Abstract
The majority of angiosperms display maternal plastid inheritance. The cytological mechanisms of this mode of inheritance have been well studied, but little is known about its genetic relationship to biparental inheritance. The angiosperm Chlorophytum comosum is unusual in that different pollen grains show traits of different modes of plastid inheritance. About 50% of these pollen grains exhibit the potential for biparental plastid inheritance, whereas the rest exhibit maternal plastid inheritance. There is no morphological difference between these two types of pollen. Pollen grains from different individuals of C. comosum all exhibited this variability. Closer examination revealed that plastid polarization occurs, with plastids being excluded from the generative cell during the first pollen mitosis. However, the exclusion is incomplete in 50% of the pollen grains, and the few plastids distributed to the generative cells divide actively after mitosis. Immunoelectron microscopy using an anti-DNA antibody demonstrated that the plastids contain a large amount of DNA. As there is a considerable discrepancy between the exclusion and duplication of plastids, resulting in plastids with opposite fates occurring simultaneously in C. comosum, we propose that the species is a transitional type with a mode of plastid inheritance that is genetically intermediate between the maternal and biparental modes.
The non-Mendelian genetics of extracellular genomes were first reported nearly a century ago (Baur, 1909; Correns, 1909). Plastids have an independent genome and display maternal inheritance in the majority of angiosperms (for reviews, see Kirk and Tilney-Bassett, 1978; Sears, 1980; Whatley, 1982; Corriveau and Coleman, 1988; Hagemann and Schröder, 1989; Kuroiwa, 1991; Mogensen, 1996). In maternal inheritance systems, paternal transmission of plastids is impeded during either the first pollen mitosis via unequal plastid distribution (Lycopersicon type), or during generative or sperm cell development via plastid degeneration (Solanum type; Hagemann and Schröder, 1989; Mogensen, 1996). Therefore, the generative and sperm cells in mature pollen tend to be free of plastids. Conversely, cells in the pollen grains of species that display biparental plastid inheritance reserve plastids and transmit plastids paternally (Pelargonium type; Hagemann and Schröder, 1989; Mogensen, 1996).
About 85% of angiosperm species display maternal plastid inheritance, and the rest exhibit the potential for biparental inheritance (Corriveau and Coleman, 1988; Zhang et al., 2003). As the maternal mode is dominant in angiosperms, it is believed that this mode is an advanced form of plastid inheritance. To date, no information has been published on the relationships between the mechanisms of maternal and biparental plastid inheritance. This is understandable, since there are few transitional species available for study. Likewise, due to the independent phylogeny of cytoplasmic inheritance (as suggested by Zhang et al., 2003), transitional species, which are crucial in evolutionary studies, are not likely to be found based on phylogenetic relationships. In fact, no plant has ever been reported that is a transitional type between the biparental and maternal modes of plastid inheritance.
The mode of plastid inheritance in Chlorophytum comosum has been studied for many years. Using sexual crosses between Chlorophytum elatum and C. comosum, Collins (1922) first observed the maternal inheritance of leaf variegation in these species. Extensive data indicated that a low rate of biparental plastid transmission occurs regularly, at about 2% to 8% (Collins, 1922). A similar result was found for C. elatum (Pandey and Blaydes, 1957). This low rate of biparental plastid transmission is nevertheless at least 10 times higher than the occasional biparental transmission of plastids in species that exhibit strict maternal inheritance. Therefore, Kirk and Tilney-Bassett (1978) classified C. comosum as a species with traces of biparental plastid transmission. The unusual mode of plastid inheritance in C. comosum later resurfaced in conflicting data from different cytological studies. Electron microscopy of C. comosum pollen ultrastructure revealed plastids in the mature generative cell, consistent with biparental transmission (Vaughn et al., 1981; Zhang and Sodmergen, 2003). However, studies of early generative cells showed that plastids were excluded from these cells, leading to the conclusion that C. comosum possesses Lycopersicon-type maternal plastid inheritance (Schröder, 1986; Hagemann and Schröder, 1989).
As discrepancies in the mode of plastid inheritance within a single angiosperm species are highly unusual, we examined more closely the behavior of paternal plastids in C. comosum. Quantitative examination of C. comosum generative cells revealed heterogeneous pollen types. This indicates that this species displays a mode of plastid inheritance that is a transitional type between Pelargonium-type biparental and Lycopersicon-type maternal plastid inheritance.
