Abstract
Context
Inflammation after spinal cord injury (SCI) may be responsible for further neural damages and therefore inhibition of inflammatory processes may exert a neuroprotection effect.
Objectives
To assess the efficacy of some non-conventional herbal medications including sulforaphane, tanshinone IIA, and tetramethylpyrazine in reducing inflammation and compare them with a known effective anti-inflammatory agent (interleukin-10 (IL-10)).
Methods
We searched relevant articles in Ovid database, Medline (PubMed) EMBASE, Google Scholar, Cochrane, and Scopus up to June 2013. The efficacy of each treatment and study powers were compared using random effects model of meta-analysis. To our knowledge, no conflict of interest exists.
Results
Eighteen articles entered into the study. The meta-analysis revealed that exogenous IL-10 was more effective in comparison with the mentioned herbal extracts. The proposed pathways for each medication's effect on reducing the inflammation process are complex and many overlaps may exist.
Conclusion
IL-10 has a strong effect in the induction of neuroprotection and neurorecovery after SCI by multiple pathways. Tetramethylpyrazine has an acceptable influence in reducing inflammation through the up-regulation of IL-10. Outcomes of sulforaphane and tanshinone IIA administration are acceptable but still weaker than IL-10.
Keywords: Cytokines, Spinal cord, Pharmacology, Neuroinflammation
Introduction
Neurological improvement in spinal cord injury (SCI) has always been a challenge. The cascade of inflammatory processes that occur after injury has been shown to play an important role in inducing further nerve damage.1,2 Many treatment strategies have focused on inhibiting these inflammatory processes and some involved pathophysiological pathways have been described so far.3 After traumatic SCI, morphological changes in tissues may occur due to hemorrhage and edema and is followed by secondary damages by activations of inflammatory responses.4,5 By considering the important role of inflammation in the induction of neural injuries, anti-inflammatory medications have been proposed to have beneficial effects.6 Major pro-inflammatory cytokines that are detected widely at the site of lesion are tumor necrosis factor-a (TNF-α), interleukin-1b (IL-1b), and IL-67 and the most effective medications function by targeting these agents. Moreover, the deficiency of some factors such as IL-10 has been shown to enhance inflammatory response.8 So the hypothesis of administration of exogenous IL-10 was proposed to induce neuroprotection in SCI through its anti-inflammatory effects.9 Previously, Kwon et al.10 summarized the neuroprotective effect of many administered medications including erythropoietin, non-steroid anti-inflammatory drugs (NSAIDs), antiCD11d antibodies, minocycline, progesterone, estrogen, magnesium, riluzole, polyethylene glycol, atorvastatin, inosine, and pioglitazone in the animal models. Although this study did not compare the efficacy of these agents, it illustrated a valuable context of medications and their mechanisms in inducing neuroprotection which is an essential and vital achievement after SCI. Here, we assessed the efficacy of some new herbal extracts that have been used in pre-clinical investigations on animal models and have shown promising outcomes. In this field, meta-analysis can perform a proper comparison between these medications and assess the power of each study. Although there are noticeable variations in experimental designs and applied methods in evaluating medications’ efficacy, by using random model effects, comparison of heterogeneous studies is possible. In the present study, we tried to compare the effect of some non-conventional medications which are shown to have anti-inflammatory influence and may induce neuroprotection and neurorecovery effects after SCI. The effects of IL-10, tanshinone IIA (TIIA; an important lipophilic diterpene extracted from Salvia miltiorrhiza), tetramethylpyrazine (TMP; a pure compound derived from a Chinese herb called Ligusticum chuanxiong), and sulforaphane (a potent anti-inflammatory extract of cruciferous vegetables) were compared. IL-10 is a known effective anti-inflammatory agent which blocks T-cells’ functions. Our purpose was to compare the anti-inflammatory efficacy of the mentioned herbal extracts with a known effective agent (IL-10) in pre-clinical investigations. By considering the fact that the patients in acute phase of SCI need the most effective anti-inflammatory medication in a gold time interval, it becomes necessary to provide evidences in introducing the most effective agent so that the pharmaceutical processes are focused on developing the most appropriate medication.
The induction of neuroprotection is mediated through many complex pathways that involve antioxidative, antiapoptotic, and anti-inflammatory processes. Along with comparing the neuroprotective effect of sulforaphane, TIIA, and TMP with a known effective agent (IL-10), here we also summarized the proposed mechanism of each medication.
Methods
This paper is a systematic review using meta-analysis to determine the difference between some medications’ effect in the induction of neuroprotection after SCI in pre-clinical studies. We like to mention that no individuals and organizations beyond the mentioned authors and affiliations have contributed in the analysis and writing this manuscript. The searching process of relevant articles was performed by using keywords of “IL-10”, “interleukin-10”, “tanshinone IIA”, “tetramethylpyrazine”, “sulforaphane”, and “spinal cord injury” in various databases including Medline (Pubmed), EMBASE, Google Scholar, Ovid, Cochrane, and Scopus. The references of retrieved articles were also scanned to detect any relevant articles. All potential relevant articles published up to July 2013 were reviewed. The searching process was conducted by two different reviewers separately and no language limitation was applied.
Study selection
Inclusion criteria consisted of: exogenous administration of IL-10, TIIA, TMP, and sulforaphane, studies on animal subjects with SCI in acute phase, assessment of the neurological recovery, and existence of a control group for proper comparison. Exclusion criteria were: evaluation of endogenous changes of IL-10 (and not exogenous administration), lack of comparable data, and evaluating other medication effects other than neurological assessment.
