Abstract
Jasmonic acid and methyl jasmonate play an essential role in plant defense responses and pollen development. Their levels are temporarily and spatially controlled in plant tissue. However, whereas jasmonate biosynthesis is well studied, metabolic pathways downstream of jasmonic acid are less understood. We studied the uptake and metabolism of jasmonic acid and methyl jasmonate in tobacco (Nicotiana tabacum) Bright Yellow-2 suspension culture. We found that upon uptake, jasmonic acid was metabolized to its Glc and gentiobiose esters, and hydroxylation at C-11 or C-12 occurred. Free hydroxylated jasmonates were the preferential fraction of the culture medium. Upon hydrolysis of methyl jasmonate to jasmonic acid, a similar set of conversions occurs. In contrast to jasmonic acid, none of its derivatives interfere with the G2/M transition in synchronized tobacco Bright Yellow-2 cells.
At the time of its first description (Demole et al., 1962), the methyl ester of jasmonic acid (MeJA) was considered as one of many plant secondary metabolites with a possible application in the perfume industry. Plant extracts were quickly replaced by synthetic MeJA and its cheaper analogs, such as methyl dihydrojasmonate, which is now a very popular commercial fragrance component under the name of hedione. In parallel, studies on the physiological role of jasmonates revealed the complex nature of the plant defense systems and promoted jasmonates to the rank of true plant hormones, which mediate in various aspects of development and stress responses (Creelman and Mullet, 1995). The biosynthetic pathway of jasmonic acid (JA) consists of four major steps (release of the precursor, linolenic acid, from cellular membranes by phospholipase; its oxidation by lipoxygenase [LOX] to a 13-hydroperoxide; and subsequent dehydration and cyclization by allene oxide synthase (AOS) and allene oxide cyclase [AOC]), which yields a cyclopentenone product: 12-oxophytodienoic acid. This compound is reduced to a cyclopentanone by12-oxophytodienoate reductase and the resulting product is converted into JA by three rounds of β-oxidation (Vick and Zimmerman, 1984). The initial steps of this cascade presumably take place in the chloroplasts, since phospholipase A1, LOX, AOS, and AOC were shown to be localized there (Bell et al., 1995; Maucher et al., 2000; Ziegler et al., 2000; Froehlich et al., 2001; Ishiguro et al., 2001).
Wounding (Creelman et al., 1992), insect feeding, or fungal elicitors (Blechert et al., 1995) induce a rapid and transient accumulation of JA. This suggests that besides the biosynthetic rate, catabolism also plays an important role in the control of free JA levels. However, in contrast to JA biosynthesis, its metabolic fate has been less extensively studied. Barley (Hordeum vulgare) shoots immersed in a (±)-[2-14C]JA solution accumulated a mixture of several compounds, among which 12-hydroxyjasmonic acid (tuberonic acid [TA]), its glucoside, 11-hydroxyjasmonic acid, and amino acid conjugates of JA and 11- and 12-hydroxylated jasmonates were identified (Meyer et al., 1989). In a cell culture of tomato (Lycopersicon esculentum), on the other hand, the most abundant metabolite was the Glc ester of JA (Meyer et al., 1989). Custom-synthesized (±)-[2-14C]JA delivered to tips of potato leaves yielded TA, which was subsequently converted into a β-glucoside (Yoshihara et al., 1996).
The function of particular modifications remains unclear, as there are many contradictory data on the biological activity of JA metabolites. For example, both JA and TA acid induce tuber formation in potato (Solanum tuberosum; Koda et al., 1991), but TA fails to activate the JA-sensitive element in the promoter of cathepsin D inhibitor (Ishikawa et al., 1994). TA is also inactive in the assay for tendril coiling, a typical response to octadecanoids (Blechert et al., 1999). JA and certain amino acid conjugates of JA, such as N-jasmonylleucine, N-jasmonylisoleucine, N-jasmonylvaline, N-jasmonylphenylalanine, and N-jasmonyltyrosine induce the expression of JIP-23 and JIP-6, whereas TA does not (Miersch et al., 1999). Therefore, not all the activities associated with JA are shared by its metabolites.
Zhang and Baldwin (1997) reported that application of (±)-[2-14C]JA to the leaves of Nicotiana sylvestris seedlings resulted in the transport of detectable amounts of radioactivity to untreated young leaves and roots. The major fraction of the radioactive compounds recovered from the untreated leaves contained compounds that were less polar than JA. The radioactive components in the roots, however, were initially attributed to JA, which after 2 h was gradually metabolized to less-polar compounds. They also observed that the pool of free JA in the aqueous extracts of plants could be increased by treatment with β-glucosidase. Unfortunately, the nature of compounds related to storage, inactivation, or transport of JA remained enigmatic.
Recent experiments on tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) cultures (Swiatek et al., 2002) already pointed to the importance of either the rate of uptake or metabolism of JA with relation to the JA-induced arrest of the cell cycle in G2. This idea was further supported by the fact that the mutation that confers partial JA insensitivity, jar1, was recently identified as a defect in the JA metabolism (Staswick et al., 2002). This opens the possibility that some responses previously attributed to JA are, in fact, caused by one or more JA metabolites produced downstream of JAR1. Therefore, with this study we readdress the correlation between uptake and metabolism of JA and its inhibitory effect on the cell-cycle progression of tobacco BY-2 cells.
RESULTS
Uptake of JA and Metabolism in BY-2
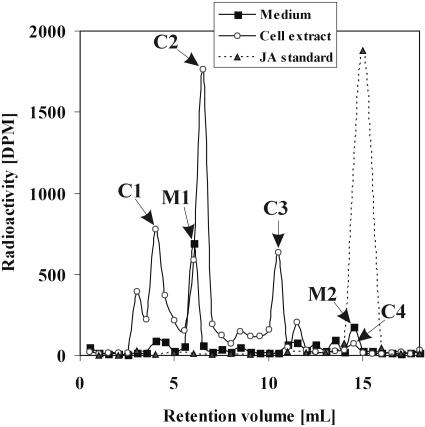
In an initial experiment, the metabolic fate of exogenously supplied JA was examined by the addition of 100 μm of cold JA spiked with 833 Bq/mL of (±)-[2,3-3H2]JA to a freshly transferred culture of BY-2 cells over 24 h. Reverse phase (RP)-HPLC analysis of culture medium and cell extracts revealed that JA was taken up from the medium and converted into several compounds. Judging from the retention times, all these compounds appeared to be more polar than JA. In the cell extract, three major peaks corresponding to radioactive compounds could be clearly distinguished (Fig. 1; C1 [tr = 4 min], C2 [tr = 6.5 min], and C3 [tr = 11 min]; tr, retention time), whereas 3H-JA (C4, tr = 15 min) was barely detectable. In the medium of the same culture, we found two other major peaks corresponding to radioactive compounds M1 (tr =6 min) and M2 (tr = 15 min). The latter is consistent with the tr of JA (Fig. 1). JA was not metabolized when incubated in a culture medium after filtering out the cells (data not shown). Omitting auxin 2,4-dichlorophenoxyacetic acid from the medium had, at least for 24 h, no effect on the metabolism of added JA (data not shown).