RESULTS
Plastid DNA Is Not Amplified from All Generative Cells
Since previous results regarding plastid inheritance in C. comosum were contradictory, we determined whether plastid DNA is present in mature generative cells of this plant. Nested PCR was performed upon individual generative cells that were released from mature pollen grains by osmotic shock, which were collected, and washed with micromanipulation until free of visible contamination (Fig. 1, A–C). To avoid possible commingling of DNA molecules, the cells were treated with deoxyribonuclease (DNase) during the washes. Single cells were placed into tubes for PCR amplification. In the first PCR round, two pairs of primers were used to amplify both plastid (rbcL, Rubisco large subunit) and mitochondrial (cox I, cytochrome c oxidase subunit I) DNA fragments in each reaction. In the second PCR round, plastid and mitochondrial DNA fragments were amplified separately. As positive controls, protoplasts from young leaves of C. comosum were isolated (Fig. 1D), and one protoplast was transferred to each tube for the first round of PCR.
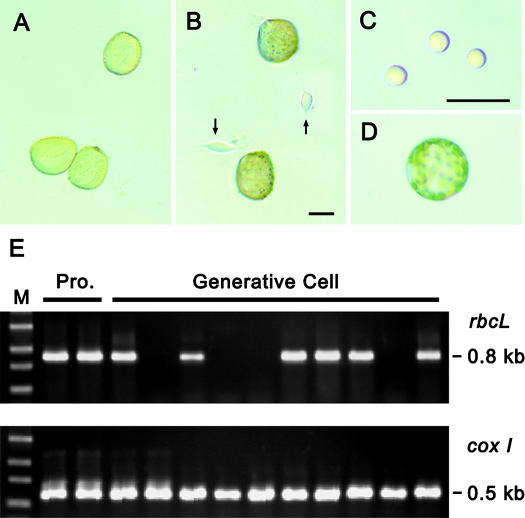
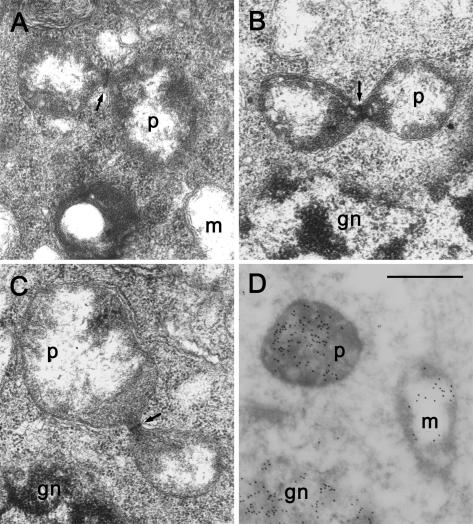
Figure 1.
Isolation of mature generative cells from pollen and detection of plastid and mitochondrial DNA from the isolated cells. A, Mature pollen grains of C. comosum collected from dehisced anthers. B, Pollen grains after osmotic shock. Generative cells (arrows) were released into the osmotic solution. C, Generative cells after DNase treatment and washing. The cells became spherical and were not associated with visible debris. D, A leaf protoplast treated and washed as for the generative cells. E, Detection by nested PCR of plastid (upper gel) and mitochondrial (lower gel) DNA fragments from single cells. The rbcL fragment from plastids was not detected from several generative cells. Pro., protoplast. Bar in B = 20 μm; bar in C = 40 μm.
Only the second round of PCR produced visible DNA bands, corresponding to the expected plastid (0.8 kb) and mitochondrial (0.5 kb) DNA fragments (Fig. 1E). However, although the mitochondrial DNA fragment was amplified from all of the cells, the plastid DNA fragment was amplified from protoplasts but not from all of the generative cells. The lack of amplification of the plastid fragment from some of the generative cells indicates that these cells did not contain plastid DNA. This indicates that the generative cells of C. comosum are heterogeneous, in that they either contain or lack plastids.
