Abstract
Whereas ifenprodil has been used as a selective GluN1/GluN2B (NR1/NR2B, B-type) receptor antagonist to distinguish between GluN2B (NR2B) and GluN2A (NR2A)-containing N-methyl-d-aspartate receptors (NMDARs), TCN 201 (3-chloro-4-fluoro-N-[4-[[2-(phenylcarbonyl)hydrazino]carbonyl]benzyl]benzenesulphonamide) and TCN 213 [N-(cyclohexylmethyl)-2-[{5-[(phenylmethyl)amino]-1,3,4-thiadiazol-2-yl}thio]acetamide] have been found to be selective GluN1/GluN2A (NR1/NR2A, A-type) antagonists. Based on the premise that A- and B-types are major synaptic NMDARs, we examined whether inhibition of NMDAR excitatory postsynaptic potentials (EPSPs) by the TCN compounds and ifenprodil are complementary. Contrary to this prediction, inhibition of NMDAR EPSPs by the TCN compounds and ifenprodil were largely overlapping in the CA1 region of hippocampal slices from 30-day-old rats. After partial inhibition by ifenprodil, TCN compounds produced little further suppression of NMDAR EPSPs. Similarly, after partial inhibition by TCN compounds ifenprodil failed to further suppress NMDAR EPSPs. However, low micromolar d-2-amino-5-phosphonovalerate, a competitive NMDAR antagonist, which alone only partially inhibits NMDAR EPSPs, markedly suppresses residual NMDAR responses in the presence of ifenprodil or the TCNs, suggesting that low 2-amino-5-phosphonovalerate antagonizes both ifenprodil- and TCN-insensitive synaptic NMDARs. These observations can be most readily interpreted if ifenprodil and TCNs act on a similar population of synaptic NMDARs. Recent lines of evidence suggest that the majority of hippocampal synaptic NMDARs are triheteromers. If so, modulation of GluN2A, and not just GluN2B NMDARs, could dampen long-term depression (LTD). Indeed, both TCNs, like ifenprodil, blocked LTD, suggesting the involvement of ifenprodil- and TCN-sensitive NMDARs in LTD induction. However, the TCNs plus ifenprodil failed to inhibit long-term potentiation (LTP), suggesting that neither ifenprodil- nor TCN-sensitive NMDARs are essential for LTP induction.
Introduction
N-Methyl-d-aspartate (NMDA) receptors (NMDARs) are tetrameric glutamate-gated ion channels containing two GluN1 (NR1) subunits and two GluN2 (NR2) subunits (Traynelis et al., 2010; Paoletti at al., 2013). Because these subunits are thought to assemble in a dimer-of-dimers configuration (Schorge and Colquhoun, 2003), NMDARs have been assumed to be predominantly diheteromers of GluN1/GluN2A (A-type) or GluN1/GluN2B (B-type) (Al-Hallaq et al., 2007).
Ifenprodil, which binds to the N terminus of GluN2B (Williams, 1993), has been used as a selective B-type antagonist to distinguish between GluN2B- and GluN2A-containing NMDARs in synaptic and extrasynaptic membranes (Cull-Candy and Leszkiewicz 2004). By contrast, selective antagonists against GluN2A-containing NMDARS have not been available until recently. Although NVP-AAM077 (NVP; [[[(1S)-1-(4-bromophenyl)ethyl]amino](1,2,3,4-tetrahydro-2,3-dioxo-5-quinoxalinyl)methyl] phosphonic acid tetrasodium hydrate) (Auberson et al., 2002) has been used to isolate GluN2A-containing NMDAR responses, NVP only weakly discriminates GluN2A from GluN2B (Frizelle et al., 2006; Izumi et al., 2006; Neyton and Paoletti 2006; Wyllie and Chen 2007), underscoring the need for more selective antagonists.
Recently, TCN 201 (3-chloro-4-fluoro-N-[4-[[2-(phenylcarbonyl)hydrazino]carbonyl]benzyl]benzenesulphonamide) and TCN 213 [N-(cyclohexylmethyl)-2-[{5-[(phenylmethyl)amino]-1,3,4-thiadiazol-2-yl}thio]acetamide] have been found to be selective A-type antagonists (Bettini et al., 2010). GluN1/GluN2A receptor currents elicited by 30 μM NMDA with 3 μM glycine in HEK 293T cells are inhibited by 3 μM TCN 201 (Bettini et al., 2010). Moreover, currents recorded from oocytes expressing GluN1/GluN2A subunits are also blocked by 10 μM TCN 201. Similar effects are observed with TCN 213, although TCN 201 is a more potent antagonist. Furthermore, TCN 201 has no effects in oocytes expressing GluN1/GluN2B, confirming its relative selectivity (Edman et al., 2012; Hansen et al., 2014).
In the CA1 hippocampal region, we previously observed that 10 μM ifenprodil only partially inhibits NMDAR-mediated excitatory postsynaptic potentials (EPSPs) and that residual responses are suppressed completely by low micromolar concentrations of 2-amino-5-phosphonovalerate (APV), a broad spectrum, competitive NMDAR antagonist. Similarly, residual NMDAR EPSPs in the presence of low APV are completely inhibited by ifenprodil (Izumi et al., 2006). These observations suggest that low micromolar APV may serve as an antagonist for ifenprodil-insensitive NMDAR subtypes. If A- and B-type NMDARs are the exclusive NMDARs in the hippocampus, these observations suggest that low concentrations of APV may preferentially act as an A-type antagonist. If so, we hypothesize that the effects of APV should be mimicked by the TCN compounds, and that these agents should block residual NMDAR EPSPs in the presence of ifenprodil.
In the present study, we examined TCN 201 and TCN 213 on NMDAR-mediated EPSPs in the absence and presence of ifenprodil and low concentrations of APV. We also examined these agents against long-term potentiation (LTP) and long-term depression (LTD), based on prior studies showing that ifenprodil inhibits LTD but not LTP (Izumi et al., 2006).
Materials and Methods
Hippocampal Slice Preparation.
Hippocampal slices were prepared from postnatal day (P) 28–32 Sprague-Dawley male albino rats using standard methods (Izumi and Zorumski, 2012). Rats were anesthetized with isoflurane and decapitated. Dissected hippocampi were placed in ice-cold artificial cerebrospinal fluid containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2/5% CO2 at 4–6°C, and cut into 500 µm slices using a rotary slicer. Acutely prepared slices were placed in an incubation chamber containing gassed artificial cerebrospinal fluid for at least 2 hours at 30°C before further experimentation.
