Abstract
Objective
Explore whether electromyography (EMG) control of electrical stimulation for walking after incomplete spinal cord injury (SCI) can affect ability to modulate speed and alter gait spatial-temporal parameters compared to cyclic repetition of pre-programmed stimulation.
Design
Single case study with subject acting as own concurrent control.
Setting
Hospital-based biomechanics laboratory.
Participants
Single subject with C6 AIS D SCI using an implanted neuroprosthesis for walking.
Interventions
Lower extremity muscle activation via an implanted system with two different control methods: (1) pre-programmed pattern of stimulation, and (2) EMG-controlled stimulation based on signals from the gastrocnemius and quadriceps.
Outcome measures
Gait speed, distance, and subjective rating of difficulty during 2-minute walks. Range of walking speeds and associated cadences, stride lengths, stride times, and double support times during quantitative gait analysis.
Results
EMG control resulted in statistically significant increases in both walking speed and distance (P < 0.001) over cyclic stimulation during 2-minute walks. Maximum walking speed with EMG control (0.48 m/second) was significantly (P < 0.001) faster than the fastest automatic pattern (0.39 m/second), with increased cadence and decreased stride and double support times (P < 0.000) but no change in stride length (z = −0.085; P = 0.932). The slowest walking with EMG control (0.25 m/second) was virtually indistinguishable from the slowest with automatic cycling (z = −0.239; P = 0.811).
Conclusion
EMG control can increase the ability to modulate comfortable walking speed over pre-programmed cyclic stimulation. While control methods did not differ at the lowest speed, EMG-triggered stimulation allowed significantly faster walking than cyclic stimulation. The expanded range of available walking speeds could permit users to better avoid obstacles and naturally adapt to various environments. Further research is required to definitively determine the robustness, generalizability, and functional implications of these results.
Keywords: Electromyography, Functional electrical stimulation, Gait training, Neural prosthesis, Rehabilitation, Spinal cord injury, Walking
Introduction
There are ∼273 000 individuals living with spinal cord injuries (SCI) in the USA.1 Due to improved medical management, there are an increasing number of individuals with incomplete SCI (iSCI), with ∼40.6% of the population presenting with incomplete tetraplegia and 18.7% with incomplete paraplegia.1 Reciprocal ambulation is a high priority for individuals with iSCI, who uniformly desire to improve their walking ability.2 Functional electrical stimulation (FES), which applies electrical impulses to peripheral nerves to elicit contractions of the otherwise paralyzed muscles, is a powerful enabling technology with the potential to satisfy this need.
Stepping can be achieved with multichannel surface stimulation by activating the quadriceps to lock the knee during stance and electrically eliciting a withdrawal reflex to generate swing.3,4 Additional channels of surface stimulation have been applied to gluteus maximus for hip extension and gluteus medius or tensor fasciae latae for hip abduction.5,6 A 6-channel commercial surface FES system known as Parastep® (Sigmedics Inc., Northfield, IL, USA) has allowed individuals with injury levels of T4 or below to stand and take steps at speeds and physiological costs similar to walking in braces.7–9 Nonetheless, it is difficult or impossible to selectively activate individual muscles deep into the skin surface (such as the hip flexors) with surface stimulation or to obtain repeatable stimulated responses from day to day.10–12 As the number of channels increases, surface stimulation systems can also become impractical and inconvenient, making them generally best suited for short-term therapeutic applications within a clinical setting.
Fully implanted, pacemaker-like systems offer numerous advantages over surface stimulation for long-term clinical use, including improved convenience, cosmesis, reliability, and repeatability.13 Muscle or nerve-based electrodes are installed surgically and connected to an implanted stimulation device, so no material crosses the skin. Power and stimulus control information is transmitted through the skin via an inductive link. Early multichannel implants have allowed people with paraplegia to stand and perform swing-through gait maneuvers.14–16 Exercise and standing functions have also been reported with a cochlear implant modified to deliver 22 channels of stimulation to the lower extremities17,18 and a 12-channel system for intradural stimulation of the L2–S2 motor roots.19 Standing and reciprocal walking have been restored to volunteers with low cervical or thoracic level SCI via an 8-channel implantable receiver-stimulator with surgically implanted epimysial or intramuscular electrodes.20–25 In these systems, pre-programmed patterns of stimulation were optimized for each subject, and then intentionally selected from a menu of options. Reciprocal stepping was achieved by triggering successive steps by repeatedly pressing ring- or walker-mounted buttons, detecting floor contact with insole or crutch-tip switches, or allowing the pre-programmed pattern to cycle automatically until deactivated by the user.
