Abstract
Pancreatic cancer is an aggressive disease with limited therapeutic options. Melanoma differentiation–associated gene-7/interleukin-24 (mda-7/IL-24), a potent antitumor cytokine, shows cancer-specific toxicity in a vast array of human cancers, inducing endoplasmic reticulum stress and apoptosis, toxic autophagy, an antitumor immune response, an antiangiogenic effect, and a significant “bystander” anticancer effect that leads to enhanced production of this cytokine through autocrine and paracrine loops. Unfortunately, mda-7/IL-24 application in pancreatic cancer has been restricted because of a “translational block” occurring after Ad.5-mda-7 gene delivery. Our previous research focused on developing approaches to overcome this block and increase the translation of the MDA-7/IL-24 protein, thereby promoting its subsequent toxic effects in pancreatic cancer cells. We demonstrated that inducing reactive oxygen species (ROS) after adenoviral infection of mda-7/IL-24 leads to greater translation into MDA-7/IL-24 protein and results in toxicity in pancreatic cancer cells. In this study we demonstrate that a novel chimeric serotype adenovirus, Ad.5/3-mda-7, displays greater efficacy in delivering mda-7/IL-24 compared with Ad.5-mda-7, although overall translation of the protein still remains low. We additionally show that d-limonene, a dietary monoterpene known to induce ROS, is capable of overcoming the translational block when used in combination with adenoviral gene delivery. This novel combination results in increased polysome association of mda-7/IL-24 mRNA, activation of the preinitiation complex of the translational machinery in pancreatic cancer cells, and culminates in mda-7/IL-24–mediated toxicity.
Introduction
Pancreatic cancer is a deadly disease with a 5-year survival of less than 5%. The lack of early stage symptoms often leads to advanced disease at the time of diagnosis. Additionally, it exhibits intrinsic resistance to chemotherapy and radiation (Hezel et al., 2006). Consequently, there is a dire need for the development of novel therapies for this devastating disease.
Melanoma differentiation–associated gene-7/interleukin-24 (mda-7/IL-24) is an anticancer cytokine originally cloned in our laboratory during studies of terminal differentiation of human melanoma cells (Jiang and Fisher, 1993; Jiang et al., 1995, 1996). This gene is a member of the interleukin-10 family of cytokines and functions as a tumor suppressor (Dash et al., 2010a). Reintroduction of this cytokine into cancer cells leads to significant endoplasmic reticulum (ER) stress, apoptosis, and anticancer immune effects, but shows no toxicity in normal cells (Jiang et al., 1996; Su et al., 1998; Dash et al., 2010a). Additionally, mda-7/IL-24 exhibits a potent “bystander effect,” wherein cells that express mda-7/IL-24 can also secrete it and further activate its expression through autocrine and paracrine loops in both normal and cancer cells (Dash et al., 2010a). Clinically, this suggests that mda-7/IL-24 has the capability of traveling from the original source of treatment to affect distant tumors and metastases (Sauane et al., 2003, 2008; Dash et al., 2010a).
Gene therapy studies using replication-incompetent adenovirus containing the full-length mda-7 gene (Ad.mda-7) have been shown to be extremely effective in multiple cancer types in both laboratory and clinical settings (Fisher, 2003; 2005, 2007; Tong et al., 2005; Cunningham et al., 2005; Eager et al., 2008). Although mda-7/IL-24 has ubiquitous activity in multiple cancer types, it is difficult to fully exploit these in pancreatic cancer owing to a block in mda-7/IL-24 translation after delivery into these cells. Infected cells accumulate mda-7/IL-24 mRNA, which exhibits a reduced ability to interact with polysomes and create MDA-7/IL-24 protein (Su et al., 2001; Lebedeva et al., 2005, 2006, 2007, 2008a,b; Sarkar et al., 2013).
Our previous work has demonstrated that the “translational block” seen after Ad.5-mda-7 treatment can be abrogated by two strategies: inhibition of oncogenic Kirsten-ras (K-ras) or in combination with a reactive oxygen species (ROS)–inducing agent (Su et al., 2001; Lebedeva et al., 2005, 2006, 2007, 2008a,b). We have shown that agents such as arsenic trioxide or perillyl alcohol (POH) are able to induce MDA-7/IL-24 protein translation and subsequent toxic effects in pancreatic cancer cells. The creation of a clinically useful drug that specifically targets K-ras has been a long-standing and complex problem that still remains unresolved. Additionally, targeting K-ras only enhances MDA-7/IL-24 expression in cells that express mutated forms of K-ras. Because of this, we have focused on identifying novel ROS-inducing agents that could translate easily into a clinical setting.
Furthermore, the use of adenoviruses (Ad) in pancreatic cancer cells is often hampered by poor infectivity rates. Serotype 5 Ad (Ad.5) viruses use Coxsackie and adenovirus receptors (CAR) to infect cells. Unfortunately, cancer cells, including pancreatic cancer cells, often downregulate these receptors, which leads to inefficient viral infectivity (Pearson et al., 1999). Using alternate viral serotypes to create chimeric viruses can help with this issue (Dash et al., 2010b, 2011; Hamed et al., 2010, 2013; Azab et al., 2012, 2014). The Ad.3 serotype relies on expression of CD46 and desmoglein for viral entry into cells (Wang et al., 2011). The creation of Ad.5/3 chimeric Ad combines both of these serotypes to create a virus that is capable of using CAR, CD46, and/or desmoglein for infection. We demonstrated that Ad.5/3-mda-7 is able to infect prostate, renal, glioblastoma multiforme, and colorectal cancer cells with greater efficiency compared with Ad.5-mda-7 and that this translates into greater toxicity (Dash et al., 2010b, 2011; Eulitt et al., 2010; Hamed et al., 2010; Azab et al., 2012).
In this study, we determined if Ad.5/3-mda-7 would improve the transduction efficiency in pancreatic cancer cells compared with Ad.5-mda-7. We also evaluated the ability of d-limonene, a dietary monoterpene, to increase the translation of mda-7/IL-24 mRNA and lead to toxicity in combination with Ad.mda-7. Limonene is a major component of citrus oil, shows minimal toxicity in low doses, and, like POH, is also able to induce ROS (Rabi and Bishayee, 2009). We hypothesized that it would function just as well as would increasing mda-7/IL-24 mRNA translation following Ad.mda-7 infection.
Materials and Methods
Cell Lines and Generating Stable Clones.