Only Some Generative Cells Contain Plastids
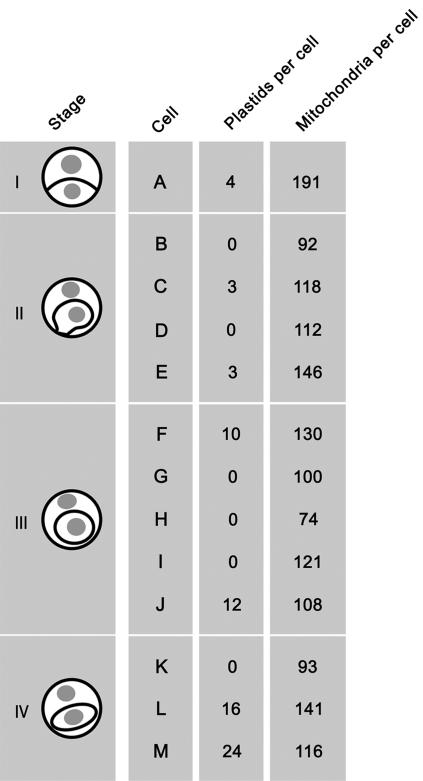
Previous studies have led to contradictory theories on the mode of plastid inheritance in C. comosum. However, both of the theories are reasonable if some generative cells contain plastids and others do not, as presumed above. To evaluate this presumption, we performed quantitative analyses using continuous sectioning microscopy. Consecutive sections were made from whole cells and observed under an electron microscope. This method is labor-intensive but reveals cell components in greater detail than ordinary electron microscopy. We obtained data from 13 generative cells named A to M, which were at stages I to IV (Fig. 2). Six of the 13 generative cells contained no plastids, and the remaining seven contained plastids (Fig. 2). Therefore, plastids were present in the generative cells of about 50% of the pollen grains and absent from the generative cells of the other 50%.
Figure 2.
Plastids and mitochondria in 13 generative cells. Cells were examined using continuous sectioning microscopy. The generative cells were divided into four groups according to the developmental stage: I, Lenticular cells attached to the intine; II, Tadpole-shaped cells with a tail attached to the intine; III, Spherical cells; IV, Elongated cells with tapering ends. Increased numbers of plastids were seen in cells that were at the later stages.
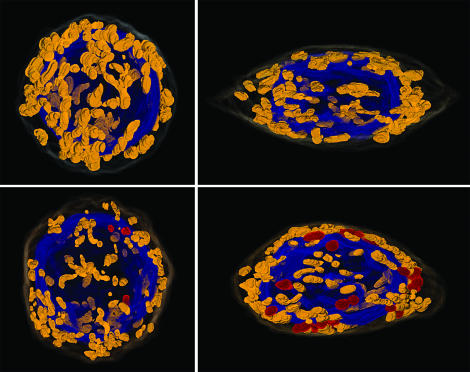
Microscopic images were combined for four of the cells that were examined with continuous sectioning microscopy (Fig. 3). The cells shown are at either the early or later stages and with or without plastids. Of the cells that contained plastids, the cell at the later stage contained several times as many plastids as the cell at the earlier stage (see also Fig. 2), and the plastids in the later generative cells tended to be clumped. One possible explanation for this observation is that plastids multiply during the later cell stages.
Figure 3.
Three-dimensional images of generative cells reconstructed from continuous sections. The colored objects represent plastids (red), mitochondria (yellow brown), and the cell nuclei (blue). The cell membrane and cell nucleus are transparent. Cells are oriented to show as many plastids as possible. Left, Generative cells at stage II. Right, Generative cells at stage IV. Cells either contain (lower) or do not contain (upper) plastids, depending on the pollen grain. The plastids in the cell at the later stage (lower right) appear in clumps.
Incomplete Exclusion of Plastids during the First Pollen Mitosis
It is known that during the first pollen mitosis, plastids are apportioned between the generative and vegetative cells, a process that differs in plants with different modes of plastid inheritance. In species that display Lycopersicon-type maternal plastid inheritance, plastids are retained in the vegetative cell during the first pollen mitosis. This unequal apportionment of plastids causes the formation of a generative cell that lacks plastids. The opposite phenomenon occurs in species with Pelargonium-type biparental plastid inheritance, in which plastids are apportioned randomly to both the generative and vegetative cells. Interestingly, only about 50% of the generative cells in C. comosum contain plastids, as demonstrated above; this is the first report to our knowledge of such a phenomenon in angiosperms. To investigate the production of heterogeneous generative cells in C. comosum, we examined the distribution of plastids during the first pollen mitosis.
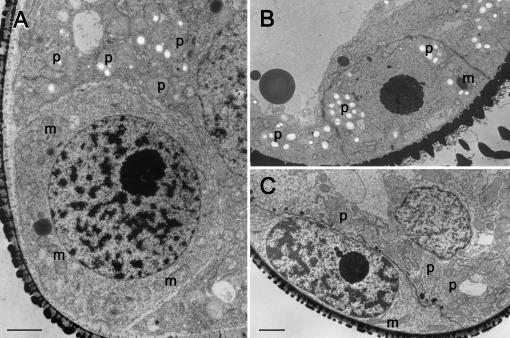
In general, there were no plastids in sections of early generative cells at the stage just after mitosis (Fig. 4A), but many plastids appeared in vegetative cells at this stage. For comparison, we examined generative cells at the same stage from Pelargonium zonale (Fig. 4B), a species with Pelargonium-type biparental plastid inheritance, and a generative cell of Gasteria verrucosa (Fig. 4C), which has Lycopersicon-type maternal plastid inheritance. The P. zonale generative cell contained plastids at a density equivalent to that in the vegetative cell, and the G. verrucosa cell did not contain plastids. This comparison suggests that C. comosum displays Lycopersicon-type maternal plastid inheritance.