In this systematic review, only studies on animal models were selected. Studies on patients with SCI are mostly in chronic phase of SCI. SCI is usually accompanied with multiple trauma and these patients experience critical conditions usually in intensive care unit. Because of vast variability in degree of injuries and critical conditions of these patients, studies on acute phase of SCI in human face difficulties (whether ethical complications or complexity in study designs as it is difficult to define two matched groups because of broad diversities in severity of injuries and clinical conditions among these patients). When we take a look at the literatures evaluating the efficacy of new drugs, barely, we observe a study on human models in acute SCI. The majority of the literatures are performed on the animal models and as we know, the existence of adequate literatures is essential in running a meta-analysis. Moreover, by considering the peak level of inflammation in acute phase, it is expected that these medications show their maximum efficacy at this phase but studies on human models mostly involve patients in chronic phase in which the coincidental complications are controlled and patients experience a stable phase.
Study selection was also performed by two independent reviewers and the rate of agreement was described with Cohen kappa. Basic information which was drawn from literatures included: the type of the study subject, existence of the control group, the type of used medications, a brief explanation of methods, P-values which indicated significant difference between the case and the control groups, the method of neurological assessment, and major findings.
Statistical analysis
All the analysis was performed by using PASW Statistics for Windows version 18 (SPSS, Inc., Chicago, IL, USA) and Comparative Meta-analysis version 2 (Biostat, Englewood, NJ, USA). Statistical homogeneity was checked by χ2 test and I2 using Cochran heterogeneity statistic as Q.11 As I2 was higher than 75%, random effects model was used to calculate the weighted mean difference and 95% confidence interval (CI). The effect size was determined by using comparison of P-values and identifying study powers which was expressed as z score. By considering the various experimental designs and different assessment methods in each study, we used random effects model. A random effects model involves an assumption that the effects being estimated in the different studies are not identical, but follow some distribution. However, in this process, the establishment of validity is difficult which is a common criticism of random effects model in the meta-analyses but still simulations have shown that this method is reliable even under extreme distributional assumptions in estimating the heterogeneity.12 When the procedures of assessed investigations are different, the proper comparison is possible only when the control group in each study exists. The medications which have induced more significant difference between the treatment and the control groups (with a more significant P-value) are considered as “more effective”, regardless of applied evaluation and measurement methods.
Results
Among literatures indicating effects of IL-10, seven articles illustrated the neurological improvement after SCI. While some other investigations were close to our purpose, they were removed from the analysis as they met our exclusion criteria. The most important excluded literatures were Milligan et al. studies13–15 which mostly illustrated the anti-inflammatory effect of IL-10 in subjects without SCI. It is noticeable that the dosage and the method of IL-10 administration were vastly different between these studies as some of them used insertion of genetic vectors to release IL-10 while some others used direct intra-spinal or intravenous injections. The basic characteristics of the involved studies are summarized in Table 1. Three studies on sulforaphane, two studies on TIIA, and six literatures on TMP were selected based on the inclusion and exclusion criteria and were entered into analysis. Total of 18 articles went through the process of meta-analysis and the Cohen kappa coefficient was acceptable (0.8).
Table 1 .
Basic characteristics of involved articles
| Study | Medication | Dosage | Subjects | Subjects (no.) | Control | Assessments |
|---|---|---|---|---|---|---|
| Zhou et al.16 | IL-10 | 2 µl of vector | Female Sprague–Dawley rats | 10 | Yes | Basso–Beattie–Bresnahan (BBB) motor rating scale17 |
| Oruckaptan et al.18 | IL-10 | 100 mg/kg | Single-strain female Albino rats | 32 | Yes | 6 point lower extremity walking scale19 |
| Jackson et al.20 | IL-10 | 200 pg/ml | Adult mice | 20 | Yes | Basso–Beattie–Bresnahan (BBB) motor rating scale |
| Brewer et al.21 | IL-10 | 5 µg | Female Sprague–Dawley rats | 24 | Yes | Histological assessment of tissue inflammation |
| Pearse et al.22 | IL-10 | 30 mg/kg | Adult rats | Yes | Basso–Beattie–Bresnahan (BBB) motor rating scale | |
| Takami et al.23 | IL-10 | 15–30 µg/kg | Adult Fischer rats | Yes | Basso–Beattie–Bresnahan (BBB) motor rating scale | |
| Bethea et al.24 | IL-10 | 5 µg/kg | Adult rats | 18 | Yes | Basso–Beattie–Bresnahan (BBB) |
| Mao et al.25 | Sulforaphane | 5 mg/kg | Male ICR rats | 96 | Yes | Measurement of TNF-α and MMP-9 by ELISA |
| Miller et al.26 | Sulforaphane | 5 mg/kg | Young CF-1 mice | Yes | Measurement of Nrf2-ARE-activitating compounds gene expression | |
| Mao et al.27 | Sulforaphane | 5 mg/kg | Wild-type mice | 288 | Yes | Neurological function assessment by Basso open-field motor score (BMS)28 |
| Yin et al.29 | TIIA | 50 mg/kg | Sprague–Dawley male adult rats | 16 | Yes | Basso–Beattie–Bresnahan (BBB) motor rating scale |
| Zhang et al.30 | TIIA | New Zealand rabbits | 54 | Yes | Assessment of expression of NF-κB and VCAM-1 genes | |
| Fan et al.31 | TMP | 30 mg/kg | Male Sprague–Dawley rats | 90 | Yes | Basso–Beattie–Bresnahan (BBB) motor rating scale |
| Liang et al.32 | TMP | 50 mg/kg | Male Sprague–Dawley rats | 24 | Yes | Neurological function by Tarlov criteria33 |
| Fan et al.34 | TMP | 30 mg/kg | Adult male New Zealand white rabbits | 36 | Yes | Neurological function by Johnson's score35 |
| Chen et al.36 | TMP | 30 mg/kg | Male New Zealand white rabbits | 45 | Yes | Neurological function by Tarlov criteria |
| Xiao et al.37 | TMP | SD rats | 110 | Yes | Modified Rivilin loxotic plate degree | |
| Basso–Beattie–Bresnahan (BBB) motor rating scale | ||||||
| Combined behavioral score (CBS) | ||||||
| Shen et al.38 | TMP | Adult Sprague–Dawley rats | 80 | Yes | Modified Rivilin loxotic plate degree | |
| Basso–Beattie–Bresnahan (BBB) motor rating scale |
All these articles used animal models and tried to find the potential efficacies of tested medication. Major findings of these articles which present the most important mechanisms of anti-inflammatory effects of these medications are summarized in Table 2. It is noticeable that different pathways have been proposed for the efficacy of each medication. In fact, the final influence of the drug is mediated through complex pathways. However, the comparison of given P-values which indicate the strength of association between two factors give us the possibility to compare these pathways to show which one is more powerful and effective in inducing neuroprotection.