AsPC-1 and BxPC-3 cell lines were obtained from the American Type Culture Collection, maintained in RPMI-1640 media (Gibco; Invitrogen, Auckland, New Zealand) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO). MIA PaCa-2 and PANC-1, also obtained from the American Type Culture Collection, were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% FBS. Immortalized normal pancreatic mesenchymal cell line LT2 was obtained from EMD Millipore (Billerica, MA) and maintained per Millipore instructions. All cell lines were cultured at 37°C in a 5% CO2 and 95% air-humidified incubator. To obtain mda-7/IL-24–overexpressing clones, MIA PaCa-2 and PANC-1 cells were transfected with pc.DNA3.1-mda-7. Individual clones were selected after ∼3–4 weeks of continuous maintenance in culture medium containing hygromycin (Lebedeva et al., 2003), and were further characterized for the presence/expression of the inserted plasmid by real-time polymerase chain reaction (PCR).
Generation of Ad.5.mda-7 and Ad.5/3-mda-7.
Recombinant serotype 5 and serotype 5/3 chimeric adenoviruses expressing mda-7/IL-24 (Ad.5-mda-7 and Ad.5/3-mda-7) and control empty adenovirus (Ad.5.vec and Ad.5/3.vec) were generated as described (Su et al., 1998; Dash et al., 2010b).
Adenoviral Infection.
Pancreatic cancer cell lines were infected with Ad.mda-7 or Ad.5/3-mda-7 in medium lacking FBS for 3 hours followed by treatment with limonene (200 μM) in complete medium (10% FBS) for time points as described for particular assays.
Cell Proliferation Assays.
Pancreatic cancer cell lines were infected with Ad.mda-7 or Ad.5/3-mda-7 in medium lacking FBS for 3 hours followed by treatment with d-limonene in complete medium (10% FBS) for 72 hours, and cell proliferation assays were performed at optical density 560 nm after adding 100 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye (1 mg/ml; Sigma-Aldrich). To further confirm cell proliferation, a chemiluminescent-based BrdU Cell Proliferation Assay kit (Cell Signaling Technology, Danvers, MA) was used.
Apoptotic Assays.
Apoptotic assays were performed using an FITC Annexin V Apoptosis Detection Kit I (Pharmingen; BD Biosciences, San Diego, CA) according to the manufacturer’s instructions. Flow cytometry assays were performed immediately after staining using FACS Canto (BD Biosciences). Data were analyzed using FACSDIVA software (BD Biosciences).
ROS Measurements.
To determine ROS production, cells were stained with 100 μl of 10 μM carboxy-2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes; Life Technologies, Grand Island, NY) in phosphate-buffered saline for 30 minutes followed by treatment with limonene, and fluorescence was measured with a fluorometer using a green filter at the indicated time points.
Preparation of Whole-Cell Lysates and Western Blotting Analyses.
Cells were treated for 48 hours, lysed using cell lysis buffer (Cell Signaling Technology) supplemented with 1 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich) with protease inhibitor cocktail, phosphatase inhibitor (Roche, Indianapolis, IN). Whole-cell lysates were collected after centrifugation at 12,000 rpm at 4°C. For Western blotting analyses, the primary antibodies used were mouse monoclonal anti-MDA-7/IL-24 (1:2000; Gen Hunter Corporation, Nashville, TN), anti-EF1α (1:5000; EMD Millipore), mouse monoclonal anti-human K-Ras (1:1000; Bio-Rad Laboratories, Raleigh, NC), rabbit monoclonal anti–Bcl-xL, anti-PARP, anti–Mcl-1, anti–phospho-p70S6K (Thr-389), anti–phospho-eIF4E (Ser-209), anti–phospho-4EBP1 (Thr-37/46) (1:1000; Cell Signaling Technology), rabbit polyclonal anti–BiP/GRP-78 (1:500; Santa Cruz Biotechnology, Inc., Dallas, TX). The secondary antibodies used were polyclonal goat anti-mouse IgG (1:1000; Dako, Carpinteria, CA) and polyclonal swine anti-rabbit IgG (1:3000; Dako).
Polysome Studies.
Cells (2 × 106) were infected with Ad.mda-7 and 48 hours later harvested in 500 μl buffer A (200 nM Tris-HCl pH 8.5, 50 mM KCl, 25 mM MgCl2, 2 mM EGTA, 100 mg/ml heparin, 100 mg/ml cycloheximide, 2% polyoxyethylene 10-tridecyl ether, and 1% sodium deoxycholate supplemented with cOmplete Mini protease inhibitor cocktail and RNase inhibitor (Roche). Cells were centrifuged at 12,000 rpm for 10 minutes at 4°C to clear cell debris. The supernatant was loaded on top of a 10–50% sucrose gradient prepared in buffer B (50 nM Tris-HCl, 25 nM KCl, and 10 mM MgCl2) and centrifuged at 40,000 rpm for 1 hour at 4°C. Fractions of 500 μl were collected, the optical density at 260 nm was monitored, and polysome fractions were identified (typically fractions 10–20). The fractions were pooled into two separate tubes: fraction I (1–10 fraction number) and fraction II (polysome-enriched fraction; 11–20 fraction number). RNA was extracted by adding equal volume of 8 M guanidine hydrochloride, and precipitated by adding 2 volumes of ethanol. RNA was extracted from each fraction with a Qiagen RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was prepared and reverse transcription PCR was performed using a probe for mda-7/IL-24 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for relative quantification of mRNA.
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Student’s t test or 1-way analysis of variance was used as indicated, to study the level of significance (P < 0.05).
Results
Enhanced Delivery of mda-7/IL-24 Using Serotype Ad.5/3-mda-7 in Pancreatic Cancer Cells.