Figure 1.
Cell extracts and media 24 h after application of 100 μm JA spiked with (±)-[2,3-3H2]JA. The samples were separated by RP-HPLC, with subsequent radioactivity detection.
Initial Characterization of Metabolites
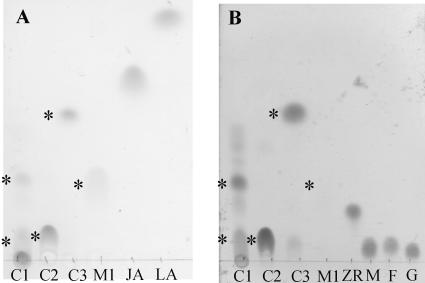
Fractions C1, C2, C3, and M1 were purified for further analysis on a larger scale by solid-phase extraction and RP-HPLC. Methylation with diazomethane had no effect on the retention time of the radioactive compounds present in fractions C1, C2, and C3, whereas the retention time of the radioactive components of M1 shifted from 6 min to 12 min (Table I). This experiment indicated that only radioactive compounds present in M1 carry a free carboxy group. Acetylation with acetic acid anhydride dramatically increased the retention time of all radioactive compounds, thus indicating the presence of free hydroxy or amino groups in all structures (Table I). Separation of the purified compounds by thin-layer chromatography (TLC) revealed spots detectable with either iodine vapor (Fig. 2A) or orcein (Bial's) reagent (Fig. 2B). In fractions C1, C2, and C3, spots were detected that stained with both reagents (Rf = 0.33, 0.19, and 0.58, respectively). When they were cut out of the TLC plates and analyzed by scintillation counting, it turned out that they were also radioactive. Fraction M1, on the other hand, gave a spot at Rf = 0.31, where the radioactivity colocalized with the iodine staining, but no coloring was observed upon visualization with the orcein reagent. Therefore, it was concluded that M1 contained one or more compounds characterized by the presence of at least one double bond and the absence of a sugar moiety.
Table I.
The HPLC retention time (min) of the radioactive component after the sample was subjected to one of the following treatments
| Sample | Untreated | Methylated | Acetylated | Digested with β-Glucosidase | Digested with Esterase |
|---|---|---|---|---|---|
| M1 | 6 | 12 | 13 | 6 | 6 |
| C1 | 3.5 | 3.5 | 17 | 6 | 6 |
| C2 | 6.5 | 6.5 | 19 | 15 | 15 |
| C3 | 11 | 11 | 17 | 15 | 15 |
| JA | 15 | 18 | 15 | 15 | 15 |
| MeJA | 18 | 18 | 18 | 18 | 15 |
Figure 2.
HPTLC analysis of JA metabolites (mobile phase: 95% CH3CN (v/v) in water). Location of the radioactivity is indicated with an asterisk; each lane contains approximately 3 nmol of substance. A, Double bonds detected with iodine vapor: C1; C2; C3; M1, samples; LA, linolenic acid. B, Carbohydrates detected with Bial's reagent: C1; C2; C3; M1, samples; ZR, zeatin riboside; M, Man; F, Fru; G, Glc.
The presence of carbohydrates and the increased polarity of the metabolites suggested the possibility of conjugate formation. In order to determine their relation to the original JA molecule, all metabolites were treated with either an esterase or a β-glucosidase. Subsequently, reaction products were separated by HPLC, and the distribution of radioactive components was recorded (Table I). The retention time of the radioactive components of M1 was not influenced by any of the treatments, whereas the radioactive compounds in fractions C1, C2, and C3 were affected by treatment with both esterase and β-glucosidase. Fraction C1 gave a product with a retention time identical to that of M1, whereas C2 and C3 produced peaks with retention times identical to that of JA.
Identity of JA Metabolites
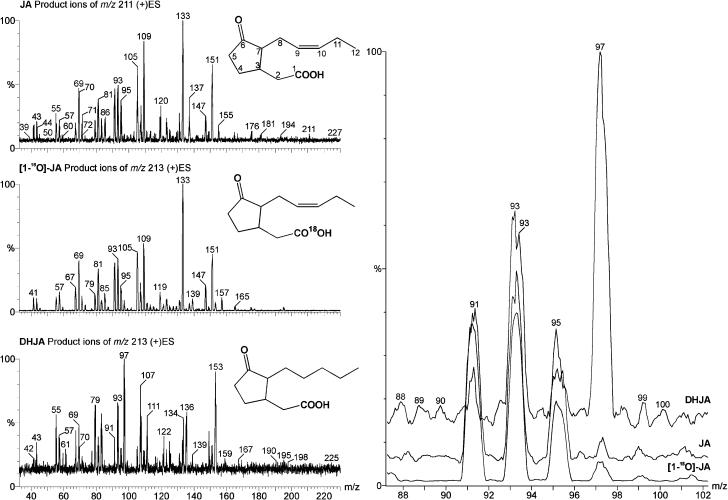
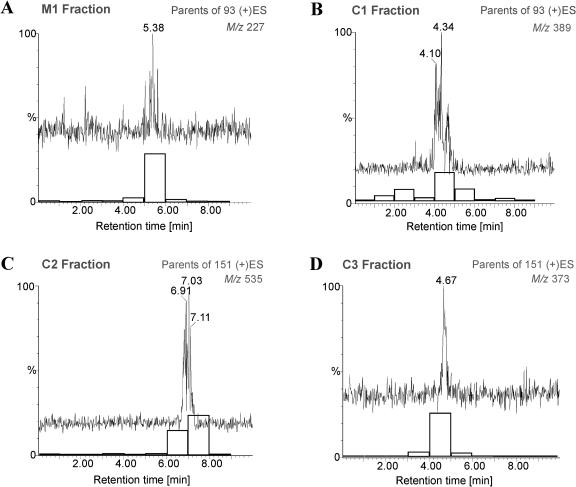
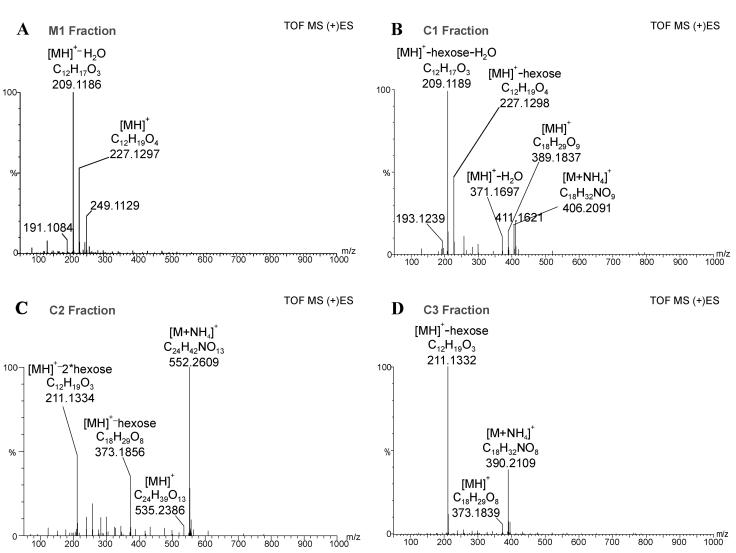
Further structural analysis was performed by combined liquid chromatography/electrospray tandem mass spectrometry (LC/(+)ES-MS/MS) applying a parent-ion scan mode. In order to determine which mass-to-charge ratio (m/z) value to use for parent-ion scanning, the low-energy collisional-activated dissociation (CAD) product ion spectra of JA, dihydrojasmonate (DHJA), and [1-18O]JA were recorded (Fig. 3). As expected, m/z 151 (unsaturated side chain at C-7) was present only in the spectra of JA and [1-18O]JA, whereas DHJA produced an ion at m/z 153, which implies the presence of a saturated side chain at C-7. Therefore, m/z 151 could serve as a marker for an unaltered side chain at C-7. The most prominent common product ion in the three spectra was found at m/z 93, which was also observed in the product spectrum of m/z 151 and corresponded to a common substructure in all three molecules. When fraction M1 was analyzed further by selecting m/z 151 for the parent-ion scan, it did not yield any significant peaks, whereas with the selection of m/z 93 gave rise to peaks with m/z values 209, 227, and 453 (Fig. 4A; Table II) in a chromatographic peak with tr = 5.38 min. This retention time was within the retention window of a radioactivity profile (Fig. 4A). The elemental composition of m/z 227 was verified by accurate mass measurement with a microbore liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry (LC/(+)ESI-QTOF) system. The measured mass was 227.1297, which was a deviation of 6 ppm from the theoretical value of 227.1283, corresponding to the protonated molecule [MH]+ (C12H19O4) of hydroxyjasmonic acid (OH-JA; Fig. 5A). The ion at m/z 209 can be explained as the dehydration (loss of water) of m/z 227, whereas m/z 453 can most likely be attributed to a [2M]H+ cluster (Fig. 5A). The identity of M1 was further confirmed by gas chromatography/mass spectrometry (GC/MS) analysis under EI conditions, which led to the detection of two peaks. Their spectra were identical to those of 11- and 12-OH-JA published by Helder et al. (1993). According to the integrated intensity values in the GC/MS chromatogram, both compounds were present in a ratio of approximately 1:1.
