volume of 1 ml/kg body weight. Lactic acid was purchased from Sigma-Aldrich (St. Louis, MO), diluted in sterile water, and administered intraperitoneally in a volume of 1 ml/kg body weight.
Results
Effects of μ-Agonists in the Assay of Acid-Stimulated Stretching
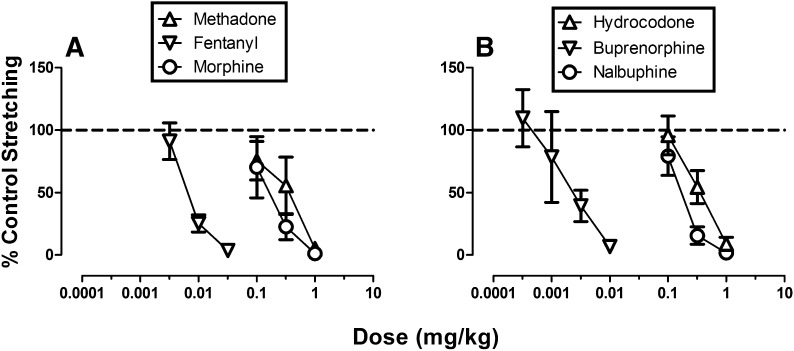
Across all 35 rats used for studies of acid-stimulated stretching, intraperitoneal administration of 1.8% lactic acid (1.0 ml/kg) after drug vehicle pretreatments elicited a mean ± S.E.M. of 13.1 ± 4.1 stretches. The baseline number of control stretches elicited by acid after vehicle pretreatment in each group is reported in the legend of Fig. 1. All six μ-opioid receptor agonists produced a dose-dependent decrease in acid-stimulated stretching, and ED50 values are shown in Table 1.
Fig. 1.
Effects of μ-opioid agonists in the assay of acid-stimulated stretching. Abscissae: dose in milligrams per kilogram (log scale). Ordinates: percent control stretching. All points show mean data ± S.E.M. from 5–8 rats, and ED50 values are reported in Table 1. The mean ± S.E.M. numbers of control stretches for each group were as follows: (A) methadone, 13.3 ± 4.3; fentanyl, 13.9 ± 2.7; morphine, 14.0 ± 7.6; (B) hydrocodone, 12.7 ± 3.1; buprenorphine, 12.3 ± 4.1; and nalbuphine, 12.9 ± 2.8.
TABLE 1.
ED50 values for μ-opioid agonists to produce antinociception in the assays of acid-stimulated stretching or acid-induced depression of ICSS
Data are presented as ED50 values with 95% confidence limits.
| Agonist | Acid-Stimulated Stretching | Acid-Depressed ICSS |
|---|---|---|
| mg/kg | ||
| 1.8% Lactic acid | ||
| Methadone | 0.272 (0.145–0.511) | 0.051 (0.030–0.085)a |
| Fentanyl | 0.008 (0.006–0.011) | 0.004 (0.003–0.006)a |
| Morphine | 0.171 (0.097–0.301) | 0.124 (0.070–0.218) |
| Hydrocodone | 0.343 (0.236–0.501) | 0.239 (0.161–0.355) |
| Buprenorphine | 0.002 (0.001–0.005) | 0.004 (0.002–0.008) |
| Nalbuphine | 0.217 (0.152–0.309) | 0.328 (0.130–0.826) |
| 5.6% Lactic acid | ||
| Methadone | Not tested | 0.51 (0.08–3.46) |
| Nalbuphine | Not tested | 4.90 (1.52–15.86)b |
Indicates significantly different from acid-stimulated stretching as indicated by nonoverlapping confidence limits.
Indicates significantly lower potency to block depression of ICSS by 5.6% than 1.8% lactic acid as indicated by nonoverlapping confidence limits.
Effects of μ-Agonists in the Assay of Acid-Depressed ICSS
Effects of the Lactic Acid Noxious Stimulus on ICSS.
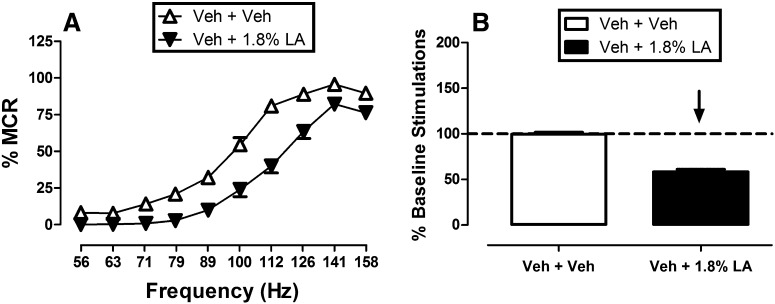
Figure 2 shows effects of the same noxious stimulus (intraperitoneal injection of 1.8% lactic acid) on ICSS. During each test session, a ‘‘baseline’’ frequency-rate curve was determined before experimental treatments to permit determination of the MCR for that session. Over the course of the entire study, the mean ± S.E.M. MCR was 61.49 ± 8.91 stimulations per trial. Reinforcement rates during each frequency trial of a session were then expressed as a percentage of that session’s MCR, and the average frequency-rate curve for all studies with drug vehicle plus acid vehicle is shown in Fig. 2. Maximum reinforcement rates were usually observed at the highest stimulation frequencies (112–158 Hz), and responding generally decreased in a frequency-dependent manner. Administration of 1.8% lactic acid depressed ICSS, producing a rightward shift in the frequency-rate curve. Figure 2 also shows summary data for the total number of stimulations delivered across all 10 frequencies during each component. The overall mean ± S.E.M. baseline number of stimulations per component for all rats in the study was 319 ± 79.5. Total ICSS after treatment with vehicle plus acid vehicle was nearly identical to baseline preinjection ICSS, but acid treatment decreased the number of stimulations per component. This acid-induced depression of ICSS provided a measure of pain-related behavioral depression, and opioids were evaluated for their ability to block this acid-induced depression of ICSS.
