Abstract
Objective
To document the effects of underwater treadmill training (UTT) on leg strength, balance, and walking performance in adults with incomplete spinal cord injury (iSCI).
Design
Pre-test and post-test design.
Setting
Exercise physiology laboratory.
Participants
Adult volunteers with iSCI (n = 11).
Intervention
Participants completed 8 weeks (3 × /week) of UTT. Each training session consisted of three walks performed at a personalized speed, with adequate rest between walks. Body weight support remained constant for each participant and ranged from 29 to 47% of land body weight. Increases in walking speed and duration were staggered and imposed in a gradual and systematic fashion.
Outcome measures
Lower-extremity strength (LS), balance (BL), preferred and rapid walking speeds (PWS and RWS), 6-minute walk distance (6MWD), and daily step activity (DSA).
Results
Significant (P < 0.05) increases were observed in LS (13.1 ± 3.1 to 20.6 ± 5.1 N·kg−1), BL (23 ± 11 to 32 ± 13), PWS (0.41 ± 0.27 to 0.55 ± 0.28 m·s−1), RWS (0.44 ± 0.31 to 0.71 ± 0.40 m·s−1), 6MWD (97 ± 80 to 177 ± 122 m), and DSA (593 ± 782 to 1310 ± 1258 steps) following UTT.
Conclusion
Physical function and walking ability were improved in adults with iSCI following a structured program of UTT featuring individualized levels of body weight support and carefully staged increases in speed and duration. From a clinical perspective, these findings highlight the potential of UTT in persons with physical disabilities and diseases that would benefit from weight-supported exercise.
Keywords: Underwater treadmill, Partial body weight-supported treadmill training, Incomplete spinal cord injury, Aquatic gait training, Water therapy, Gait training
Introduction
The use of partial body weight support during overground or treadmill training with manual assistance, robotic assistance, or functional electrical stimulation has been shown to aid in the recovery of walking following spinal cord injury (SCI).1–7 In a recent study by Field-Fote and Roach6 involving four different training approaches (treadmill-based training with manual assistance, treadmill-based training with stimulation, overground training with stimulation, and treadmill-based training with robotic assistance), both overground and treadmill training produced improvements in walking speed, but overground training was found to be most effective in improving walking distance in persons with chronic motor incomplete SCI. However, the presence of methodological constraints associated with existing forms of gait training, including the need for multiple therapists and the labor-intensive nature of training, use of a harnessing system which can be uncomfortable and sometimes contraindicated due to adverse cardiovascular and orthostatic responses, generation of abnormal patterns of muscle recruitment and force production, and the challenge of coordinating voluntary walking efforts with electrical stimulation patterns7–13 highlights the need to evaluate the potential of emerging therapies to increase the mobility of persons with incomplete spinal cord injury (iSCI).
A self-initiated walking intervention which has remained largely unexamined as a means of improving ambulation in individuals with iSCI is underwater treadmill training (UTT). The use of a treadmill submerged in a self-contained, water-filled tank allows for the precise control of water depth, walking speed, and water temperature, a trio of variables that can markedly influence training responses. Treadmill training performed in a water environment can also serve as an effective alternative or support to land-based physical activity and walking programs in adults who experience balance problems and lower-limb muscle weakness.14–18 In a typical land-based unweighting system, the weight of the body can be unloaded, but the weight of the legs remains unchanged. Consequently, if leg strength is inadequate, some form of external facilitation is required to move the lower extremities during walking. Conversely, use of water as an unloading medium reduces core weight and the weight of the legs, thus decreasing strength levels needed to move the lower extremities during self-initiated gait. Other potential benefits of walking on an underwater treadmill include improved balance, increased muscle strength caused by overcoming water resistance and turbulence, generation of muscle activity and gait patterns similar to those seen in overground walking,14–21 and enhanced venous return and cardiac preload associated with the effects of hydrostatic pressure in an aquatic environment.22
Against this backdrop, the purpose of this exploratory study was to document the impact of UTT on locomotor function in adults with iSCI. Based on the relationship between balance deficits and limited functional ambulation following SCI23 and recent findings indicating that leg strength and preferred walking speed explain a large and significant proportion of variation in daily step activity (DSA) among persons with iSCI,24 we hypothesized that UTT would lead to improvements in lower-extremity strength, balance, and walking performance.
Method
Participants
Twelve adults with iSCI (males, n = 7; females, n = 5) volunteered to participate in this investigation. One participant, who experienced transportation issues, was not able to complete the training program within the allotted time period and her results were not included in the statistical analysis. Hence, data from 11 participants were analyzed in this paper. Participants were recruited through flyers, local newspaper stories describing the study, and contacts with local clinicians and SCI community groups. Study enrollment criteria included being 21 years of age or older, the absence of complex co-morbidity (e.g. health issues that might limit participants’ ability to complete the training protocol), the ability to walk at least 10 m with or without an assistive device, and being more than 1-year post-accident. All study volunteers supplied physician documentation of their SCI and American Spinal Cord Injury Impairment Scale rating and provided medical clearance to engage in laboratory testing and UTT. Table 1 provides descriptive information for study participants. This project was approved by the university Institutional Review Board and participants provided informed written consent before the start of data collection.
Table 1 .
Descriptive characteristics of study participants
| Sex | Age in years | Level of lesion | AIS | Years post-injury | Primary mode of locomotion |
|---|---|---|---|---|---|
| M | 56 | T5 | C | 3 | Wheelchair |
| M | 62 | C4 | D | 2.5 | Ambulation |
| M | 62 | L2 | C | 6 | Wheelchair |
| F | 51 | C3 | C | 3 | Ambulation |
| M | 43 | T8 | C | 2 | Wheelchair |
| M | 28 | L2 | C | 28 | Wheelchair |
| M | 23 | C6 | C | 1.5 | Wheelchair |
| F | 64 | C4 | C | 1 | Ambulation |
| M | 50 | C2 | C | 1 | Wheelchair |
| F | 40 | T6 | D | 3 | Wheelchair |
| F | 46 | L2 | C | 2 | Wheelchair |
Note: M, male; F, female; ASI, American Spinal Injury Association Impairment Scale.