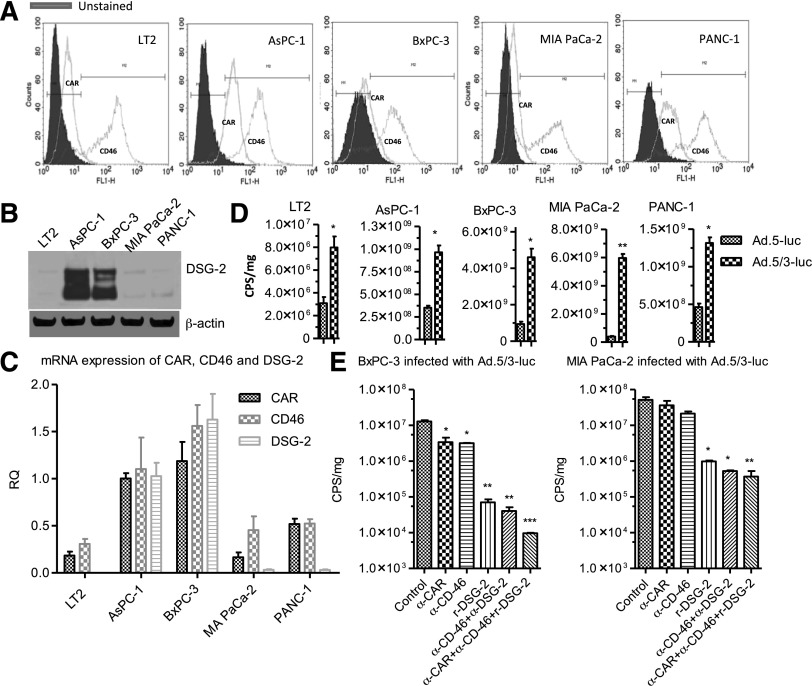
Initial studies with mda-7/IL-24 focused on gene delivery using the Ad.5 serotype, which utilizes CAR for infection. Pancreatic cancer cells express relatively low levels of CAR, making infection with Ad.5 inefficient. To circumvent this problem, a serotype chimeric recombinant adenovirus, Ad.5/3, was generated. Ad.5/3, while able to use CAR receptors, is also reliant on expression of CD46 and desmoglein for infection. Pancreatic cancer cell lines AsPC-1, PANC-1, MIA PaCa-2, and BxPC-3, as well as an immortal pancreas fibroblast cell line, LT2, all express CD46 protein at much higher levels compared with CAR (Fig. 1A). AsPC-1 and BxPC-3 cells also express high levels of desmoglein (Fig. 1B). These results were also confirmed by measuring mRNA transcript levels (Fig. 1C). To compare Ad.5/3 infection to that of Ad.5, we used viruses expressing a luciferase reporter gene. In all pancreatic cell lines, higher luciferase levels were found using the Ad.5/3 serotype compared with the Ad.5 serotype (Fig. 1D). Pretreatment of cells with blocking antibodies to CAR, CD46, desmoglein, or a combination of the three confirmed the importance of all three receptors in determining infectivity of pancreatic cancer cells, with infection being inhibited the most when antibodies to all three receptors were used in combination (Fig. 1E). These results support the use of the Ad.5/3 virus as a means of effectively delivering mda-7/IL-24 to pancreatic cancer cells.
Fig. 1.
Enhanced gene delivery using a serotype chimeric modified recombinant Ad.5/3. (A) Expression of CAR and CD46 in pancreatic cancer cells as measured by flow cytometry. Cells were stained with anti-CAR, anti-CD46, and unstained or isotype-specific primary antibody. (B) Western blotting of desmoglein-2 (DSG-2) expression. (C) mRNA levels of CAR, CD46, and DSG-2 determined by real-time PCR. RQ, relative quotient. (D) Pancreatic cell lines were infected with Ad.5-luc and Ad.5/3-luc and level of luciferase (luc) expression was measured 48 hours postinfection using a luminometer (CPS, counts per second). (E) Pancreatic cancer cell lines BxPC-3 and MIA PaCa-2 were pretreated with anti-CAR (α-CAR), anti-CD46 (α-CD46) and recombinant DSG-2 for 2 hours followed by Ad.5/3-luc infection, and luciferase expression was quantified using a luminometer (CPS, counts per second). Level of significance: *P < 0.05; **P < 0.01; ***P < 0.001.
d-Limonene Inhibits Growth of Pancreatic Cancer Cells and Induces ROS.
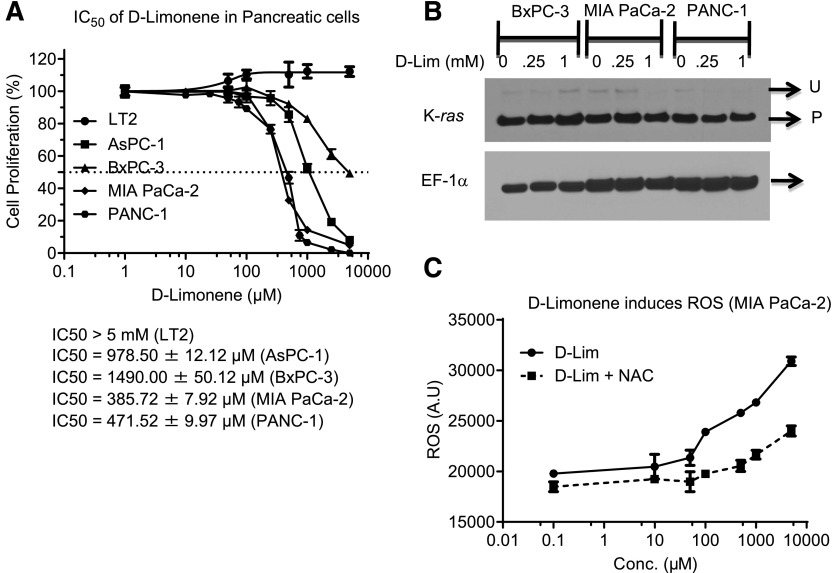
d-Limonene is able to inhibit the proliferation of multiple pancreatic cancer cells but does not affect growth of an immortalized pancreatic mesenchymal cell line, LT2 (Fig. 2A). d-Limonene is a monoterpene and capable of inhibiting the prenylation of GTPases, such as K-ras (Chen et al., 1999). However, the doses of d-limonene we used in our study did not affect the normal prenylation of K-ras (Fig. 2B). We next evaluated the ability of d-limonene to induce ROS and found that even at nontoxic or sublethal doses d-limonene promoted ROS induction, specifically peroxides, and this increase was abrogated with N-acetyl cysteine (NAC) pretreatment (Fig. 2C).
Fig. 2.
d-Limonene (d-Lim) inhibits cellular proliferation by inducing ROS. (A) Pancreatic cells were treated with increasing doses of d-Lim, and MTT assays were performed after 72 hours to measure cell proliferation. (B) Western blotting analysis of K-ras prenylation status after treatment with d-Lim. (U, unprenylated, P, prenylated.) (C) MIA PaCa-2 cells were treated with d-Lim with or without NAC pretreatment and stained with dihydrodichlorofluorescein to detect ROS. ROS induction was quantified using fluorescence measurements. A.U., arbitrary units; Conc., concentration.
d-Limonene Enhances Translation of mda-7/IL-24 mRNA and Promotes Toxicity of Ad.mda-7.
