Abstract
Gravistimulation of tree stems affects wood development by unilaterally inducing wood with modified properties, called reaction wood. Commonly, it also stimulates cambial growth on the reaction wood side. Numerous experiments involving applications of indole-3-acetic acid (IAA) or IAA-transport inhibitors have suggested that reaction wood is induced by a redistribution of IAA around the stem. However, in planta proof for this model is lacking. Therefore, we have mapped endogenous IAA distribution across the cambial region tissues in both aspen (Populus tremula, denoted poplar) and Scots pine (Pinus sylvestris) trees forming reaction wood, using tangential cryosectioning combined with sensitive gas chromatography-mass spectrometry analysis. Moreover, we have documented the kinetics of IAA during reaction wood induction in these species. Our analysis of endogenous IAA demonstrates that reaction wood is formed without any obvious alterations in IAA balance. This is in contrast to gravitropic responses in roots and shoots where a redistribution of IAA has been documented. It is also of interest that cambial growth on the tension wood side was stimulated without an increase in IAA. Taken together, our results suggest a role for signals other than IAA in the reaction wood response, or that the gravitational stimulus interacts with the IAA signal transduction pathway.
Displacement of stems and branches by wind or mechanical stress in woody species results in the formation of reaction wood. This response is unilateral and beneficial for the tree in that it creates physical strains in the wood that force the stem or branch back toward its original orientation in space (Scurfield, 1973; Wilson and Archer, 1977; Timell, 1986). Angiosperm and gymnosperm trees differ in their nature of reaction wood. In angiosperm trees, such as poplar, reaction wood is called tension wood and forms on the upper side of stems that have been displaced. Tension wood characteristically has few, small vessels, and fibers with an inner gelatinous cell wall layer (the G-layer) that consists of almost pure cellulose with microfibrils that are parallel to the long cell axis (Haygreen and Bowyer, 1996; Jourez et al., 2001). In gymnosperm trees, such as pine, reaction wood is called compression wood and forms at the lower side of displaced stems. Compression wood is characterized by short, rounded tracheids that have thick walls with increased lignin content and increased microfibril angles (Timell, 1969). The formation of reaction wood is often (but not always) accompanied by a stimulation of cambial cell division, whereas the cell division at the opposite side is more or less inhibited.
The physiology and development of reaction wood formation has been extensively explored (particularly in gymnosperms) and reviewed in great detail by Timell (1986). The induction of reaction wood by gravistimuli rather than by mechanical stimulation has been deduced from a large number of bending, leaning, and clinostat experiments. Reaction wood can also be induced by internal mechanisms different from gravistimulation. It is, for example, responsible for the upward bending of side shoots after loss of the leader. The molecular mechanisms underlying the reaction wood response interact with cells at all stages of the developmental sequence of wood formation. In early developmental stages, the frequency of cambial cell divisions is increased, and, in angiosperms, the determination of xylem mother cells to vessel element is inhibited. During cell expansion the shape of tracheary elements is altered, and in late development the chemistry and structure of the secondary wall is strongly modified. This raises the question whether the inductive signal acts irreversibly on xylem mother cells early in development, or alternatively, if all cells along the developmental gradient can respond to the inductive signal(s). In conifers the latter has been shown to be the case, i.e. developing xylem cells can be reversible induced during the whole differentiation process (see Timell, 1986). Consequently, there are xylem cells that exhibit only some of the characteristics typical of reaction wood. From our own unpublished experiments, the same seems to hold true for poplar. Moreover, the enhanced growth rate seen in the reaction wood response is uncoupled from the modification of xylem cell differentiation, although these events normally occur in parallel. These observations imply that the different traits of the reaction wood are independently regulated. Thus, reaction wood formation offers an experimental system for exploring the molecular control of several important aspects of wood development.