Figure 4.
Apportionment of plastids between vegetative and generative cells after the first pollen mitosis. A, C. comosum. Plastids are visible in the vegetative cell but not the generative cell. B, P. zonale. Plastids are visible at equal frequency in both generative and vegetative cells. C, G. verrucosa. As in C. comosum, plastids are visible in the vegetative cell but not in the generative cell. Sections were 75 nm thick. p, plastid; m, mitochondria. Bars = 2 μm.
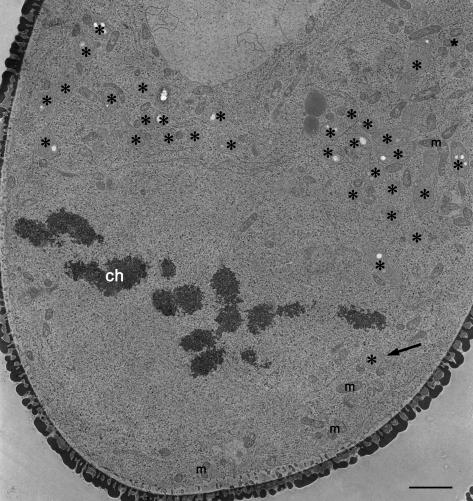
To understand how plastids enter the generative cell, we examined the distribution of plastids at an earlier stage of mitosis. At metaphase, most plastids were near the area where the vegetative cell forms (Fig. 5). This polarization of plastids is also reminiscent of the behavior of plastids in species with Lycopersicon-type plastid inheritance. However, the polarization of plastids in these pollen grains was not absolute; one plastid was observed in the region where the generative cell forms (Fig. 5). Plastids such as this likely remain in the generative cell during the mitosis that follows. Consequently, the exclusion of plastids from the generative cell occurs during the first pollen mitosis, but is not absolute in about one-half of the pollen grains, resulting in the presence of plastids in some generative cells.
Figure 5.
Polarization of plastids at metaphase of the first pollen mitosis. Plastids are denoted with an asterisk. Most plastids were distributed in the region of the forming vegetative cell. In one case, an isolated plastid was seen in the region of the forming generative cell (arrow). ch, metaphase chromosome; m, mitochondrion. Bar = 2 μm.
Plastids Distributed to the Generative Cell Divide Actively and Contain DNA
In angiosperm species that display Solanum-type maternal plastid inheritance, the mode of plastid inheritance is not determined by the first pollen mitosis. Instead, plastids are apportioned to the generative cell during mitosis but are rapidly destroyed. Therefore, to understand how plastids that are apportioned to the generative cell in C. comosum contribute to plastid inheritance, it is important to know the fate of the plastids during the later stages of pollen development.
A comparison of generative cells containing plastids revealed that cells in the later stages (III and IV) contained several times the number of plastids found in the earlier stages (I and II; Fig. 2), suggesting that the plastids multiplied after the generative cell detached from the intine. This prediction was confirmed with the observation of many dividing plastids in continuous sections of generative cells at later stages (Fig. 6, A–C). In addition, immunoelectron microscopy analysis of the cells showed that the multiplying plastids contain large amounts of DNA (Fig. 6D). It is apparent that the strength of paternal plastid transmission is enhanced in C. comosum pollen grains with generative cells that contain plastids, as in plants with the Pelargonium-type biparental plastid inheritance (discussed below). The plastid exclusion that occurs at the early stage and the multiplication of DNA-containing plastids in the generative cell is apparently an inconsistency in the mode of plastid transmission and inheritance. Therefore, C. comosum appears to be a transitional species, with a mixture of the Pelargonium-type biparental and Lycopersicon-type maternal plastid inheritance modes.
Figure 6.
Plastids in generative cells. A–C, Dividing plastids in generative cells. Images were acquired from 120-nm sections obtained using continuous sectioning microscopy. D, Localization of anti-DNA immunogold labeling on a plastid, a mitochondrion, and the generative nucleus. Large amounts of gold particles are visible on the plastid. Arrows indicate the plastid division rings. gn, generative nucleus; p, plastid; m, mitochondrion. Bar = 0.5 μm.