Fig. 2.
Depression of ICSS by 1.8% lactic acid. (A) The left panel compares effects of pretreatment with vehicle plus vehicle and vehicle plus 1.8% lactic acid on full frequency-rate curves for all 35 rats used in the first phase of ICSS experiments. Abscissa: frequency of electrical brain stimulation in hertz (log scale). Ordinate: ICSS rate expressed as %MCR. Two-way ANOVA indicated a significant main effect of frequency [F(9,306) = 295.4, P < 0.001] and acid treatment [F(1,34) = 86.8, P < 0.001], and the interaction was also significant [F(9,306) = 10.2, P < 0.001]. The acid noxious stimulus significantly depressed ICSS at all frequencies (Holm–Sidak post hoc test, P < 0.05). (B) The right panel shows summary data for lactic acid effects on the total number of stimulations per component. Abscissa: pretreatment condition. Ordinate: percent baseline number of stimulations per component. The downward arrow indicates that lactic acid produced a significant decrease in ICSS at one or more frequencies in the full frequency-rate curve. LA, lactic acid; Veh, vehicle.
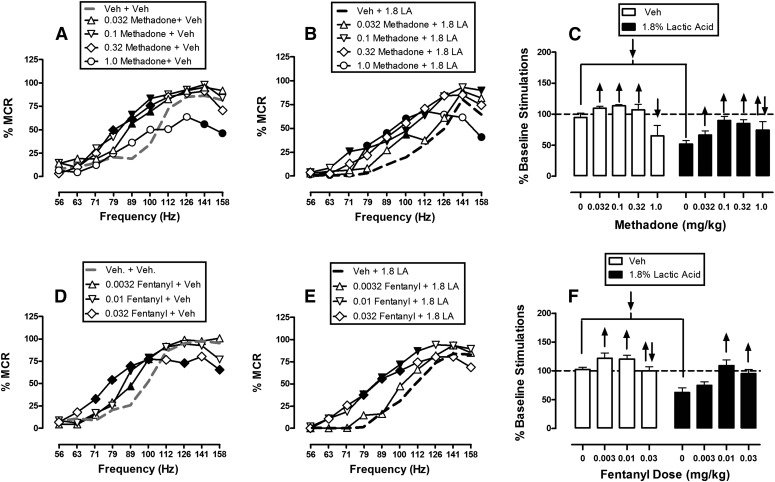
Methadone and Fentanyl.
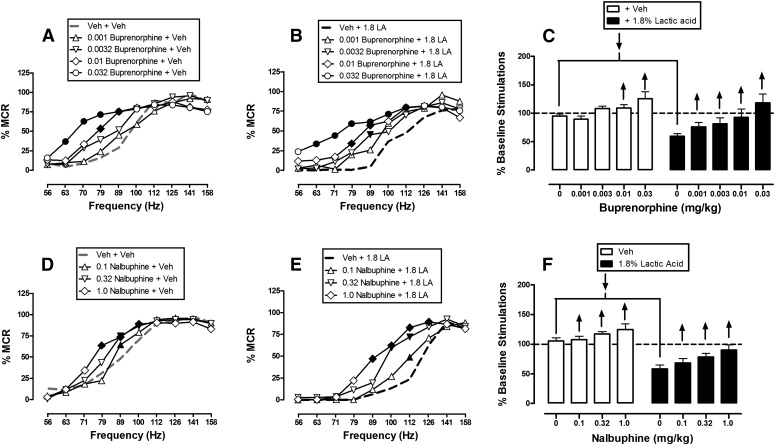
Figure 3 shows that methadone and fentanyl dose-dependently and completely blocked 1.8% acid-induced depression of ICSS at or near doses that also facilitated control ICSS in the absence of the noxious acid stimulus. When administered as a pretreatment to acid vehicle, methadone doses of 0.032–0.32 mg/kg produced leftward shifts in the ICSS frequency-rate curve and significant facilitation of ICSS at intermediate frequencies of brain stimulation (71–100 Hz), whereas the highest dose of 1.0 mg/kg methadone only depressed ICSS at the highest two frequencies (141–158 Hz) (Fig. 3A). Similarly, when administered as a pretreatment to 1.8% lactic acid, methadone increased ICSS responding and ameliorated acid-induced depression of ICSS (Fig. 3B). Significant increases in ICSS responding were observed after pretreatment with all methadone doses at a broad range of frequencies ranging from 71 to 112 Hz. The highest dose of 1.0 mg/kg methadone also decreased ICSS at 158 Hz after acid pretreatment. Figure 3C shows summary data for methadone effects, and 0.1 mg/kg was the dose that produced the maximal attenuation of acid-induced depression of ICSS.