Table 2 .
Major findings in the selected literatures
| Study | Year of publication | Medication | P-value | Major findings |
|---|---|---|---|---|
| Zhou et al.16 | 2009 | IL-10 | <0.05 | Improved motor function, increased expression of Bcl-2 and Bcl-xL |
| Inhibition of cytochrome c release and caspase 3 cleavage | ||||
| Oruckaptan et al.18 | 2009 | IL-10 | <0.026 | Decreased lipid peroxidation and myeloperoxidase activity, attenuation of the early ischemic response, and restriction of the tissue damage |
| Jackson et al.20 | 2005 | IL-10 | <0.05 | Greater functional recovery in the first 24 hours after injury |
| Brewer et al.21 | 1999 | IL-10 | – | Significantly decreased lesion volume, reduction in neuronal damage |
| Pearse et al.22 | 2004 | IL-10 | – | Improved gross locomotor performance, worsened behavioral outcome |
| Takami et al.23 | 2002 | IL-10 | – | Improved gross locomotor performance, increased the volume of spared spinal gray matter 3 months after a moderate contusion |
| Bethea et al.24 | 1999 | IL-10 | – | Reduction of lesion volume by ∼49% |
| Mao et al.25 | 2010 | Sulforaphane | <0.01 | Lower expression and activity of MMP and decreased tumor necrosis factor-α |
| Miller et al.26 | 2013 | Sulforaphane | <0.05 | Increased expression of hemeoxygenase-1mRNA, attenuation of (4-hydroxy-2-Nonenal) 4-HNE-induced inhibition of mitochondrial respiration for complex I |
| Mao et al.27 | 2011 | Sulforaphane | <0.01 | Activation of Nrf2, improved hind limb locomotor function assessed by BMS, reduced inflammatory damage, histologic injury, dying neurons count, and spinal cord edema |
| Yin et al.29 | 2012 | TIIA | <0.05 | Improved motor function, reduced tissue injury (histological score), reduced myeloperoxidase activity, inhibition of NF-κB and MAPK signaling pathways, decreased production of pro-inflammatory cytokines (TNF-α, IL-1b, and IL-6) |
| Zhang et al.30 | 2012 | TIIA | <0.01 | Reduced expression of NF-κB and VCAM-1 |
| Fan et al.31 | 2011 | TMP | <0.05 | Significant improved neurological outcome, decreased infarct volume, alleviated neutrophil infiltration |
| Liang et al.32 | 2011 | TMP | <0.05 | Suppressed glutamate level, suppressed the expression of mGluR-1 mRNA, better neurological function |
| Fan et al.34,39 | 2006 | TMP | <0.05 | Significantly better neurological outcomes, decreased spinal cord malondialdehyde levels, reduced the loss of motoneurons, and reduced apoptotic cell death through Bcl-2 up-regulation parallel to Bax down-regulation |
| Chen et al.36 | 2002 | TMP | <0.05 | Better neurological status and histopathology, significantly reduced neurological injury |
| Xiao et al.37 | 2012 | TMP | <0.05 | Significantly higher BBB score, significantly low number of MIF-positive cells |
| Shen et al.38 | 2008 | TMP | <0.05 | Reduced expression of caspase-3 and increased expression of neurofilament protein (NF-L, NF-H, and NF-M) |
ELISA, enzyme-linked immunosorbent assay; MIF, macrophage migration inhibitory factor; MMP-9, metalloproteinase-9; Nrf2-ARE, NF-E2-related factor 2-antioxidant-response element; TNF-α, tumor necrosis factor-α; BMS, Basso open-field motor score.