An important aspect of functional ambulation is gait speed and the range of achievable walking speeds. Many activities of daily living (ADL) require a full range of gait velocities, from slow walking in crowds to fast walking for crossing a street. Being able to ambulate over a range of speeds is important to transition between various environments and surfaces. In addition, gait speed can be an indicator of overall health and independence. Geriatric patients with walking speeds <0.25 m/second were more likely to be dependent in at least one ADL, while those with gait speeds between 0.35 and 0.55 m/second were more likely to be independent in all ADL functions.26
Some surface stimulation systems for walking after hemiplegia have attempted to alter FES-assisted walking speed by scaling temporal patterns of stimulation based on previous step times as measured by insole-mounted foot switches.27 Patterns of stimulation delivered to the involved extremity were compressed or expanded by a constant factor depending on the times between successive past steps with the uninvolved leg, therefore making the system indirectly responsive to user intent. Problems with this approach include all of the issues related to surface stimulation, as well as the poor reliability and inconvenience associated with insole switches and their cabling. Even with a single cyclic pattern of stimulation, it is possible to modify gait speed by increasing stride length through the interactions of the upper body with the walker or assistive device. At the same pre-programmed cadence, walking speed can be changed by modifying walker placement and the effort exerted to pull the body forward during swing phase. The effectiveness of this strategy has not been quantified, and relies on more intentional upper extremity (UE) effort, concentration, and coordination of volitional upper body actions with cyclic pre-programmed stimulation.
An alternative control scheme to smoothly vary gait speed in walking neuroprostheses is to integrate stimulation and voluntary function with real-time feedback of the electromyographic (EMG) activity of the partially paralyzed musculature. In stroke survivors, surface EMG has been used to initiate electrical stimulation of the tibialis anterior to correct for foot drop.28–31 Other studies have shown that it is feasible to use intramuscular EMG from voluntarily controlled muscles of the lower extremity (LE) to detect gait events after cerebral palsy32 or to trigger FES-assisted steps in individuals with iSCI using surface EMG signals.33 After iSCI, the gait resulting from surface EMG control of an implanted stimulator was found to be more coordinated and dynamically stable than automatically cycling through successive steps or manually initiating every step with a pushbutton.34 In our study, permanently implanted EMG recording electrodes were used for the first time to detect gait events and trigger stimulation coordinated with voluntary movements via a single comprehensive implanted walking neuroprosthesis.
The purpose of this single-subject exploratory study was to quantify and assess the ability of EMG control to modulate walking speed and determine how it alters spatial-temporal parameters as compared to a single cyclic pattern of pre-programmed stimulation. Synchronizing with voluntary movements via the EMG provided a novel way to alter the speed of FES-assisted walking that is capable of responding to intention without requiring direct conscious interaction. This control should allow easy and automatic adjustments of walking speed in open spaces or when approaching environmental barriers.
Methods
The subject was a 42-year-old male who sustained a C6 incomplete SCI ASIA Impairment Scale (AIS) D with significant left LE weakness and moderate weakness of the trunk, upper extremities, and right LE. After approval by the Institutional Review Board of the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, informed consent was obtained prior to installation of an EMG-controlled neuroprosthesis consisting of a 12-channel implanted stimulator-telemeter (IST-12),35 12 stimulating electrodes,36 and 2 surgically implanted intramuscular EMG recording electrodes.37 The implanted stimulator-receiver was powered and controlled by a wearable external control unit (ECU) that transmitted information to the IST-12 and recovered signals from the implanted EMG electrodes via an inductive communication link maintained by a coil affixed to the skin. The subject had previously received an 8-channel implanted gait assist system during participation in other studies in our laboratory,25 which was removed and replaced with the advanced EMG-controlled neuroprosthesis in a single surgical procedure. He was independent using the 8-channel system to exercise and was routinely able to ambulate within his home environment without assistance. His baseline average volitional walking speed before implantation was 0.12 m/second, which plateaued at 0.23 m/second after gait training with stimulation. Walking speed with stimulation was consistently greater than maximal voluntary walking and averaged 0.28 m/second prior to upgrade to the IST-12.25
Due to the subject having more volitional control over his right LE than left, the following stimulating electrodes were implanted to address his primary gait deficits: (1, 2) left and right iliopsoas for hip flexion, (3) left tensor fasciae latae for hip flexion and abduction, (4) left gluteus medius for hip abduction, (5) left and (6, 7) right gluteus maximus for hip extension, (8) left posterior portion of the adductor magnus for hip extension and adduction, (9) left vastus lateralis for knee extension, (10) left semimembranosus for hip extension, and (11, 12) left and right tibialis anterior for ankle dorsiflexion. Two intramuscular myoelectric signal (IM-MES) recording electrodes were implanted in the right medial gastrocnemius and the right vastus lateralis for use in gait detection (Fig. 1). These muscles were chosen specifically for this subject after collecting surface EMG from a variety of muscles during over ground walking trials with his original 8-channel gait assist neuroprosthesis because they exhibited the greatest amount of volitional control and largest, most repeatable cyclic signals that correlated well with events in the gait cycle.