DISCUSSION
In this study, plastid DNA could not be amplified by PCR from a portion of the generative cells of C. comosum (Fig. 1). Since mitochondrial DNA could be amplified from the same cells, we suspected that these pollen grains were heterogeneous, with the generative cells either containing or lacking plastids. This presumption was confirmed using continuous sectioning microscopy with approximately 100 sections per cell of 13 cells at different developmental stages (Fig. 2). About one-half of the cells contained plastids, and the other one-half did not. These data are more reliable than the PCR data on the proportion of cells that contained plastids, because with PCR there is a possibility of false positive detection of a plastid due to contamination, despite careful washing and DNase treatment. The shortcoming of continuous sectioning microscopy is the inability to examine large numbers of samples because of the great amount of work involved for each sample. However, since the technique yields reliable results, we used it to examine as many cells as possible. We believe that the proportion of cells that contains plastids determined using this technique reflects the natural population, because the data were derived from 13 randomly selected cells.
For our studies, we purchased more than 10 pots of flowering plants from two well-separated flower markets. Pollen grains were collected from one or two pots for continuous sectioning electron microscopy examination of individual developmental stages (I–IV), and the pollen grains used for isolation of generative cells for nested PCR were collected from a plant grown in the university greenhouse. As all samples gave similar results, the results were not specific to individual plants or flowers.
We demonstrated that the heterogeneity of the generative cells was due to an incomplete exclusion of plastids during the first pollen mitosis (Figs. 4 and 5). Since most of the plastids were apportioned to the vegetative cell, we conclude that C. comosum has traces of Lycopersicon-type maternal plastid inheritance, agreeing with the conclusion of a previous study (Schröder, 1986). However, the few plastids apportioned to the generative cells were shown to multiply after mitosis via active division, and contained large amounts of DNA (Fig. 6). It is apparent that the strength of paternal plastid transmission is enhanced in pollen grains with generative cells that contain plastids. A previous study also found DNA-containing plastids in the generative cells of C. comosum (Zhang and Sodmergen, 2003). According to Nagata et al. (1999), the amount of plastid DNA increases in the generative cells of species that display biparental plastid inheritance, but it degrades rapidly after mitosis in the cells of species that exhibit maternal inheritance. The authors suggested that biparental inheritance is achieved primarily by the selective increase of plastid DNA in male reproductive cells. Therefore, the later behavior of plastids in the generative cells of C. comosum cancels the effect of plastid exclusion during mitosis and appears similar to that in the cells of species that exhibit biparental plastid inheritance. No other angiosperm has ever been shown to have such a contradiction in cell and plastid behavior. We therefore presume that C. comosum is a transitional species, with a mode of plastid inheritance that is intermediate between the biparental and maternal modes.
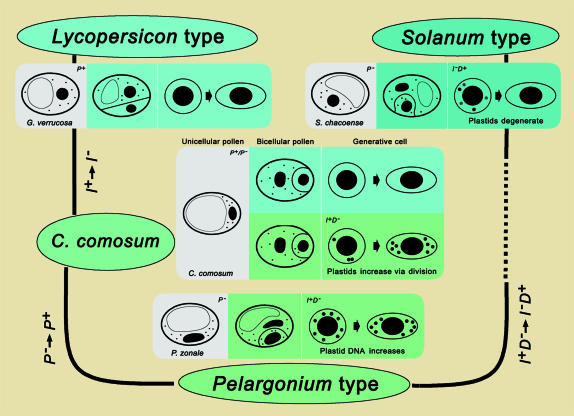
The details of our results are summarized in Figure 7. Shown are diagrams of the development of the generative cells of G. verrucosa, a species representing Lycopersicon-type maternal plastid inheritance; Solanum chacoense, a species representing Solanum-type maternal plastid inheritance; and P. zonale, a species representing Pelargonium-type biparental plastid inheritance. These constitute the dominant models of plastid inheritance in angiosperms. Plastid polarization (P+) resulting in the exclusion of plastids from the generative cell and plastid degeneration (D+) in the generative cell are the mechanisms responsible for maternal plastid inheritance in the Lycopersicon- and Solanum-type species. Such controls on plastids do not act (P−D−) in Pelargonium-type species, and there is an increase in plastid DNA (I+). Since C. comosum exhibits incomplete plastid polarization (P+/P−) during mitosis and plastid duplication (I+) during pollen development, it is intermediate between the Lycopersicon and Pelargonium types. This intermediate appearance suggests that C. comosum is a transitional species between plants that display the Pelargonium and the Lycopersicon modes of plastid inheritance. We postulate that the genetic transition from the Pelargonium type to the Lycopersicon type is achieved by promoting genes related to plastid polarization (P− to P+) in the unicellular pollen and blocking plastid-duplication-related genes (I+ to I−) in the generative cells. Similarly, we presume that Solanum-type maternal plastid inheritance deviates from Pelargonium-type biparental plastid inheritance owing to the promotion of genes related to plastid degeneration and the suppression of plastid-duplication-related genes (I+D− to I−D+) in the generative cells. Parallel to these presumptions, we also suggest that both Lycopersicon- and Solanum-type maternal plastid inheritance are achieved through the control of at least two genes. In the Lycopersicon type, one gene (P+) acts in unicellular pollen and another (I−) acts in the generative cell. However, in the Solanum type, both of the genes (I−D+) act in the generative cell.