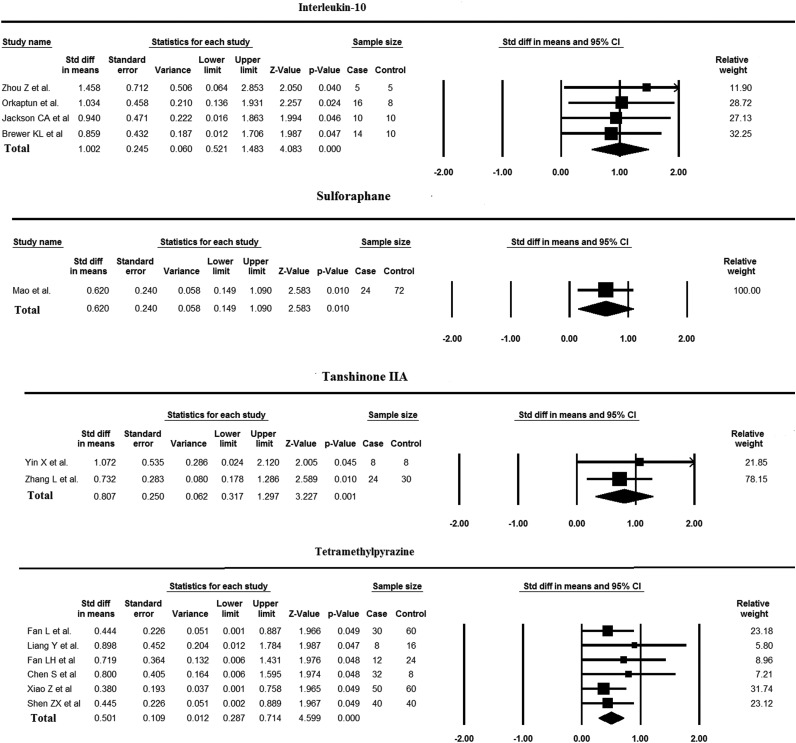
Standard deviation differences (SDD) in means of the case and the control groups in each study with CI of 95% are shown in Fig. 1. The total results in four studies on IL-10 showed a SDD of 1.002 (0.521–1.483, z-value: 4.083), which was higher in comparison with other tested medications. Studies on sulforaphane in SCI were so limited or did not contain adequate comparative data and consequently our analysis on sulforaphane was limited to Mao et al. investigation (SDD of 0.620 (0.149–1.090, z-value: 2.58)). Because of the lack of available comparative literatures on sulforaphane efficacy in SCI, these results should be interpreted cautiously. TIIA showed a SDD in means of 0.807 (0.317–1.297, z-value: 3.227) and TMP had SDD of 0.501 (0.287–0.714, z-value: 4.59). The comparison of all these medications shows that these herbal extracts have acceptable efficacy in inducing neuroprotection or neurorecovery after SCI (P-values: 0.010, 0.001, and <0.0001 for sulforaphane, TIIA, and TMP, respectively) but still their influence is relatively weaker than IL-10 (Fig. 1).
Figure 1 .
Meta-analysis of several medications’ efficacy including IL-10, sulforaphane, TIIA, and TMP on inflammation reduction after SCI. Five studies were excluded due to the lack of comparative data.
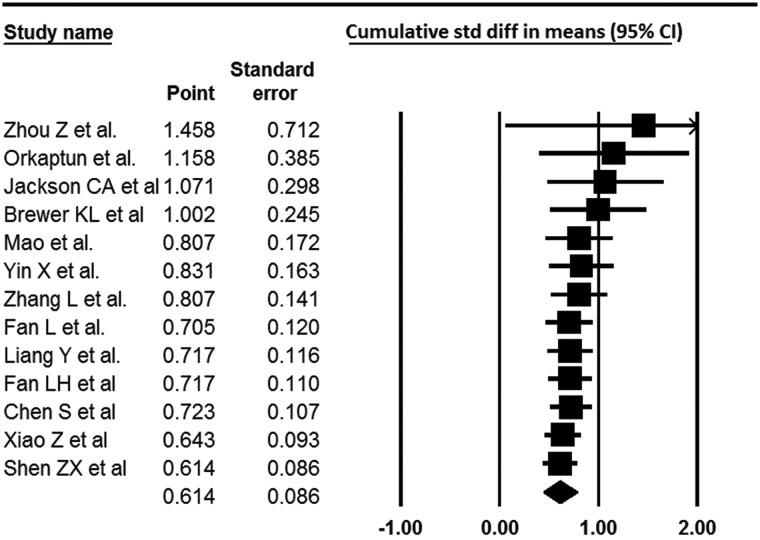
Figure 2 .
Cumulative study of 13 studies; 5 studies were excluded due to the lack of comparative data.
Various mechanisms have been shown to play a role in the reduction of inflammation. When considering the combined pathways, the interconnections are so complex. This meta-analysis shows that administration of IL-10 was the most powerful anti-inflammatory agent than other tested drugs (Fig. 1). Both methods of releasing IL-10 (inserting genetic vectors or direct intra-spinal/intravenous injection) induced neuroprotection more effectively in comparison with sulforaphane, TIIA, and TMP.
The results over TMP efficacy are controversial, while a very strong power in inflammation reduction was reported by Liang et al., Fan LH et al., and Chen et al. (Fig. 1), the lower strength of its effect in Fan L et al., Xio et al., and Shen et al. studies, led to a moderate total efficacy (SDD in means: 0.501). However, it is noticeable that mechanisms which were evaluated in these literatures were different which shows that TMP efficacy is more powerful in some specific pathways.