Pre-training assessments
Each participant completed a pre-training test battery during a single laboratory visit. Participants were transported from their cars using a golf cart or power wheelchair to limit the amount of walking prior to testing.
Leg strength
Lower-limb strength was measured using a handheld dynamometer (JTech Commander PowerTrack II). A physical therapist trained in the use of handheld dynamometry in persons with physical disabilities performed all muscle strength assessments. Our decision to use handheld dynamometry to assess muscle strength was partly based on the inability of manual muscle testing methods to sufficiently detect small or moderate gains in strength over the course of rehabilitation.25 Moreover, handheld dynamometry has been shown to be highly reliable (r > 0.91) when assessing the impaired limbs of persons with neurological disabilities26 and demonstrate good validity in all force ranges, with validity and reliability increasing when stabilization techniques are employed and trained examiners are present.27
Participants were securely positioned in a chair or on a mat table, with proximal stabilization provided to minimize muscle substitution and ensure appropriate force application. Maximal isometric leg strength during (a) hip flexion, extension, and abduction; (b) knee flexion and extension; and (c) ankle dorsiflexion and plantar flexion was measured in both legs. These muscle groups were selected based on research demonstrating the individual and collective relationship between muscle strength determined by manual muscle tests and functional walking measures in adults with iSCI.28 A minimum of three trials were completed for each muscle group in both legs26 and participants were instructed to exert as much force as possible against the dynamometer, which was held in place by the primary investigator. When maximal effort appeared to be generated (∼3 to 5 seconds), participants were instructed to relax. The highest achieved values during strength measurements obtained for each leg were summed to establish an overall strength score for a given muscle group.29–31 Overall strength scores for the seven muscle groups were then added to derive a total lower-extremity muscle strength score expressed relative to body mass. This summative approach to quantifying muscle strength has been implemented in previous studies of strength and neurological impairment in the SCI population.32–34
Balance
Balance was measured using the Berg Balance Scale (BBS). The BBS, which has been validated in persons with SCI23 and used in iSCI intervention research,32 evaluates balance during static and dynamic activities performed while seated and standing. This balance evaluation required participants to complete 14 tasks, ranging from sitting with feet on the floor with no back support to standing on one leg. Performances on these tasks were combined to provide a numeric balance score ranging from 0 to 56.
Walking speed
Walking speed was determined using the 10-m walk test. In this test, participants walked in a straight line for 14 m at a normal, comfortable pace in an indoor gymnasium. Using two photoelectric cells, walking time was recorded during the central 10 m of the course to account for potential acceleration and deceleration effects. Participants completed the test using assistive devices typically employed while walking in their natural environment. Each participant performed three walking trials and was allowed to rest for as long as needed between trials. From knowledge of distance and time, walking speed was calculated. Mean preferred walking speed for each participant was obtained by averaging speeds calculated for each walking trial. Participants who were able to walk at velocities exceeding their typical pace also completed three additional walks over the same course at the fastest pace they could safely sustain. Speeds for these trials (expressed in meters per second) were averaged to derive mean rapid walking speed.
Walking endurance
The 6-minute walk test, which has been validated in the iSCI population,35 was performed to assess the walking endurance of our sample. In this test, participants walked for 6 minutes around an oval course in a gymnasium. Participants were instructed to cover as much distance as possible in the time allotted and allowed to select their preferred method of assistive device use during the walking trial. Participants were followed with a wheelchair and provided with an opportunity to rest during the test, if necessary. Total walking distance was recorded using a calibrated measuring wheel and expressed in meters traveled.
Daily step activity
Step activity at home and in community settings was monitored using an Orthocare Step Activity Monitor (SAM). The SAM has been reported to be 97% accurate when compared to hand-tallied step counts during 10-m and 6-minute walk tests.36 Additional validation was conducted in a published SCI case study demonstrating 98% accuracy between manual counts and those recorded with the SAM.37 Initial calibration of the SAM involved establishing settings for the expected sensitivity, cadence, threshold, and motion characteristics of each participant. Following this calibration procedure, the SAM was attached with a Velcro strap to the less-involved leg based on previous research validating the use of the less-involved lower limb in patients with SCI.36 Participants were observed during overground walking to ensure that all step activity was captured. If step counts were missed, or if non-step activity was registered, initial sensitivity, cadence, threshold, and motion settings were adjusted until all valid step activities were registered. Prior to leaving the laboratory, participants were instructed in the care of and wearing schedule for the SAM.
Step monitoring began the day after calibration of the SAM. Over seven consecutive days, step activity data were collected in 1-minute epochs during waking hours, except while showering or bathing. The activity monitors were returned to the primary investigator following the 7-day assessment period for downloading and processing of stored step data.