Figure 7.
Models of plastid inheritance and their genetic relationships. The increase in plastid DNA during generative cell development is based on Nagata et al. (1999). Maternal plastid inheritance is achieved by promoting genes related to plastid polarization (P+) in unicellular pollen in Lycopersicon-type species, or by promoting genes related to plastid degeneration (D+) in the generative cells of Solanum-type species. Biparental plastid inheritance in Pelargonium-type species is achieved by blocking these genes (P−D−) and simultaneously promoting genes related to increases in the number of plastids or the amount of plastid DNA (I+) in the generative cells. Incomplete polarization of plastids (P+/P−) and an increase in the number of plastids in C. comosum generative cells leads to the formation of pollen with a heterogeneous potential for plastid inheritance, intermediate between the Lycopersicon-type maternal plastid inheritance and the Pelargonium-type biparental plastid inheritance.
The genetic relationships shown in Figure 7 are based on the assumption that maternal plastid inheritance evolved from biparental plastid inheritance. This assumption is based on the fact that maternal inheritance is the dominant mode in angiosperms, and because biparental plastid inheritance occasionally occurs in species that display maternal inheritance. This opinion is also based on the assumption that biparental transmission of plastids occurs naturally during sexual reproduction when two equiform gametes fuse. It is biologically and evolutionarily significant that maternal transmission of plastids apparently reduces the chance of the plant obtaining plastid genetic variation, benefiting the nuclear control of plastid duplication and function. This clear advantage suggests that the advancement of maternal plastid inheritance should not be limited to higher plants. It is possible that the evolution of plastid inheritance accompanied the formation of eukaryotic cells. Eukaryotic cells that displayed uniparental plastid inheritance might have had an evolutionary advantage because of their increased cell stability. We propose that the development of uniparental plastid inheritance in higher plants (maternal in angiosperms and paternal in gymnosperms) is an extension of the mechanism in other eukaryotic cells, i.e. maternal plastid inheritance evolved from biparental plastid inheritance under the evolutionary pressure of cell (plant) stability. The fact that the modes of plastid inheritance in angiosperms are not correlated with phylogenetic relationships (Corriveau and Coleman, 1988; Zhang et al., 2003) supports this idea.
The results of this study explain the discrepancies in the theories on plastid inheritance in C. comosum. These inconsistencies are observed because only some of its generative cells contain plastids. As plastids in generative cells multiply during pollen development, more sections of mature generative cells contain plastids than do sections of early generative cells. This may explain why microscopic studies of mature generative cells show the presence of plastids (Vaughn et al., 1981; Zhang and Sodmergen, 2003), whereas those of early generative cells indicate the absence of plastids (Schröder, 1986; Hagemann and Schröder, 1989). In addition, we demonstrated the presence of mitochondrial DNA in generative cells (Figs. 1E and 6D), and therefore the potential biparental mitochondrial inheritance of C. comosum, as suggested by Zhang and Sodmergen (2003).
Previous genetic analyses using sexual crossing revealed biparental plastid transmission in C. comosum at a rate of 2% to 8% (Collins, 1922; Pandey and Blaydes, 1957). This biparental transmission is not likely to be merely sporadic, since occasional transmission of organelles occurs at a distinctly lower rate (Yu and Russell, 1994a, 1994b). We have shown that about 50% of the pollen grains in natural populations have the potential for biparental plastid transmission, a rate higher than the 2% to 8% previously detected. This discrepancy could be explained by the fact that paternal organelles may be eliminated after fertilization via insufficient replication in the embryo or partial distribution to the suspensor (Whatley, 1982). Further examination will be required to answer this crucial question regarding organelle transmission.
MATERIALS AND METHODS
Plant Materials
Chlorophytum comosum, Gasteria verrucosa, and Pelargonium zonale were either grown in the greenhouse at the College of Life Sciences at Peking University or purchased from local flower markets. Pollen grains at different developmental stages were collected from these plants.