A widely accepted model describing the role of auxin in reaction wood formation has been developed from a large number of application experiments using auxin and auxin-transport inhibitors. The model postulates that tension wood requires a difference in auxin concentration around the stem and forms in the region deficient in indole-3-acetic acid (IAA), whereas compression wood is induced by an increase in auxin concentration (for review, see Timell, 1986; Little and Savidge, 1987; Srivastava, 2002). However, for the correct interpretation of application experiments direct information on the resulting internal hormone status is required. But in the only case where this has been documented in reaction wood formation, the previous assumption about the auxin status was shown to be incorrect (Sundberg et al., 1994). Moreover, to advance hypotheses developed from application experiments we need to know the balance of endogenous hormones under natural conditions in the processes they are supposed to control. In reaction wood formation, reliable measurements of endogenous IAA are scarce, contradictory, and limited because small numbers of samples were evaluated (Wilson et al., 1989; Funada et al., 1990; Moyle et al., 2002). Interpretation of endogenous IAA levels in cambial region tissues is further complicated by its large variation in concentrations across the developing wood tissues (Uggla et al., 1996, 1998). In this study we set out to test the dogma that reaction wood is induced by differential endogenous IAA levels in both a gymnosperm (Scots pine, Pinus sylvestris) and an angiosperm (aspen, Populus tremula, denoted poplar) tree species. We could not detect the kind of changes in the IAA balance postulated by current models. This finding emphasizes the importance of factors other than differences in auxin concentrations, such as auxin perception mechanisms and/or additional signals.
RESULTS
Induction of Reaction Wood
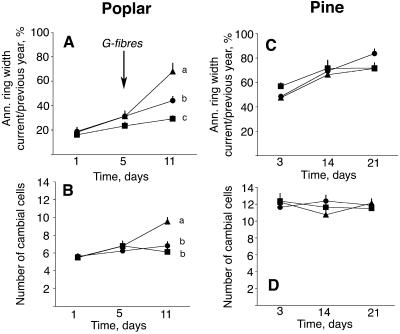
Reaction wood was induced in a number of independent experiments where field-grown trees were displaced from their upright position by bending. In poplar, all features of typical tension wood were induced along the developmental gradient of wood formation, i.e. increased growth, reduction in vessel size and density, and the formation of G-fibers (Fig. 1, A–C; Fig. 2A). The stimulation of cambial cell division on the upper side was associated with an increase in the number of cambial zone cells (Fig. 2B). At the opposite side, growth was inhibited, and the number of cambial zone cells remained the same throughout the experimental period. In Scots pine, bending induced typical compression wood tracheids at the lower side of the stem characterized by a rounded shape and intracellular spaces (Fig. 1, D and E). Cambial growth in this experiment was unaffected by bending (Fig. 2, C and D), reflecting the fact that the differentiation of reaction wood is not necessarily coupled to a growth stimulation.
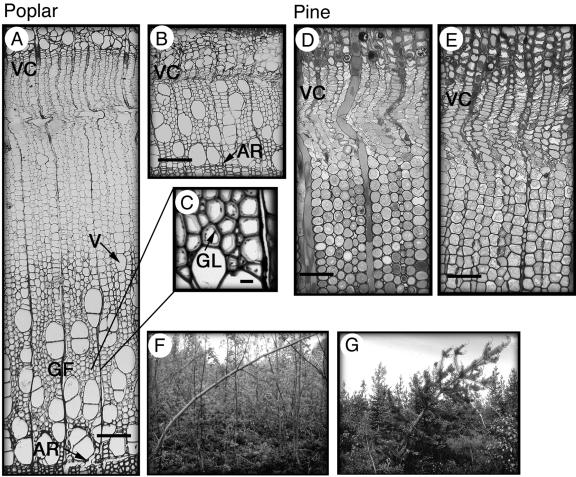
Figure 1.
Anatomy of the reaction wood side and the opposite side of gravistimulated poplar and pine trees. A, Formation of tension wood at the upper side of the poplar stem. Growth is vigorous with a wide zone of primary walled, dividing, and expanding cells. Vessel size and density are reduced and, as shown in C, the fibers form a gelatinous cell wall layer. B, Formation of wood at the opposite side in poplar. Note the difference in growth between the tension wood and opposite wood sides. D, The formation of compression wood at the lower side of the pine stem. Tracheids are rounded and have intercellular spaces. E, The formation of wood at the opposite side in pine. F and G, Reaction wood was induced by bending the poplar (F) and pine (G) trees and fixing the stem with a string. Scale bars represent 100 μm in A, B, D, and E, and 10 μm in C. VC, vascular cambium; V, vessel; GF, gelatinous fibers; AR, annual ring; GL, gelatinous layer.
Figure 2.