Fig. 3.
Effects of methadone (A–C, n = 6) and fentanyl (D–F, n = 5) on control and 1.8% acid-depressed ICSS. Left and center panels show drug effects on full frequency-rate curves when drugs were administered as a pretreatment to vehicle (left panels in A and D) or 1.8% lactic acid (center panels in B and E). Abscissae: frequency of electrical brain stimulation in hertz (log scale). Ordinates: %MCR. Right panels (C and F) show summary data for drug effects on the total number of stimulations per component when drugs were administered as a pretreatment to vehicle (open bars) or acid (filled bars). Abscissae: dose of drug in milligrams per kilogram. Ordinate: percent baseline number of stimulations per component. Statistical results for two-way ANOVA of full frequency-rate curves are as follows. (A) Significant main effects of frequency [F(9,45) = 41.2, P < 0.001] and dose [F(4,20) = 5.9, P = 0.003], and a significant interaction [F(36,180) = 3.0, P < 0.001]. (B) Significant main effects of frequency [F(9,45) = 41.2, P < 0.001] and dose [F(4,20) = 3.8, P = 0.018], and a significant interaction [F(36,180) = 4.2, P < 0.001]. (D) Significant main effect of frequency [F(9,36) = 72.8, P < 0.001], but not dose [F(3,12) = 1.1, P = 0.384]; the interaction was significant [F(27,108) = 4.3, P < 0.001]. (E) Significant main effects of frequency [F(9,36) = 63.5, P < 0.001] and dose [F(3,12) = 6.1, P = 0.009], and a significant interaction [F(27,108) = 1.7, P = 0.030]. Filled symbols indicate a significant difference from vehicle plus vehicle (A and D) or vehicle plus lactic acid (B and E) (Holm–Sidak post hoc test, P < 0.05). Upward/downward arrows indicate that the drug dose produced a significant increase/decrease in ICSS at one or more frequencies when comparing the full frequency-rate curves. LA, lactic acid; Veh, vehicle.
Pretreatment with fentanyl also facilitated ICSS responding in the absence of the noxious stimulus (Fig. 3D) and blocked acid-induced depression of ICSS (Fig. 3E). Doses of 0.01 and 0.032 mg/kg fentanyl significantly increased ICSS under both conditions across a broad range of frequencies from 79 to 112 Hz, and these effects of fentanyl are summarized in Fig. 3F.
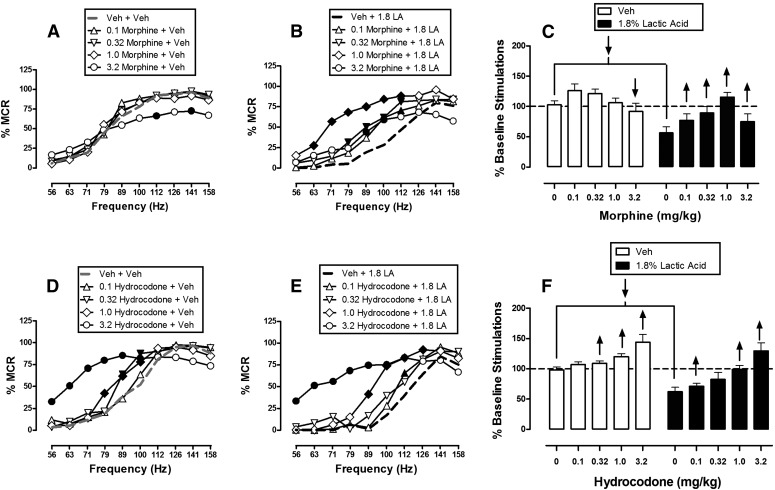
Morphine and Hydrocodone.
Figure 4 shows effects of morphine and hydrocodone on ICSS in the absence or presence of the acid noxious stimulus. When administered as a pretreatment to acid vehicle, morphine doses of 0.1–1.0 mg/kg had no significant effect on ICSS, but a higher dose of 3.2 mg/kg significantly depressed high rates of ICSS maintained by some high brain stimulation frequencies (Fig. 4A). When administered as a pretreatment to lactic acid, morphine doses of 0.1–1.0 mg/kg dose-dependently blocked acid-induced depression of ICSS; the higher dose of 3.2 mg/kg also attenuated acid-induced depression of ICSS, although to a lesser degree than 1.0 mg/kg (Fig. 4B). In contrast with morphine, hydrocodone doses of 0.1–3.2 mg/kg dose-dependently facilitated ICSS in the absence of the noxious stimulus (Fig. 4D) and also dose-dependently blocked acid-induced depression of ICSS (Fig. 4E). No dose of hydrocodone depressed ICSS at any frequency in the absence or presence of the noxious stimulus. Overall, both morphine and hydrocodone blocked acid-induced depression of ICSS, although with hydrocodone, this was accompanied by facilitation of ICSS in the absence of the noxious stimulus (Fig. 4, C and F).
Fig. 4.