Accommodation to underwater treadmill walking
Participants completed a walking session in the treadmill tank (HydroTrack® Underwater Treadmill System, Conray, Inc., Phoenix, AZ, USA) before commencing UTT. Prior to this session, each participant was fitted with a non-weight-bearing body harness worn loosely around the mid-section of the body, which served to restrain participants if they stumbled while walking in the water tank. Absolute levels of water height were established for each participant based on the minimum water height necessary to produce and maintain an upright position consisting of knees and hips in a maximally extended position while standing in the tank without support from the body harness or upper extremities. Once an appropriate water height was determined, participants walked for 1 minute at a speed that was either 50% slower than their mean preferred overground walking speed or 0.20 m·s−1 (the slowest speed setting on the treadmill), whichever was faster. If the personalized water height level allowed for an appropriate balance between support and loading during the 1-minute walk, it was recorded and used during UTT. This height was also marked on the body and used when calculating the percentage of body weight unloading for each participant. Following the 1-minute walk, participants were lifted completely off the bottom of the tank using a hydraulic suspension system, water level was raised to the previously marked location on the body, and body mass was recorded using load cells embedded within a weight-unloading system (LiteGait BiSym Suspension System, Model PBS 1637, Mobility Research, Tempe, AZ, USA) located next to the tank. The difference in body mass measured on land and in the water was divided by the participant's land body mass to determine the relative level of body mass that was unloaded. For our sample, the mean percentage of land body mass unloaded in the water tank was 38% (range = 30–47%), a value similar to that employed in other investigations of body weight-supported treadmill training.7,38,39
During the treadmill accommodation session, participants completed three 5-minute walking trials, during which standing and exercise heart rates were recorded using a Polar heart rate monitor. Participants were also asked to identify their rating of perceived exertion (RPE) at the conclusion of each walk.40 At the beginning of the first 5-minute walk, walking speed was initially set as described previously and gradually adjusted during the first minutes of exercise until an increase in heart rate was observed from standing heart rate, an RPE of at least “3” (moderate exertion) was reported, no adverse responses were observed (e.g. dizziness, changes in muscle tone, shortness of breath, pain), and appropriate levels of hip and knee extension were maintained. This final speed setting was used during the remaining walking trials and served as the initial training speed during the first week of UTT. Rest periods of at least 5 minutes occurred between walking trials. While resting, participants were provided with a choice of standing in water or sitting in a flotation chair. Despite their limited capacity to sustain overground ambulation, all participants were able to successfully complete three 5-minute underwater treadmill walks. Consequently, the duration of exercise trials in week 1 of UTT was set at 5 minutes to build upon this initial stage of walking success and avoid overtaxing or exceeding the functional capabilities of our participants.41
Water temperature for the treadmill accommodation session was initially established at 90°F. Participants were observed during the walking trials to evaluate muscle tone (e.g. facilitation of a crossed extension pattern interfering with stepping) and pain levels. Water temperature was adjusted if a negative response was noted or if requested by the participant. The final water temperature selected was used throughout the UTT program unless the participant requested a change or adverse physiological reactions (e.g., excessive sweating, abnormal rise in heart rate) were observed by the trainer.
Underwater treadmill training
Following the treadmill accommodation session, each participant underwent a 2-month period of UTT consisting of three training sessions per week for 8 weeks. Each training session consisted of three walks of equal duration with ample rest periods (5–10 minutes) between walking bouts. In the first week of UTT, participants completed three 5-minute walking bouts at the personalized water height, walking speed, and water temperature established during the treadmill accommodation session. For all training sessions, heart rate was monitored continuously using a Polar heart rate monitor and participants verbally reported their RPE value. All participants declined the option of wearing the non-weight-bearing harness used in the treadmill accommodation session.
As training progressed, staggered increases in walking speed and duration were imposed in a systematic and incremental fashion. The general outline of adjustments in training parameters is shown in Table 2. As displayed in this table, walking speed was raised by 10% in weeks 2, 4, 6, and 8, resulting in a 40% increase in walking speed over the 2-month training program. All but one study participant was able to tolerate scheduled biweekly increments in walking speed, and this person accommodated to speed increases ranging from 5 to 10%. The length of each walking bout was also lengthened by 1-minute increments in weeks 3, 5, and 7, resulting in biweekly increases of 3 minutes in overall walking duration in a given training session. Consequently, at the beginning of UTT (weeks 1 and 2), participants completed three 5-minute walks and accumulated 15 minutes of total walking. However, towards the end of training (weeks 7 and 8), all participants were able to perform three 8-minute walks for a total of 24 minutes of walking, resulting in a 60% increase in walking duration.
Table 2 .
Overview of weekly increments in walking speed and duration
| Variable | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
|---|---|---|---|---|---|---|---|---|
| Speed | TA speed | 10% increase | No change | 10% increase | No change | 10% increase | No change | 10% increase |
| Duration | 5-minute walks | No change | 6-minute walks | No change | 7-minute walks | No change | 8-minute walks | No change |
Note: TA speed, treadmill accommodation speed.
Post-training assessments and data analysis
Following UTT, participants completed a post-training evaluation identical to that conducted during the pre-training phase of the study. Data were analyzed using SPSS Version 17 (SPSS Inc., Chicago, IL, USA). Repeated-measures analysis of variance (ANOVA) was conducted to measure training-related changes in leg strength, balance, preferred and rapid walking speeds, 6-minute walk distance, and DSA. Additional ANOVA tests were performed to quantify differences in primary outcome variables as a function of level of walking independence and to compare relative strength gains between the stronger and weaker legs and across individual muscle groups.