Figure 3.
Determination of characteristic m/z ion peaks for elements of the JA structure. Left, Low-energy CAD product ion spectra of JA, DHJA, and [1-18O]JA were recorded and compared. Right, Magnified range from m/z 88 to102. The most abundant common ion in the three spectra is m/z 93.
Figure 4.
Parent scan for ions that have m/z 93 in their fragmentation pattern. Chromatograms for [MH]+ ions overlaid with the corresponding radioactivity profiles (white bars). A, M1 sample. B, C1 sample. C, C2 sample. D, C3 sample.
Table II.
Summary of the spectra obtained in MS/MS experiments in the (+)ES mode
| Sample | Parent-Ion Scan of m/z 151 | Parent-Ion Scan of m/z 93 | CNL of 162 | CNL of 324 | Product Ions |
|---|---|---|---|---|---|
| M1 | ND | 453 (100), 227 (30), 209 (31) | ND | ND | 227→227 (100), 209 (70), 191 (39) |
| C1 | ND | 389 (100), 371 (63) | 389 (69), 377 (100), 371 (63) | ND | 389→389 (17), 371 (8), 227 (100), 209 (28) |
| C2 | 535 (100), 373 (89), 308 (45), 211 (32) | 535 (100), 373 (63) | 373 (100) | 535 (100) | 535→535 (14), 373 (65), 325 (26), 211(100) |
| C3 | 373 (9), 252 (11), 211 (100) | 373 (100), 252 (20) | 373 (100) | ND | 373→211(100), 193 (22) |
m/z, % relative intensity; [MH]+ ions indicated in bold; ND, None detected.
Figure 5.
Accurate mass spectra obtained on a microbore LC/(+)ESI-QTOF. Spectra centered on reference lock mass. Major peaks annotated with best matching empirical formulae (for details, see “Materials and Methods”). A, M1 sample, lock mass 283.17568. B, C1 sample, lock mass 344.22844. C, C2 sample, lock mass 344.22844. D, C3 sample, lock mass 283.17568.
Fraction C3 was analyzed with a microbore LC/(+)ESI-MS/MS system analogously to fraction M1. When the ion at m/z 151 was selected for the parent scan, a single chromatographic peak eluted at tr = 4.67 min, which correlated with the offline measurement of the radioactivity. Parent ions were found at m/z 373 and 211 (Fig. 4D; Table II). The ion at m/z 373 was also identified as a parent of constant neutral loss (CNL) of 162, the mass of a hexose unit (Table II). The accurate mass measurement of the [MH]+ ion present in the spectrum (Fig. 5D) matched well with an empirical formula expected for a hexose conjugate of JA: C18H29O9 (theoretical value 373.1862, experimental value 373.1839). The carbohydrate moiety was identified as d-Glc by analysis of the reaction by TLC and GC/MS (see “Materials and Methods”). Therefore, in agreement with all experimental data, the structure of jasmonyl-1-β-Glc was assigned to C3. This compound was previously described as a JA metabolite by Meyer et al. (1989).
Similarly to C3, a chromatographic peak was detected in C2 when using m/z 151 in a parent-ion scan.The following m/z values were present: 535, 373, 308, and 211 (Fig. 4C; Table II). The difference between m/z 535 and 211 is 324, which reflects the loss of a disaccharide. This loss was confirmed further by a CNL scan of 324, for which ion m/z 535 was detected as a parent (Table II). The accurate mass measurement of the [MH]+ ion in the spectrum indicated a formula C24H39O13 (theoretical value 535.2391, experimental value 535.2386), which could correspond to JA linked to two hexose units (Fig. 5C). GC/MS analysis of the carbohydrate released upon treatment with esterase led to its identification as gentiobiose. Therefore, in agreement with all experimental data, we proposed C2 to be jasmonyl-1-β-gentiobiose, a compound mentioned earlier by Sembdner et al. (1994) as a JA metabolite.
In contrast to fractions C2 and C3, C1 gave no clear response for m/z 151 in a parent-ion scan. However, when m/z 93 was used, m/z 389 and m/z 371 were detected as parent ions (Fig. 4B; Table II). The chromatographic peak containing those ions coeluted with the major fraction of the radioactive compounds (Fig. 4B). When the C1 fraction was analyzed under CNL (Δ162) conditions, m/z 377 and 251 were detected in addition to the already mentioned ions m/z 371 and 389 (Table II). Because both m/z 377 and 251 were absent in the parent-ion scan, we regarded them as structurally unrelated to JA. Furthermore, separation of this sample by TLC already indicated that it contained a mixture of carbohydrate conjugates that were not all radioactive (Fig. 2A). In order to verify the relationship between m/z 389 and 371, we recorded a low-energy CAD product-ion spectra of the m/z 389 ion, which contained m/z 371, 227, and 209 as major ions (Table II). This result allowed us to conclude that m/z 371 in the parent-ion and CNL spectrum was a product of in-source fragmentation of m/z 389.