Cambial growth patterns in gravistimulated and upright poplar and pine trees. A and C, Time course of annual ring width development. B and D, Number of cambial zone cells counted under the microscope, at the tension wood/compression wood side (▴), opposite wood side (▪), and in upright controls (•). Mean ± se (n = 8). The significance of treatments was tested for all time points by analysis of variance followed by the Duncan test at P ≤ 0.05. Different letters indicate significant differences between the treatments at the indicated time point. No letters means no significant differences were observed between the treatments at that time.
Distribution and Dynamics of IAA during Reaction Wood Formation
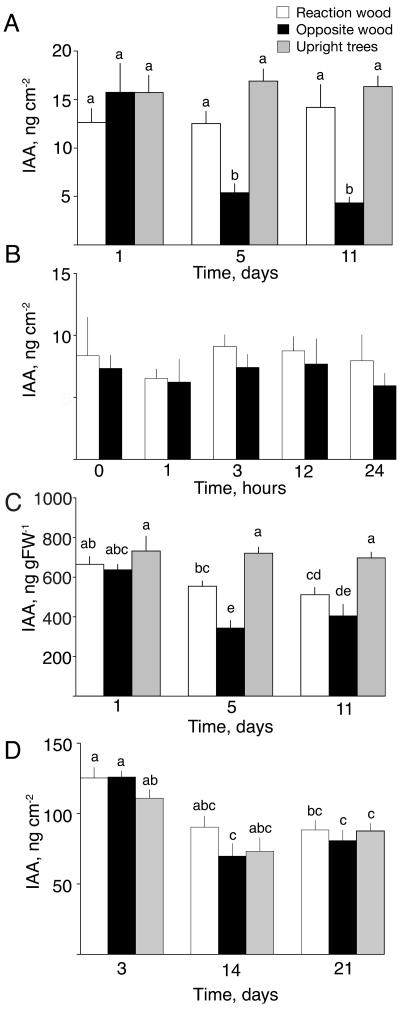
Gravistimulation of poplar trees did not affect the total amount of IAA in the cambial region on the upper tension wood side as compared to upright control trees, whereas large reductions in amounts of IAA were observed at the lower side of the stem (Fig. 3A). This decrease in IAA levels was slow and was not detectable within the first 24 h of treatment, as confirmed in an additional independent experiment (Fig. 3B). Furthermore, the decrease was not a result of IAA conjugation, as no significant amounts of hydrolyzable IAA conjugates were detected in the cambial tissues, either in the bent trees or in the upright control trees (Table I). The absence of IAA conjugates in cambial tissues agrees with earlier observations in poplar stems (Tuominen et al., 1995).
Figure 3.
Levels of IAA in the cambial region tissues during the formation of reaction wood. Time courses of IAA levels in poplar are shown (A) expressed as the total amount of IAA per unit stem area during the first 11 d after induction and (B) during 24 h of induction, and (C) expressed as concentration per unit tissue weight. D, Time course of total amount of IAA per unit stem area in pine during 21 d of induction. Mean ± se (n = 6). White bars, reaction wood; black bars, opposite side wood; gray bars, upright control trees. The significance of treatments was tested for all time points by analysis of variance followed by the Duncan test at P ≤ 0.05. Different letters indicate significant differences.
Table I.
Conjugated IAA in the cambial region of bent trees forming reaction wood and opposite wood, and in upright trees forming normal wood
| Poplar | 1 d
|
5 d
|
11 d
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| TW | OW | Upright | TW | OW | Upright | TW | OW | Upright | |
| Before hydrolysis | 692 | 619 | 709 | 522 | 358 | 687 | 472 | 443 | 682 |
| After hydrolysis | 758 | 655 | 734 | 563 | 397 | 716 | 494 | 449 | 654 |
| Pine | 3 d
|
14 d
|
21 d
|
||||||
| CW | OW | Upright | CW | OW | Upright | CW | OW | Upright | |
| Before hydrolysis | 1659 | 1711 | 1760 | 1818 | 1777 | 1713 | 2026 | 2010 | 1836 |
| After hydrolysis | 1548 | 1596 | 1685 | 1727 | 1847 | 1721 | 1914 | 2053 | 1822 |
IAA was measured in poplar and pine trees before and after hydrolysis with 7 n NaOH at 100°C for 3 h and is given as nanogram per gram FW. For each tissue and date 5 independent trees were measured and the difference in IAA before and after hydrolysis was tested by pairwise t-test. In no case a significant difference was found (P ≤ 0.01). TW, tension wood; CW, compression wood; OW, opposite wood.