Isolation of Generative Cells and Nested PCR
Pollen grains collected from naturally dehisced anthers were immersed in a 12% (w/v) mannitol solution at room temperature. About 10 min later, most of the pollen grains began to burst and the mature generative cells were released from the pollen grains. The generative cells were immediately transferred individually by micromanipulation into a solution containing 50 units/mL DNase I (TAKARA BIO, Shiga, Japan), 40 mm Tris-HCl (pH 7.4), 5 mm MnCl2, and 12% (w/v) mannitol. After 30 min of incubation, the cells were washed three times in fresh mannitol solution to remove contaminations, carrying as little solution as possible during the transfer. After the washes, each cell was inspected under a microscope, and only intact cells without any associated granules were used for PCR. Protoplasts were used as a positive control for the presence of both plastids and mitochondria. An enzyme solution containing 4% (w/v) cellulase RS, 1% (w/v) macerozyme (Yakult, Tokyo), 0.1% (w/v) CaCl2, and 0.4 m mannitol was used for the separation of protoplasts from young C. comosum leaves. The treatment and washing of the protoplasts were the same as for the generative cells.
The nested PCR procedure to amplify DNA fragments from individual cells was based on Nishimura et al. (1999). PCR primers were designed to amplify an rbcL gene fragment from the plastid genome and a cox I gene fragment from the mitochondrial genome of C. comosum generative cells. The primers used for amplifying the rbcL fragment were: rbcL_F1, 5′-CAGCATTCCGAGTAACTCCTCAAC-3′; rbcL_R1, 5′-GAATAACACCTGGCATAGAAACCC-3′; rbcL_F2, 5′-TGCTACCACATTGATCCCGTTCTT-3′; and rbcL_R2, 5′-ATACCGCGACTTCGGTCTTCTTTA-3′. These primer sequences were based on the published sequence of the C. comosum rbcL gene (GenBank accession number L05031). The primers used to amplify the cox I fragment, designed according to cox I sequences from eight dicotyledonous and monocotyledonous species, were: cox I_F1, 5′-CCTGACATGGCATTTCCACGAT-3′; cox I_R1, 5′-TACCCGAAAGCCCTAAGAAATG-3′; cox I_F2, 5′-TCCGCCCTTAAGTGGTATTACCAG-3′; and cox I_R2, 5′-GCCCACAGTAAACATATGATGAGCC-3′.
The expected sizes of the fragments amplified with the rbcL gene primers were 1.01 kb from the first round of PCR and 0.84 kb from the second round, and for the fragments amplified with the cox I primers, 1.02 kb from the first round and 0.50 kb from the second round. All PCR reaction volumes were 25 μL. The first round of PCR was performed with 30 cycles of 1 min at 94°C, 1 min at 65°C, and 1 min at 72°C, with a single generative cell per reaction as the template. The two outer primer pairs (rbcL_F1 and rbcL_R1; cox I_F1 and cox I_R1) were used in the first PCR round to amplify both gene fragments simultaneously. One microliter of the first PCR product was then used as the template for the second round of PCR, in which the inner primer pairs (rbcL_F2 and rbcL_R2; cox I_F2 and cox I_R2) were used to amplify the rbcL and cox I fragments in separate reactions. PCR conditions for the second round of PCR were the same as for the first round.
Electron Microscopy
Transmission electron microscopy of pollen cells was performed according to standard methods. Pollen grains were fixed with 3% (v/v) glutaraldehyde and 1% (w/v) paraformaldehyde in 0.1 m sodium cacodylate buffer (pH 7.4) and postfixed in 2% (w/v) osmium tetroxide. The samples were embedded in Epon 812 resin. Sections were cut 75 nm thick for regular microscopy and 120 nm thick for continuous sectioning microscopy. Both types of section were stained with 1% (w/v) uranyl acetate and lead citrate, and observed with a JEOL electron microscope. For quantitative examination of plastids in generative cells, entire cells were cut into continuous sections, which were carefully collected in single-slot grids. Images of each section were captured with a high-resolution CCD camera. To determine the number of organelles in individual cells, plastids and mitochondria were numbered in each section, and matching organelles in different sections were counted as one. As indicated by the volumes of the plastids and mitochondria, no more than three consecutive sections were lost per cell.