Results
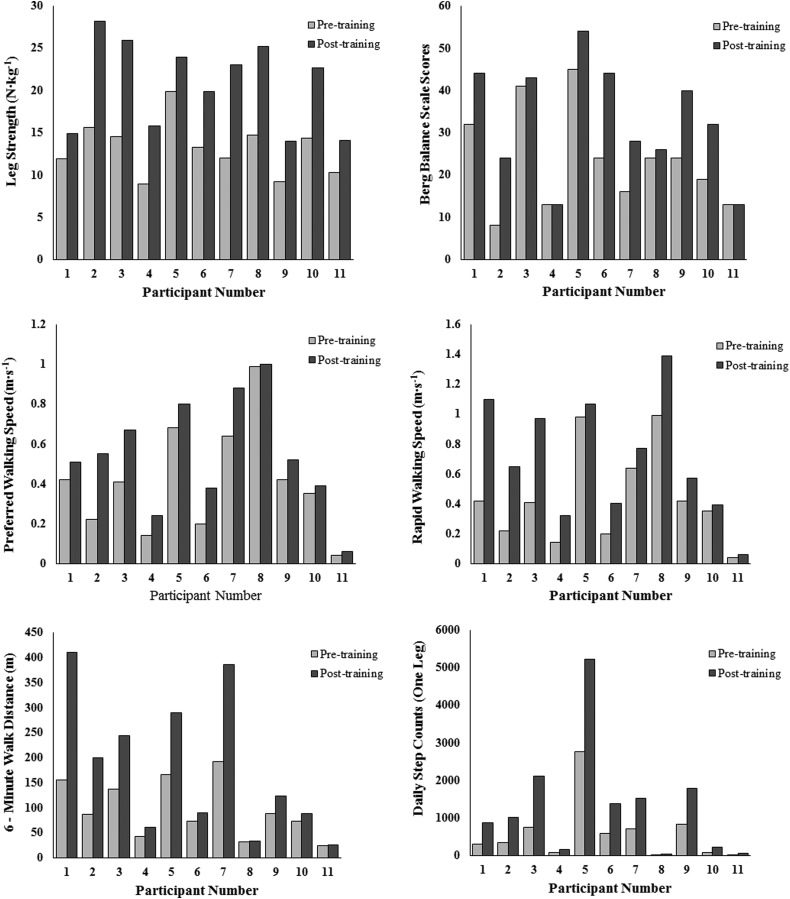
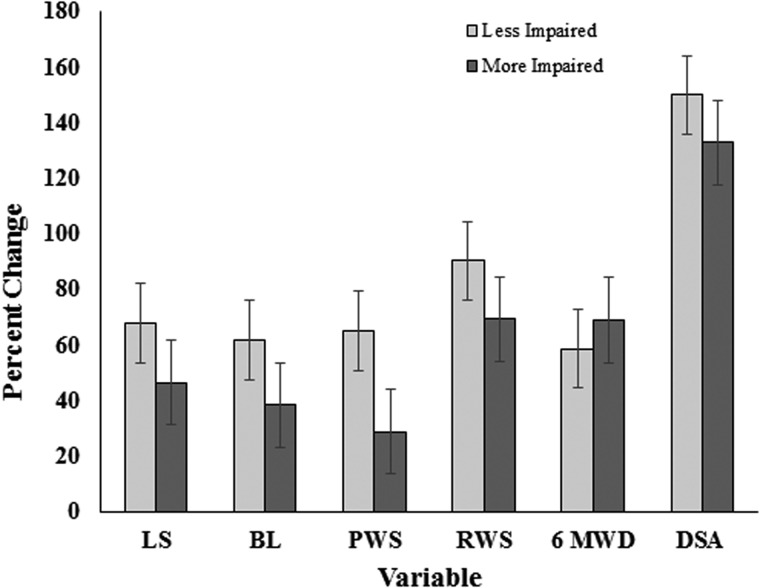
Descriptive group statistics for pre-and post-training measurements of leg strength, balance, and functional mobility are presented in Table 3. Participants exhibited significant (P ≤ 0.05) improvements in leg strength (57%), balance (39%), preferred walking speed (34%), rapid walking speed (61%), 6-minute walk distance (82%), and DSA (121%) following UTT. Effect sizes (partial η2) for these primary outcome measures varied from 0.51 to 0.84, indicating that training effects were medium to large in magnitude. Fig. 1 depicts pre- and post-training values for each participant. Compared to pre-training data, all 11 participants exhibited improvements in leg strength, preferred and rapid walking speed, 6-minute walk distance, and DSA and nine participants displayed an increase in balance following UTT. Furthermore, no differences in any of the primary outcome variables were observed when comparisons were made between participants with lower or higher WISCII-II scores (Fig. 2).
Table 3 .
Pre- and post-training values of leg strength, balance, preferred walking speed, rapid walking speed, 6-minute walk distance, and daily step activity
| Pre-training |
Post-training |
|||||
|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | P | Partial η2 |
| Leg strength (N·kg−1) | 13.17 | 3.19 | 20.69 | 5.18 | <0.001* | 0.84 |
| Balance | 23.55 | 11.74 | 32.82 | 13.37 | 0.002* | 0.65 |
| Preferred walking speed (m·s−1) | 0.41 | 0.27 | 0.55 | 0.28 | 0.002* | 0.64 |
| Rapid walking speed (m·s−1) | 0.44 | 0.31 | 0.71 | 0.40 | 0.002* | 0.64 |
| 6-minute walk distance (m) | 97.3 | 80.2 | 177.0 | 122.33 | 0.009* | 0.51 |
| Daily step activity (one leg) | 593 | 782 | 1310 | 1258 | 0.01* | 0.51 |
Note: Values are mean and standard deviation; * = P < 0.05; partial η2 = effect size.
Figure 1 .
Pre- and post-training values of primary outcome variables for individual participants.
Figure 2 .
Relative changes in primary outcome variables in less-impaired and more-impaired participants following UTT. Less-impaired participants (n = 6) achieved WISCI-II scores from 13 to 18 and more-impaired participants (n = 5) achieved WISCI-II scores from 6 to 11. LS, leg strength; BL, balance; PWS, preferred walking speed; RWS, rapid walking speed, 6MWD, 6-min walk distance, and DSA, daily step activity.