Because of the large difference in IAA concentration across the cambial tissues, expressing IAA as a total amount per unit stem area and as a concentration of IAA per unit tissue weight may give rise to different patterns of IAA changes when samples with different proportions of specific types of tissue are compared (see Uggla et al., 1998). In such cases, IAA should be calculated both as an amount and as a concentration to give a more detailed picture of any changes in the tissues. In the poplar trees, for example, a much wider zone of developing xylem cells was present when tension wood was formed than in the formation of normal wood. Hence, the amount of sampled tissue per unit area was larger (data not shown). Consequently, the total amount of IAA, which was unaffected by the tension wood induction, was distributed across a wider region of tissues. Thus, the concentration of IAA in these tissues decreased when tension wood was formed (Fig. 3C). Similarly, a decrease in the number of developing cells at the opposite side means that samples will contain less tissue per unit area, so the decrease in IAA concentration was not as large as the decrease in the total amount of IAA.
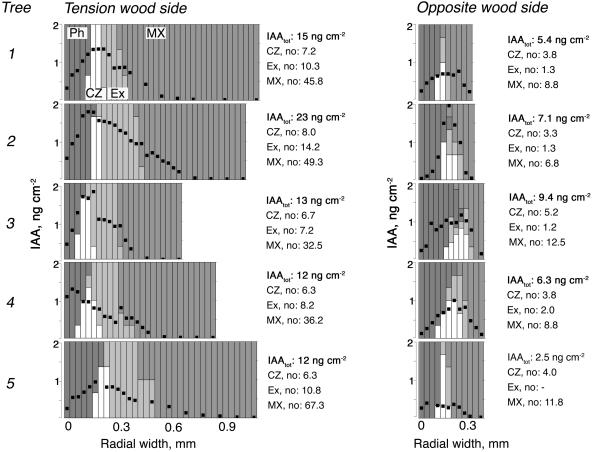
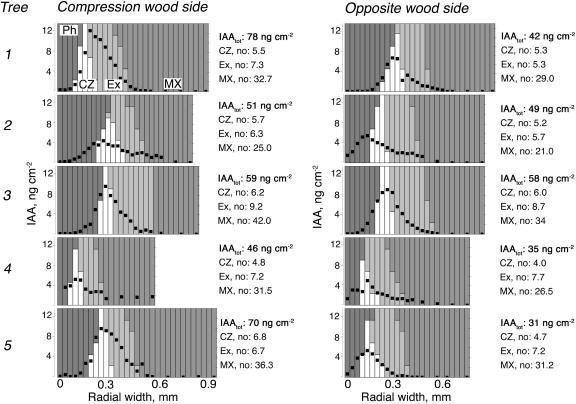
The large size of the cambial meristem in trees gives an opportunity to map the pattern of IAA concentrations across the cambial region tissues by using tangential cryosectioning combined with sensitive gas chromatography-mass spectrometry (GC-MS) analysis (Uggla and Sundberg, 2001). This was done in trees sampled after 15 d of gravistimulation, at a time when tension wood was actively developing at the upper side and growth at the lower side was inhibited (Fig. 4). The distribution pattern of IAA at both the tension wood side and the opposite wood side was similar to patterns earlier observed in upright trees (Tuominen et al., 1995, 2000), i.e. there was a peak concentration in the cambial zone and steep gradients toward the xylem and phloem sides. The slight shoulder at the transition between primary and secondary walled cells has also been observed earlier in upright trees (Tuominen et al., 2000). By mapping the IAA distribution, we clearly observed that not only the amount of IAA, but also the peak concentration, was lower at the opposite side. The extent of the decrease in the amount of IAA and the concentration varied between different trees, but it is notable that the decrease in peak concentration was less dramatic than the decrease in the amount. In fact, in one of the five trees the peak concentration was similar at both sides.
Figure 4.
Levels and distribution patterns of IAA across the cambial region at the tension wood side and the opposite wood side in gravistimulated poplar trees. The radial distribution pattern of IAA was measured across the cambial region tissues at the tension wood and opposite wood sides of 5 field-grown trees actively forming tension wood after 15 d of bending. IAA was measured in 30 μm tangential sections obtained by a cryomicrotome. Each column represents one sample. The amount of IAA per centimeter squared section is indicated with a black dot, and the shading of the column reflects the composition of tissues in the sample, as explained in the figure. The total amount of IAA per centimeter squared stem area (i.e. the integrated area under the gradient) and the number of cells in different tissues for each tree are indicated to the right of each graph. Ph, phloem; CZ, cambial zone; Ex, expanding fibers; MX, maturing and mature xylem.