Previous studies indicate that the translational block seen with mda-7/IL-24 mRNA in pancreatic cancer cells can be overcome with ROS induction (Lebedeva et al., 2008a,b). Because d-limonene showed significant ROS induction, we infected cells with Ad.5-mda-7 or Ad.5/3-mda-7 in the presence or absence of d-limonene to determine if this compound could enhance mda-7/IL-24 mRNA translation and promote toxicity. In LT2 cells, we found no effect on growth (Fig. 3A). However, in pancreatic cancer cell lines PANC-1 (Fig. 3B) and MIA PaCa-2 (Fig. 3C), the addition of d-limonene to Ad.mda-7-infected cells was able to significantly enhance growth inhibition. Additionally, in agreement with prior results comparing Ad.5 versus Ad.5/3, Ad.5/3-mda-7 alone was able to induce a greater growth inhibition compared with Ad.5-mda-7 (Dash et al., 2010b; Azab et al., 2012, 2014). Western blotting showed similar results, with Ad.5/3-mda-7 inducing greater MDA-7/IL-24 protein expression, both with and without the addition of d-limonene compared with Ad.5-mda-7 (Fig. 3, D and E). This correlated with an increase in cell death, as indicated by increased poly(ADP-ribose) polymerase cleavage in these cells. This effect correlated with decreases in the expression of the Bcl-2 antiapoptotic proteins Mcl-1 and Bcl-xL.
Fig. 3.
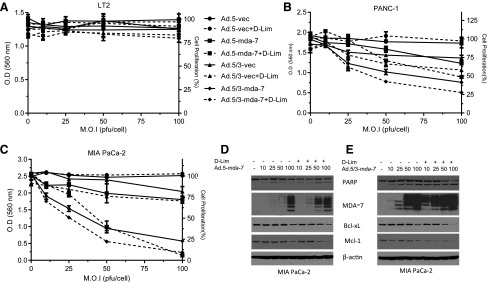
d-Limonene (d-Lim) synergizes with mda-7/IL-24, in inducing cancer-specific apoptosis in pancreatic cancer cells. Pancreatic cells were infected with increasing m.o.i (multiplicity of infection; pfu/cell) of Ad.5-mda-7 and its recombinant chimeric Ad.5/3-mda-7, followed by 100 μM d-Lim treatment. MTT assays were performed in (A) LT2 cells, (B) PANC-1 and (C) MIA PaCa-2 cells 72 hours after treatment. (D and E) Western blotting of MIA PaCa-2 lysates for MDA-7 and markers of apoptosis. O.D., optical density.
Effects of d-Limonene in Inducing Cancer-Specific Cell Death Are Dependent on ROS Induction.
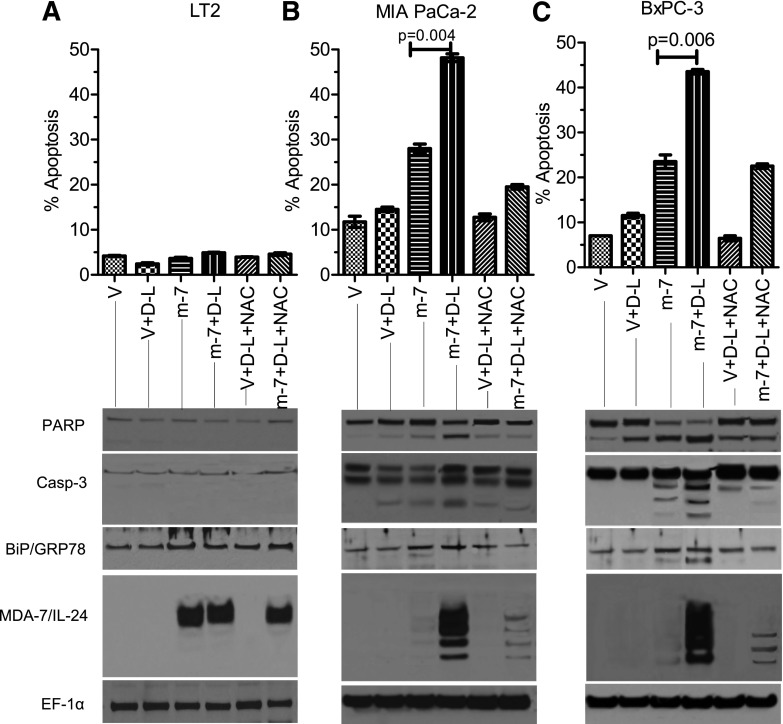
To evaluate whether cell death induction and growth inhibition following treatment with d-limonene are related to its ability to induce ROS, we pretreated cells with NAC before Ad infection and d-limonene treatment. As expected, this addition did not show any change in proliferation in LT2 cells (Fig. 4A). However, in both mutant K-ras–bearing MIA PaCa-2 (Fig. 4B) and wild type K-ras–bearing BxPC-3 cells (Fig. 4C) pretreatment with NAC significantly rescued cells from the growth-inhibitory effects of Ad.5/3-mda-7 plus d-limonene. To further confirm that these changes were reflective of alterations in proliferation, bromodeoxyuridine (5-bromo-2′-deoxyuridine) incorporation assays were done in both LT2 and MIA PaCa-2 cells. These studies confirmed the observations seen by MTT, showing very similar results (Supplemental Fig. 1). The observation of ROS levels (specifically peroxide) confirmed that NAC pretreatment prevented d-limonene–induced ROS generation (Fig. 4D). Pyruvate, another ROS scavenger, was also shown to inhibit this increase in ROS (Fig. 4D). Similar results were obtained when monitoring apoptosis induction, with LT2 cells showing no increase in apoptosis, but MIA PaCa-2 and BxPC-3 cells infected with Ad.5/3-mda-7 and treated with d-limonene showing increases in Annexin V staining, cleaved poly(ADP-ribose) polymerase, and cleaved caspase-3 (Fig. 5). Furthermore, these cells also showed increased mda-7/IL-24 protein expression as well as an increase in an ER stress marker, BiP/GRP-78, which is induced by mda-7/IL-24. These effects were also prevented by pretreatment with NAC (Fig. 5).
Fig. 4.