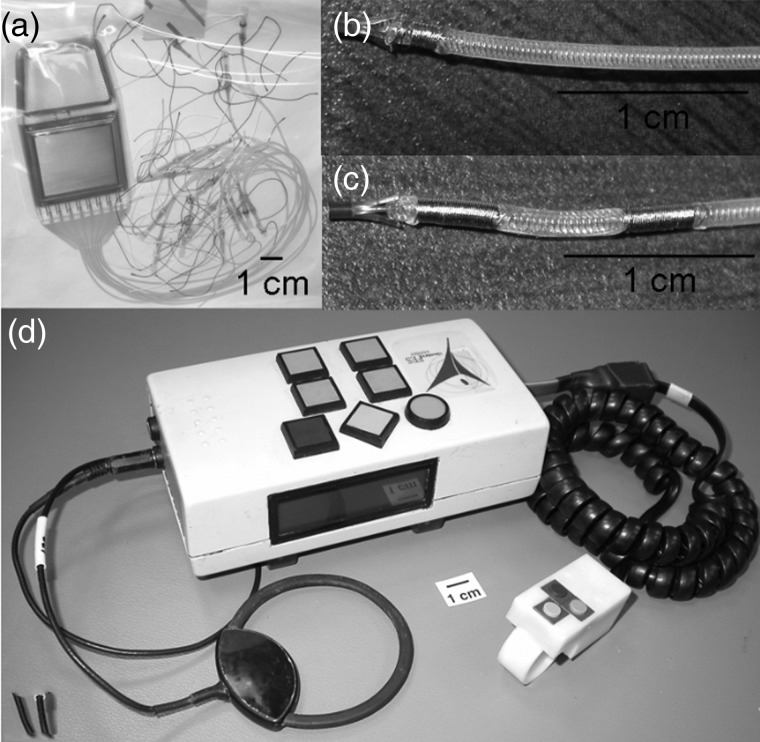
Figure 1 .
Components of the neuroprosthesis: (A) 12-channel implantable stimulator-telemeter, (B) intramuscular stimulating electrode, (C) IM-MES recording electrode; and (D) external components: Universal ECU with attached transmitting/receiving coil and hand-held or walker-mounted finger switch buttons.
After surgery, the subject underwent 6 weeks of limited activity to promote healing and encapsulation of the new implanted components. Because he was a well-conditioned and experienced user of an earlier 8-channel implanted pulse generator,25 he quickly progressed through gait training to walk with the IST-12-based neuroprosthesis. A pattern of continuously cycling, “automatic”, stimulation (Fig. 2) was constructed according to our standardized published tuning protocol and adjusted for his self-selected speed.38,39 The pattern was set to the fastest speed that he could comfortably and safely maintain walking. The subject reported using this pattern routinely at home and in the community, regularly walking in his warehouse environment of his workplace with cyclic stimulation. Due to the additional muscles stimulated with the new system, his average walking speed increased to 0.33 m/second. Usage patterns monitored automatically by the ECU during the 2 months prior to EMG testing indicate that the subject utilized his IST-12 system for exercise or walking outside of the laboratory on 85% (52 of 61) of days surveyed, as compared to 67% (41 of 61) of days during a similar monitoring period when he only had an automatic pattern of simulation available. Average walking times per day were similar with both patterns.
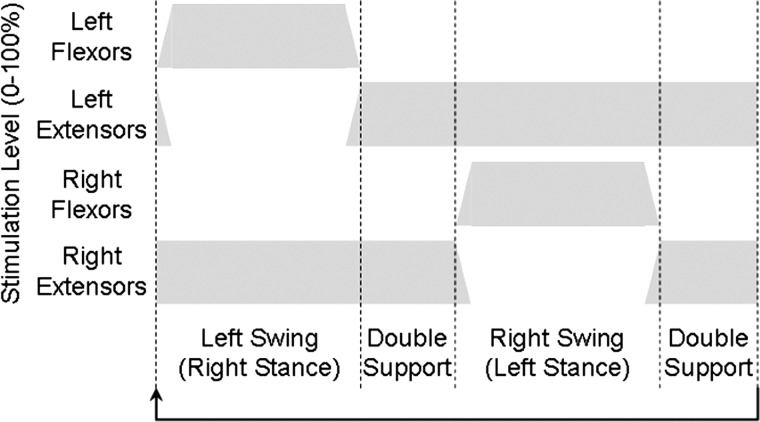
Figure 2 .
Automatic control flow diagram showing changes in stimulation levels during the phases of gait with stimulation cyclic as shown with no input from the user.