In Scots pine, the induction of compression wood by bending did not cause any changes in IAA amounts between the compression wood side and the opposite wood side, or between bent and upright trees, during the time course experiment (Fig. 3D). Because the proportion of tissues in different developmental stages, and hence the amount of sampled tissue per unit area, was not affected, the calculation of IAA concentration per unit fresh weight (FW) in this case gave the same results (data not shown). No significant amounts of hydrolyzable IAA conjugates were detected in compression wood-forming trees or in control trees (Table I), which agrees with earlier observation in Scots pine (Sundberg et al., 1990). During the experimental period a general decline in IAA content was observed for all treatments, which we attribute to its normal pattern of seasonal variation (see Sundberg et al., 2000). We also applied the tangential sectioning technique to the pine trees that had been bent for 15 d and were forming compression wood at their lower side. This showed that the pattern of IAA distribution across the cambial region tissues is not affected when compression wood is formed. The typical pattern, with a peak in the cambial zone and steeply decreasing levels toward the xylem and phloem as earlier described in Scots pine (Uggla et al., 1996, 1998), was found on both the compression wood side and the opposite side (Fig. 5). In this experiment, some trees exhibited identical amounts and concentrations of IAA across the cambial tissues on both sides, whereas other trees showed a slight increase in both the amounts and concentrations at the compression wood side.
Figure 5.
Levels and distribution patterns of IAA across the cambial region at the compression wood side and the opposite wood side in gravistimulated pine trees. The radial distribution pattern of IAA was measured across the cambial region tissues at the compression wood and opposite wood sides of 5 field-grown trees actively forming compression wood after 15 d of bending. IAA was measured in 30 μm tangential sections obtained by a cryomicrotome. Each column represents one sample. The amount of IAA per centimeter squared section is indicated with a black dot, and the shading of the column reflects the composition of tissues in the sample, as explained in the figure. The total amount of IAA per centimeter squared stem area (i.e. the integrated area under the gradient) and the number of cells in different tissues for each tree are indicated to the right of each graph. Ph, phloem; CZ, cambial zone; Ex, expanding fibers; MX, maturing and mature xylem.
In two cases (in pine tree no. 2 on the opposite side, and in poplar tree no. 4 on the tension wood side) the peak of the IAA gradient did not coincide with the cambial meristem. Similar phenomena have occasionally been observed in earlier studies with pine trees (Uggla et al., 2001), and we attribute this to the longitudinal waviness of the cambial zone.
DISCUSSION
When reaction wood is induced by gravistimulation developing xylem cells at all stages of differentiation respond to the stimuli, and if the induction is not maintained all throughout the differentiation process the cells reverts to their normal pattern of development (Timell, 1986). Thus, substances involved in transducing the induction signals must act on all developing xylem cells in a radial file, and the induction needs to be maintained in these cells as long as reaction wood is being formed. This could conceivably be caused by signaling substance(s) acting across all developing cells. When evaluating the function of plant hormones in reaction wood formation it is therefore important to assay their abundance at specific developmental stages, in addition to measuring their overall amounts in the combined tissues of the cambial region. It has been hypothesized that induction of reaction wood is due to a radial redistribution of auxin across the developing wood tissues rather than to changes in its overall amount (Morey and Cronshaw, 1968a; Sundberg et al., 1994). A shift in IAA concentration from differentiating to dividing cells, for example, was used to explain the anomaly in the idea that tension wood differentiation requires an auxin deficiency, whereas the growth stimulation that occurs during tension wood formation should require an increase in auxin. However, our data do not support the idea of a radial auxin redistribution because the concentration pattern of IAA across the reaction-wood-forming tissues, in both gravistimulated poplar and pine trees, resembles the pattern found in vertical trees forming normal wood (Figs. 4 and 5; Uggla et al., 1996; Tuominen et al., 1997). These data were collected after 15 d of gravistimulation when reaction wood was being formed, and early transient changes in the radial auxin distribution, which may be of importance in the induction process, cannot be excluded. But, nevertheless, repatterning of the auxin concentration gradient seems not to be the signaling system that maintains the cells into the mode of reaction wood development.