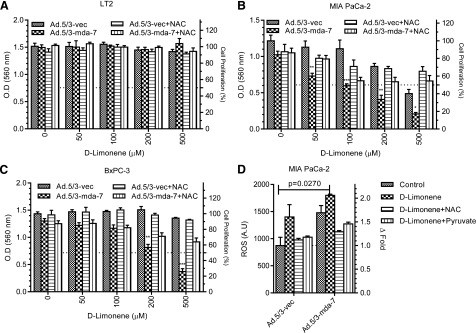
d-Limonene enhances mda-7/IL-24–mediated inhibition of cell growth by inducing ROS (specifically peroxide). Pancreatic cells were pretreated with 10 mM NAC prior to treatment with Ad.5/3-mda-7 (25 pfu/cell) and d-Lim. MTT assays were performed on (A) LT2 cells, (B) MIA PaCa-2, and (C) and BxPC-3 cells 72 hours after treatment. (D) MIA-PaCa-2 cells were pretreated with either NAC or peroxide scavenger, pyruvate, and then stained with DCF to detect ROS induction (specifically peroxide). A.U., arbitrary units; O.D., optical density.
Fig. 5.
d-Limonene enhances mda-7/IL-24–mediated apoptosis. (A) LT2, (B) MIA PaCa-2, and (C) BxPC-3 cells were treated with NAC prior to treatment with Ad.5/3-mda-7 (m-7) (25 pfu/cell) and d-limonene (D-L) (100 μM). Apoptosis was measured after 48 hours by Annexin V/PI staining and flow-cytometer analysis. Western blotting was performed from the whole-cell lysates 48 hours post-treatment. V, Annexin V.
ROS Induction by d-Limonene Activates Translational Machinery in Cells.
The effects of d-limonene when used in conjunction with Ad.mda-7 infection are similar to what we have observed previously with other ROS-inducing agents (Lebedeva, et al., 2005, 2008a,b). In an effort to enhance our understanding of the mechanism behind these observations, we evaluated the effects of d-limonene further. We found that d-limonene–induced ROS generation occurs in both a dose- and time-dependent manner (Fig. 6A). We know that without this compound, mda-7/IL-24 mRNA is very weakly translated when introduced into pancreatic cancer cells via Ad infection and that the addition of d-limonene results in greater translation of mda-7/IL-24 mRNA into protein. To further confirm that d-limonene is able to increase the translation of this mRNA into protein, we looked at the expression of the preinitiation complex proteins after d-limonene treatment. In accordance with our observed time-dependent ROS induction, we found that d-limonene is able to activate the transient translational machinery within a few hours of treatment. Increases in phosphorylated eIF4E were found to peak at around 3 hours post-treatment. Translational repressor 4EBP-1, which inhibits eIF4E in its unphosphorylated form, also demonstrated an increase in phosphorylation that peaked at 3 hours after d-limonene treatment. We also saw activation of upstream kinase P70S6K in this same time frame (Fig. 6, B and C). The dose of limonene used in these experiments showed potent induction of ROS at 3 hours after treatment (Fig. 6A), supporting a potential role of ROS in the activation of the translational machinery. To confirm that this activation of the preinitiation complex was related to the induction of ROS, we pretreated cells with NAC and also included the potent ROS-inducer arsenic trioxide as a positive control. d-Limonene was able to induce the phosphorylation of P70S6K, eIF4E, and 4EBP-1 to the same extent as arsenic trioxide. Importantly, these increases in phosphorylation were completely abrogated if cells were pretreated with NAC (Fig. 6D).
Fig. 6.
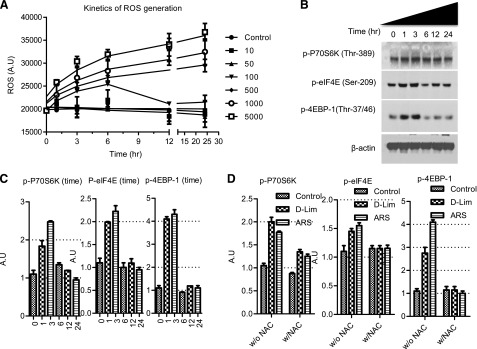
d-Limonene (d-Lim) activates the translational machinery in the cell through the induction of ROS. (A) Dose and time kinetics of ROS generation by d-Lim. (B) Western blotting analysis of phosphorylated P70S6K, eIF4E, and 4EBP-1 after 100 μM limonene treatment. (C) Western blotting quantification of (B). (D) Quantification of Western blotting analysis of phosphorylated P70S6K, eIF4E, and 4EBP-1 after limonene (100 μM) or arsenic trioxide (ARS) (1 μM) treatment with or without NAC pretreatment. A.U., arbitrary units.
d-Limonene Increases mda-7/IL-24 mRNA Polysome Association.
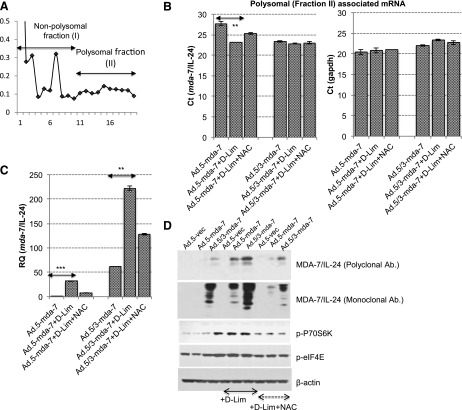
These experiments provide a novel role for d-limonene, showing that this compound induces ROS that can subsequently activate the translational machinery in pancreatic cancer cells. We have also shown that the overall effect of this phenomenon is increased mda-7/IL-24 translation, thereby resulting in cancer-specific toxicity. We finally wanted to determine if the activation of the translational machinery actually resulted in increased association of mda-7/IL-24 mRNA with the polysomes. Although we have demonstrated that MDA-7/IL-24 protein levels increase, showing increased polysome association would confirm our hypothesis that the ROS generated by d-limonene not only activated the preinitiation complex of the translational machinery but also subsequently facilitated the association of mda-7/IL-24 mRNA with polysomes. As a proof-of-principle, MIA PaCa-2 cells were infected with either Ad.5-mda-7 or Ad.5/3-mda-7, with or without subsequent d-limonene treatment. Polysomal fractions from these cells were separated using sucrose gradient gels (Fig. 7A) and then assayed for the presence of mda-7/IL-24 mRNA using real-time PCR. We found that with both Ad serotypes, the addition of d-limonene resulted in a significantly increased polysome association of mda-7/IL-24 mRNA as observed by a decreased Ct value (Fig. 7B). There was no apparent change in the association of the housekeeping gene GAPDH mRNA with polysomes (Fig. 7, B and C), indicating that d-limonene induces the translation of weakly translated mRNAs such as mda-7/IL-24. In order to see the fold-change in polysomal-associated mda-7/IL-24 mRNA with respect to polysomal-associated GAPDH mRNA following treatment with Ad.mda-7/IL-24 and/or d-limonene, the relative quotient was calculated. It was found that d-limonene significantly increased the association of weakly translated mda-7/IL-24 mRNA with polysomes when MIA PaCa-2 cells were treated with mda-7/IL-24 using either serotype of Ad (Fig. 7C). Furthermore, pretreating cells with NAC prevented increased polysome association, supporting our conclusion that it is indeed the induction of ROS that ultimately leads to increased mda-7/IL-24 translation. Western blots confirmed that this combination resulted in increased MDA-7/IL-24 protein expression following phosphorylation of translation machinery proteins P70S6K and eIF4E, which leads to the formation of the preinitiation complex. All of these changes were inhibited with NAC pretreatment (Fig. 7D).