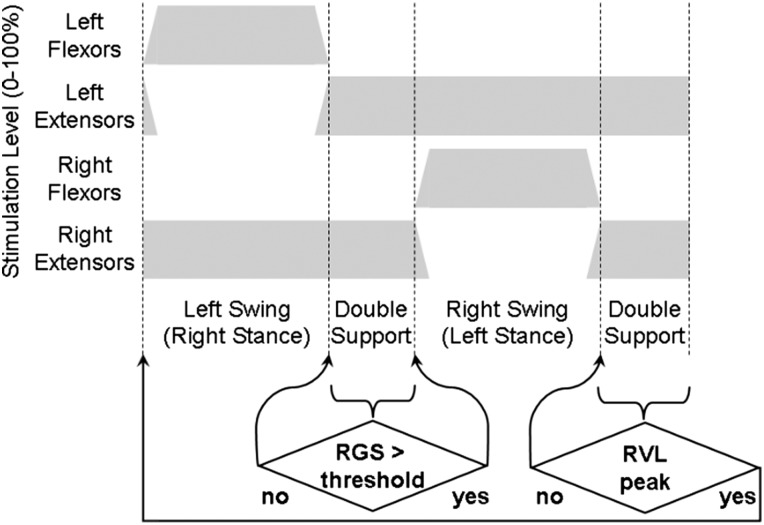
Once comfortable with and accomplished at walking with a pattern of continuous cycling, he began training with a pattern using EMG to detect gait events and trigger stimulation. The implanted IM-MES signals were sampled by the IST-12, bandpass filtered at 100–1000 Hz to remove movement artifact and 60 Hz noise, integrated over a window of 20 ms at a frequency of 16 Hz, and transmitted back to the ECU for use in the control algorithm described here. To begin the program, the subject initiated the first (left) step in the stimulated walking pattern by depressing a finger switch connected to the ECU. Once the swing phase of the left step was completed, left double stance stimulation was maintained while the algorithm looked for the right gastrocnemius EMG signal to rise above a threshold to indicate the subject was initiating a right step by pushing off with the plantarflexors. After the signal reached the threshold, the right step stimulation pattern was initiated. Once the swing phase of the right step was completed, right double stance stimulation was maintained while the algorithm looked for a peak of the right vastus lateralis EMG signal to indicate a loading response of the right leg, therefore signifying the user was ready to take a left step. Upon detecting the peak vastus lateralis activity corresponding to right leg weight acceptance, the left swing stimulation pattern was activated. To reduce the potential for tripping, the algorithm waited to begin the EMG event detection for each step until swing phase stimulation was finished and the extensors had turned on in order to ensure that the subject was in proper position to take the next step. If no EMG event detection occurred, the subject would remain standing with stimulation to maintain stance in double limb support (Fig. 3).
Figure 3 .
EMG control flow diagram showing changes in stimulation levels during the phases of gait with stimulation holding at each double support phase until the user provides a required trigger condition.
Data collection sessions to quantify the ability to modulate speed with EMG triggering and an automatic pattern of cyclic stimulation, and to determine the range of speeds using each control method, were conducted after he was independent with both systems. Two-minute walk tests were performed on 4 days. Each day, two trials of EMG and two trials of automatic walking were collected for a total of eight trials per condition. Conditions alternated between EMG and cyclic control on successive walks on the same day, and the control method applied first alternated between days to avoid confounding interactions. Ten minutes of rest separated each walk to minimize the effects of fatigue. The subject was instructed to walk as far as possible in 2 minutes, and permitted to slow down or stop to rest while standing if necessary. The subject used a wheeled walker and left ankle foot orthosis due to weakness of the stimulated left tibialis anterior contractions for all data collections. Walking distances were measured with a calibrated distance measuring wheel, and timed with a stop watch. In order to determine subjective perception of effort, the subject was asked to rate each walk by applying the Usability Rating Scale (URS), a 7-point ordinal scale ranging from “very difficult” (−3) to “very easy” (+3)40.
Quantitative gait analysis with EMG and automatic control was also performed over 2 days using a 16-camera Vicon MX motion capture system (West Way, Oxford, UK). Fifteen markers were placed on the pelvis and lower extremities according to Vicon's Lower Extremity Plug-In Gait model to obtain spatial-temporal data. A total of four walking conditions were tested, including walking as quickly as possible with (1) EMG control (EMG fast) and (2) automatic control (Auto fast), and walking as slowly as possible with (3) EMG control (EMG slow) and (4) automatic control (Auto slow). Because the timing of the automatic pattern of stimulation was fixed, the subject modulated gait speed by altering the coordination of his upper body with the stimulation. Eight trials for each condition on each day were collected in random order, with 5 minutes of rest between every other trial to minimize fatigue. Gait speed, cadence, stride length, stride time, and double support time were derived from the spatial-temporal data acquired over a 10 m walkway.
The data were checked for normality using Shapiro–Wilk test. The data from the 2-minute walk tests were found to be normally distributed, so a paired sample t-test (P < 0.05) was used to determine differences in distance and speed between the two control methods. Tests for significant differences in subjective impressions of difficulty between automatic and EMG walking were determined with the non-parametric Wilcoxon-signed rank sum test applied to the ordinal URS scores for the 2-minute walk trials. Some non-normality was detected in the spatial-temporal data, so the non-parametric Wilcoxon-signed rank sum test (P < 0.05) was applied to determine (1) if EMG allowed the subject to modulate walking speed and, if so, (2) which gait parameters contributed to the change in speed. To adjust for multiple comparisons, a Bonferroni correction was utilized (P < 0.0025).
Results
Two-minute walk tests
Walking with an EMG-controlled pattern resulted in a significant (P < 0.001) increase in both walking distance and speed over 2 minutes. Walking distance was 6.7 m farther and gait speed was 0.05 m/second faster (∼15% increase) with EMG triggering (Table 1). There was no difference in subjective rating of walking with an EMG-controlled pattern or an automatic pattern (z = −1.134, P = 0.257) at the self-selected speed and nominal pre-programmed speed, respectively. The median URS score for both control methods was +2 (moderately easy).
Table 1 .
Average distance and speed with standard deviation for 2-minute walk tests
| Distance (m) | Speed (m/second) | |
|---|---|---|
| Auto | 41.7 ± 1.1 | 0.35 ± 0.1 |
| EMG | 48.4 ± 1.9 | 0.40 ± 0.2 |
Quantitative gait analysis
The key outcome measures for both methods of control were (1) speed, (2) cadence, (3) stride length, (4) stride time, and (5) double support time (Table 2). Because the subject had no volitional control over his left LE, only spatio-temporal data from the right LE are reported with the exception of gait speed. Left strides were consistent across trials and conditions because stimulation controlled the length and time of each step. With EMG control, the participant was able to readily change the timing of his overall gait pattern, as evidenced by the variation in cadences and double support times.