When fraction C1 was analyzed by microbore LC/(+)ESI- QTOF under full-scan conditions, the following ions were observed upon lock mass correction: m/z 406.2091, 389.1837, 371.1967, 227.1298, and 209.1189. When those values were matched with empirical formulas, the following in-source fragmentation pattern emerged: when [MH]+ is assigned to m/z 389 (theoretical value for formula C18H29O9, 389.1812; experimental value, 389.1837) then m/z 371 could be explained by the loss of water from m/z 389 (theoretical value for formula C18H27O8, 371.1706; experimental value, 371.1697), whereas m/z 227 and 209 could be explained by the subsequent loss of hexose and water (Fig. 5B). At this point it might be worthwhile mentioning that m/z 227 and 209 were also observed in the corresponding OH-JA spectrum (Fig. 5A).
The carbohydrate moiety released upon treatment with esterase was identified as d-Glc by GC/MS (see “Materials and Methods”). Therefore, in order to be in agreement with all experimental data, we propose C1 to be a mixture of 11- and 12-hydroxy-jasmonyl-1-β-glucopyranose. The fact that C1 is a d-Glc ester rather than a β-glucoside was further corroborated by its spectrum. Besides the protonated molecule at m/z 389, m/z 371 was also present (Fig. 5B). The ion m/z 371 represents the loss of the free hydroxy group in the hydrocarbon chain as water. The loss of water was not observed in the spectrum of jasmonyl-1-β-Glc, which has no free hydroxy group in the hydrocarbon chain. Moreover, the m/z 398 product-ion spectrum recorded at a collision energy of 20 eV contained an ion at m/z 167 (data not shown), which matches the mass expected from the loss of the substituent at C-3 from the parent molecule containing a free hydroxy group in the unsaturated side chain at C-7. To the best of our knowledge, this is the first time that hydroxy-jasmonyl-1-β-glucopyranose has been identified as a metabolite of JA.
Kinetics of Formation of JA Metabolites
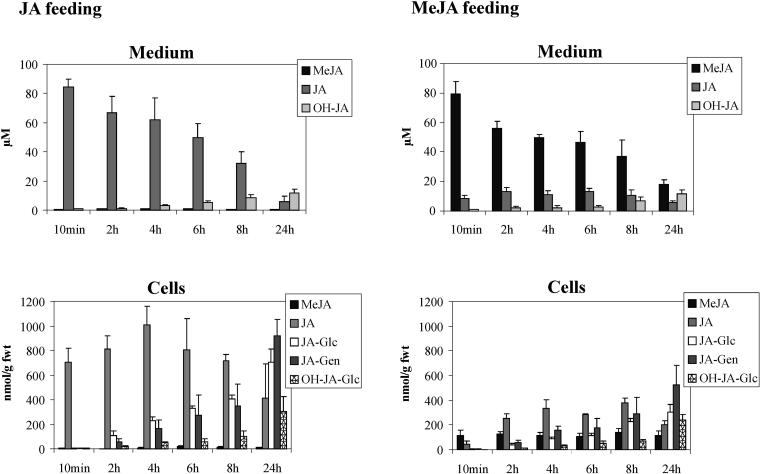
The four major products of the metabolism of JA in tobacco BY-2 cells were identified after 24 h of feeding. However, cell cycle-related effects of exogenously supplied JA could be observed already 2 to 6 h after treatment with JA (Swiatek et al., 2002). In order to determine whether any of the metabolites could be involved in the inhibitory action of JA, the rate of their formation was monitored during the cell-cycle progression of a synchronized tobacco BY-2 cell culture. Upon aphidicolin release, the cells were treated with 100 μm JA or MeJA, spiked with 72.5 kBq of (±)-[2,3-3H2]JA or 55 kBq (±)-[2,3-3H2]MeJA (14.5 Bq/nmol JA and 11 Bq/nmol MeJA). Samples were taken 10 min and 2, 4, 6, and 24 h after aphidicolin release. Cell extracts and media were analyzed separately by HPLC, and each metabolite was identified by its specific retention time and quantified by isotope dilution. The results of this kinetic study are presented in Figure 6. JA was taken up very rapidly and more efficiently than MeJA. After the time necessary to collect the first sample (approximately 10 min), the concentration of JA recovered in the JA-treated cells (700 nmol/g fresh weight [fwt]) exceeded multifold its concentration in the surrounding medium. The accumulation of MeJA in the MeJA-treated cells was near that in the medium (111 nmol/g fwt) after the first 10 min of incubation. Upon application, MeJA was readily hydrolyzed to JA, but the amount of MeJA formed from exogenous JA (<20 nmol/g fwt) was very low relative to other metabolites. After 8 h of treatment, the concentration of original JA or MeJA in the medium decreased to less than 50 μm; hydroxylated jasmonates started to accumulate in the medium but were absent in the cell extracts. In JA-treated cells, the Glc ester of JA started to accumulate 2 h after the treatment; after 24 h, the gentiobiose ester of JA was a major metabolite, and substantial levels of Glc esters of JA and OH-JA were also present. In MeJA-treated cells, JA was the major product until 24 h after treatment, at which time the gentiobiose ester of JA became the most abundant metabolite, and substantial levels of the Glc esters of JA and OH-JA were detected. In contrast to case in the medium, the free acid forms of 11- and 12-OH-JA in the cell extracts were present below the detection limit of 2 nmol/g fwt (data not shown).
Figure 6.
Kinetics of JA and MeJA metabolism during a synchronization experiment. Samples from medium and cell extracts from the synchronized culture were separated by HPLC. Radioactivity was monitored, and the peaks were assigned to the appropriate metabolites on the basis of the retention time and were quantified by isotope dilution. The data represent the average from three independent experiments (error bars = sd). JA-Glc, jasmonyl-1-d-glucopyranose; JA-Gen, jasmonyl-1-d-gentiobiose; OH-JA-Glc, d-Glc ester of OH-JA.
The Effect of the JA Derivatives on the G2/M Transition
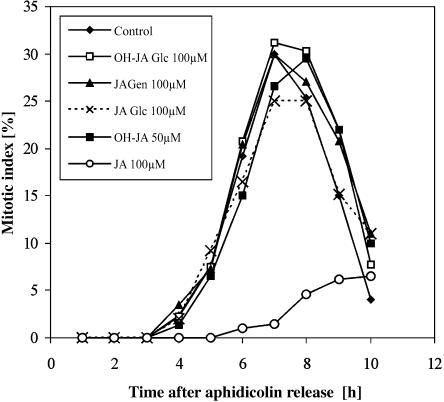
As mentioned in the introduction, JA blocks the G2/M transition when applied during the S phase, but it has less effect when applied at the late G2 phase. We detected that all the metabolites could already be present during G2 when JA is applied during the S phase, immediately after aphidicolin release (Fig. 6). To investigate whether any of the main metabolites could interfere with the G2/M transition, they were purified by HPLC and reapplied to the cells. The concentrations used were even higher than those that occur naturally during JA feeding: 100 μm for cell-derived compounds and 50 μm for medium-derived hydroxylated jasmonates. Surprisingly, when the effect of the exogenously applied JA derivatives on mitosis was tested, not one of the metabolites appeared to be active (Fig. 7). This may indicate that the release of JA from its carbohydrate esters is too slow to amount to the accumulation of a sufficient amount of JA to effectively block the cell division at that point. Long term, however, there is clearly an effect. Indeed, when BY-2 callus was cultivated on a medium containing the metabolites at a 10 μm concentration, they all had a negative effect on the culture growth (data not shown).