Fig. 7.
d-Limonene (d-Lim) enhances the binding of weakly translated mda-7/IL-24 mRNA with polysomes. (A) MIA PaCa-2 cells were infected with Ad.5-mda-7 or Ad.5/3-mda-7 (25 pfu/cell) and subsequently treated with 100 μM d-Lim. Polysomal fractions were separated using a 10–40% sucrose gradient as indicated by graph. Fractions were pooled into two sets, i.e., fraction I (1–10 fraction number) and fraction II (enriched with mRNA associated with polysomes). (B) mRNA was isolated and reverse transcription PCR was performed using a probe for mda-7/IL-24 and GAPDH. (C) The relative quotient of mda-7/IL-24 with respect to GAPDH. (D) Western blotting was performed on lysates from cells infected with Ad.5-mda-7 or Ad.5/3-mda-7 (25 pfu/cell), with or without d-Lim and/or NAC, using antibodies for preinitiation complex markers and MDA-7/IL-24. Level of significance: **P < 0.01; ***P < 0.001.
d-Limonene Induces mda-7/IL-24 Expression and Toxicity in Cell Lines That Stably Overexpress mda-7/IL-24 mRNA.
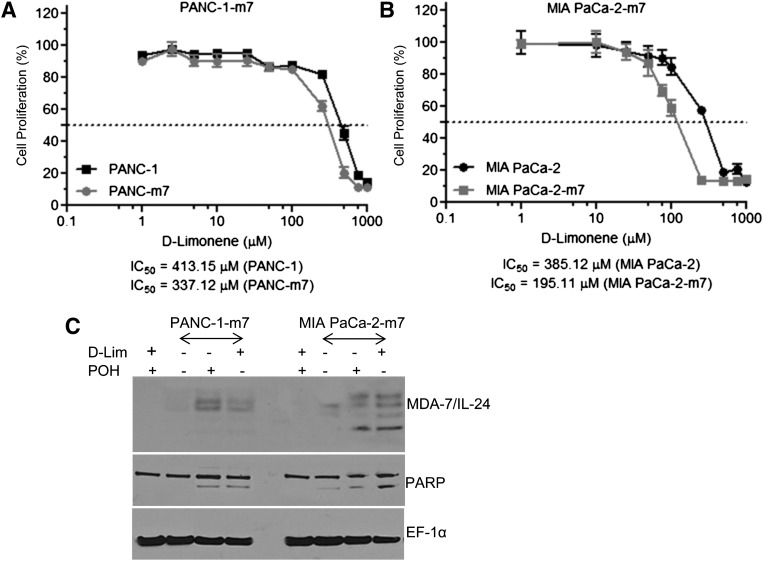
To ensure that the effects of d-limonene on mda-7/IL-24 mRNA translation were not dependent on the adenoviral delivery of mda-7/IL-24, we evaluated this compound in pancreatic cancer cell lines that stably overexpress mda-7/IL-24 mRNA, without detectable protein. PANC-1 (Fig. 8A) and MIA PaCa-2 (Fig. 8B) stable mda-7/IL-24 mRNA–expressing clones were treated with increasing doses of d-limonene for 3 days and evaluated for effects on proliferation by MTT assays. We found that compared with parental cell lines containing an empty vector, the mda-7/IL-24–overexpressing clones exhibited greater growth inhibition by d-limonene, with left-shifting dose curves and lower IC50 values. The IC50 values of PANC-1 and MIA PaCa-2 treated with d-limonene were 413.15 and 385.12 μM, respectively. The IC50 values were decreased to 337.12 and 195.11 μM, respectively, for PANC-1 overexpressing mda-7/IL-24 (PANC-1-m7) and MIA PaCa-2 overexpressing mda-7/IL-24 (MIA PaCa-2-m7), respectively. Western blotting results confirmed that both d-limonene and POH were able to induce MDA-7/IL-24 protein expression in pancreatic cancer cells overexpressing mda-7/IL-24, and this expression resulted in cell death as demonstrated by poly(ADP-ribose) polymerase cleavage (Fig. 8C).
Fig. 8.
d-Limonene (d-Lim) induces mda-7/IL-24 expression and toxicity in cell lines that stably overexpress mda-7/IL-24 mRNA. (A) PANC-1 stably transfected with pcDNA3.1-mda-7/IL-24 (PANC-1-m7) and (B) MIA PaCa-2 stably transfected with pcDNA3.1-mda-7 (MIA PaCa-2-m7) were treated with d-Lim (100 μM) and MTT assays were used to assess the effect on cellular proliferation 72 hours after treatment. (C) Western blotting of whole cell lysates of PANC-1-m7 and MIA PaCa-2-m7 with or without d-Lim (100 μM) or POH (100 μM).