Effects of morphine (A–C, n = 6) and hydrocodone (D–F, n = 6) on control and 1.8% acid-depressed ICSS. Details are as in Fig. 3. Statistical results for two-way ANOVA of full frequency-rate curves are as follows. (A) Significant main effect of frequency [F(9,45) = 91.9, P < 0.001], but not dose [F(4,20) = 1.2, P = 0.356]; the interaction was significant [F(36,180) = 2.2, P < 0.001]. (B) Significant main effects of frequency [F(9,45) = 56.8, P < 0.001] and dose [F(4,20) = 8.2, P < 0.001], and a significant interaction [F(36,180) = 2.7, P < 0.001. (D) Significant main effects of frequency [F(9,45) = 71.0, P < 0.001] and dose [F(4,20) = 8.0, P < 0.001], and a significant interaction [F(36,180) = 5.6, P < 0.001]. (E) Significant main effects of frequency [F(9,45) = 70.0, P < 0.001] and dose [F(4,20) = 9.4, P < 0.001], and a significant interaction [F(36,180) = 5.8, P < 0.001]. LA, lactic acid; Veh, vehicle.
Buprenorphine and Nalbuphine.
Figure 5 shows that, similar to hydrocodone, the lower-efficacy μ-agonists buprenorphine and nalbuphine produced dose-dependent facilitation of ICSS in the absence of the noxious stimulus (Fig. 5, A and D) and also dose-dependently blocked acid-induced depression of ICSS (Fig. 5, B and E). No dose of buprenorphine or nalbuphine depressed ICSS at any frequency in the absence or presence of the noxious stimulus. Summary data for buprenorphine and nalbuphine are shown in Fig. 5, C and F, respectively.
Fig. 5.
Effects of buprenorphine (A–C, n = 6) and nalbuphine (D–F, n = 6) on control and 1.8% acid-depressed ICSS. Details are as in Fig. 3. Statistical results for two-way ANOVA of full frequency-rate curves are as follows. (A) Significant main effect of frequency [F(9,45) = 70.6, P < 0.001] and dose [F(4,20) = 2.9, P = 0.049], and a significant interaction [F(36,180) = 3.8, P < 0.001]. (B) Significant main effects of frequency [F(9,45) = 37.9, P < 0.001] and dose [F(4,20) = 4.2, P = 0.012], and a significant interaction [F(36,180) = 2.1, P < 0.001]. (D) Significant main effect of frequency [F(9,45) = 91.5, P < 0.001], but not dose [F(3,15) = 1.5, P = 0.261]; the interaction was significant [F(27,135) = 2.9, P < 0.001]. (E) Significant main effects of frequency [F(9,45) = 152.2, P < 0.001] and dose [F(3,15) = 5.1, P = 0.012], and a significant interaction [F(27,135) = 2.9, P < 0.001]. LA, lactic acid; Veh, vehicle.
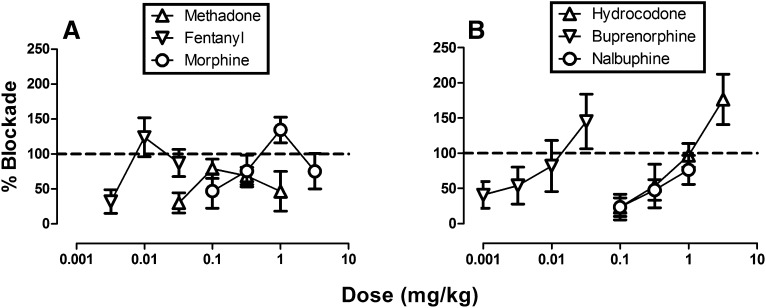
Opioid ED50 Values to Block Acid-Induced Depression of ICSS.
Figure 6 shows effects of all six opioids expressed as ‘‘percent blockade” in the assay of acid-induced depression of ICSS. For the highest-efficacy drugs methadone, fentanyl, and morphine, an inverted U-shape curve was produced such that peak blockade of acid-induced ICSS depression was achieved with intermediate doses (Fig. 6A). Conversely, hydrocodone, buprenorphine, and nalbuphine produced dose-dependent blockade of acid-induced depression of ICSS across the dose range examined (Fig. 6B). Table 1 shows ED50 values for effects of each drug to block acid-induced depression of ICSS.
Fig. 6.
Dose-effect curves for μ-agonist blockade of acid-induced depression of ICSS. Abscissae: dose in milligrams per kilogram (log scale). Ordinates: percent blockade of acid-induced depression of ICSS. All points show mean data ± S.E.M. from five to six rats, and ED50 values are reported in Table 1.
Effects of Noxious Stimulus Intensity.