Figure 7.
The effect of JA and its metabolites on the mitotic index of the synchronized BY-2 cells. All compounds were applied at t = 0 h. JA-Glc, jasmonyl-1-d-glucopyranose; JA-Gen, jasmonyl-1-d-gentiobiose; OH-JA-Glc, d-Glc ester of OH-JA.
Conversions between the Derivatives
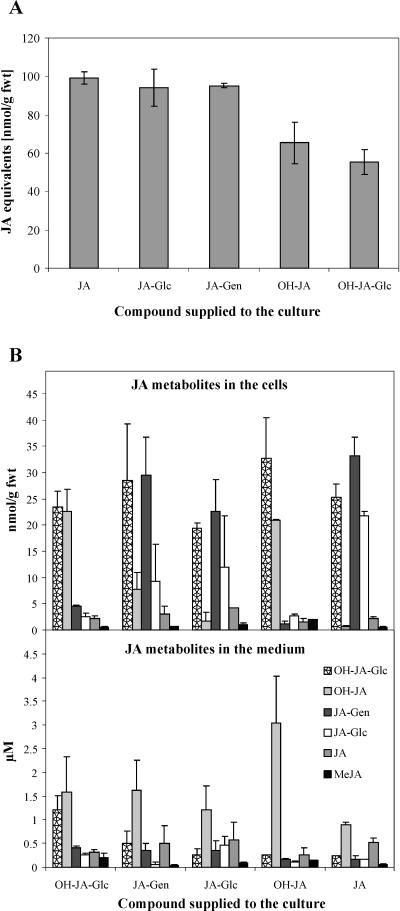
In order to study the possibility that some of the JA metabolites are involved in inactivation, storage, and transport of JA, the conversions between particular metabolites, their uptake, and the reversibility of the reactions of the catabolic pathway of JA were examined. Taking into account that endogenous JA content in unchallenged tobacco BY-2 cells varies between 0.2 and 3 nmol/g fwt (A. Swiatek, unpublished data), we used a 10 μm concentration of racemic JA and of each of the four metabolites (jasmonyl-1-β -Glc, jasmonyl-1-β-gentiobiose, isomers of OH-JA, and hydroxyjasmonyl-1-β-Glc). The total accumulation of radioactive components in the cells (Fig. 8A) and the concentration of the specific metabolites in both cells and media were analyzed 24 h after application (Fig. 8B). Approximately 50% of the radioactivity was recovered in the cells when JA or its esters were supplied, leading to the accumulation of about 100 nmol/g fwt. The hydroxylated derivatives were taken up less readily, so that approximately 60 nmol/g fwt were recovered in the cells. Isomers of OH-JA were always a dominant fraction in the medium, irrespectively of the parent molecule (Fig. 8B). In the cells, however, a different pattern was observed. Treatment with JA and its Glc and gentiobiose esters resulted in the accumulation of the Glc ester of OH-JA and the Glc and gentiobiose esters of JA. Treatment with OH-JA and its Glc ester led to their accumulation in the cells, and dehydroxylation to JA was barely observed.
Figure 8.
Conversions between particular metabolites of JA. All compounds were applied in a concentration of 10 μm. Samples from media and cell extracts were collected, the radioactivity was quantified, and the distribution analyzed by HPLC. On the basis of retention time, the peaks were assigned to the appropriate metabolites, which were quantified by isotope dilution. The data represent the average from three independent experiments (error bars = sd). JA-Glc, jasmonyl-1-d-glucopyranose; JA-Gen, jasmonyl-1-d-gentiobiose; OH-JA-Glc, d-Glc ester of OH-JA. A, Uptake of JA and its metabolites after treatment for 24 h. B, Contribution of the particular metabolites in the pool of known compounds after treatment for 24 h with the metabolites.
DISCUSSION
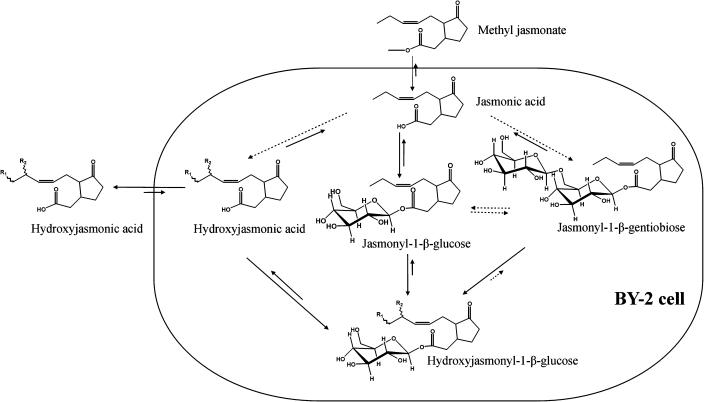
After the publication of the sequence of the JAR1 gene and identification of the corresponding protein as a JA-adenylate-forming enzyme (Staswick et al., 2002), the metabolism of JA and the function of its metabolites deserved thorough attention. We used microbore LC/(+)ESI-MS/MS, microbore LC/(+)ESI-QTOF, and GC/MS to identify several metabolites formed within 24 h after treatment of tobacco BY-2 cells with JA. Besides the known metabolites, such as the 11-OH-JA, 12-OH-JA, JA-1-β-Glc, and 1-β-gentiobiose esters (Meyer et al., 1989), OH-JA-Glc ester was identified for the first time as a JA metabolite. The compounds were purified and reused in the feeding experiments in order to determine their position in the JA catabolic pathway (Fig. 9). The first step in the JA metabolism in tobacco BY-2 is most likely to be the conjugation to d-Glc and formation of a jasmonyl-1-β-Glc ester. The second step involves formation of the 1-jasmonyl-β-gentiobiose, possibly by the extension of the carbohydrate chain by a further Glc unit. Both compounds were shown to release free JA and might therefore serve as a JA-storage or -transport form because of their excellent solubility in water. Another conversion step involves the introduction of a hydroxy group at C-11 or C-12.
Figure 9.
Schematic representation of the metabolic conversions of jasmonates in tobacco BY-2 cells. Reversible steps are marked with opposing arrows. The steps for which insufficient information was available to allow verification are marked with dotted lines.