Discussion
IL-10 is a powerful agent that can block T-cell function via antigen-presenting cells.40 Excessive inflammation damages that occur after SCI can be attenuated by proper interventions through inhibition of pro-inflammatory and cytotoxic release. The time interval of initiating these anti-inflammatory medications is also important and it has been shown that cellular events that occur right after SCI are regulated by the expression of pro-inflammatory cytokines including TNF-α and IL-1β at the site of injury.41 While the absence of IL-10 invigorates neural damage by increasing the expression of iNOS,8,42 its exogenous consumption leads to significant neurological improvement.16,18,20–22 The mechanisms of IL-10 efficacy have been well-described up to now. Therefore, IL-10 is a known effective agent in reducing inflammation and subsequently inducing neuroprotection. For instance, TNF-α, which is inhibited by IL-10, is one of the most important agents that induces oligodendrocytic apoptosis;43 so if IL-10 is initiated immediately after SCI, it can prevent further neural damages. Moreover, its antiapoptotic effect has been shown not only in spinal cord neurons, but also in cerebellar granule cells44 and ganglion cell line.45 Many mechanisms have been proposed for this antiapoptotic effect. Zhou et al.16 described that apoptotic cascade is inhibited by IL-10 through cytochrome c release and caspase 3 cleavage along with an increased expression of Bcl-2 and Bcl-xL. Up-regulation of caspase 3 expression along with the activation of caspase 9 were the proposed apoptotic pathways46,47 which are shown to be efficiently inhibited by IL-10. Clinical investigations did not show any differences in IL-10 level between the patients with SCI and the able-bodied patients while the increased concentration of pro-inflammatory cytokines was observed in the SCI group48 which shows that despite the neuroprotective effect of IL-10, it is not autonomously released at injury site itself in human bodies and is not in line with animal models of reperfusion injuries.49,50 By considering the vast majority of literatures showing that IL-10 decreases the infarct size in ischemic damages,51 it seems that not only the anti-inflammatory effect but also other mechanisms exist that mediate the influence of IL-10 on ischemic/reperfusion tissue damages. In this regard, Oruckaptan et al.18 showed decreased lipid peroxidation and myeloperoxidase activity which was mediated by IL-10.
It is shown that IL-10 leads to the inhibition of cytochrome c release and caspase 3 cleavages while it increases the expression of Bcl-2 and Bcl-xL. Meanwhile, it can also decrease the lipid peroxidation and myeloperoxidase activity. It seems that these pathways are the most powerful and effective pathways of inflammatory reduction. Moreover, worsening of behavioral scores was only reported by treatment with IL-10.
Recently, some new herbal medications (sulforaphane, TIIA, and TMP) have been proposed to be effective in reducing inflammation and inhibiting neural damage. Here, we compared IL-10 efficacy in neurological improvement and anti-inflammatory strength with sulforaphane, TIIA, and TMP. Our results show that although these herbal extracts have acceptable efficacy, their influence is still weaker than IL-10. However, it is considerable that worsening of the behavioral outcome was only reported by IL-10 administration.22 Although this complication was reported in experimental animal models, its clinical administration must be exerted cautiously.
By considering the fact that the inhibition of inflammation results in preventing further neural damage, the administration of some conventional medications such as NSAIDs has been proposed so far. In this regard, Kopp et al.52 showed that ibuprofen which is a Food and Drug Administration-approved NSAID can enhance axonal regeneration by inhibiting the small GTPase RhoA molecule. Furthermore, Kwon et al.10 has expressed the efficacy of various medications including erythropoietin, NSAIDs, antiCD11d antibodies, minocycline, progesterone, estrogen, magnesium, riluzole, polyethylene glycol, atorvastatin, inosine, and pioglitazone as neuroprotective treatments for acute SCI in a systematic review on animal models. Although Kwon did not present any comparison among these medications, the study is considerable as it summarizes the available treatments in inhibiting neural damage after SCI. Here, we tried to assess other new medications. Our vast searches showed the mentioned herbal drugs as potential extracts that are trying to enter into pharmaceutical process. Hence, it was essential to know if these drugs can influence as effective as previous known anti-inflammatory agents (such as IL-10). Besides, we decided not to compare the efficacy of these extracts with conventionally used steroids. The usage of steroids after SCI is not only indicated because of its anti-inflammatory effect, but mostly because of adrenal shock and extremely low blood pressure to maintain circulation. Due to vast indications of steroid administrations after SCI, we did not perform any comparison of the mentioned medications with steroids and we tried to focus only on neuroprotection and anti-inflammatory aspects of some specific drugs.
Sulforaphane (1-isothiocyanato-4-methylsulfinyl-butane), which is a member of isothiocyanate family and is derived from cruciferous vegetables, have been shown to exert anti-inflammatory activities.53,54 The mechanism was first reported in leukemic cell where it was observed that sulforaphane inhibited TNF-α-induced nuclear factor-κB (NF-κB) activation in pathways involving metalloproteinase-9 (MMP-9).55 Mao et al.25 investigated this effect in individuals with SCI and showed lower expression and activity of MMP-9. MMPs are known to mediate the degradation of gelatin (denatured collagens), collagen IV, V, and XI, and myelin basic protein.56,57 It has been reported that MMP-9 production can be induced by TNF-α58,59 and therefore inhibition of MMP-9 may block one of the activity pathways of TNF-α. Moreover, TNF-α and MMP-9 are up-regulated during SCI60 and although their inhibition may exert beneficial influences, our meta-analysis shows that this effect is weaker than IL-10 efficacy. It is noticeable that sulforaphane acts as an anti-inflammatory agent by activating NF-erythroid 2-related factor 2 (Nrf2)27,61 and thus it protects cells from oxidative damage by the induction of detoxification enzymes.62 In conclusion, it seems that administration of sulforaphane in SCI has beneficial effects but other additive medications may be required to invigorate the anti-inflammatory effect. Unfortunately, we could not detect any investigations on combined therapies of sulforaphane and thus we cannot conclude a proper comparison with multidrug regimens efficacy.