Hippocampal Slice Physiology.
At the time of study, slices were transferred individually to a submersion-recording chamber. Experiments were done at 30°C with continuous artificial cerebrospinal fluid perfusion at 2 ml/min. Extracellular recordings were obtained from the apical dendritic layer (stratum radiatum) of the CA1 region for analysis of EPSPs using electrodes filled with 2 M NaCl (5–10 MΩ resistance).
Input/output curves were generated using stimuli of six different intensities to allow determination of half-maximal responses. The smallest stimulus was set to evoke a response less than half-maximal while the largest stimulus was designed to evoke a fully saturated response. During an experiment, evoked EPSPs were monitored by applying single stimuli to the Schaffer collateral pathway every 60 seconds at an intensity that elicited half-maximal responses. After establishing a stable baseline for at least 10 minutes, LTD was induced by applying low-frequency stimulation (LFS) at 1 Hz using the same intensity stimulus to the Schaffer collateral pathway for 15 minutes. LTP was induced by high-frequency stimulation (HFS) consisting of a single 100 Hz × 1 second train using the same intensity stimulus. Input/output curves were repeated after 60 minutes following HFS or LFS to determine the magnitude of LTP and LTD based on changes in the half-maximal responses. Isolated NMDAR synaptic responses were studied in bath solution containing 2.5 mM calcium and 0.1 mM magnesium in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione to inhibit α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor–mediated components, and responses were evoked once per minute (Izumi et al., 2006).
Chemicals.
TCN 201 and TCN 213 were purchased from Tocris (St. Louis, MO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). According to the instructions from the supplier, TCN 201 was dissolved in dimethylsulfoxide as a stock solution (50 mM), while TCN 213 was dissolved in dimethylsulfoxide or ethanol as stock solutions (50 and 10 mM, respectively).
Statistical Analysis.
Data were collected and analyzed using PClamp software (Axon Instruments, Union City, CA). LTP and LTD data are expressed as the mean ± S.E.M. measured 60 minutes following LFS or HFS, and are normalized with respect to initial baseline recordings (set at 100%). A two-tailed Student’s t test was used for comparisons between groups. In cases of non-normally distributed data, the nonparametric Wilcoxon rank sum test was used. Statistical comparisons were based on input/output curves at baseline and 60 minutes following LFS or HFS, with P < 0.05 considered significant, and were done using commercial software (SigmaStat; Systat Software, Inc., Richmond City, CA). Changes within the same slice in isolated NMDAR synaptic responses by multiple drug perfusions were compared with the paired Student’s t test using SigmaStat.
Results
Effects of APV and Ifenprodil on NMDA EPSPs.
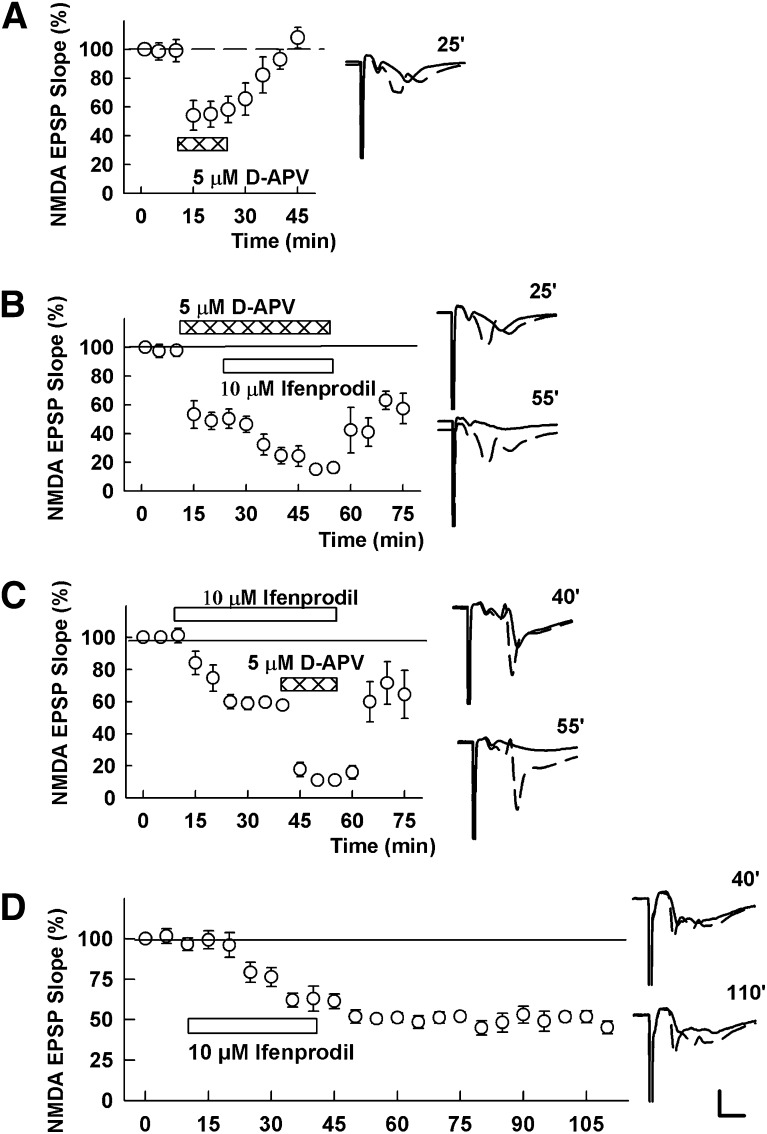
In the initial experiments, we re-examined the effects of low micromolar concentrations of ifenprodil and d-2-amino-5-phosphonovalerate (D-APV) on NMDAR-mediated EPSPs in the CA1 region of hippocampal slices from postnatal day 30 (P30) rats. As found previously, 10 μM ifenprodil and 5 μM D-APV each depress NMDAR-mediated EPSPs by 50% or less (Izumi et al., 2006). Administration of 5 μM D-APV alone promptly but partially depressed NMDAR EPSPs in a reversible manner (NMDAR-EPSP slope: 58.2 ± 9.2% of baseline following 15 minute administration, N = 5; Fig. 1A). In another set of slices treated with 5 μM D-APV, 10 μM ifenprodil further suppressed NMDAR responses (50.6 ± 6.6% of baseline with D-APV alone, 16.3 ± 3.6% with the combination, N = 5, P < 0.01 compared before and after ifenprodil administration, N = 5; Fig. 1B). When ifenprodil was administered first, NMDAR EPSPs were slowly depressed (57.8 ± 1.9% after 30 minute administration) and the addition of D-APV further suppressed these responses (11.1 ± 2.3%, P < 0.01 compared before and after D-APV administration, N = 5; Fig. 1C). The depression induced by ifenprodil is very slow to reverse and persists after washout of the drug (51.9 ± 4.4% after 30 minute administration and 51.9 ± 3.7% 60 minutes after washout, N = 5; Fig. 1D).