Table 2 .
Summary of gait speed, right stride cadence, stride length, stride time, and double support time for all four conditions: (1) Auto slow, (2) EMG slow, (3) Auto fast, (4) EMG fast
| Auto fast | EMG fast | Difference (%) | P-value | Auto slow | EMG slow | Difference (%) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Cadence (steps/minute) | 39.03 | 46.24 | 19 | 0.001 | 39.22 | 42.41 | 8 | 0.006 |
| Double support time (second) | 1.44 | 1.06 | 26 | 0.001 | 1.85 | 1.71 | 8 | 0.038 |
| Stride length (m) | 1.22 | 1.22 | <1 | 0.932 | 0.79 | .73 | 8 | 0.393 |
| Stride time (second) | 3.08 | 2.60 | 16 | 0.001 | 3.06 | 2.83 | 8 | 0.008 |
| Gait speed (m/second) | 0.39 | 0.48 | 23 | 0.001 | 0.26 | .25 | 2 | 0.811 |
With percent differences and P-values to compare the two slow conditions and the two fast conditions.
Automatic slow versus EMG slow
There was no significant (P ≥ 0.006) difference in overall gait speed, right cadence, right stride length, right stride time, or right double support time when the subject walked as slowly as possible with both an automatic- and an EMG-controlled pattern. All showed <10% difference between the two conditions.
Automatic fast versus EMG fast
When comparing walking as quickly as possible with both an automatic pattern and an EMG-controlled pattern, there was a significant (P < 0.001) increase in overall walking speed (0.09 m/second, 23%) and right cadence (7 steps/minute, 19%) with the EMG-controlled pattern. In addition, there was a significant (P < 0.001) decrease of 0.48 seconds (16%) in right stride time and 0.38 seconds (26%) in right double support time with the EMG-controlled pattern. There was no difference (z = −0.085; P = 0.932) in right stride length between the automatic- and the EMG-controlled patterns.
Range of speeds with automatic versus EMG control
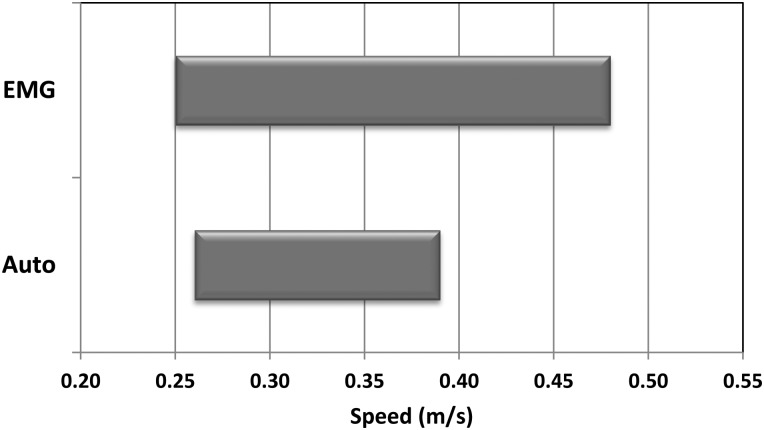
The ability to modulate gait speed from the slow to the fast walking and the range of available walking speeds with both the automatic and the EMG-controlled patterns were also examined. The difference between maximum and minimum speeds with EMG control was 0.23 m/second, while the difference between maximum and minimum walking speed with automatic control was 0.13 m/second. This represents a 59% increase in the range of available walking speeds with EMG control (z = −4.747; P < 0.001). Since slow speeds were comparable and did not differ significantly between conditions, the main effect of EMG control was to afford the subject with the ability to walk ∼0.10 m/second faster than automatic control and vary his gait speed from 0.25 to 0.48 m/second (Fig. 4).
Figure 4 .
Range in speed from slow to fast with EMG control versus automatic control.
Discussion
The slowest walking with both an EMG-controlled stimulation and the automatic pattern during gait analysis was indistinguishable with <10% differences between any single outcome measure. There was only a 2% difference in overall gait speed between the two methods of control when the subject was walking as slow as possible. This indicates that although the automatic pattern was set at the fastest speed that the subject was able to consistently and safely maintain, he was still able to modulate his speed and walk more slowly with cyclic stimulation. In contrast, the fastest walking possible with EMG control far exceeded that possible with automatic cycling of the stimulation pattern. In addition, gait speed during the 2-minute walk test significantly increased with the EMG-controlled pattern by >15%. While these results are statistically significant, they may also be clinically relevant. While there is a lack of consensus on what constitutes a clinically significant increase in walking speed in individuals with iSCI, others41 have looked for guidance in populations with similar strength and coordination deficits in the hip, knee, and ankle function and have adapted the modified Hoffer scale for this purpose.42 According to this scale, the increase in speed with EMG triggering would allow the subject to be classified as a limited community ambulatory (0.4–0.8 m/second) instead of a household ambulator (<0.4 m/second). In addition, the increase in speed using EMG over automatic-controlled pattern (0.09 m/second) exceeds the minimal threshold (0.06 m/second) to define a clinically important difference for individuals with iSCI.43
Examination of the spatio-temporal gait parameters indicate that increased gait speed with the EMG-controlled stimulation was achieved primarily by significant increases in cadence and decreases in stride and double support times. Stride length, however, was not significantly different between the two control schemes. These suggest that walking with EMG control is potentially more dynamic than automatic cycling with the user able to exert more direct control over the timing of the gait pattern.