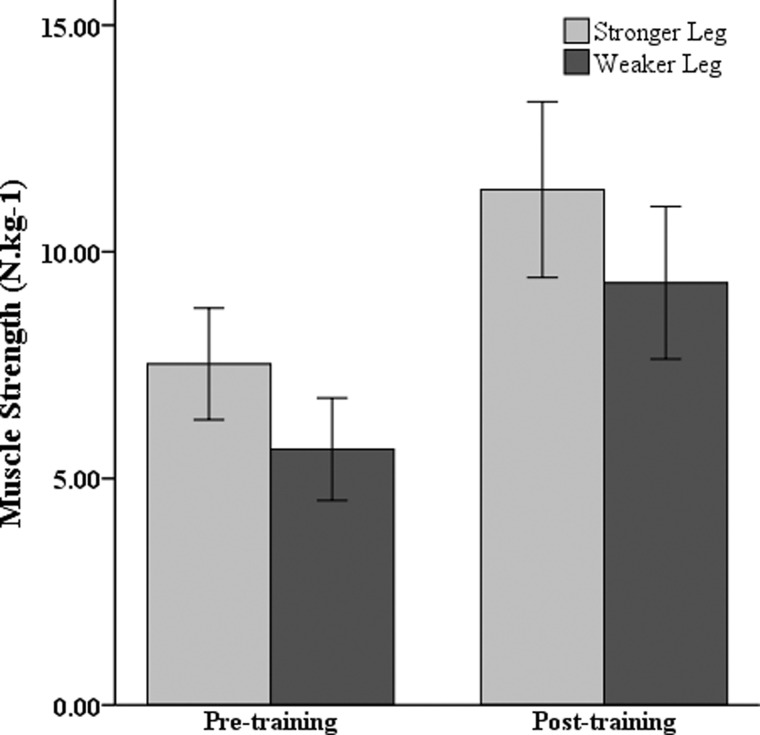
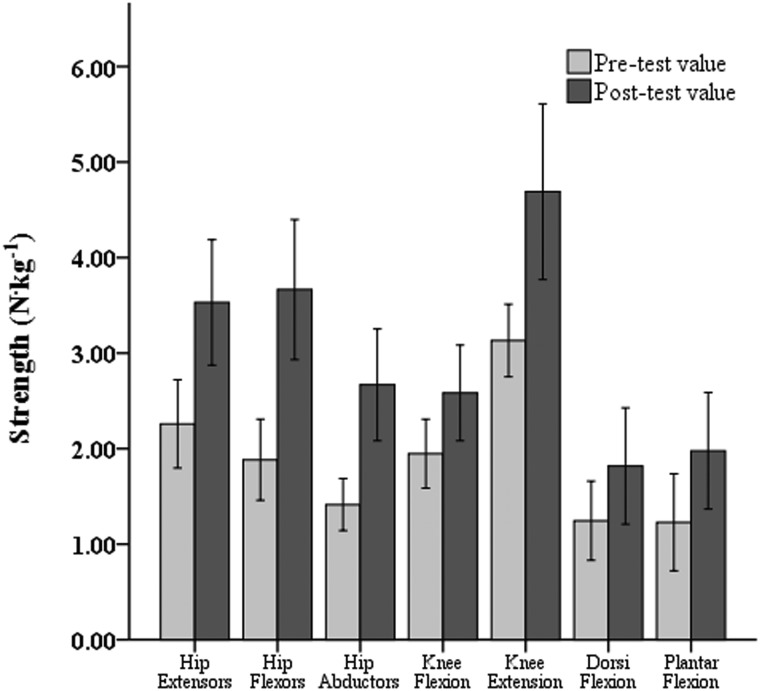
Post-hoc tests were conducted to evaluate overall strength training responses of both legs, as well as specific lower-extremity muscle groups. These analyses revealed no significant difference (P > 0.05) in the training response between the stronger and weaker legs. Strength improvements averaging 51 and 65% were measured in the stronger and weaker legs, respectively (Fig. 3). Fig. 4 depicts muscle strength levels, averaged across legs, before and after UTT. When training-related strength increases were expressed in relative terms, similar percentage gains in mean strength (P > 0.05) were noted for all seven lower-limb muscle groups.
Figure 3 .
Changes in lower-extremity strength of the stronger and weaker leg following UTT.
Figure 4 .
Strength gains achieved in each lower-limb muscle group following UTT.
Discussion
Limitations in mobility and physical activity status take on a heightened sense of importance for persons with SCI, as sedentary living is associated with a variety of negative outcomes in this population, including skin breakdown, osteoporosis, muscle atrophy, respiratory and cardiovascular problems, urinary tract infections, and a host of comorbidities associated with diminished health and decreased lifespan.42,43 Consequently, the aim of this exploratory investigation was to quantify the effects of UTT on leg strength, balance, and walking performance in adults with iSCI. Overall, our findings demonstrate that partial body weight-supported treadmill training performed in a controlled aquatic environment led to significant improvements of moderate to large magnitude in physical function and walking ability in our sample of physically challenged individuals.
Lower-extremity strength
Significant improvements were observed in all leg strength measures, with individual muscle groups contributing in a similar fashion to the overall increase in leg strength. In addition, the UTT protocol was sufficient to strengthen both the stronger and weaker legs of our participants (Fig. 3). It is difficult to compare lower-extremity strength values measured in the present investigation with data collected in other studies of persons with SCI because researchers have typically employed scale-based measures of muscle strength.32,34 However, previous research has identified recovery of leg strength as the best predictor of functional ambulation following SCI28 and Stevens et al.24 have reported a strong positive association (r = 0.83) between lower-extremity strength and daily stepping activity in adults with iSCI.
To compensate for muscle atrophy, which occurs following spinal cord damage, more motor units are recruited than would normally be needed to perform daily tasks requiring submaximal efforts, resulting in a greater energy demand and early fatigue.44 This level of motor unit recruitment is typically seen in higher-threshold units, which fatigue rapidly due to their inability to support aerobic metabolism44 and usually remain inactivated during activities such as slow walking.44 Although speculative, interventions like UTT, which feature sustained periods of walking and gains in lower-extremity strength, may rely to a greater extent on the recruitment of lower-threshold motor units and lead to reduced fatigue levels during standing and low- to moderate-intensity walking in persons with iSCI.