Fig. 1.
Effects of APV and ifenprodil on NMDAR-mediated EPSPs (NMDA EPSPs). (A) D-APV (5 µM; hatched bar) reversibly depressed NMDA-EPSPs by about 50%. (B) In the presence of D-APV (hatched bar), 10 µM ifenprodil (open bar) depressed NMDA EPSPs almost completely. (C) Application of ifenprodil (open bar), only partially depressed NMDA EPSPs. In the presence of ifenprodil, D-APV almost completely depressed NMDA EPSPs. (D) The depression induced by ifenprodil persists after washout of ifenprodil. Traces to the right of the graph show NMDAR EPSPs at the times denoted with initial control responses shown as dashed lines. Values are expressed as the mean ± S.E.M. N = 5 for each experiment. Calibration: 1 mV; 5 milliseconds.
Effects of TCN Compounds on NMDA EPSPs.
These observations indicate that ifenprodil-insensitive NMDARs are inhibited by low micromolar concentrations of APV. Based on the premise that GluN1/GluN2A (A-type) and GluN1/GluN2B (B-type) are the major diheteromeric NMDAR subtypes in the hippocampus, we hypothesized that ifenprodil and the TCN compounds, which have been described as selective inhibitors of GluN2A-containing receptors (Hansen et al., 2014), may also discriminate these receptor subtypes at synapses in the native hippocampus. Specifically, we examined whether the TCN compounds mimic the actions of 5 μM APV and suppress residual NMDAR EPSPs in the presence of ifenprodil.
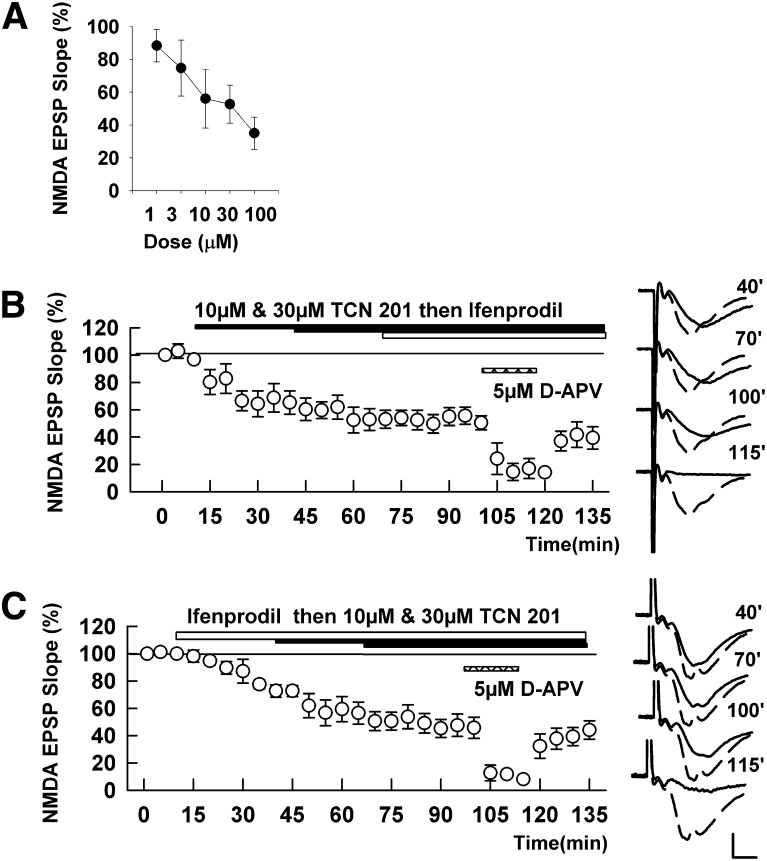
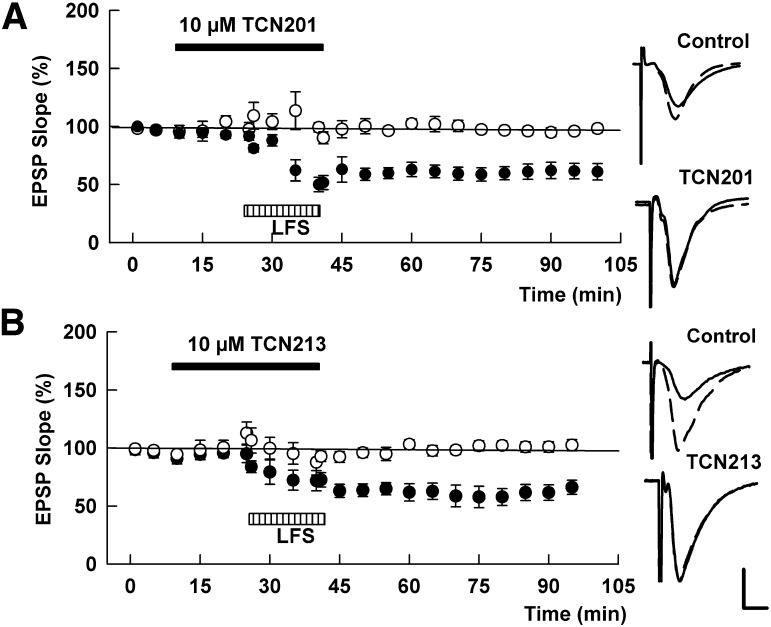
TCN 201 depressed isolated NMDAR EPSPs in a concentration-dependent fashion (Fig. 2A). Thirty minute administration of 10 μM TCN 201 depressed NMDAR EPSPs only partially (65.3 ± 8.6% of baseline, N = 5). Increasing the concentration to 30 µM depressed NMDAR-mediated EPSPs to 53.0 ± 6.6%; however, this increase was not statistically significant (P = 0.27). Against our hypothesis, addition of 10 µM ifenprodil failed to further suppress these responses (50.5 ± 5.1%). In contrast, addition of 5 µM D-APV quickly suppressed the residual responses in a reversible manner (14.1 ± 2.1%, P < 0.01; Fig. 2B).