Another important finding in this study is the range over which the subject could change walking speed with the two control schemes. With the automatic pattern, his maximum gait speed was 57% faster than his slowest walking speed. With the EMG-controlled pattern, his maximum speed was 91% faster than his slowest speed. Therefore, he was able to achieve a greater range of speeds with the EMG-controlled pattern, which would allow him to modulate his speed better when facing environmental obstacles or unexpected situations in the home or community.
It is important to note that even with an automatic program that has preset stimulation timing, the subject was still able to change his walking speed when asked to walk as slowly and as quickly as possible. There was no significant difference in cadence or stride time between the fast and the slow speeds; however, there was a 54% increase in stride length and a 22% decrease in double support time with the faster speed. Clinical observation suggested that the subject required greater UE support on the walker to pull himself forward to increase his stride length; however, UE loading was not explored during this study. In contrast, the subject showed a statistically significant increase in cadence and decrease in stride time with the EMG-controlled pattern when walking quickly compared to walking slowly.
Although these preliminary results indicate that this subject was able to vary walking speed to a greater extent with EMG control than an automatic pattern, the study was limited by only having one subject and a limited number of trials. Another limitation was that it was necessary to use EMG from the right vastus lateralis to control the left step due to limited volitional control on the subject's left side. Using EMG from the ipsilateral side would allow detection of the intent to step, rather than completion of the contralateral step, as a control source.
In future work, a larger, heterogeneous population of subjects with iSCI would allow results to be more generalizable. UE loading and cognitive burden should also be thoroughly examined to quantify interactions with the walker and the attention required for each control method. Comparisons with other methods of modulating speed, such as scaling temporal patterns of stimulation or selecting individual patterns programmed for different walking speeds, is also indicated, since in this study we only looked at the fastest automatic pattern that he was comfortable and safe using. In the future, when more advanced implantable hardware is available it may also be advantageous and make the system more robust by using more EMG signals to better detect gait events or speed-dependent changes in muscle activation patterns. Due to limitations of the IST-12 device used in the current study, only two channels of EMG were available for this purpose.
Although the subject did not report a high rate of false positives or negatives, the reliability of the EMG-based control was not studied. In addition, the changes to the voluntary strength of muscles used to volitionally trigger steps with the EMG could be explored in the future. It is plausible that they would exhibit gains in strength and measures of coordination due to training with EMG-controlled walking, and the therapeutic effects of reinforcing voluntary patterns of muscle activity could be quantified. Patterns of home and community use of various control schemes and user preferences for automatic versus EMG-controlled stimulation also remain to be determined. Finally, new rehabilitation and training protocols need to be developed to better time volitional contractions with the gait cycle, which may improve reliability and/or control of speed in EMG-triggered walking.
Conclusion
EMG-controlled stimulation has the potential to allow a user to have access to larger range of walking speeds than pre-programed cyclic patterns of stimulation. When comparing an EMG-controlled pattern to an automatic pattern, the slow speeds were similar; however, statistically significant and clinically relevant increases in walking speed with the EMG-controlled pattern were possible. Faster gait speed was achieved primarily by increasing cadence and decreasing stride and double support times, resulting in more dynamic walking. In this single case study, using EMG control appears to have the potential to be an effective way to modulate FES-assisted walking speed in an individual with iSCI. Further research is required to definitively determine the robustness, generalizability, therapeutic effect, and clinical impact of this control modality.
Disclaimer statements
Contributors Conceiving and designing the study: RJT; obtaining funding and/or ethics approval: RJT, GP; collecting the data: LML, SNB, KMF, MEM; analyzing the data: LML, RJT, SNB; interpreting the data: LML, RJT, SNB; drafting of the manuscript: LML; critical revision of the manuscript for important intellectual content: RJT, SNB, KMF, MEM; technical, clinical, or material support: LML, SNB, KMF, MEM, GP.
Funding This research was supported by Merit Review from the Rehabilitation Research & Development Service of the U.S. Department of Veterans Affairs (grant no. B4451R).
Conflicts of interest None.
Ethics approval This study was approved by the Institutional Review Board of the Louis Stokes Cleveland Department of Veterans Affairs Medical Center.
Acknowledgments
We acknowledge the support of merit review grant B4451R from the VA Rehabilitation Research and Development Service and the many contributions of the research volunteer in this single-subject case study.