TIIA is a lipophilic diterpene extracted from S. miltiorrhiza and is used in Chinese traditional herbal medicine. Literatures have shown not only its anti-inflammatory effects63,64 but also its antiapoptotic65 and antioxidant66 activities. Yin et al.29 showed that TIIA leads to improved motor function, reduced tissue injury (histological score), reduced myeloperoxidase activity, and inhibition of NF-κB and MAPK signaling pathways in SCI. TIIA has been shown to have antitoxicity activity and its potential effect in preventing cancer has been described in human;67,68 however, no clinical investigation on patients with SCI was detected so it is considerable that most of side effects of this medication are not yet known and more citations are needed to recommend this drug in clinical practices in spinal cord injured individuals.
TMP is the main ingredient of a Chinese herb called Ligusticum wallichii Franchat (Chuan Xiong) which has been shown to protect tissues from ischemic/reperfusion injuries.69,70 One of the involved pathways in reducing apoptosis is exerted through the regulation of Bcl-2 and Bax expression.39 Other proposed mechanisms are: down-regulation of nitric oxide production,64 blockage of H2O2-induced apoptosis,39 increased transcription of thioredoxin,70 suppression of glutamate level,32 inhibition of NF-κB activation in the ischemic tissue,32 decreased spinal cord malondialdehyde levels,34 reduced apoptotic cell death through Bcl-2 up-regulation,34 and scavenging oxygen free radicals.71 All these mechanisms provide a strong neuroprotection benefit of using this medication. Although its effect in investigations by Fan L et al., Xio Z et al., and Shen et al. was statistically significant, it was weaker than IL-10 efficacy. Moreover, it seems that TMP is more effective in SCIs with the underlying mechanism of ischemic/reperfusion damage. In fact, the mechanism of trauma influences on the outcomes of administration of this medication. Plus, it is known that some biochemical pathways are common as it has been reported that TMP up-regulates endogenous IL-10 and both act as inhibitors of NF-κB. It has also been demonstrated that TMP scavenges superoxide anion dose-dependently, decreases the production of nitric oxide in human leukocytes72 and its antiplatelet activity in human has been described as well73; however, it seems that although TMP is an effective agent with multiple functions on different human cells, its neuroprotective effect is still weaker than IL-10.
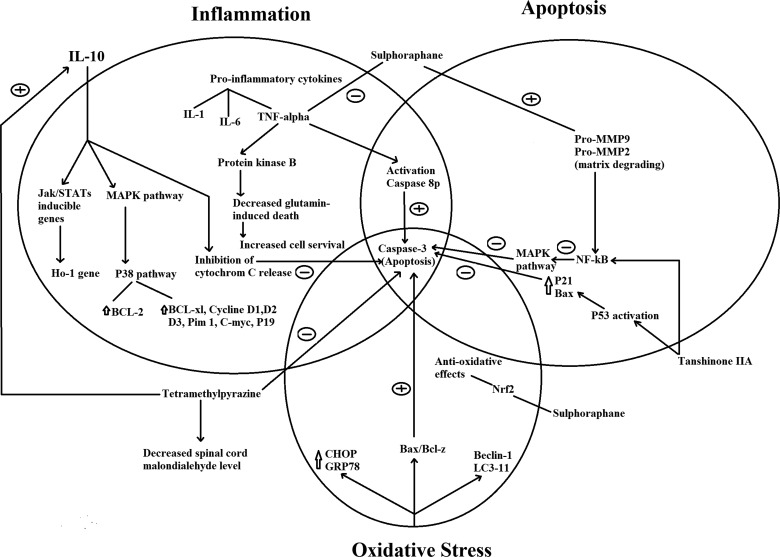
There are complex pathways among inflammatory, apoptotic, and oxidative pathways. It seems that all these medications (IL-10, sulforaphane, TIIA, and TMP) inhibit caspase 3 and subsequently prevent cell apoptosis (Fig. 3). Although the mechanisms of these medications’ functions are different, it seems that caspase 3 is the common agent among their pathways. Fig. 3 shows that caspase 3 (which is an apoptotic agent) is inhibited by all these drugs. Although these medications have different efficacy and power, some parts of their function may overlap with each other through inhibition of caspase 3.
Figure 3 .
Proposed pathways in which effects of IL-10, sulforaphane, TIIA, and TMP are mediated through.
Conclusion
IL-10 has a strong effect in the induction of neuroprotection and neurorecovery after SCI by multiple pathways. TMP has an acceptable influence on inflammation reduction through up-regulation of IL-10. The results on sulforaphane and TIIA are acceptable but still weaker than IL-10.
Disclaimer statements
Contributors DK contributed in study selection process, data drafting from the literatures and analyzing the data. SL contributed in drafting data from manuscript, analyzing and writing the manuscript. ANJ contributed in editing the paper. MM contributed in writing and editing the manuscript.
Funding None.
Conflicts of interest None.
Ethics approval None.