Balance
Changes in balance resulting from partial body weight-supported treadmill training have typically not been evaluated, primarily because trunk and pelvic support are provided. In the current study, balance was significantly improved following UTT, with participants increasing their scores on the BBS by an average of nine points. According to the developers of this balance assessment instrument, a change of eight points or more is needed to observe a genuine change in postural balance and function.45 The magnitude of change in BBS score reported in the present investigation also exceeds the minimal clinically important difference (MCID) suggested by Ditunno et al.46 of five to seven points for individuals with SCI and the MCID of six points for persons post-stroke.47 While the reasons underlying improvement in balance remain to be elucidated, the use of water as the unloading mechanism during UTT may have allowed for greater excursions of weight shift than that provided in a land-based harnessing system, lengthened reaction time, or enabled small, corrective positional adjustments to be made on a real-time basis, all of which would serve as positive stimuli to maintain an upright posture and enhance balance while walking.
With respect to other published work documenting the impact of partial body weight-supported training (PBWST) on locomotor status, Behrman and Harkema,48 reported that a 43-year-old male who was eight months post-injury (injury at C6; AIS D classification) progressed from PBWST to overground training in an 11-week period and displayed a 23% increase in his BBS score. In comparison, participants in the current investigation displayed a 39% improvement in the BBS score despite the presence of a longer mean post-injury time frame (5 years) and a shorter training period (8 weeks). Moreover, Harkema et al.49 reported an average improvement in BBS of 9.6 points in 196 participants with iSCI who engaged in an intensive locomotor training program featuring body weight-supported step training with manual facilitation on a treadmill followed by overground assessments and community integration, a degree of improvement nearly identical to that observed in our sample.
Walking speed
Significant increases in walking speed were noted following UTT. Compared to pre-training values, preferred and rapid walking speeds rose by an average of 0.14 and 0.27 m·s−1, respectively. The magnitude of increase in preferred walking speed was 27% greater than that reported by Wirz et al.7 after an 8-week program of robotic gait training involving 20 adults with iSCI and exceeded the MCID of 0.05–0.06 m·s−1 reported by Mussleman.47 Additionally, the increase in rapid walking speed noted in the current project was larger than the MCID of >0.16 m·s−1 reported by Tilson et al.50 for persons with severe gait speed impairment. In relating our findings to ecological measures of walking ability, a recent investigation conducted by Stevens et al.24 demonstrated the existence of a curvilinear association between freely chosen walking speed and the number of daily steps taken in home and community settings by adults with SCI. Results drawn from this study of 21 adults with iSCI indicated that once walking velocity exceeds ∼0.42 m·s−1, greater improvement in daily stepping activity occurs with relatively small increments in walking velocity. In our participant sample, mean pre- and post-training preferred walking speeds were 0.41 and 0.55 m·s−1, respectively. Based on the walking speed–step activity relationship established previously by Stevens et al.,24 this training-related increase in preferred walking speed should result in an average increase in DSA of approximately 875 steps. Interestingly, this predicted gain in daily step count comports reasonably well with the actual mean increase of 717 steps per day observed following UTT in the current investigation.
Six-minute walk test
An 82% increase in 6-minute walk distance was observed following 2 months of UTT. The magnitude of improvement in locomotor performance is substantially greater than the 53% increase in 6-minute walk distance reported by Wirz et al.7 in 20 persons with iSCI who completed an 8-week, robotically assisted body weight-supported treadmill training program consisting of three therapy sessions per week. In practical terms, the mean increase in distance observed was approximately 80 m, a value that exceeds the MCID of 54 m reported in a series case study by Mussleman47 and the increase of 63 m noted by Harkema et al.49 in a large sample of adults with iSCI who completed a rigorous program of locomotor training. In addition, while none of the 11 participants in our study were able to complete the 6-minute walk test without resting prior to UTT, nine of them were able to complete the test with no rest break after UTT. Based on these findings, we hypothesize that the increase we recorded in 6-minute walk distance may reflect central and peripheral adaptations associated with the aerobic nature of the UTT program, including an expanded stroke volume and increases in mitochondrial capacity, muscle capillarization, and fat and carbohydrate oxidation.51 Improvements in metabolic gait economy, maximal aerobic power, and the ability to incur a higher percentage of maximal aerobic power may have also contributed to the greater distance covered during the 6-minute walk test.52
Daily step activity
Participants more than doubled their mean DSA in home and community settings following UTT. In the current study, participants exhibited an average daily step count (for one leg) of 593 steps prior to training, with individual step count values ranging from 15 to 2760 steps. In contrast, Bowden and Behrman36 reported a mean DSA of 1604 steps (range = 68–4468 steps) for nine participants (age 21–59 years) with iSCI and Saraf et al.53 noted that the average DSA of their sample of 91 people with iSCI was 2658 (±2745) steps per day, with a range of 0–11 660. Although our sample of adults with iSCI was less ambulatory compared to participants in the Bowden and Behrman36 and Saraf et al.53 studies, the wide range of individual step count activity displayed in all three investigations clearly highlights the pronounced heterogeneity in the community-based walking activity of adults with iSCI. This degree of variability in daily step count may be linked to factors such as the extent of neural sparing, residual leg strength, and the availability of opportunities to engage in ambulatory physical activity.
When considering the health benefits of walking, it is important to recognize that health-related cutpoints for DSA have yet to be established for adults with various neurological conditions. However, relative to the general adult activity guideline of 10 000 steps per day established by the Centers for Disease Control and Prevention54 or published step count values for sedentary behavior (<5000 steps per day for both legs),55 it is clear that participants in the present investigation exhibited low levels of daily walking activity. Viewed in this context, the large relative increase in step activity documented in the present study represents a gain in functional mobility which, if maintained or expanded over time, might be expected to lower the risk of morbidity and mortality from cardiovascular disease and other lifestyle-related health disorders.
While positive benefits in physical performance and functional mobility were observed in adults with iSCI following UTT, limitations of this exploratory investigation include a limited sample size, the absence of a matched control group, and involvement by study personnel in both pre- and post-training assessments and the training program. Future studies featuring larger samples sizes and appropriate control groups should be performed to compare the effectiveness of UTT with existing neurorehabilitation approaches. Additional research should also be conducted to optimize UTT protocols in persons with iSCI and assess the interactive effects of UTT, disability level, and injury timeline on walking ability, physical function and health, and social participation.