Immunoelectron microscopy to detect cellular DNA was performed as described previously (Johnson and Rosenbaum, 1990). Briefly, pollen grains were fixed with 3% (v/v) glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.2) and embedded in Lowicryl K4M resin. Sections were first incubated with a monoclonal antibody specific to single- and double-stranded DNA from mouse-mouse hybrid cells (Boehringer Mannheim GmbH, Mannheim, Germany), and then with commercial goat anti-mouse IgM conjugated to 10-nm colloidal gold particles (British BioCell International, Cardiff, UK). As a negative control, sections were pretreated with DNase and processed as above.
Three-Dimensional Reconstruction
Cells were reconstructed from continuous section images. AVS/Express software (Advanced Visual Systems Inc., Waltham, MA) was used to generate the three-dimensional images.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number L05031.
Acknowledgments
We thank Dr. Sun MX of Wuhan University (China) for his technical assistance in the separation of the generative cells.
This study was supported by the National Science Fund for Distinguished Young Scholars of China (grant no. 30025004) and by the State Key Basic Research and Development Plan of China (grant no. G1999011700).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036657.
References
- Baur E (1909) Das Wesen und die Erblichkeitsverhältnisse der “Varietates albomarginatae hort” von Pelargonium zonale. Z Indukt Abstammungs-Vererbungsl 1: 330–351 [Google Scholar]
- Collins EJ (1922) Variegation and its inheritance in Chlorophytum elatum and C. cernosum. J Genet 12: 1–17 [Google Scholar]
- Correns C (1909) Vererbungsversuche mit blass(gelb)grünen und buntblättrigen Sippen bei Mirabilis jalapa, Urtica pilulifera und Lunaria annua. Z Indukt Abstammungs-Vererbungsl 1: 291–329 [Google Scholar]
- Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot 75: 1443–1458 [Google Scholar]
- Hagemann R, Schröder M-B (1989) The cytological basis of the plastid inheritance in angiosperms. Protoplasma 152: 57–64 [Google Scholar]
- Johnson KA, Rosenbaum JE (1990) The basal bodies of Chlamydomonas reinhardtii do not contain immunologically detectable DNA. Cell 62: 615–619 [DOI] [PubMed] [Google Scholar]
- Kirk JTO, Tilney-Bassett RAE (1978) The Plastids: Their Chemistry, Structure, Growth and Inheritance. Elsevier/North Holland Biomedical Press, Amsterdam
- Kuroiwa T (1991) The replication, differentiation and inheritance of plastids with emphasis on the concept of organelle nuclei. Int Rev Cytol 128: 1–62 [Google Scholar]
- Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83: 383–404 [Google Scholar]
- Nagata N, Saito C, Sakai A, Kuroiwa H, Kuroiwa T (1999) The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis one controls cytoplasmic inheritance. Planta 209: 53–65 [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Misumi O, Matsunaga S, Higashiyama T, Yokota A, Kuroiwa T (1999) The active digestion of uniparental chloroplast DNA in a single zygote of Chlamydomonas reinhardtii is revealed by using the optical tweezer. Proc Natl Acad Sci USA 96: 12577–12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KK, Blaydes GW (1957) Cytoplasmic inheritance of plastids in Impatiens sultanii Hook F, Petunia violacea Lindl and Chlorophytum elatum R Br. Ohio J Sci 57: 135–147 [Google Scholar]
- Schröder M-B (1986) Ultrastructural studies on plastids of generative and vegetative cells in Liliaceae. 5. The behavior of plastids during pollen development in Chlorophytum comosum (Thunb) Jacques. Theor Appl Genet 72: 840–844 [DOI] [PubMed] [Google Scholar]
- Sears BB (1980) Elimination of plastids during spermatogenesis and fertilization in plant kingdom. Plasmid 4: 233–255 [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Kimpel DL, Wilson KG (1981) Control of organelle transmission in Chlorophytum. Curr Genet 3: 105–108 [DOI] [PubMed] [Google Scholar]
- Whatley JM (1982) Ultrastructure of plastid inheritance: Green algae to angiosperms. Biol Rev 57: 527–569 [Google Scholar]
- Yu HS, Russell SD (1994. a) Occurrence of mitochondria in the nuclei of tobacco sperm cells. Plant Cell 6: 1477–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Russell SD (1994. b) Population of plastids and mitochondria during male reproductive cell maturation in Nicotiana tabacum L.: a cytological basis for occasional biparental inheritance. Planta 193: 115–122 [Google Scholar]
- Zhang Q, Liu Y, Sodmergen (2003) Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol 44: 941–951 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sodmergen (2003) Cytological evidence for preservation of mitochondrial and plastid DNA in the mature generative cells of Chlorophytum spp. (Liliaceae). Protoplasma 221: 211–216 [DOI] [PubMed] [Google Scholar]