Fig. 2.
Effects of TCN 201 on NMDA EPSPs. (A) In three slices, concentrations of TCN 201 were increased every 15 minutes to create a concentration-response curve. (B) TCN 201 (10 µM, then 30 µM; solid bar) only partially depressed NMDA EPSPs. Addition of 10 µM ifenprodil (open bar) failed to further depress NMDA EPSPs. Addition of 5 µM D-APV (hatched bar) almost completely suppressed NMDA EPSPs in the presence of TCN 201 and ifenprodil. (C) In the presence of 10 µM ifenprodil (open bar) TCN 201 (10 µM, then 30 µM; solid bars) produced only a small further increase in NMDA EPSP suppression. D-APV (5 µM; hatched bar) depressed NMDA-EPSPs almost completely in the presence of ifenprodil and TCN 201. Traces to the right of the graph show NMDA EPSPs at the times denoted with initial control responses shown as dashed lines. Values are expressed as the mean ± S.E.M. N = 5 for each experiment. Calibration: 1 mV; 5 milliseconds.
To further test interactions of TCN 201 with ifenprodil and APV, we reversed the order of drug application (Fig. 2C). Thirty minute administration of 10 μM ifenprodil depressed NMDAR-mediated EPSPs to 72.7 ± 4.5% of baseline (N = 5). Addition of 10 and 30 µM TCN 201 depressed NMDAR EPSPs to 56.5 ± 5.1 and 45.8 ± 7.8%, respectively (P < 0.01 versus ifenprodil alone for both concentrations). While the effects of TCN 201 were significant in this set of experiments, we did not observe anything near a complete block of NMDAR EPSPs by the drug combination, and the effects of ifenprodil were less than typically observed. In contrast, addition of 5 μM D-APV nearly completely suppressed the residual responses (8.0 ± 2.1%, P < 0.01; Fig. 2B).
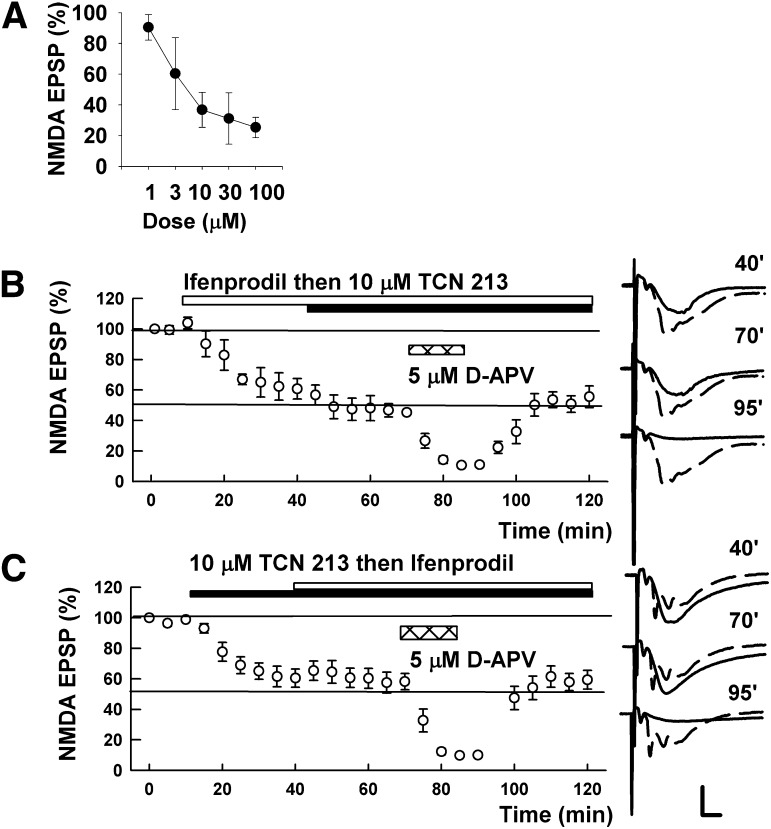
We also examined whether TCN 213, a related GluN1/GluN2A antagonist, showed similar effects on NMDAR EPSPs. As was true for TCN 201, depression of NMDAR EPSPs by ifenprodil was increased by TCN 213 but again did not result in complete NMDAR EPSP suppression (60.8 ± 6.7% with ifenprodil and 49.5 ± 1.7% by addition of 10 µM TCN 213, N = 5; Fig. 3A). In contrast, residual responses were clearly suppressed by 5 μM D-APV in a reversible manner (10.6 ± 1.3%, P < 0.001). Similarly, the depression induced by 10 µM TCN 213 (60.5 ± 6.0%, N = 5; Fig. 3B) was not clearly augmented by addition of 10 μM ifenprodil (58.1 ± 5.3%, P = 0.72); however, residual responses were nearly completely and reversibly suppressed by 5 µM D-APV (9.7 ± 1.1%, P < 0.001).
Fig. 3.
Effects of TCN 213 on NMDAR-mediated EPSPs. (A) In three slices, concentrations of TCN 213 were increased every 15 minutes to generate a concentration-response curve. (B) TCN 213 (10 µM; solid bar) only partially depressed NMDA EPSPs. Addition of 10 µM ifenprodil (open bar) failed to further depress NMDA EPSPs, but addition of 5 µM D-APV (hatched bar) almost completely suppressed NMDA EPSPs. (C) In the presence of 10 µM ifenprodil (open bar) TCN 213 (10 µM; black bar) produced only a small increase in NMDA EPSP inhibition. D-APV (5 µM; hatched bar) depressed NMDA-EPSPs about almost completely in the presence of ifenprodil and TCN 213. Traces to the right of the graph show NMDAR EPSPs at the times denoted with initial control responses shown as dashed lines. Values are expressed as the mean ± S.E.M. N = 5 for each experiment. Calibration: 1 mV; 5 milliseconds.