In conclusion, data from this preliminary study revealed that 8 weeks of UTT improved leg strength, balance, and walking performance in adults with iSCI. From a practical viewpoint, confirmation of mobility and functional adaptations resulting from UTT could lead to the eventual use of smaller, relatively inexpensive portable underwater treadmills in clinic- and community-based settings by persons with a variety of physical disabilities and diseases (e.g. stroke, knee and hip injuries, lower-limb surgery, obesity) that would benefit from weight-supported exercise.
Disclaimer statements
Contributors SS coordinated the study, wrote the original draft of the manuscript, and participated in all phases of data collection and analysis. JC assisted in project design, participant recruitment, daily project operations, and manuscript editing and revising. DF was the statistician who assisted in the design of the project, data analysis, and writing the results section of the manuscript. DM provided advisement and direction throughout the project. He assisted with recruitment, project design, and has provided contributions to the manuscript both as an author and editor. His experience in previous research provided the foundation from which this project evolved.
Funding None.
Conflicts of interest We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
Ethics approval This project was approved by the IRB of Middle Tennessee State University.
References
- 1.Alexeeva N, Sames C, Jacobs P, Hobday L, DiStasio M, Mitchell S, et al. Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. J Spinal Cord Med 2011;34(4):362–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz V.Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull 2009;78(1):I–VI. [DOI] [PubMed] [Google Scholar]
- 3.Dietz V, Colombo G. Locomotor activity in spinal man. Lancet 1994;344(8932):1260–4. [DOI] [PubMed] [Google Scholar]
- 4.Edgerton V, Roy R. Robotic training and spinal cord plasticity. Brain Res Bull 2009;78(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouad A, Pearson K. Restoring walking after spinal cord injury. Prog Neurobiol 2004;73(2):107–26. [DOI] [PubMed] [Google Scholar]
- 6.Field-Fote E, Roach K. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther 2011;91(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirz M, Zemon D, Rupp R, Scheel A, Colombo G, Dietz V, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehab 2005;86(4):672–80. [DOI] [PubMed] [Google Scholar]
- 8.Brissot R, Gallien P, Le Bot M, Beillot J, Dassonville J. Clinical experience with functional electrical stimulation-assisted gait with parastep in spinal cord injured patients. Spine 2000;25(4):501–8. [DOI] [PubMed] [Google Scholar]
- 9.Galvez J, Budovitch A, Harkema S, Reinkensmeyer D.. Quantification of therapists’ manual assistance on the leg during treadmill gait training with partial body-weight support after spinal cord injury. Proceeding of the 29th Annual International Conference of the Institute of Electrical and Electronics Engineers, Engineering in Medicine and Biology Society, Lyon, France; 2007 p. 4028–32. [DOI] [PubMed] [Google Scholar]
- 10.Israel J, Campbell D, Kahn J, Hornby T. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther 2006;86(11):1466–78. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs P, Johnson B, Mahoney E. Physiologic responses to electrically assisted and frame-supported standing in persons with paraplegia. J Spinal Cord Med 2003;26(4):384–9. [DOI] [PubMed] [Google Scholar]
- 12.Krassioukov A, Harkema S. Effects of harness application and postural changes on cardiovascular parameters of individuals with spinal cord injury. Spinal Cord 2006;44(12):780–6. [DOI] [PubMed] [Google Scholar]
- 13.Hamid S, Hayek R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: an overview. Eur Spine J 2008;17(9):1256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bocalini D, Serra A, Murad N, Levy R. Water- versus land-based exercise effects on physical fitness in older women. Geriatr Gerontol Int 2008;8(4):265–71. [DOI] [PubMed] [Google Scholar]
- 15.Harrison R, Hillman M, Bulstrode S. Loading of the lower limb when walking partially immersed: implications for clinical practice. Physiotherapy 1992;78(3):164–6. [Google Scholar]
- 16.Napoletan J, Janes P, Hicks R. The effect of underwater treadmill exercise in the rehabilitation of ACL repair 32. Med Sci Sport Exer 1992;24(5):S6. [Google Scholar]
- 17.Noh D, Lim J, Shin H, Paik N. The effect of aquatic therapy on postural balance and muscle strength in stroke survivors—a randomized controlled pilot trial. Clin Rehabil 2008;22(10–11):966–76. [DOI] [PubMed] [Google Scholar]
- 18.Thein L, Brody J. Aquatic-based rehabilitation and training for the elite athlete. J Orthop Sport Phys 1998;27(11):32–41. [DOI] [PubMed] [Google Scholar]
- 19.Byrne K, Craig J, Wilmore J. A comparison of the effects of underwater treadmill walking to dry land treadmill walking on oxygen consumption, heart rate, and cardiac output. J Aquatic Phys Ther 1996;4(3):4–11. [Google Scholar]
- 20.Giaquinto S, Ciotola E, Marqutti F. Gait in the water: a comparison between young and elderly subjects. Disabil Rehabil 2007;29(9):727–30. [DOI] [PubMed] [Google Scholar]
- 21.Napoletan J. An innovation in aquatic therapy: the underwater treadmill. Phys Ther Prod 1994;7:43–4. [Google Scholar]
- 22.Gulick D. Effects of aquatic intervention on the cardiopulmonary system in the geriatric population. Top Geriatr Rehabil 2010;26(2):93–103. [Google Scholar]
- 23.Lemay J, Nadeau S. Standing balance assessment in ASIA D paraplegic and tetraplegic participants: concurrent validity of the Berg Balance Scale. Spinal Cord 2010;48(3):245–50. [DOI] [PubMed] [Google Scholar]