References
- 1.National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance [Internet]. Birmingham, AL: University of Alabama at Birmingham; 2013. Available from: http://www.nscisc.uab.edu
- 2.Brown-Triolo DL, Roach ML, Triolo RJ, Nelson K. Consumer perspectives on mobility: implications for neuroprosthesis design. J Rehabil Res Dev 2002;39(6):659–69. [PubMed] [Google Scholar]
- 3.Bajd T, Kralj A, Turk R, Benko H, Sega J. The use of a four-channel electrical stimulator as an ambulatory aid for paraplegic patients. Phys Ther 1983;63(7):1116–20. [DOI] [PubMed] [Google Scholar]
- 4.Kralj A, Bajd T, Turk R. Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin Orthop 1988;(233):34–43 [PubMed] [Google Scholar]
- 5.Isakov E, Mizrahi J, Majenson T. Biomechanical and physiological evaluation of FES-activated paraplegic patients. J Rehabil Res Dev 1986;23(3):9–19. [PubMed] [Google Scholar]
- 6.Kralj A, Bajd T, Turk R. Electrical stimulation providing functional use of paraplegic patient muscles. Med Prog Technol 1980;7(1):3–9. [PubMed] [Google Scholar]
- 7.Chaplin E. Functional neuromuscular stimulation for mobility in people with spinal cord injuries. The Parastep® I System. J Spinal Cord Med 1996;19(2):99–105. [PubMed] [Google Scholar]
- 8.Gallien P, Brissot R, Eyssette M, Tell L, Barat M, Wiart L, et al. . Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia 1995;33(11):660–4. [DOI] [PubMed] [Google Scholar]
- 9.Winchester P, Carollo JJ, Habasevich R. Physiologic cost of reciprocal gait in FES assisted walking. Paraplegia 1994;32(10):680–6. [DOI] [PubMed] [Google Scholar]
- 10.Petrofsky JS, Phillips CA. Closed-loop control of movement of skeletal muscle. Crit Rev Biomed Eng 1985;13(1):35–75. [PubMed] [Google Scholar]
- 11.Kralj A, Bajd T, Turk R, Krajnik J, Benko H. Gait restoration in paraplegic patients: a feasibility demonstration using multichannel surface electrode FES. J Rehabil R D 1983;20(1):3–20. [PubMed] [Google Scholar]
- 12.Kralj AR, Bajd T. Functional electrical stimulation: standing and walking after spinal cord injury. Boca Raton, FL: CRC Press Inc; 1989. p. 123–38. [Google Scholar]
- 13.Kilgore KL, Peckham PH, Keith MW, Thrope GB, Wuolle KS, Bryden AM, et al. . An implanted upper extremity neuroprosthesis: a five patient follow-up. J Bone Joint Surg Am 1997;79(4):533–41. [DOI] [PubMed] [Google Scholar]
- 14.Wilemon WK, Mooney V, McNeal DR, Reswick JB. Surgically implanted peripheral neuroelectric stimulation. Internal Report of Rancho Los Amigos Hospital, Downey, CA; 1970. [Google Scholar]
- 15.Brindley GS, Polkey CE, Rushton DN. Electrical splinting of the knee in paraplegia. Paraplegia 1979;16(4):428–37. [DOI] [PubMed] [Google Scholar]
- 16.Holle J, Frey M, Gruber H, Kern H, Stohr H, Thoma H. Functional electrostimulation of paraplegics: experimental investigations and first clinical experience with an implantable stimulation device. Orthopedics 1984;7(7):1145–55. [DOI] [PubMed] [Google Scholar]
- 17.Davis R, Eckhouse R, Patrick JF, Delehanty A. Computer-controlled 22-channel stimulator for limb movement. Acta Neurochir Suppl (Wien) 1987;39:117–20. [DOI] [PubMed] [Google Scholar]
- 18.Davis R, Houdayer T, Andrews B, Emmons A, Patrick J. Paraplegia: prolonged closed-loop standing with implanted nucleus FES-22 stimulator and Andrews’ foot-ankle orthosis. Stereotact Funct Neurosurg 1997;69(1–4 Pt 2):281–7. [DOI] [PubMed] [Google Scholar]
- 19.Rushton DN, Perkins TA, Donaldson N, Wood DE, Harper VJ, Tromans AM, et al. . LARSI: how to obtain favorable muscle contractions. Proceedings of the Second Annual IFESS Conference (IFESS '97) and Neural Prosthesis: Motor Systems 5 (NP '97); Burnaby, BC, Canada; 16–21 Aug, 1997. p. 163–4. [Google Scholar]
- 20.Kobetic R, Triolo RJ, Uhlir J, Bieri C, Wibowo M, Polando G, et al. . Implanted functional electrical stimulation system for mobility in paraplegia: a follow-up case report. IEEE Trans Rehabil Eng 1999;7(4):390–8. [DOI] [PubMed] [Google Scholar]
- 21.Davis JA, Triolo RJ, Uhlir JP, Bhadra N, Lissy DA, Nandurkar S, et al. . Surgical technique for installing an eight channel neuroprosthesis for standing. Clin Orthop 2001 Apr;(385):237–52. [DOI] [PubMed] [Google Scholar]
- 22.Johnston TE, Betz RR, Smith BT, Mulcahey MJ. Implanted functional electrical stimulation: an alternative for standing and walking in pediatric spinal cord injury. Spinal Cord 2003;41(3):144–52. [DOI] [PubMed] [Google Scholar]