Discussion
Pancreatic cancer is an invariably fatal disease that currently lacks effective therapeutic options for patients (Hezel et al., 2006). The development of novel ways to combat this cancer is essential. mda-7/IL-24 has shown great promise as a cancer therapeutic (Fisher et al., 2003, 2005; Cunningham et al., 2005; Tong et al., 2005; Eager et al., 2008; Dash et al., 2010a). This tumor suppressor, when introduced back into cancer cells, is capable of inducing ER stress and apoptosis, initiating an autocrine and paracrine feedback-loop to increase mda-7/IL-24 expression, and fostering an anticancer immune response (Fisher, 2005; Dash et al., 2010a). Furthermore, this cytokine shows a bystander effect as a result of its feedback loop, whereby MDA-7/IL-24 protein secretion from cells is able to bind to receptors on neighboring normal and cancer cells and induce mda-7/IL-24 expression in those cells (Su et al., 2005; Sauane et al., 2008). This loop intensifies the overall anticancer response and contributes significantly to its efficacy as a cancer therapeutic.
Unfortunately, the use of mda-7/IL-24 in pancreatic cancer has been restricted, owing to decreased translation of its encoded mRNA into protein (Su et al., 2001; Lebedeva et al., 2008a,b; Dash et al., 2014). Adenoviral delivery of this cytokine results in robust mda-7/IL-24 mRNA expression with only low or limited levels of expressed MDA-7/IL-24 protein, and consequently minimal changes in cellular phenotype. We have studied strategies to overcome this block in protein translation of the mda-7/IL-24 mRNA (Su et al., 2001; Lebedeva et al., 2005, 2006, 2007, 2008a,b; Sarkar et al., 2013; Dash et al., 2014), and thereby increase the utility of mda-7/IL-24 for the therapy of pancreatic cancer. Our previous work has shown that inhibiting oncogenic K-ras is one mechanism of increasing the translation of mda-7/IL-24 mRNA. The mechanism through which K-ras influences MDA-7/IL-24 protein expression is currently unknown. K-ras signaling involves a multitude of downstream target proteins and it is possible that one of these K-ras effector proteins is involved in protein translation. While this is a useful strategy in vitro, it is difficult to translate this into the clinic, as inhibiting K-ras in patients is problematic because of the lack of success in the development of drugs specifically targeting this oncogene (Maurer et al., 2012; Sun et al., 2012; Hocker et al., 2013). We also found that the induction of ROS was able to increase expression of MDA-7/IL-24 protein and produce a subsequent therapeutic effect. Potent ROS inducers such as arsenic trioxide show great success when used in combination with mda-7/IL-24 in experimental settings (Lebedeva et al., 2005).
We then turned our studies to more natural compounds capable of inducing ROS. Drug toxicity is a major problem in the treatment of cancer and this only intensifies as patients are treated with increasing numbers of drugs in combination. We evaluated POH, a dietary agent known to induce ROS at nontoxic doses. It was demonstrated that the combination of mda-7/IL-24 and POH also induced MDA-7/IL-24 expression and potent cell death in pancreatic cancer cells (Lebedeva et al., 2008b).
While we had made advances in increasing the utilization of mda-7/IL-24 as a therapy for pancreatic cancer, a few outstanding questions needed to be addressed. The goal of this study was to examine some of those questions. Could we improve our viral delivery of mda-7/IL-24 as a means of increasing therapeutic efficacy? Do dietary agents other than POH also function to increase mda-7/IL-24 mRNA translation and promote subsequent phenotypic effects?
We began this study by evaluating our delivery system of mda-7/IL-24. Adenoviral delivery mechanisms can be a successful means of gene therapy but are reliant on certain factors for success. Adenoviral serotypes use different cellular receptors as a means of introducing their genetic material into target cells (Bergelson et al., 1998; Pearson et al., 1999; Dash et al., 2010b; Wang et al., 2011; Azab et al., 2012, 2014). Ad.5-mda-7, the traditional type 5 adenovirus serotype used in our prior work, is dependent on the expression of Coxsackie and adenovirus receptors (CAR) for efficacy. Our initial experiments showed that, as a whole, pancreatic cancer cells expressed relatively low levels of CAR, a factor that was most likely preventing us from successfully delivering optimum levels of mda-7/IL-24 to these cells. In contrast, the Ad.5/3 serotype uses desmoglein and CD46, as well as CAR, receptors for entry into cells. We show that pancreatic cancer cells express higher levels of these receptors, both on an mRNA and protein level and that expression of all three receptors yielded the highest rate of success in gene delivery (Fig. 1E).
However, despite being able to increase the infection efficacy of Ad, we still only observed minimal MDA-7/IL-24 protein expression. Our prior studies have shown that POH, a dietary monoterpene, is able to relieve this translational block when combined with Ad.mda-7 infection and enhance MDA-7/IL-24 protein production and subsequent expression through the induction of ROS (Lebedeva et al., 2008a,b). These observations supported the hypothesis that other monoterpenes that are capable of inducing ROS might also have similar effects on MDA-7/IL-24 protein production. In this study, we evaluated d-limonene, also a dietary monoterpene known to induce ROS. This compound has a low toxicity profile and has been shown to be safe when used in humans. Adverse effects reported include skin irritation when d-limonene was used in cosmetic products (Kim et al., 2013). As a systemic therapy, this compound has been used in humans in clinical trials at up to 15 gm/day without major toxicities being observed. Some mild side effects, such as nausea or fatigue, were seen at higher doses (Sun, 2007). Given its safe drug profile, d-limonene is a viable candidate for inclusion as adjuvant treatment in patients.
We found that, similar to POH, nontoxic doses of d-limonene are able to enhance MDA-7/IL-24 protein production in cells infected with Ad.mda-7, which translates into mda-7/IL-24–induced toxicity. Our experiments demonstrated that this ROS induction was necessary for MDA-7/IL-24 protein production and that it enhanced translation through activation of the translation machinery and hence increased polysome association of mda-7/IL-24 mRNA and subsequent protein expression. These observations correlate with studies in the literature demonstrating that ROS generated via extracellular hydrogen peroxide is capable of inducing activation of p70S6K and its downstream target 4EBP-1 (Bae et al., 1999). We observed that ROS generated through d-limonene was also able to activate these members of the preinitiation complex and that led to subsequent activation of translation. Furthermore, we showed that this strategy was successful using both an adenoviral delivery strategy as well as stable transfection of mda-7/IL-24, resulting in mRNA expression with little protein production, in pancreatic cancer cells.
As a whole, our data supports the efficacy of mda-7/IL-24 gene therapy in pancreatic cancer. The use of mda-7/IL-24 as a cancer therapeutic is exciting and promising in many cancer types, but progress in pancreatic cancer has been slow. We have made major strides in developing strategies to use this cancer-specific cytokine in this disease but continue to work on this issue. The presented data help to answer some of our outstanding questions and represent novel ways of increasing the efficacy and therapeutic use of mda-7/IL-24 in this devastating disease.