TCN 213 can also be dissolved in ethanol. To determine whether the solvent influences our results, we examined whether TCN 213 dissolved in ethanol had similar effects on NMDAR EPSPs. Again, expression of NMDAR EPSPs by ifenprodil was not altered significantly by TCN 213 (62.4 ± 6.4% with ifenprodil and 49.1 ± 10.7% by addition of 10 µM TCN 213, N = 5, P = 0.32; data not shown). Residual responses again were clearly suppressed by 5 μM D-APV in a reversible manner (7.2 ± 0.6%, P < 0.01). Also, the depression induced by 10 µM TCN 213 (79.2 ± 9.5%, N = 5; data not shown) was not clearly augmented by addition of 10 μM ifenprodil (67.0 ± 6.6%, P = 0.32); however, residual responses were reversibly and nearly completely suppressed by 5 µM D-APV (10.5 ± 1.2%, P < 0.01).
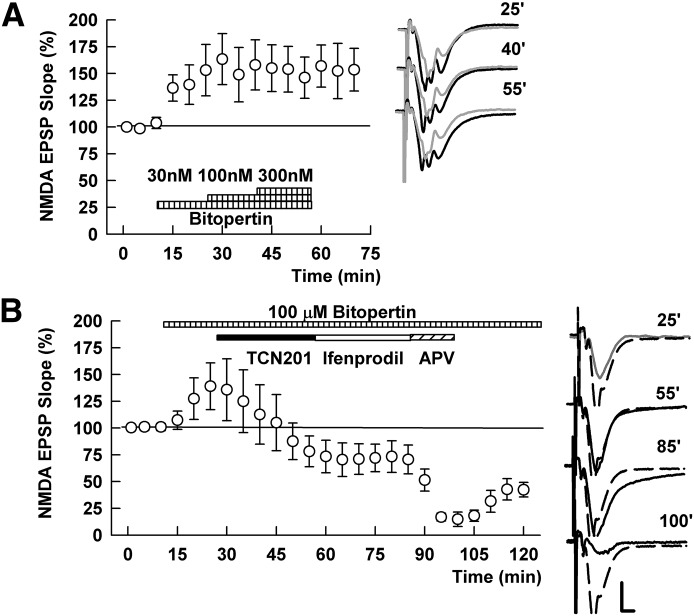
Effects of TCN 201 in the Presence of Bitopertin.
Because TCN 201 is reported to be a negative allostereic modulator of glycine binding in GluN1/2A receptors (Edman et al., 2012; Hansen et al., 2012), we examined it in the presence of bitopertin, a glycine uptake inhibitor. It has been reported that 100 nM, but not 300 nM, bitopertin facilitates LTP induction in rat slices (Alberati et al., 2012). Administration of 30 nM bitopertin augmented NMDAR EPSPs (153 ± 24%, N = 5; Fig. 4A); however, these EPSPs were not further augmented by 100 nM (158 ± 23%) or by 300 nM (146 ± 19%). In another set of experiments, 100 nM bitopertin increased NMDAR EPSPs (139.0 ± 21.8%, N = 6; Fig. 4B). In these slices 30 minute administration of 10 µM TCN 201 partially depressed NMDAR EPSPs (73.4 ± 15.1 or 60.9 + 6.3%, if compared with the response in the presence of bitopterin alone); however, administration of 10 µM ifenprodil failed to further depress NMDAR EPSPs (71.9 ± 12.9%).
Fig. 4.
Effects of bitopertin on actions of TCN 201. (A) Bitopterin augmented NMDA EPSPs in an irreversible manner. (B) After augmentation of NMDA EPSP by 100 nM bitopertin, TCN 213 (10 µM; solid bar) only partially depressed NMDA EPSPs but administration of ifenprodil (10 µM; open bar) failed to further depress NMDA EPSPs. D-APV (5 µM; hatched bar) depressed NMDA-EPSPs. Traces to the right of the graph show NMDA EPSPs at the times denoted with initial control responses shown as dashed lines. In (B), the response with bitopertin alone (at 25′) is shown as solid line. Values are expressed as the mean ± S.E.M. N = 5 for each experiment. Calibration: 1 mV; 5 milliseconds.
Effects of the TCN Compounds on LTP.
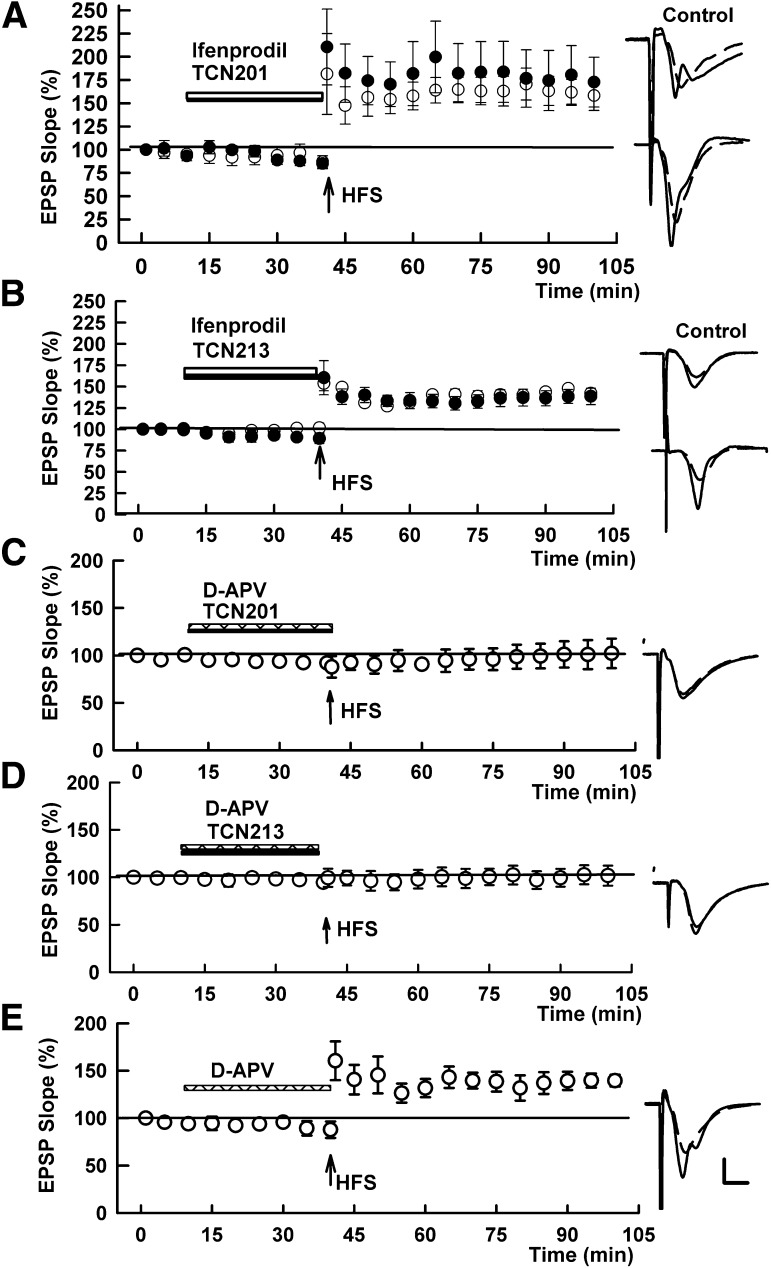
These observations suggest that the TCN compounds do not serve as selective antagonists for GluN2A-containing NMDARs, but rather have major effects on NMDARs inhibited by ifenprodil that likely express GluN2B subunits at CA1 synapses. We previously showed that ifenprodil alone, or low concentrations of APV alone, do not inhibit CA1 LTP induction, although a combination of ifenprodil with low concentrations of APV blocks LTP (Izumi et al., 2006). This strongly suggests that LTP inhibition in the CA1 region requires blocking of both ifenprodil-sensitive and ifenprodil-insensitive NMDARs. In the next set of experiments, we examined whether the combination of the TCN compounds with ifenprodil alters LTP induction. Consistent with the apparent lack of strong effects of the TCN compounds on ifenprodil-insensitive NMDARs, we found that a combination of 10 μM TCN 201 with 10 μM ifenprodil failed to block LTP induction (147.8 ± 20.8% of baseline responses after 60 minutes following HFS, N = 5; Fig. 5A). Similarly, 10 μM TCN 213 dissolved in ethanol with ifenprodil failed to block LTP (N = 5; Fig. 5B). In contrast, a combination of 10 µM TCN 201 and 5 μM D-APV completely inhibited LTP (N = 5; Fig. 5C). HFS also failed to induce LTP in the presence of 10 μM TCN 213 and 5 μM D-APV (101.7 ± 7.5%, N = 5; Fig. 5D). We also confirmed that 5 μM D-APV alone does not inhibit LTP (135.3 ± 3.7%, N = 5; Fig. 5E), as previously reported (Izumi et al., 2006).
Fig. 5.