Free hydroxylated JA was the preferential fraction in the medium of the BY-2 cells treated with different JA metabolites but not in the cell extracts. It was also taken up less efficiently than JA and its esters. This extracellular accumulation of OH-JAs makes them good candidates for long-distance transport and/or removal of superfluous jasmonates. Equilibrium favors the hydroxylated derivatives of JA because mixed isomers of OH-JA or their Glc esters are very poorly converted into JA.
Some of our findings seem to be in partial agreement with data obtained by Zhang and Baldwin (1997), who analyzed certain aspects of JA storage and transport in the related species N. sylvestris. They found that treatment of ether-insoluble fractions of root and shoot extracts with β-glucosidase released a pool of free JA from unidentified compounds. Both JA-1-β-Glc and1-β-gentiobiose esters release free JA when treated with β-glucosidase, and they are also insoluble in ether. Yet, in contrast to our findings, the radioactive compounds recovered after application of (±)-[2-14C]JA to the leaves of N. sylvestris seedlings were more hydrophobic than JA.
Surprisingly, no conjugates of JA with amino acids were found in our study, despite the fact that they were identified in various species and suggested to be possible metabolites of JA in the literature (e.g. Meyer et al., 1989). One possible explanation for this absence is that the conjugation pathway might be determined by a carbon source, as tobacco BY-2 cells are cultured in a Suc-rich medium. The availability of Glc might potentiate its incorporation into metabolic conjugates. The submillimolar concentrations used in synchronization experiments might also lead to an unspecific conjugation of the compound supplied. For example, the analogous accumulation of the Glc ester of indole-3-acetic acid (IAA) in Arabidopsis is thought to result from the treatment with the nonphysiologically high concentration of IAA used in feeding experiments. This conjugate could not be detected in experiments in which IAA ≤ 5 μm was used (Ostin et al., 1998). Another possibility is that analogously to IAA, whose amino acid conjugates have a low abundance (Kowalczyk and Sandberg, 2001), amino acid conjugates of JA might have a different function to the free acid and be present in small amounts and only in the specific tissues. Our analysis was performed on a tobaccoBY-2 cell culture in which hardly any differentiation was observed.
In our previous experiments, we established that exogenous JA could interfere with the cell division of a synchronized tobacco BY-2 cell culture by blocking cells in the G2 phase of the cell cycle (Swiatek et al., 2002). It is intriguing that JA was most effective when applied 4 h before its effect on the G2/M transition was observed. The kinetics of JA metabolism during the synchronization experiment indicated that within 4 h of treatment, small amounts of metabolites were formed. Yet all the identified derivatives of JA were far less efficient than JA in preventing mitosis when exogenously supplied (Fig. 7). Therefore, we conclude that JA is exclusively responsible for the G2/M arrest. This fact does not exclude other biological functions for the JA-derived compounds described here. Tuber formation in potatoes can be triggered by exogenous JA as well as by TA (Koda et al., 1991); the latter was recently detected in Arabidopsis, which could indicate the widespread occurrence of hydroxyjasmonates in the plant kingdom. They are probably involved in processes other than the formation of tubers. For example, the expression of the recently identified hydroxyjasmonate sulfotransferase gene in Arabidopsis is positively regulated by both JA and TA (Gidda et al., 2003). This enzyme is specific for 11- and 12-OH-JA, but only in the free acid forms. Hence, the Glc esters of those two compounds might be protected from inactivation by sulfonation. Accordingly, our initial data from growth assays indicated that Glc and gentiobiose esters of JA could inhibit root growth in Arabidopsis to the same extent as free JA. The hydroxylated derivatives of JA and their Glc esters were less effective, yet they also caused a significant decrease in the rate of root growth.
The difference between the uptake rate of JA and of MeJA in tobacco BY-2 cells was very surprising, the high accumulation of JA in particular. In order to verify whether this was due to the sampling method or unspecific interactions of JA with cell material, we prepared a negative control in which cells were killed by treatment with 1 mm salicylic acid prior to incubation with JA. In this sample, the radioactive components of the cellular fraction amounted to approximately 30 nmol/g fwt as opposed to 700 nmol/g fwt incorporated by viable cells. This in turn might indicate that JA, in contrast to MeJA, behaves similarly to fatty acids, which are easily taken up by a combination of diffusion, active transport, and flip-flop movement through the membranes. They are known to accumulate in the cells within seconds of the time of application and in quantities exceeding 10-fold the concentration of free fatty acid outside the cell (e.g. Abumrad et al., 1981).
Another issue that we addressed in this study was the ongoing debate as to whether MeJA or JA is the active form in activating jasmonate-specific responses. The accumulation pattern of MeJA upon wounding closely follows that of JA (Creelman et al., 1992). MeJA is as effective as JA in inducing the activity of the promoter of cathepsin D inhibitor (Ishikawa et al., 1994), the expression of JIP-23 and JIP-6 mRNA (Miersch et al., 1999) and tuber formation in potato (Koda et al., 1991). Overexpression of JA carboxyl methyltransferase (JIMT) in Arabidopsis leads to the induction of expression of jasmonate-responsive genes and the accumulation of MeJA without altering the JA levels in transgenic plants (Seo et al., 2001). This in turn led to a conclusion that MeJA is an active compound independently of its hydrolysis to JA. In our previous experiments, we found that both compounds were equally effective in preventing mitosis in synchronized BY-2 cells (Swiatek et al., 2002). We used the same experimental system to test whether any interconversion was possible between these two compounds under our experimental conditions. No significant amounts of MeJA was observed in the JA-feeding experiments, and when the culture was treated with MeJA, the latter was rapidly hydrolyzed to JA and further processed like JA (Fig. 6). This may point to JA as the active component that interferes with the G2/M transition in BY-2. The effect of MeJA might be due to its hydrolysis to JA, although the direct effect of MeJA cannot be excluded. This allows us to speculate on the nature of jasmonate perception. There seems to be at least two types: some, like tuber formation, are triggered by a family of structurally related compounds, whereas G2 arrest seems to be specific for JA and eventually MeJA and might involve a different signal transduction pathway.
MATERIALS AND METHODS
Chemicals
All chemicals and materials were obtained from Merck (Darmstadt, Germany), unless mentioned otherwise. (±)-[2,3-3H2]JA (specific activity 50 mCi/mmol) was obtained from American Radiolabeled Chemicals (St. Louis).
Cell Culture and Treatment
BY-2 cells were maintained as described by Nagata et al. (1992) with modifications. The culture was refreshed weekly by transfer of 0.5 mL of a 7-d-old culture into 50 mL of fresh Murashige and Skoog medium (Duchefa, Haarlem, The Netherlands), pH 5.8, containing 3% (w/v) Suc (Duchefa), 0.2 g L−1 KH2PO4, 10 mg L−1 myoinositol (Sigma, Bornem, Belgium), 1 mg L−1 thiamine hydrochloride (Sigma), and 0.2 mg L−1 2,4-dichlorophenoxyacetic acid (Serva, Hiedelberg), referred to as medium throughout the article. The culture was kept at 27°C in constant darkness at 130 rpm. In experiments, cultures were additionally treated with solutions of (±)-JA or (±)-MeJA (Apex Organics, Honiton, Devon, UK) in methanol. An appropriate volume of methanol (1:1,000, v/v) was added to the control culture. Before use in feeding experiments, radiolabeled JA was diluted with unlabeled (±)-JA to reach a specific activity of 14.5 Bq/nmol. Radiolabeled MeJA was obtained by methylation of (±)-[2,3-3H]2-JA with ethereal diazomethane. Before use, radiolabeled MeJA was diluted with unlabeled (±)-MeJA to obtain a specific activity of 11 Bq/nmol.