Supplementary Material
Acknowledgments
The authors thank the other technicians in the group for technical assistance with performing some of the experiments outlined in this paper. D.S. is a Harrison Scholar and P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Abbreviations
- Ad
adenovirus
- Ad.5
serotype 5 adenovirus
- Ad.5/3
serotype 5 and serotype 3 chimeric adenovirus
- Ad.mda-7
replication-incompetent adenovirus containing the full-length mda-7 gene
- CAR
Coxsackie and adenovirus receptors
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL
interleukin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAC
N-acetyl cysteine
- PCR
polymerase chain reaction
- POH
perillyl alcohol
- ROS
reactive oxygen species
Authorship Contributions
Participated in research design: Fisher, D. Sarkar, Das, Emdad.
Conducted experiments: S. Sarkar, Quinn, Shen.
Contributed new reagents or analytic tools: Dent.
Performed data analysis: S. Sarkar, Quinn, Das, Emdad, Fisher.
Wrote or contributed to the writing of the manuscript: S. Sarkar, Quinn, Emdad, Das, D. Sarkar, Dent, Fisher.
Footnotes
Support for the present study was provided from the National Institutes of Health National Cancer Institute [P01-CA104177, R01-CA097318, and R01-CA127641], the National Foundation for Cancer Research, and the Samuel Waxman Cancer Research Foundation.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, Sarkar S, Wang XY, Hedvat M, Dmitriev IP, et al. (2012) Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol 227:2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab BM, Dash R, Das SK, Bhutia SK, Sarkar S, Shen XN, Quinn BA, Dent P, Dmitriev IP, Wang XY, et al. (2014) Enhanced prostate cancer gene transfer and therapy using a novel serotype chimera cancer terminator virus (Ad.5/3-CTV). J Cell Physiol 229:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Seo DW, Kwon HK, Lee HY, Hong S, Lee ZW, Ha KS, Lee HW, Han JW. (1999) Hydrogen peroxide activates p70(S6k) signaling pathway. J Biol Chem 274:32596–32602. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, Wickham T, Crowell RL, Finberg RW. (1998) The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol 72:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yano Y, Hasuma T, Yoshimata T, Yinna W, Otani S. (1999) Inhibition of farnesyl protein transferase and P21ras membrane association by d-limonene in human pancreas tumor cells in vitro. Chinese Med Sci J 14:138–144. [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. (2005) Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther 11:149–159. [DOI] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, et al. (2010a) mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev 21:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, Yacoub A, Dent P, Curiel DT, Sarkar D, et al. (2010b) Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther 17:447–456. [DOI] [PubMed] [Google Scholar]
- Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, et al. (2011) Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci USA 108:8785–8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Bhoopathi P, Das SK, Sarkar S, Emdad L, Dasgupta S, Sarkar D, Fisher PB. (2014) Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction. Cancer Res 74:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager R, Harle L, Nemunaitis J. (2008) Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther 8:1633–1643. [DOI] [PubMed] [Google Scholar]
- Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. (2010) Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther 10:1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PB. (2005) Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res 65:10128–10138. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. (2003) mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther 2(4, Suppl 1)S23–S37. [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, et al. (2007) Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol 224:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, Dmitriev IP, Lesniak MS, Shah K, Grant S, et al. (2010) Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther 18:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hamed HA, Yacoub A, Park MA, Archer K, Das SK, Sarkar D, Grant S, Fisher PB, Dent P. (2013) Histone deacetylase inhibitors interact with melanoma differentiation associated-7/interleukin-24 to kill primary human glioblastoma cells. Mol Pharmacol 84:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. (2006) Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20:1218–1249. [DOI] [PubMed] [Google Scholar]
- Hocker HJ, Cho KJ, Chen CY, Rambahal N, Sagineedu SR, Shaari K, Stanslas J, Hancock JF, Gorfe AA. (2013) Andrographolide derivatives inhibit guanine nucleotide exchange and abrogate oncogenic Ras function. Proc Natl Acad Sci USA 110:10201–10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Fisher PB. (1993) Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Mol Cell Differ 1:285–299. [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. (1995) Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11:2477–2486. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. (1996) The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA 93:9160–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Kim MJ, Chung BY, Bang Y, Lim SK, Choi SM, Lim DS, Cho MC, Yoon K, Kim HS, et al. (2013) Safety evaluation and risk assessment of d-limonene. J Toxicol Environ Health B Crit Rev 16:17–38. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, Reed JC, Fisher PB. (2003) Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene 22:8758–8773. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, Dent P, Fisher PB. (2005) Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene 24:585–596. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Gopalkrishnan RV, Athar M, Randolph A, Valerie K, Dent P, Fisher PB. (2006) Molecular target-based therapy of pancreatic cancer. Cancer Res 66:2403–2413. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Washington I, Sarkar D, Clark JA, Fine RL, Dent P, Curiel DT, Turro NJ, Fisher PB. (2007) Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas. Proc Natl Acad Sci USA 104:3484–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. (2008a) Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis. Mol Cancer Ther 7:2042–2050. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. (2008b) Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol. Cancer Res 68:7439–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D, et al. (2012) Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA 109:5299–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AS, Koch PE, Atkinson N, Xiong M, Finberg RW, Roth JA, Fang B. (1999) Factors limiting adenovirus-mediated gene transfer into human lung and pancreatic cancer cell lines. Clin Cancer Res 5:4208–4213. [PubMed] [Google Scholar]
- Rabi T, Bishayee A. (2009) d -limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J Carcinog 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Azab BM, Das SK, Quinn BA, Shen X, Dash R, Emdad L, Thomas S, Dasgupta S, Su ZZ, et al. (2013) Chemoprevention gene therapy (CGT): novel combinatorial approach for preventing and treating pancreatic cancer. Curr Mol Med 13:1140–1159. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. (2003) MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev 14:35–51. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. (2008) Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci USA 105:9763–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. (2001) A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci USA 98:10332–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, et al. (2005) Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene 24:7552–7566. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. (1998) The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA 95:14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. (2007) D-limonene: safety and clinical applications. Altern Med Rev 12:259–264. [PubMed] [Google Scholar]
- Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, Lee T, Rossanese OW, Fesik SW. (2012) Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew Chem Int Ed Engl 51:6140–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, et al. (2005) Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther 11:160–172. [DOI] [PubMed] [Google Scholar]
- Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, et al. (2011) Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.