Effects of the TCN compounds and other NMDAR antagonists on LTP induction. (A) HFS (100 Hz, 1 second; arrow) successfully induced LTP (open circles) in the presence of 10 µM ifenprodil (open bar) plus 10 µM TCN 201 (solid bar). Solid circles show the control LTP in the presence of dimethylsulfoxide alone. (B) Similarly, LTP (open circles) was not inhibited in the presence of 10 µM ifenprodil (open bar) plus 10 µM TCN 213. Solid circles show the control LTP in the presence of ethanol alone. (C) HFS failed to induce LTP in the presence of 5 µM D-APV (hatched bar) plus 10 µM TCN 201. (D) Similarly, HFS failed to induce LTP in the presence of 5 µM D-APV (hatched bar) plus 10 µM TCN 213. (E) LTP was not inhibited by 5 µM D-APV alone (hatched bar). Traces to the right of the graph show EPSPs recorded 60 minutes after HFS with initial control responses shown as dashed lines. Values are expressed as the mean ± S.E.M. N = 5 for each experiment. Calibration: 1 mV; 5 milliseconds.
Effects of the TCN Compounds on LTD.
In contrast to LTP, we previously found that ifenprodil alone inhibits LTD induction (Izumi et al., 2006). If TCN compounds and ifenprodil share mechanisms of action, then these agents would also be expected to inhibit LTD. In a final set of experiments, we examined the effects of the TCN compounds alone on LTD induction. In control slices, LFS reliably induced LTD (63.1 ± 7.9%, N = 5). In the presence of 10 µM TCN 201, LFS failed to induce LTD (96.2 ± 5.6 of baseline responses, N = 5, P < 0.01 versus control; Fig. 6A). Similarly, LFS failed to induce LTD in the presence of 10 µM TCN 213 dissolved in ethanol (107.2 ± 4.0, N = 7, P < 0.01; Fig. 6B). These results suggest that TCN compounds are similar to ifenprodil in their actions on both LTP and LTD in the rat CA1 region.
Fig. 6.
TCN compounds inhibit LTD induction. (A) LFS (1 Hz, 900 pulses; vertically striped bar) induced LTD in control slices in the presence of dimethylsulfoxide alone (solid circles), but not in the presence of 10 µM TCN 201 (open circles, open bar). (B) Similarly, LFS (striped bar) failed to induce LTD in the presence of 10 µM TCN 213 (solid bar). Solid circles show the control LTD in the presence of ethanol alone. Traces to the right of the graph show EPSPs recorded 60 minutes after LFS with initial control responses shown as dashed lines. The upper traces show the control LTD. Values are expressed as the mean ± S.E.M. N = 5 for each experiment in (A) and N = 7 for each experiment in (B). Calibration: 1 mV; 5 milliseconds.
Discussion
We previously showed that 10 μM ifenprodil partially inhibits NMDAR EPSPs in hippocampal slices from 30 day old rats (Izumi et al., 2005b, 2006). Furthermore, we observed that low micromolar APV, which alone only partially inhibits NMDAR EPSPs (Fig. 1), markedly suppresses residual NMDAR responses in the presence of ifenprodil, suggesting that low APV antagonizes ifenprodil-insensitive synaptic NMDARs (Izumi et al., 2006). In the present study, we hypothesized that inhibition of NMDAR EPSPs by the TCN compounds and ifenprodil would be complementary, based on the premise that A- and B-type NMDARs are major synaptic NMDARs and that the former are blocked by TCN compounds and the latter by ifenprodil. To test this hypothesis, TCN compounds were administered in the presence of ifenprodil or vice versa. Contrary to our prediction, inhibition of NMDAR EPSPs by the TCN compounds and ifenprodil are largely overlapping. Because glycine/d-serine levels may affect inhibition by the TCN compounds, we examined effects in the presence of the selective glycine uptake inhibitor, bitopertin. Although bitopertin augmented NMDAR EPSPs in some slices, the degree of depression by TCN 201 was similar and further depression was not observed with ifenprodil. While our studies did not specifically evaluate d-serine, recent evidence indicates that both d-serine and glycine are synaptic NMDAR coagonists in the CA1 region. However, d-serine appears to be the more important coagonist for LTP induction (Rosenberg et al., 2013).