Synchronization
The synchronization protocol was based on the method of Nagata et al. (1992). The stationary culture was transferred in a proportion 14:100 to the fresh medium, supplied with 5 mg L−1 aphidicolin (ICN Biomedicals, Asse, Belgium). After 24 h of incubation, the cells were released by extensive washing with fresh medium without 2,4-dichlorophenoxyacetic acid and thiamine (4 L per 100 mL of blocked culture). Afterward, the cells were transferred into fresh medium and divided according to the number of the treatments. For mitotic index measurements, the cells were fixed in ethanol:acetic acid (3:1, v/v), stained with 4′,6-diamino-phenylindole, and examined under a fluorescence microscope.
Extraction and Purification of JA Metabolites
Samples collected from the tobacco BY-2 culture were separated into medium and cells by filtration through Whatman number 1 filter paper (Clifton, NJ), frozen in liquid N2, and stored at −70°C for further analysis. Prior to quantitative analysis, the cells were disrupted by sonication in 80% (v/v) ice-cold methanol (10 mL/g of fwt), 18 pulses (5 s each at 5-s intervals, 30% of maximal amplitude; Vibra Cell, Sonics & Materials, Danbury, CT). The samples were extracted at −20°C overnight, and the extracts were cleared by centrifugation at 33,000g for 20 min. The extracts were dried under a constant flow of nitrogen at 35°C and redissolved in 50% (v/v) CH3CN.
Several purification steps were included prior to the qualitative analysis of the samples. The methanol cell extracts were passed through a C-18 cartridge (500 mg, Bond Elut; Varian, Palo Alto, CA), and the methanol was removed under nitrogen flow. The remaining water phase was acidified with 2 mL of 0.05 m HCl and applied to a C-18 cartridge (Varian). The cartridge was subsequently washed with 0.05 m HCl and MilliQ-grade water. Bound compounds were eluted with 50% (v/v) CH3CN and dried under nitrogen flow at 35°C. The dry residue was dissolved in 100 μL of 50% (v/v) CH3CN. The samples of the medium were acidified with 10 mL of 0.05 m HCl and applied to a C-18 cartridge (Varian). The subsequent purification procedure was the same as for the cell extracts.
Quantitative HPLC
Prior to HPLC separation, samples were filtered through a 0.2-μm polyvinylidene difluoride syringe filter (Alltech, Lokeren, Belgium) and injected manually through a 20-μL loop on a C-18 Altima 5-micron column (150 mm × 4.6 mm i.d.; Alltech, Deerfield, IL) connected to a Waters 600-MS solvent-delivery system (Waters, Milford, MA). The following separation conditions were applied: constant flow rate 1 mL/min, 5 min of isocratic flow 25% (v/v) CH3CN in water containing 0.5% (v/v) acetic acid, 10 min of a linear gradient to 75% (v/v) CH3CN in water containing 0.5% (v/v) acetic acid, followed by 5 min of isocratic flow under the same conditions. Fractions of 1 mL were collected (FRAC-100; Amersham Biosciences, Uppsala) in scintillation vials and analyzed by liquid scintillation counting (Tri-Carb 1500 scintillation analyzer; Packard BioScience, Zellik, Belgium). Under these conditions, JA and MeJA showed retention times of 12 and 15 min, respectively. The media were filtered through a 0.2-μm polyvinylidene difluoride syringe filter and injected directly into the HPLC. The measured radioactivity was used to calculate the content of a particular metabolite in the sample by isotope dilution. When the identity could not be concluded from the retention time alone, the sample was reexamined by chromatography upon treatment with diazomethane, digestion with 1 unit of porcine liver esterase (Sigma) in 20 mm Tris-HCl, pH 8.0, or treatment with 1 unit of β-glucosidase (Fluka, Bornem, Belgium) in 20 mm ammonium acetate, pH 5.5.
Preparative HPLC and TLC
The media and cell samples were separated on a C-18 Prevail 5-micron column (250 mm × 4.6 mm i.d.; Alltech), under the same conditions used for the quantitative analysis [tr (JA) = 15 min, tr (MeJA) = 18 min]. Fractions of 0.5 mL were collected and tested for radioactivity. Fractions that tested positive were dried under nitrogen flow at 35°C, resuspended in 50% (v/v) CH3CN,and stored at −20°C for further analysis. Sample C1 was reexamined by chromatography on the same column with the following elution gradient: 5 min of isocratic flow of 10% (v/v) CH3CN in water containing 0.5% (v/v) acetic acid, 10 min of linear gradient to 50% (v/v) CH3CN in water containing 0.5% (v/v) acetic acid, and 5 min of isocratic flow of the same solution.
Approximately 3 nmol of each compound was applied to a high-performance TLC (HPTLC) plate (Silica gel 60, Merck, Darmstadt, Germany) with 95% (v/v) CH3CN used as mobile phase. For spot detection, either resublimed iodine (UCB, Leuven, Belgium) or Bial's reagent (Sigma) was used.
Carbohydrate Identification
Sugars were identified by comparison with our collection of standards, which included the most common natural monosaccharides and disaccharides. Approximately 5 nmol of compound per sample was subjected to TLC separation or GC/MS analysis. HPTLC was performed with Silica gel 60 and 80% CH3CN as the mobile phase. Prior to GC/MS analysis, the samples were derivatized with methoxylamine and N-Methyl-N-trifluoroacetamide according to Roessner et al. (2000). The measurements were performed on an HP5890 II GC (Agilent Technologies, Diegem, Belgium) coupled to a VG Trio 2000 quadrupole mass spectrometer (Micromass, Manchester, UK). Samples were separated on a 15-m CP-Sil 5 CB Low Bleed/MS 0.25 mm i.d. column (Varian), the temperature gradient was 2 min at 60°C, followed by an increase to 300°C at 15°C/min. The carrier gas was helium. Ionization was done under electron impact (EI+) conditions at an ionization energy of 70 eV. The source temperature was set at 200°C. A mass range between 50 and 1,000 mass units was recorded at a speed of 1 scan/s with a 0.1-s interscan delay. Rf and Tr for Glc and gentiobiose were 0.48 and 11.38 min and 0.31 and 16.69 min, respectively.
Microbore Liquid Chromatography/Mass Spectrometry
Chromatographic Conditions
LC-MS analysis was performed with a Kontron 325 pump (Kontron Instrumens, Milan) coupled to a Z-spray ion source of a Quattro II triple quadrupole mass spectrometer (Micromass). A Kontron 465 injector was used to introduce 20-μL samples into a C-18 column (Prodigy, 5-micron, 50 mm × 1 mm i.d.; Phenomenex, Torrance, CA). JA and other standards were separated through isocratic elution at a flow rate of 60 μL/min with CH3CN/0.05% formic acid (1:1, v/v) as the mobile phase.