- 24.Stevens S, Fuller D, Morgan D. Lower extremity strength and walking speed as predictors of daily step activity in adults with incomplete spinal cord injuries. Top Spinal Cord Inj Rehabil 2013;19(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noreau L, Vachon J. Comparison of three methods to assess muscular strength in individuals with spinal cord injury. Spinal Cord 1998;36(10):716–23. [DOI] [PubMed] [Google Scholar]
- 26.Riddle D, Finucaine S, Rothstein J, Walker M. Intrasession and intersession reliability of hand-held dynamometer measurements taken on brain damaged patients. Phys Ther 1998;69(3):182–94. [DOI] [PubMed] [Google Scholar]
- 27.Nollet F, Beelen A.. Strength assessment in postpolio syndrome: validity of a hand-held dynamometer in detecting change. Arch Phys Med Rehab 1999;80(10):1316–23 [DOI] [PubMed] [Google Scholar]
- 28.Kim C, Eng J, Whittaker M. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord 2004;42(3):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolber M, Cleland J. Strength testing using hand-held dynamometry. Phys Ther Rev 2005;10(2):99–112. [Google Scholar]
- 30.Merlini L, Mazzone E, Solari A, Morandi L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve 2002;26(1):64–70. [DOI] [PubMed] [Google Scholar]
- 31.Visser J, Mans E, de Visser M, van den Berg-Voss R, Franssen H, de Jong J, et al. Comparison of maximal voluntary isometric contraction and hand-held dynamometry in measuring muscle strength of patients with progressive lower motor neuron syndrome. Neuromuscul Disord 2003;13(9):744–50. [DOI] [PubMed] [Google Scholar]
- 32.Ditunno J, Scivoletto G. Clinical relevance of gait research applied to clinical trials in spinal cord injury. Brain Res Bull 2009;78(1):35–42. [DOI] [PubMed] [Google Scholar]
- 33.Dobkin B, Barbeau H, Deforge D, Ditunno J, Elashoff R, Apple D, et al. The evolution of walking-related outcomes over the first twelve weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized spinal cord injury locomotor trial. Neurorehab Neural Repair 2007;21(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisler F, Dorsey F, Coleman W. Recovery of motor function after spinal cord injury—a randomized placebo-controlled trail with GM-1 ganglioside. New Engl J Med 1991;324(26):1829–38. [DOI] [PubMed] [Google Scholar]
- 35.Hedel H, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehab 2005;86(2):190–6. [DOI] [PubMed] [Google Scholar]
- 36.Bowden M, Behrman A. Step activity monitor: accuracy and test–retest reliability in persons with incomplete spinal cord injury. J Rehabil Res Dev 2007;44(3):355–62. [DOI] [PubMed] [Google Scholar]
- 37.Behrman A, Lawless-Dixon A, Davis S, Bowden M, Nair P, Phadke C, et al. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther 2005;85(12):1356–70. [PubMed] [Google Scholar]
- 38.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehab Neural Repair 2003;17(3):153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Protas E, Holmes S, Qyreshy H. Supported treadmill ambulation training after spinal cord injury: a pilot study. Arch Phys Med Rehab 2001;82(6):825–31. [DOI] [PubMed] [Google Scholar]
- 40.Borg E, Kaijser L.. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sport 2006;16(1):57–69. [DOI] [PubMed] [Google Scholar]
- 41.Lapointe R, Lajoie Y, Serresse O, Barbeau H. Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord 2001;39(6):327–35. [DOI] [PubMed] [Google Scholar]
- 42.McDonald J, Sadowsky C. Spinal-cord injury. Lancet 2002;359(9304):417–25. [DOI] [PubMed] [Google Scholar]
- 43.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury. Am J Phys Med Rehab 2007;86(2):142–52. [DOI] [PubMed] [Google Scholar]
- 44.Lin V.Spinal cord medicine: principles and practice. New York: Demos Medical Publishing; 2003. [Google Scholar]
- 45.Berg K, Maki B, Williams J, Holliday P, Wood-Dauphinee S. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehab 1992;73(11):1073–80. [PubMed] [Google Scholar]
- 46.Ditunno J, Barbeau H, Dobkin B, Elashoff R, Harkema S, Marino R, et al. Validity of the walking scale for spinal cord injury and other domains of function in a multicenter clinical trial. Neurorehabil Neural Repair 2007;21(16):539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mussleman K.Clinical significance testing in rehabilitation research: what, why, and how? Phys Ther Rev 2007;12(4):287–96. [Google Scholar]
- 48.Behrman A, Harkema S. Locomotor training after human spinal cord injury. Phys Ther 2000;80(7):688–700. [PubMed] [Google Scholar]
- 49.Harkema S, Schmidt-Read M, Lorenz D, Edgerton V, Behrman A. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehab 2012;93(9):1508–17. [DOI] [PubMed] [Google Scholar]
- 50.Tilson J, Sullivan K, Cen S, Rose D, Koradia C, Azen S, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther 2010;90(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McArdle W, Katch F, Katch V.. Exercise physiology: energy, nutrition, and human performance. 5th ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2010. p. 464–6. [Google Scholar]
- 52.Kenney W, Wilmore J, Costill D.. Physiology of sport and exercise. 5th ed.Champaign, IL: Human Kinetics; 2012. p. 125–6, 266. [Google Scholar]
- 53.Saraf P, Rafferty M, Moore J, Kahn J, Hendron K, Leech K, et al. Daily stepping in individuals with motor incomplete spinal cord injury. Phys Ther 2010;90(2):224–35. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. Physical activity and health. A report of the surgeon general. 2007 [accessed 2010 Jun 6]. Available from: http://www.cdc.gov/nccdphp/sgr/disab.htm.
- 55.Tudor-Locke C, Ainsworth B, Thompson R, Matthews C. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sport Exer 2002;34(1):2045–51. [DOI] [PubMed] [Google Scholar]