How should we interpret these findings? Based on prior studies, it seems unlikely that the TCNs mimic ifenprodil as B-type antagonists. Bettini et al. (2010) found that TCN 201 blocks NMDA currents in HEK 293 cells expressing GluN1/GluN2A with an IC50 of 109 nM and no effect on GluN1/GluN2B below 30 μM. Other studies (Edman et al., 2012; Hansen et al., 2014) found that NMDA currents in cells expressing GluN1/GluN2A are substantially inhibited by TCN 201 and TCN 213, without effecting GluN1/GluN2B. These studies strongly indicate that TCNs preferentially affect GluN2A expressing NMDARs. Similarly, there is agreement that low micromolar ifenprodil is a selective GluN2B antagonist (Williams, 1993; Gray et al., 2011; Hansen et al., 2014), although pharmacology in slices can differ from heterologous cells.
If TCN compounds are selective for GluN2A-containing NMDARs, there is a possibility that these agents (and ifenprodil) affect a receptor subtype that expresses both GluN2A and GluN2B at hippocampal synapses. Using mouse lines expressing specific subunit combinations, Rauner and Köhr (2011) showed that GluN1/GluN2A/GluN2B (type AB) triheteromers are a major NMDAR subtype in adult CA3-CA1 synapses. Gray et al. (2011) made similar observations and emphasized that GluN2A and GluN2B are the only GluN2 subunits expressed at CA1 pyramidal neuron synapses. In cultured hippocampal neurons, about two-thirds of NMDAR EPSCs involve type AB NMDARs (Tovar et al., 2013). Other data using sequential immunoprecipitation indicate that AB triheteromers account for 15–40% of functional synaptic receptors (Al-Hallaq et al., 2007). These studies further indicate that AB triheteromers are less sensitive to ifenprodil (Blevins et al., 1997). Based on the premise that native NMDARs are largely diheteromers, the roles of GluN2A and GluN2B in synaptic plasticity have been described (Cull-Candy and Leszkiewicz, 2004; Shipton and Paulsen, 2014). However, if the majority of synaptic NMDARs are triheteromers prior studies must be reinterpreted (Tovar et al., 2013).
Our finding that ifenprodil and the TCNs are not additives against NMDAR EPSPs suggests that ifenprodil and the TCNs act on a similar population of synaptic NMDARs, although we cannot presently identify the specific subtype. We previously found that 10 μM zinc mimics ifenprodil in partially depressing NMDAR EPSPs (Izumi et al., 2006), and zinc fails to inhibit residual NMDAR EPSPs in the presence of ifenprodil. Under the assumption that antagonism of A- and B-type NMDARs are complimentary, we speculated that low micromolar zinc behaves as a B-type antagonist, although zinc preferentially but partially inhibits A-type NMDARs at lower nanomolar concentrations (Rachline et al., 2005). However, as shown in the present study, inhibition of NMDARs by ifenprodil and TCN compounds are not complimentary. If AB triheteromers are a major NMDAR in the hippocampus and are similarly antagonized by ifenprodil and TCN coupounds, it is possible that zinc, acting on GluN2A-containing NMDARs, inhibits the same NMDAR subtypes as ifenprodil. Zinc is a partial inhibitor of AB receptors at nanomolar concentrations (Hatton and Paoletti, 2005; Hansen et al., 2014), as is ifenprodil at low micromolar levels, with both inhibiting less than 50% of AB responses at saturation. Nanomolar zinc also increases ifenprodil efficacy at AB receptors, although NMDA currents are still blocked by 50% at most (Hansen et al., 2014).
We have also reported that ifenprodil sensitivity of NMDAR EPSPs is markedly dampened when rats are exposed to ethanol during synaptogenesis (Izumi et al., 2005a). This observation suggests impairment of GluN2B-containing NMDARs but does not imply that GluN2A-expressing NMDARs are unaffected. Similarly, neuroprotection by ifenprodil does not exclude GluN2A if ifenprodil also blocks AB triheteromers. Here, we used ifenprodil at 10 µM, a concentration that saturates B-type NMDARs but likely has some effects (approximately 15% block) on A-type diheteromers (Gray et al., 2011; Hansen et al., 2014). We used this concentration based on earlier observations in which we found that CA1 NMDAR EPSPs are only weakly sensitive to lower ifenprodil concentrations (data not shown), which is consistent with evidence that AB NMDARs have dampened ifenprodil sensitivity (Hansen et al., 2014).
NMDARs play key roles in LTP and LTD, and NMDAR subtypes may drive these forms of plasticity. Inhibition of LTD by ifenprodil supports the involvement of receptors expressing GluN2B (Izumi et al., 2005b, 2006). Furthermore, ifenprodil insensitivity of NMDAR EPSPs in some circumstances has correlated with LTD failure. We observed this when early postnatal rats are exposed to ethanol (Izumi et al., 2005a) or when the immediate early gene Egr3 is deleted during development (Gallitano-Mendel et al., 2007). These findings suggest that GluN2B-containing NMDARs are susceptible to developmental insults but do not indicate that GluN2A-containing NMDARs are immune to these insults. Thus, the sensitivity of LTD induction to ifenprodil does not exclude a role for GluN2A subunits in LTD induction. If triheteromeric NMDARs are the major CA1 synaptic receptors, then modulation of GluN2A could also dampen LTD induction. Indeed, in the present study, LTD was blocked by both TCNs. The finding that LTD is sensitive to both ifenprodil and TCNs could suggest the involvement of ifenprodil- and TCN-sensitive triheteromeric NMDARs rather than the possibility that LTD requires both A- and B-type NMDARs.
The role of NMDAR subtypes in LTP is more complicated. Several studies found that LTP requires GluN2A-containing NMDARs, but not GluN2B-containing NMDARs (Sakimura et al., 1995; Sprengel et al., 1998; Liu et al., 2004; von Engelhardt et al., 2008). However, some studies relied on NVP, an antagonist that only weakly discriminates A- and B-type NMDARs (Frizelle et al., 2006; Neyton and Paoletti, 2006; Wyllie and Chen, 2007), and even moderate NVP concentrations block CA1 NMDAR EPSPs completely (Izumi et al., 2006). Other investigators found that NMDAR-mediated LTP is not subtype specific (Berberich et al., 2005; Weitlauf et al., 2005), and we observed that LTP was not blocked by NVP at a concentration that was selective for GluN2A receptors (Izumi et al., 2006). In the present study, we found that the TCNs failed to inhibit LTP at concentrations that are effective against LTD. Moreover, in the presence of 5 μM D-APV, the same concentrations of the TCNs blocked LTP. These results suggest that LTP induction can be independent from TCN-sensitive NMDARs, and likely reflect a role for triheteromeric but not diheteromeric NMDARs that are TCN sensitive. Alternatively, LTP induction could depend on TCN-sensitive NMDARs but with a different threshold. Posttranslational modifications could also affect the sensitivity of native NMDAR subtypes to TCNs and ifenprodil.