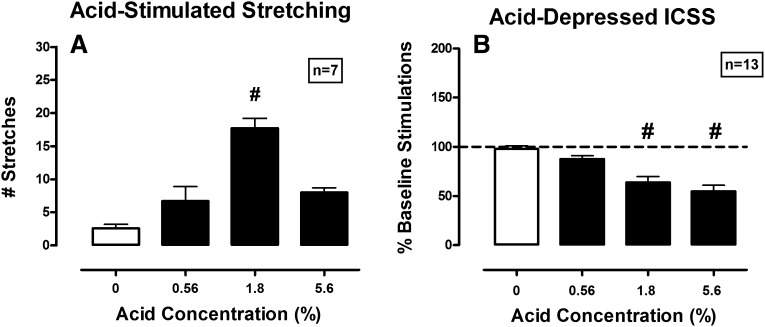
Figure 7 shows the effect of different concentrations of lactic acid on acid-stimulated stretching and acid-induced depression of ICSS. Vehicle produced 2.57 ± 1.72 stretches during the 30-minute observation period, and vehicle did not facilitate or depress ICSS compared with baseline. Lactic acid produced a bitonic effect on acid-stimulated stretching, such that 0.56% and 5.6% acid did not produce significant stimulation of stretching relative to vehicle, whereas 1.8% acid did produce significant stimulation of stretching (Fig. 7A). Because the high concentration of 5.6% lactic acid did not stimulate a significant stretching response, opioid effects were not examined. By contrast, lactic acid produced a concentration-dependent depression of ICSS that was significant after 1.8% and 5.6% acid (Fig. 7B). Table 1 shows the effect of increasing noxious stimulus intensity on potency of methadone and nalbuphine to block acid-induced depression of ICSS. For methadone, the ED50 value increased 10-fold, but 95% confidence limits overlapped, so the change in potency did not meet the criterion for statistical significance. For nalbuphine, the ED50 value increased 15-fold, and this reduction in potency was significant as indicated by nonoverlapping confidence intervals.
Fig. 7.
Effect of acid concentration on acid-stimulated stretching (left) and acid-depressed ICSS (right). Abscissae: lactic acid concentration administered intraperitoneally. Left ordinate: number of stretches during the 30-minute observation period. Right ordinate: percent baseline number of stimulations per component. One-way ANOVA (with Dunnett post hoc test; P < 0.05) revealed the following results. (A) Significant effect of acid concentration [F(6,18) = 16.7, P < 0.001]. (B) Significant effect of acid concentration [F(12,36) = 22.3, P < 0.001]. The pound sign indicates significantly different from 0% acid as indicated by Dunnett post hoc test (P < 0.05).
Discussion
This study compared antinociceptive effects of six μ-opioid agonists in assays of acid-stimulated stretching and acid-depressed ICSS in rats. There were three main findings. First, each μ-agonist, regardless of its efficacy at the μ-receptor, produced antinociception in both assays when the noxious stimulus intensity was 1.8% lactic acid. These results provide further evidence for sensitivity of acid-induced depression of ICSS to blockade by a broad range of clinically effective opioid analgesics. Second, both the high-efficacy agonist methadone and the low-efficacy agonist nalbuphine retained antinociceptive effectiveness in the assay of acid-depressed ICSS when the noxious stimulus intensity was increased to 5.6% lactic acid. This increase in noxious stimulus intensity tended to reduce the potency of both compounds, although this reduction in potency was significant only for nalbuphine. Finally, with the notable exception of morphine, doses of μ-agonists that produced antinociception in the assay of acid-depressed ICSS also tended to facilitate ICSS in the absence of the noxious stimulus. Drug-induced facilitation of ICSS is often interpreted as an abuse-related effect (Negus and Miller, 2014), and as a result, these findings are consistent with the abuse liability of μ-opioid analgesics.
μ-Agonist Effects on Acid-Stimulated Stretching.
In this study, all μ-opioid agonists produced a dose-dependent decrease in acid-stimulated stretching. Previous studies have shown similar results with morphine and other opioids in assays of acid-stimulated stretching in rats and mice (Cowan et al., 1977; Fürst, 1991; Stevenson et al., 2006; Pereira Do Carmo et al., 2009). Previous studies have also shown that μ-opioid agonists block other examples of pain-stimulated behavior, such as tail- or paw-withdrawal responses from noxious thermal stimuli (Paronis and Holtzman, 1991, 1992; Gringauz et al., 2001; Taracha et al., 2009) or withdrawal responses in subjects rendered hypersensitive to thermal or mechanical stimuli by inflammatory or neuropathic manipulations (Wang et al., 2006; Cobos et al., 2012; Jagla et al., 2014). μ-Agonist effects on pain-stimulated behaviors are often interpreted as evidence consistent with clinical analgesic efficacy (Negus et al., 2006). For example, μ-agonists effects on acid-stimulated stretching in this study agree with evidence for the efficacy of μ-agonists to treat visceral pain in humans (Yuan et al., 2010; Carter and Green, 2011; O’Connor and Rao, 2012). However, assays of pain-stimulated behavior, such as this assay of acid-stimulated stretching in rats, often yield “false positive” effects with drugs that lack analgesic efficacy in humans. Assays of pain-depressed behavior provide one strategy to address this discrepancy in translational evaluation of analgesics (Negus, 2013), and a major goal of this study was to compare μ-agonist effects in parallel assays of pain-stimulated and pain-depressed behavior.
μ-Agonist Effects on Acid-Depressed ICSS.