- 23.Triolo RJ, Bailey SN, Miller ME, Rohde L, Anderson J, Davis JA, et al. . Longitudinal performance of a surgically implanted neuroprosthesis for lower extremity exercise, standing, and transfers after spinal cord injury. Arch Phys Med Rehabil 2012;93(5):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardin E, Kobetic R, Murray L, Corado-Ahmed M, Pinault G, Sakai J, et al. . Walking after incomplete spinal cord injury using an implanted FES system: a case report. J Rehabil Res Dev 2007;44(3):333–46. [DOI] [PubMed] [Google Scholar]
- 25.Bailey SN, Hardin EC, Kobetic R, Boggs LM, Pinault G, Triolo RJ. Neurotherapeutic and neuroprosthetic effects of implanted electrical stimulation for ambulation after incomplete spinal cord injury. J Rehabil Res Dev 2010;47(1):7–16. [DOI] [PubMed] [Google Scholar]
- 26.Potter JM, Evans AL, Duncan G. Gait speed and activities of daily living function in geriatric patients. Arch Phys Med Rehabil 1995;76(11):997–9. [DOI] [PubMed] [Google Scholar]
- 27.Stanic U, Acimovic-Janezic R, Gros N, Trnkocky A, Bajd T, Kljajic M. Multichannel electrical stimulation for correction of hemiplegic gait. Scand J Rehabil Med 1977;10(25):175–92. [PubMed] [Google Scholar]
- 28.Byrne CA, O'Keeffe DT, Donnelly AE, Lyons GM. Effect of walking speed changes on tibialis anterior EMG during healthy gait for FES envelope design in foot drop correction. J Electromyogr Kinesiol 2007;17(5):605–16. [DOI] [PubMed] [Google Scholar]
- 29.Chen WL, Chen SC, Chen CC, Chour CH, Shih YY, Chen YL, et al. . Patient-driven loop control for ambulation functional restoration in non-invasive functional electrical stimulation system. Disabil Rehabil 2010;32(1):65–71. [DOI] [PubMed] [Google Scholar]
- 30.Dutta A, Khattar B, Banerjee A. Nonlinear analysis of electromyogram following gait training with myoelectrically triggered neuromuscular electrical stimulation in stroke survivors. EURASIP J Adv Signal Process 2012;2012(153):1–8. [Google Scholar]
- 31.Kordjazi N, Kobravi HR. Control of tibialis anterior FES envelope for unilateral drop foot gait correction using NARX neural network. 34th Annual International Conference of the IEEE EMBS; California; 2012 p. 1880–3. [DOI] [PubMed] [Google Scholar]
- 32.Coiro D, Smith B, Betz R. Gait event detection using intramuscular electromyography to trigger functional electrical stimulation in the child with cerebral palsy. 6th Annual Conference of International Functional Electrical Stimulation Society; Cleveland, OH; 2001. [Google Scholar]
- 33.Dutta A, Kobetic R, Triolo R. Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Trans Biomed Eng 2008;55(2):791–4. [DOI] [PubMed] [Google Scholar]
- 34.Dutta A, Kobetic R, Triolo R. Gait initiation with electromyographically triggered electrical stimulation in people with partial paralysis. J Biomech Eng 2009;131(8):1–9. [DOI] [PubMed] [Google Scholar]
- 35.Smith B, Tang M, Johnson W, Pourmehdi S, Gazdik MM, Buckett JR, et al. . An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle. IEEE Trans Biomed Eng 1998;45(4):463–75. [DOI] [PubMed] [Google Scholar]
- 36.Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Biomed Eng 1994;2(2):80–91. [Google Scholar]
- 37.Hart RL, Bhadra N, Montague FW, Kilgore KL, Peckham PH. Design and testing of an advanced implantable neuroprosthesis with myoelectric control. IEEE Trans Neural Syst Rehabil Eng 2011;19(1):45–53. [DOI] [PubMed] [Google Scholar]
- 38.Kobetic R, Marsolais EB. Synthesis of paraplegic gait with multichannel functional neuromuscular stimulation. IEEE Trans Rehabil Eng 1994;2(2):66–79. [Google Scholar]
- 39.Kobetic R, Triolo RJ, Marsolais EB. Muscle selection and walking performance of multichannel FES systems for ambulation in paraplegia. IEEE Trans Rehabil Eng 1997;5(1):23–9. [DOI] [PubMed] [Google Scholar]
- 40.Dittmer S, Gresham G. Functional assessment and outcome measures for the rehabilitation health professional. Gaithersburg, MD: Aspen Publishers; 1997. [Google Scholar]
- 41.Saraf P, Rafferty MR, Moore JL, Kahn JH, Hendron K, Leech K, et al. . Daily stepping in individuals with motor incomplete spinal cord injury. Phys Ther 2010;90(2):224–35. [DOI] [PubMed] [Google Scholar]
- 42.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26(6):982–9. [DOI] [PubMed] [Google Scholar]
- 43.Rehabilitation Measures Database [Internet]. Chicago: Rehabilitation Institute of Chicago Center for Rehabilitation Outcomes Research Northwestern University Feinberg School of Medicine Department of Medical Social Sciences Informatics group; 2010. Available from: http://www.rehabmeasures.org