Importantly, unlike their effects on diheteromeric NMDARs, the TCNs and ifenprodil are partial inhibitors of AB triheteromers (Hansen et al., 2014). The reasons for partial antagonism are uncertain; however, this leaves a significant percentage of unblocked NMDARs that can drive LTP but not LTD. These residual receptors are sensitive to low micromolar APV, raising the possibility that there may be subtypes of AB triheteromers, at least one of which is sensitive to the TCN compounds and ifenprodil and another that is insensitive to these agents. LTP is inhibited when low micromolar APV is coadministered with ifenprodil (Izumi et al., 2006) or the TCN compounds, but not by low micromolar APV alone. If ifenprodil- and TCN-sensitive NMDARs are dysfunctional, then activation of remaining NMDARs appears sufficient for LTP induction.
In summary, we find that TCN 201 and TCN 213, known GluN1/GluN2A antagonists, do not antagonize ifenprodil-insensitive NMDARs at CA1 synapses in P30 rat hippocampal slices. Thus, ifenprodil and TCN compounds behave similarly in this system. This presents an enigma. What is the residual NMDAR component that is insensitive to ifenprodil and TCN compounds? Are there novel NMDAR subtypes? A specific and full antagonist against triheteromeric NMDARs is needed to clarify the role that AB receptors play in hippocampal function.
Acknowledgments
The authors thank Kazuko O’Dell for technical assistance.
Abbreviations
- APV
2-amino-5-phosphonovalerate
- D-APV
d-2-amino-5-phosphonovalerate
- EPSP
excitatory postsynaptic potential
- HFS
high-frequency stimulation
- LFS
low-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- NVP/NVP-AAM077
[[[(1S)-1-(4-bromophenyl)ethyl]amino](1,2,3,4-tetrahydro-2,3-dioxo-5-quinoxalinyl)methyl] phosphonic acid tetrasodium hydrate
- TCN 201
3-chloro-4-fluoro-N-[4-[[2-(phenylcarbonyl)hydrazino]carbonyl]benzyl]benzenesulphonamide
- TCN 213
N-(cyclohexylmethyl)-2-[{5-[(phenylmethyl)amino]-1,3,4-thiadiazol-2-yl}thio]acetamide
Authorship Contributions
Participated in research design: Izumi, Zorumski.
Conducted experiments: Izumi.
Performed data analysis: Izumi.
Wrote or contributed to the writing of the manuscript: Izumi, Zorumski.
Footnotes
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants R01-MH077791 and R01-MH101874]; the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant R01-AA017413], and the Bantly Foundation. C.F.Z. is a member of the Scientific Advisory Board of Sage Therapeutics. There are no other competing financial interests.
References
- Alberati D, Moreau JL, Lengyel J, Hauser N, Mory R, Borroni E, Pinard E, Knoflach F, Schlotterbeck G, Hainzl D, et al. (2012) Glycine reuptake inhibitor RG1678: A pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology 62:1152–1161. [DOI] [PubMed] [Google Scholar]
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. (2007) NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci 27:8334–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. (2002) 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett 12:1099–1102. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby Ø, Köhr G. (2005) Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci 25:6907–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, et al. (2010) Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B-containing receptors. J Pharmacol Exp Ther 335:636–644. [DOI] [PubMed] [Google Scholar]
- Blevins T, Mirshahi T, Chandler LJ, Woodward JJ. (1997) Effects of acute and chronic ethanol exposure on heteromeric N-methyl-d-aspartate receptors expressed in HEK 293 cells. J Neurochem 69:2345–2354. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004:re16. [DOI] [PubMed] [Google Scholar]
- Edman S, McKay S, Macdonald LJ, Samadi M, Livesey MR, Hardingham GE, Wyllie DJ. (2012) TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology 63:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. (2006) Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-d-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol 70:1022–1032. [DOI] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. (2007) The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience 148:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. (2011) Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: Single-cell NMDA receptor subunit deletion in vivo. Neuron 71:1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Traynelis SF. (2012) Subunit-selective allosteric inhibition of glycine binding to NMDA receptors. J Neurosci 32:6197–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Yuan H, Traynelis SF. (2014) Distinct functional and pharmacological properties of triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 81:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. (2005) Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46:261–274. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. (2006) Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci 26:7181–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Yuede C, Hartman RE, Wozniak DF, Zorumski CF. (2005a) A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, N-methyl-d-aspartate receptor function and ethanol sensitivity. Neuroscience 136:269–279. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. (2005b) Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136:509–517. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. (2012) NMDA receptors, mGluR5, and endocannabinoids are involved in a cascade leading to hippocampal long-term depression. Neuropsychopharmacology 37:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304:1021–1024. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. (2006) Relating NMDA receptor function to receptor subunit composition: Limitations of the pharmacological approach. J Neurosci 26:1331–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. (2013) NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383–400. [DOI] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. (2005) The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci 25:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Köhr G. (2011) Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-d-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286:7558–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard JM, et al. (2013) Neuronal d-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci 33:3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. (1995) Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature 373:151–155. [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. (2003) Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci 23:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, Paulsen O. (2014) GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos Trans R Soc Lond B Biol Sci 369:20130163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, et al. (1998) Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92:279–289. [DOI] [PubMed] [Google Scholar]
- Tovar KR, McGinley MJ, Westbrook GL. (2013) Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci 33:9150–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. (2010) Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol Rev 62:405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Doganci B, Jensen V, Hvalby Ø, Göngrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JN, Seeburg PH, et al. (2008) Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron 60:846–860. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. (2005) Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci 25:8386–8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. (1993) Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: Selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44:851–859. [PubMed] [Google Scholar]
- Wyllie DJ, Chen PE. (2007) Taking the time to study competitive antagonism. Br J Pharmacol 150:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]