Consistent with previous findings, morphine produced a dose-dependent blockade of acid-induced depression of ICSS (Pereira Do Carmo et al., 2009; Negus et al., 2010b). This study extends these previous findings by showing that acid-induced depression of ICSS was also blocked by other μ-agonists that are used clinically in humans as analgesics (Sunshine et al., 1997; Gutstein and Akil, 2006; Anderson, 2011; Calderon and Copenhaver, 2013; Chen et al., 2014). These findings are also consistent with the ability of μ-agonists to block pain-related depression of other behaviors produced by other types of noxious stimuli (Negus and Altarifi, 2013). For example, morphine has been shown to block depression of 1) feeding and locomotion by intraperitoneal acid in mice (Stevenson et al., 2006, 2009), 2) wheel running by intraplantar complete Freund’s adjuvant in mice (Cobos et al., 2012), 3) food-maintained operant responding by laparotomy or capsaicin treatment in rats (Martin et al., 2004; Neubert et al., 2006), and 4) locomotion by intra-articular formalin treatment in dogs (Rodríguez, 1974). These findings suggest considerable generality in the effectiveness of μ-agonists to block pain-related depression of behavior.
The μ-agonists tested in this study were selected because they vary along a continuum of efficacy to activate G protein signaling pathways coupled to μ-receptors (Selley et al., 1997, 1998; Thompson et al., 2004). Despite these differences in efficacy at the receptor level, all six μ-agonists were fully effective to block both acid-stimulated stretching and acid-induced depression of ICSS. μ-Agonist efficacy appeared to influence only the relative potency of drugs in the two assays, in that the high-efficacy μ-agonists methadone and fentanyl were more potent to produce antinociception in the assay of acid-depressed ICSS, whereas the other μ-agonists displayed similar potencies in the assays of acid-stimulated and acid-depressed behavior. The antinociceptive effectiveness of these compounds in this study agrees with their effectiveness in other preclinical antinociception procedures that use relatively low-intensity noxious stimuli (Morgan et al., 1999; Negus et al., 2012a) and with the analgesic effectiveness of all six compounds to treat pain in humans (Gutstein and Akil, 2006). A more prominent behavioral correlate of μ-receptor efficacy in this study was effectiveness of high doses to reduce ICSS rates. Thus, the relatively high-efficacy agonists methadone, fentanyl, and morphine produced inverted U-shaped dose-effect curves in the assay of acid-depressed ICSS, such that peak blockade of acid-induced depression of ICSS was produced by intermediate doses, but rate-decreasing effects were produced by high doses, which can be explained by their sedative effects at high doses (for review, see Altarifi and Negus, 2011). Conversely, lower-efficacy agonists produced only a monotonic and dose-dependent blockade of acid-induced ICSS depression across the dose range tested. Higher doses of hydrocodone, buprenorphine, and nalbuphine were tested in a previous study of ICSS in the absence of noxious stimulation (Altarifi et al., 2012), and although a higher dose of hydrocodone (10 mg/kg) did reduce ICSS, higher doses of buprenorphine (1.0 mg/kg) and nalbuphine (10 mg/kg) still produced little or no ICSS depression. Taken together, these results suggest that μ-receptor efficacy is a more important determinant of ICSS rate-decreasing effects than of antinociception in this assay of acid-induced ICSS depression.
In addition to their different efficacies at the μ-receptor, the six test drugs in this study also have different selectivities for μ- versus κ- and δ-opioid receptors. For example, the high-efficacy μ-agonists methadone and fentanyl are relatively selective for μ-receptors versus other opioid receptor types, whereas the low-efficacy μ-agonist nalbuphine has little selectivity for μ- versus κ-receptors (Emmerson et al., 1994; Raynor et al., 1994; Butelman et al., 1998) and also has low but significant efficacy at κ-receptors (Remmers et al., 1999). However, it is unlikely that κ-receptors contributed to effects of nalbuphine or other opioid agonists in this study. Selective κ-agonists only depress ICSS and exacerbate acid-induced depression of ICSS (Todtenkopf et al., 2004; Negus et al., 2010b, 2012b); however, in this study, ICSS depression was produced only by high doses of the higher efficacy and more selective opioids, and all opioids blocked rather than exacerbated acid-induced depression of ICSS.
μ-Agonist Effects on ICSS after 5.6% Lactic Acid.
Preclinical studies that used assays of pain-stimulated behavior previously found that increases in noxious stimulus intensity were more likely to reduce antinociceptive potency and/or effectiveness of low-efficacy than of high-efficacy agonists (Walker et al., 1993; Morgan and Picker, 1996; Morgan et al., 1999; Negus and Mello, 1999). This finding has also been supported by clinical data, which suggest that high-efficacy μ-agonists are preferred over low-efficacy ligands in cases of severe pain (Prommer and Ficek, 2012). Consistent with these observations, an increase in lactic acid concentration from 1.8% to 5.6% in this study produced a significant reduction in potency (increase in ED50 values) of the low-efficacy μ-agonist nalbuphine but not the high-efficacy agonist methadone. However, the increase in ED50 values was only slightly smaller for methadone than for nalbuphine, and both drugs retained effectiveness. The relatively modest dissociation by stimulus intensity of antinociceptive effects produced by low- and high-efficacy μ-agonists in this study contrasts with the greater dissociation produced by increases in thermal stimulus intensity on thermal nociception. For example, in rats tested in a warm-water tail-withdrawal procedure, an increase in stimulus intensity from 52 to 56°C produced only a 3-fold reduction in the potency of morphine, but the same increase in stimulus intensity produced a >30-fold reduction in nalbuphine potency (Morgan and Picker, 1996). The reasons for this discrepancy are currently unclear, but may be related to the different types of, and increments in, noxious stimuli, or the different dependent measures of nociception. Opioid effects could not be examined with the higher acid concentration in the assay of acid-stimulated stretching in this study, because as reported previously (Pereira Do Carmo et al., 2009), intraperitoneal acid stimulates stretching with an inverted U-shaped dose-effect curve, and the highest acid concentration failed to significantly increase stretching.