Fractions C1, C2, and M1 were analyzed isocratically for 2 min with CH3CN/0.05% HCOOH (1:9, v/v), followed by a linear gradient to 50% (v/v) CH3CN in 8 min. The flow rate was 60 μL/min.
Fraction C3 was separated isocratically with CH3CN/0.05% HCOOH (1:3, v/v) for 2 min, followed by a linear gradient to 80% (v/v) CH3CN in 8 min. The flow rate was 60 μL/min.
All gradients were selected to obtain on-column focusing of the sample.
Mass Spectrometric Conditions
(+)ESI mass spectra were recorded on a Quattro II triple-quadrupole mass spectrometer at a source temperature 80°C and a cone voltage of 12 to 15 V. The flow rate of the drying gas was 250 L/min, and the cone gas flow was set at 90 L/min.
Low-energy, CNL product and parent-ion scans were recorded at collision energies of 12, 5, and 20 eV, respectively, with Ar as the collision gas at a pressure of 0.004 mbar.
Accurate Mass Measurement
Accurate mass measurement was performed on a QTOF II triple-quadrupole instrument (Micromass) under (+)ESI conditions. The instrument was calibrated with a solution of 55 ng/μL polyethylene glycol in 50% (v/v) CH3CN/2 mm ammonium acetate (1:1, v/v). The samples were delivered by a microbore LC (Waters) solvent-delivery system at a flow rate of 40 μL/min on a C-18 column (Prodigy, 5-micron 50 mm × 1 mm i.d.; Phenomenex). The calibration solution was delivered postcolumn through a T junction by an infusion pump (Harvard Apparatus, Holliston, MA) at a constant flow rate of 0.5 μL/min. The following instrument settings were applied: 80° source temperature, 20 V cone voltage, TOF 9, reflectron 35 kV, flow rate of nitrogen gas 250 L/min. Spectrum analysis and empirical formula predictions were performed with MassLynx 3.5 software (Micromass). Spectra of samples M1 and C3 were analyzed with a lock mass of 283.17568; samples C2 and C2 were analyzed with a lock mass of 344.2844.
Acknowledgments
We thank Dr. Otto Miersch for a generous gift of 12-hydroxyjasmonic acid and Dr. Els Prinsen and Sevgi Öden for their kind help in the development of sample-preparation and -extraction methods. We also thank Dr. José Oliveira for his patient and critical reading of the manuscript.
This work was supported by the Interuniversity Pools of Attraction financed by the Belgian State.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040501.
References
- Abumrad NA, Perkins RC, Park JH, Park CR (1981) Mechanism of long-chain fatty-acid permeation in the isolated adipocyte. J Biol Chem 256: 9183–9191 [PubMed] [Google Scholar]
- Bell E, Creelman RA, Mullet JE (1995) A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA 92: 8675–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert S, Bockelmann C, Fusslein M, Von Schrader T, Stelmach B, Niesel U, Weiler EW (1999) Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta 207: 470–479 [Google Scholar]
- Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH (1995) The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA 92: 4099–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA 92: 4114–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE (1992) Jasmonic acid and methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene-expression. Proc Natl Acad Sci USA 89: 4938–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demole E, Lederer E, Mercier D (1962) Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant charactéristique de l'essence de jasmin. Helv Chim Acta 45: 645–685 [Google Scholar]
- Froehlich JE, Itoh A, Howe GA (2001) Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome p450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol 125: 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidda SK, Miersch O, Levitin A, Schmidt J, Wasternack C, Varin L (2003) Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J Biol Chem 278: 17895–17900 [DOI] [PubMed] [Google Scholar]
- Helder H, Miersch O, Vreugdenhil D, Sembdner G (1993) Occurrence of hydroxylated jasmonic acids in leaflets of Solanum demissum plants grown under long-day and short-day conditions. Physiol Plant 88: 647–653 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The Defective in Anther Dehiscence1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Yoshihara T, Nakamura K (1994) Jasmonate-inducible expression of a potato Cathepsin-D inhibitor-GUS gene fusion in tobacco cells. Plant Mol Biol 26: 403–414 [DOI] [PubMed] [Google Scholar]
- Koda Y, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T (1991) Potato tuber-inducing activities of jasmonic acid and related-compounds. Phytochemistry 30: 1435–1438 [Google Scholar]
- Kowalczyk M, Sandberg G (2001) Quantitative analysis of indole-3-acetic acid metabolites in Arabidopsis. Plant Physiol 127: 1845–1853 [PMC free article] [PubMed] [Google Scholar]
- Maucher H, Hause B, Feussner I, Ziegler J, Wasternack C (2000) Allene oxide synthases of barley (Hordeum vulgare cv. Salome): tissue specific regulation in seedling development. Plant J 21: 199–213 [DOI] [PubMed] [Google Scholar]
- Meyer A, Gross D, Vorkefeld S, Kummer M, Schmidt J, Sembdner G, Schreiber K (1989) Metabolism of the plant-growth regulator dihydrojasmonic acid in barley shoots. Phytochemistry 28: 1007–1011 [Google Scholar]
- Miersch O, Kramell R, Parthier B, Wasternack C (1999) Structure-activity relations of substituted, deleted or stereospecifically altered jasmonic acid in gene expression of barley leaves. Phytochemistry 50: 353–361 [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell-line as the Hela-cell in the cell biology of higher-plants. Int Rev Cytol 132: 1–30 [Google Scholar]
- Ostin A, Kowalyczk M, Bhalerao RP, Sandberg G (1998) Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol 118: 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 23: 131–142 [DOI] [PubMed] [Google Scholar]
- Sembdner G, Atzorn R, Schneider G (1994) Plant hormone conjugation. Plant Mol Biol 26: 1459–1481 [DOI] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD (2001) Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA 98:4788–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14:1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek A, Lenjou M, Van Bockstaele D, Inzé D, Van Onckelen H (2002) Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol 128: 201–211 [PMC free article] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC (1984) Biosynthesis of jasmonic acid by several plant-species. Plant Physiol 75: 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Amanuma M, Tsutsumi T, Okumura Y, Matsuura H, Ichihara A (1996) Metabolism and transport of (±)-[2-14C] jasmonic acid in the potato plant. Plant Cell Physiol 37: 586–590 [Google Scholar]
- Zhang ZP, Baldwin IT (1997) Transport of (±)-[2-14C] jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203: 436–441 [Google Scholar]
- Ziegler J, Stenzel I, Hause B, Maucher H, Hamberg M, Grimm R, Ganal M, Wasternack C (2000) Molecular cloning of allene oxide cyclase. The enzyme establishing the stereochemistry of octadecanoids and jasmonates. J Biol Chem 275: 19132–19138 [DOI] [PubMed] [Google Scholar]