References
- 1.Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and spinal cord injury: infiltrating leukocytes as determinants of injury and repair processes. Clin Neurosci Res 2006;6(5):283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 2008;209(2):378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 2004;4(4):451–64. [DOI] [PubMed] [Google Scholar]
- 4.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med 1993;11Suppl 1:13–22. [PubMed] [Google Scholar]
- 5.Taoka Y, Okajima K. Role of leukocytes in spinal cord injury in rats. J Neurotrauma 2000;17(3):219–29. [DOI] [PubMed] [Google Scholar]
- 6.Tator CH, Hashimoto R, Raich A, Norvell D, Fehlings MG, Harrop JS, et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury. J Neurosurg Spine 2012;17(1 Suppl):157–229. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J 1990;4(11):2860–7. [PubMed] [Google Scholar]
- 8.Genovese T, Esposito E, Mazzon E, Di Paola R, Caminiti R, Bramanti P, et al. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J Neurochem 2009;108(6):1360–72. [DOI] [PubMed] [Google Scholar]
- 9.Howard M, O'Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol 1992;12(4):239–47. [DOI] [PubMed] [Google Scholar]
- 10.Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma 2011;28(8):1545–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One 2013;8(7):e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol 2006;2(4):293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005;21(8):2136–48. [DOI] [PubMed] [Google Scholar]
- 15.Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, et al. , Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 2006;126(1–3):294–308. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol 2009;220(1):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12(1):1–21. [DOI] [PubMed] [Google Scholar]
- 18.Oruckaptan HH, Ozisik P, Atilla P, Tuncel M, Kilinc K, Geyik PO, et al. Systemic administration of interleukin-10 attenuates early ischemic response following spinal cord ischemia reperfusion injury in rats. J Surg Res 2009;155(2):345–56. [DOI] [PubMed] [Google Scholar]
- 19.Kanellopoulos GK, Kato H, Hsu CY, Kouchoukos NT. Spinal cord ischemic injury: development of a new model in the rat. Stroke 1997;28(12):2532. [DOI] [PubMed] [Google Scholar]
- 20.Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology 2005;336(2):173–83. [DOI] [PubMed] [Google Scholar]
- 21.Brewer KL, Bethea JR, Yezierski RP. Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp Neurol 1999;159(2):484–93. [DOI] [PubMed] [Google Scholar]
- 22.Pearse DD, Marcillo AE, Oudega M, Lynch MP, Wood PM, Bunge MB. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J Neurotrauma 2004;21(9):1223–39. [DOI] [PubMed] [Google Scholar]
- 23.Takami T, Oudega M, Bethea JR, Wood PM, Kleitman N, Bunge MB. Methylprednisolone and interleukin-10 reduce gray matter damage in the contused Fischer rat thoracic spinal cord but do not improve functional outcome. J Neurotrauma 2002;19(5):653–66. [DOI] [PubMed] [Google Scholar]
- 24.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma 1999;16:851–63. [DOI] [PubMed] [Google Scholar]
- 25.Mao L, Wang HD, Wang XL, Qiao L, Yin HX. Sulforaphane attenuates matrix metalloproteinase-9 expression following spinal cord injury in mice. Ann Clin Lab Sci 2010;40(4):354–60. [PubMed] [Google Scholar]
- 26.Miller DM, Singh IN, Wang JA, Hall ED. Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic Biol Med 2013;57(10):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao L, Wang H, Wang X, Liao H, Zhao X. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J Surg Res 2011;170(1):e105–15. [DOI] [PubMed] [Google Scholar]
- 28.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006;23(5):635–59. [DOI] [PubMed] [Google Scholar]
- 29.Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, et al. Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One 2012;7(6):e38381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, An GY, Zhang WG, Chen K. Effects of tanshinone-II A sulfonate on expression of nuclear factor-kappaB, vascular cell adhesion molecule-1 and hemorrheology during spinal cord ischemia reperfusion injury. Zhongguo Gu Shang 2012;25(12):1016–20. [PubMed] [Google Scholar]
- 31.Fan L, Wang K, Shi Z, Die J, Wang C, Dang X. Tetramethylpyrazine protects spinal cord and reduces inflammation in a rat model of spinal cord ischemia-reperfusion injury. J Vasc Surg 2011;54(1):192–200. [DOI] [PubMed] [Google Scholar]
- 32.Liang Y, Yang QH, Yu XD, Jiang DM. Additive effect of tetramethylpyrazine and deferoxamine in the treatment of spinal cord injury caused by aortic cross-clamping in rats. Spinal Cord 2011;49(2):302–6. [DOI] [PubMed] [Google Scholar]
- 33.Zea-longa E, Weinstein PR, Carlson S. Reversible middle cerebral artery occlusion without craniectomy in rat. Stroke 1989;20(1):84–91. [DOI] [PubMed] [Google Scholar]
- 34.Fan LH, Wang KZ, Cheng B, Wang CS, Dang XQ. Anti-apoptotic and neuroprotective effects of tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci 2006;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson SH, Kraimer JM, Graeber GM. Effects of flunarizine on neurological recovery and spinal cord blood flow in experimental spinal cord ischemia in rabbits. Stroke 1993;24(10):1547–53. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Xiong L, Wang Q, Sang H, Zhu Z, Dong H, et al. Tetramethylpyrazine attenuates spinal cord ischemic injury due to aortic cross-clamping in rabbits. BMC Neurol 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Z, Hu J, Lu H, Zhuo X, Xu D, Wang S, et al. Effect of tetramethylpyrazine on the expression of macrophage migration inhibitory factor in acute spinal cord injury in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2012;37(10):1031–6. [DOI] [PubMed] [Google Scholar]