μ-Agonist Effects on ICSS in the Absence of Acid.
In this study, opioid effects on ICSS were also examined in the absence of noxious stimulation. As previously reported, morphine blocked acid-induced depression of ICSS at doses that did not alter control ICSS (Pereira Do Carmo et al., 2009; Negus et al., 2010b). However, morphine effects on ICSS are highly influenced by factors that include not only dose, but also pretreatment time and regimen of prior opioid exposure, and even modest prior exposure to opioids can increase morphine-induced ICSS facilitation (Altarifi and Negus, 2011). Moreover, we previously reported that intermittent dosing with the other μ-agonists tested here can also facilitate ICSS (Altarifi et al., 2012). Insofar as drug-induced facilitation of ICSS is predictive of abuse potential (Negus and Miller, 2014), these results are consistent with the abuse liability of opioid analgesics (Gutstein and Akil, 2006). These results are also consistent with other data that suggest reciprocal effects of μ-opioid analgesics and pain states on brain reward circuitry and behaviors such as ICSS that rely on brain reward circuitry (Leknes and Tracey, 2008; Leitl et al., 2014).
Abbreviations
- ANOVA
analysis of variance
- ICSS
intracranial self-stimulation
- MCR
maximum control rate
- U69,593
(+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide
Authorship Contributions
Participated in research design: Altarifi, Negus.
Conducted experiments: Altarifi.
Contributed new reagents or analytic tools: Rice.
Performed data analysis: Altarifi, Negus.
Wrote or contributed to the writing of the manuscript: Altarifi, Negus.
Footnotes
This research was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS070715]; and by a training grant from the Jordan University of Science and Technology. A portion of this work was also supported by the Intramural Research Program of the National Institutes of Health [National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism].
References
- Altarifi AA, Miller LL, Negus SS. (2012) Role of µ-opioid receptor reserve and µ-agonist efficacy as determinants of the effects of µ-agonists on intracranial self-stimulation in rats. Behav Pharmacol 23:678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. (2011) Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 22:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. (2013) Abuse-related effects of µ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol 24:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Pain Society (2000) Pain assessment and treatment in the managed care environment. A position statement from the American Pain Society. Case Manager 11:50–53. [DOI] [PubMed] [Google Scholar]
- Anderson D. (2011) A review of systemic opioids commonly used for labor pain relief. J Midwifery Womens Health 56:222–239. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. (2003) Depression and pain comorbidity: a literature review. Arch Intern Med 163:2433–2445. [DOI] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Coyne JC, Farrar JT. (2008) Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 233:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton L, Chabner B, Knollman B. (2011) Goodman & Gilman’s The Pharemacological Basis of Therapeutics, 12th ed, McGraw-Hill Professional, New York. [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. (1998) kappa-Opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J Pharmacol Exp Ther 285:595–601. [PubMed] [Google Scholar]
- Calderon R, Copenhaver D. (2013) Buprenorphine for chronic pain. J Pain Palliat Care Pharmacother 27:402–405. [DOI] [PubMed] [Google Scholar]
- Carter MR, Green BR. (2011) Renal calculi: emergency department diagnosis and treatment. Emerg Med Pract 13:1–17; quiz 18. [PubMed] [Google Scholar]
- Chen KY, Chen L, Mao J. (2014) Buprenorphine-naloxone therapy in pain management. Anesthesiology 120:1262–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23:129–138. [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. (2012) Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153:876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. (1977) Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. (2009) The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol 604:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. (1994) Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271:1630–1637. [PubMed] [Google Scholar]
- Fürst S. (1991) Pharmacological interaction of opiates with various classes of centrally acting dopaminergic drugs. Drug Metabol Drug Interact 9:77–102. [DOI] [PubMed] [Google Scholar]
- Gringauz M, Rabinowitz R, Stav A, Korczyn AD. (2001) Tolerance to the analgesic effect of buprenorphine, butorphanol, nalbuphine, and cyclorphan, and cross-tolerance to morphine. J Anesth 15:204–209. [DOI] [PubMed] [Google Scholar]
- Gutstein H, Akil H. (2006) Opioid analgesics, in Goodman and Gilman’s the Pharmacological Basis of Therapeutics (Brunton L, Lazo J, Parker K.) pp 547–590, McGraw-Hill, New York. [Google Scholar]
- Jagla G, Mika J, Makuch W, Obara I, Wordliczek J, Przewlocka B. (2014) Analgesic effects of antidepressants alone and after their local co-administration with morphine in a rat model of neuropathic pain. Pharmacol Rep 66:459–465. [DOI] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. (2014) Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology 39:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Tracey I. (2008) A common neurobiology for pain and pleasure. Nat Rev Neurosci 9:314–320. [DOI] [PubMed] [Google Scholar]
- Lépine JP, Briley M. (2004) The epidemiology of pain in depression. Hum Psychopharmacol 19 (Suppl 1):S3–S7. [DOI] [PubMed] [Google Scholar]
- Lethbridge-Cejku M, Schiller JS, Bernadel L. (2004) Summary health statistics for U.S. adults: National Health Interview Survey, 2002. Vital Health Stat 10 222:1–151. [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. (2004) Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 101:191–203. [DOI] [PubMed] [Google Scholar]
- Mogil JS. (2009) Animal models of pain: progress and challenges. Nat Rev Neurosci 10:283–294. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Picker MJ. (1999) Sensitivity to the discriminative stimulus and antinociceptive effects of mu opioids: role of strain of rat, stimulus intensity, and intrinsic efficacy at the mu opioid receptor. J Pharmacol Exp Ther 289:965–975. [PubMed] [Google Scholar]
- Morgan D, Picker MJ. (1996) Contribution of individual differences to discriminative stimulus, antinociceptive and rate-decreasing effects of opioids: importance of the drug’s relative intrinsic efficacy at the mu receptor. Behav Pharmacol 7:261–284. [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed, National Academies Press, Washington, DC. [Google Scholar]
- Negus SS. (2013) Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 42:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Altarifi AA. (2013) Mu, delta and kappa opioid agonist effects in novel assays of pain-depressed behavior, in Research and Development of Opioid-Related Ligands (Ko H, Husbands S, eds) pp 163–176, American Chemical Society, Washington, DC. [Google Scholar]
- Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. (2010a) Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia, in Methods in Molecular Biology: Analgesia (Szallasi A, ed) pp 79–91, Humana Press, New York. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. (1999) Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther 290:1132–1140. [PubMed] [Google Scholar]
- Negus SS, Miller LL. (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66:869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Folk JE, Rice KC. (2012a) Interaction between mu and delta opioid receptor agonists in an assay of capsaicin-induced thermal allodynia in rhesus monkeys. Pain Res Treat 2012:867067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. (2010b) Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. (2012b) Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther 319:507–514. [DOI] [PubMed] [Google Scholar]
- Neubert JK, Rossi HL, Malphurs W, Vierck CJ, Jr, Caudle RM. (2006) Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behav Brain Res 170:308–315. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. (2009) Forebrain pain mechanisms. Brain Res Brain Res Rev 60:226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AB, Rao A. (2012) Why do emergency providers choose one opioid over another? A prospective cohort analysis. J Opioid Manag 8:403–413. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. (1991) Increased analgesic potency of mu agonists after continuous naloxone infusion in rats. J Pharmacol Exp Ther 259:582–589. [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. (1992) Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther 262:1–9. [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. (2009) Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prommer E, Ficek B. (2012) Management of pain in the elderly at the end of life. Drugs Aging 29:285–305. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. (1994) Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45:330–334. [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. (1999) Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther 288:827–833. [PubMed] [Google Scholar]
- Rodríguez R. (1974) [Analgesic properties of narcotic antagonists]. Arch Invest Med (Mex) 5 (Suppl 1):207–214. [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. (2013) Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain 14:246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR. (1998) Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther 285:496–505. [PubMed] [Google Scholar]
- Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR. (1997) mu-Opioid receptor-stimulated guanosine-5′-O-(gamma-thio)-triphosphate binding in rat thalamus and cultured cell lines: signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol 51:87–96. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. (2006) Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain 7:408–416. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. (2009) Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci 85:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine A, Olson NZ, O’Neill E, Ramos I, Doyle R. (1997) Analgesic efficacy of a hydrocodone with ibuprofen combination compared with ibuprofen alone for the treatment of acute postoperative pain. J Clin Pharmacol 37:908–915. [DOI] [PubMed] [Google Scholar]
- Taracha E, Mierzejewski P, Lehner M, Chrapusta SJ, Kała M, Lechowicz W, Hamed A, Skórzewska A, Kostowski W, Płaźnik A. (2009) Stress-opioid interactions: a comparison of morphine and methadone. Pharmacol Rep 61:424–435. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE. (2004) Activation of G-proteins by morphine and codeine congeners: insights to the relevance of O- and N-demethylated metabolites at mu- and delta-opioid receptors. J Pharmacol Exp Ther 308:547–554. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172:463–470. [DOI] [PubMed] [Google Scholar]
- Walker EA, Butelman ER, DeCosta BR, Woods JH. (1993) Opioid thermal antinociception in rhesus monkeys: receptor mechanisms and temperature dependency. J Pharmacol Exp Ther 267:280–286. [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. (2006) Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol 291:R300–R306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Adedoyin A, Leventhal L. (2008) Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 54:767–775. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Chen JY, Guo H, Zhang Y, Liang DM, Zhou D, Zhao H, Lin F. (2010) Relief of abdominal pain by morphine without altering physical signs in acute appendicitis. Chin Med J (Engl) 123:142–145. [PubMed] [Google Scholar]