- 38.Shen ZX, Lü HB, Li XM, Xu DQ, Hu JZ, Wang XY. Tetramethylpyrazine accelerated spinal cord repair through regulation of caspase-3 and neurofilament protein expression: an acute spinal cord injury model in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008;33(8):693–9. [PubMed] [Google Scholar]
- 39.Fan LH, Wang KZ, Cheng B, Wang CS, Dang XQ. Anti-apoptotic and neuroprotective effects of tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci 2006;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991;146(10):3444–51. [PubMed] [Google Scholar]
- 41.Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol 1998;152(1):74–87. [DOI] [PubMed] [Google Scholar]
- 42.Matsuyama Y, Sato K, Kamiya M, Yano J, Iwata H, Isobe K. Nitric oxide: a possible etiologic factor in spinal cord cavitation. J Spinal Disord 1998;11(3):248–52. [PubMed] [Google Scholar]
- 43.Muzio M, Salvesen GS, Dixit VM. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem 1997;272(5):2952–6. [DOI] [PubMed] [Google Scholar]
- 44.Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, et al. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci 2001;21(9):3104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyd ZS, Kriatchko A, Yang J, Agarwal N, Wax MB, Patil RV. Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Invest Ophthalmol Vis Sci 2003;44(12):5206–11. [DOI] [PubMed] [Google Scholar]
- 46.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med 1999;5(8):943–6. [DOI] [PubMed] [Google Scholar]
- 47.Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, et al. Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 2000;166(2):213–26. [DOI] [PubMed] [Google Scholar]
- 48.Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 2007;88(11):1384–93. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000;101(9):1019–26. [DOI] [PubMed] [Google Scholar]
- 50.Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem 1999;72(4):1466–71. [DOI] [PubMed] [Google Scholar]
- 51.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett 1998;251(3):189–92. [DOI] [PubMed] [Google Scholar]
- 52.Kopp MA, Liebscher T, Niedeggen A, Laufer S, Brommer B, Jungehulsing GJ, et al. Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res 2012;349(1):119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose P, Huang Q, Ong CN, Whiteman M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol 2005;209(2):105–13. [DOI] [PubMed] [Google Scholar]
- 54.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 2001;276(34):32008–15. [DOI] [PubMed] [Google Scholar]
- 55.Choi WY, Choi BT, Lee WH, Choi YH. Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed Pharmacother 2008;62(9):637–44. [DOI] [PubMed] [Google Scholar]
- 56.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993;4(2):197–250. [DOI] [PubMed] [Google Scholar]
- 57.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004;16(5):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lempinen M, Inkinen K, Wolff H, Ahonen J. Matrix metalloproteinases 2 and 9 in indomethacin-induced rat gastric ulcer. Eur Surg Res 2000;32(3):169–76. [DOI] [PubMed] [Google Scholar]
- 59.Mori N, Sato H, Hayashibara T, Senba M, Geleziunas R, Wada A, et al. Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor κB. Gastroenterology 2003;124(4):983–92. [DOI] [PubMed] [Google Scholar]
- 60.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci 2002;22(17):7526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 2002;62(18):5196–203. [PubMed] [Google Scholar]
- 62.Brandenburg LO, Kipp M, Lucius R, Pufe T, Wruck CJ. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm Res 2010;59(6):443–50. [DOI] [PubMed] [Google Scholar]
- 63.Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol 2007;568(1–3):213–21. [DOI] [PubMed] [Google Scholar]
- 64.Zhao BL, Jiang W, Zhao Y, Hou JW, Xin WJ. Scavenging effects of Salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochem Mol Biol Int 1996;38(6):1171–82. [PubMed] [Google Scholar]
- 65.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res 2002;137:37–47. [DOI] [PubMed] [Google Scholar]
- 66.Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci 2005;25(5):1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kan S, Cheung WM, Zhou Y, Ho WS. Enhancement of doxorubicin cytotoxicity by tanshinone IIA in HepG2 human hepatoma cells. Planta Med 2014;80(1):70–6. [DOI] [PubMed] [Google Scholar]
- 68.Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL, Pang CY. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis 2013;16(4):315–22. [DOI] [PubMed] [Google Scholar]
- 69.Chen SY, Hsiao G, Hwang HR, Cheng PY, Lee YM. Tetramethylpyrazine induces heme oxygenase-1 expression and attenuates myocardial ischemia/reperfusion injury in rats. J Biomed Sci 2006;13(5):731–40. [DOI] [PubMed] [Google Scholar]
- 70.Zhu XL, Xiong LZ, Wang Q, Liu ZG, Ma X, Zhu ZH, et al. Therapeutic time window and mechanism of tetramethylpyrazine on transient focal cerebral ischemia/reperfusion injury in rats. Neurosci Lett 2009;449(1):24–7. [DOI] [PubMed] [Google Scholar]
- 71.Kwan CY, Daniel CE, Chen MC. Inhibition of vasoconstriction by tetramethylpyrazine: does it act by blocking the voltage-dependent Ca2+ channel? J Cardiovasc Pharmacol 1990;15(1):157–62. [PubMed] [Google Scholar]
- 72.Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W. Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci 2003;72(22):2465–72. [DOI] [PubMed] [Google Scholar]
- 73.Sheu JR, Kan YC, Hung WC, Ko WC, Yen MH. Mechanisms involved in the antiplatelet activity of tetramethylpyrazine in human platelets. Thromb Res 1997;88(3):259–70. [DOI] [PubMed] [Google Scholar]