In poplar, induction of tension wood caused no difference in the amount of IAA in the cambial region tissues as compared with amounts found in upright trees, whereas the IAA level at the opposite side was greatly reduced (Fig. 3A). The reduction at the opposite side may be due to an active lateral redistribution toward the tension wood side by auxin transporters recently demonstrated in poplar cambial tissues (Schrader et al., 2003) and cause the growth inhibition observed on the opposite side. But it is equally likely that cambial cell division is inhibited by other mechanisms, and the decrease in auxin is secondary, merely reflecting a lower capacity for polar auxin transport in cambial tissues displaying little or no growth. The rather high concentrations of IAA in the cambial meristem at the opposite side (Fig. 4), the slow response in the decrease of the IAA pool, and the lack of increase of IAA at the tension wood side (Fig. 3A) favors this hypothesis. The notion that the unilateral cambial growth inhibition in gravistimulated shoots is due to unresponsiveness to IAA rather than an IAA deficiency is also supported by experiments where IAA was applied to horizontal shoots of both gymnosperm and angiosperm shoots, and only stimulated growth at the lower and upper sides, respectively (Wareing et al., 1964). This unresponsiveness to IAA would be in analogy to the cessation of cambial growth during the induction of dormancy; another developmental event that is not mediated by a decrease in IAA concentrations (Uggla et al., 2001).
Although the amount of IAA was not affected at the tension wood side as compared to upright trees, IAA was lower in these tissues when expressed as a concentration per unit FW (Fig. 3C). This was due to the growth stimulation that increased the radial width of the wood-forming tissues (and the amount of tissue sampled per unit area) at the tension wood side. It is not likely, however, that this small decrease in IAA concentration induced the differentiation of tension wood, as much larger reductions in both the amount and concentration of IAA took place on the opposite side (Fig. 3, A and B). Thus, the idea that tension wood is induced at the position of the stem circumference where the concentration is lowest (Cronshaw and Morey, 1968; Morey and Cronshaw, 1968a, 1968b), was not supported. It is also noted that strong growth stimulation at the tension wood side took place without an increase in IAA. This is an interesting observation because IAA is considered to control the extent of cambial growth both in gymnosperm and angiosperm trees (Sundberg et al., 2000). However, it is also well established that other hormones, such as gibberellins and ethylene, can act in synergy with auxin in promoting cambial growth (Little and Savidge, 1987; Little and Pharis, 1995). The enhanced growth during tension wood formation may be mediated through signals other than IAA and/or through a change in IAA sensitivity.
Compression wood tracheids differentiated in gravistimulated Scots pine trees without any changes in endogenous IAA levels as compared to those in either upright control trees or the opposite side in the same tree (Fig. 3D). This lack of growth response provided an opportunity to specifically evaluate the role of auxin in the differentiation of compression wood tracheids. Other studies have found lower IAA levels associated to compression wood formation (Wilson et al., 1989; Sundberg et al., 1994), although the study by Wilson only measured IAA in tissues of differentiating tracheids (xylem scrapings), thus excluded most of the auxin, which is present in the cambial meristem and its recent derivatives (Uggla et al., 1996). Funada and coworkers (1990), on the other hand, found higher IAA contents in cambial region tissues forming compression wood compared to tissues forming normal wood after 1 week, but not after 4 weeks, of gravistimulation in Cryptomeria trees, but limited their studies to a single tree. Our results show that compression wood tracheids induced by gravistimulation can differentiate under similar endogenous auxin concentrations as normal tracheids.
Gravistimulation of primary roots and shoots resulting in differential growth responses and geotropism is mediated by auxin efflux regulators and a lateral transport of IAA toward the lower side in coleoptiles, shoots, and roots as demonstrated in maize coleoptiles, snapdragon, and Arabidopsis (Philippar et al., 1999; Philosoph-Hadas et al., 2001; Friml et al., 2002). In line with these findings, experiments where radiolabeled IAA was applied to woody horizontal shoots of both gymnosperms and angiosperms species have shown that transport is also favored toward the lower side (Leach and Wareing, 1967; Starbuck and Phelps, 1986; Lachaud, 1986). Endogenous levels of IAA were not measured in these experiments, but in the much older stems of pine and poplar used in our study, gravistimulation did not seem to induce any downward redistribution of IAA. Neither could we detect any major radial redistribution of IAA across the cambial region tissues (Figs. 4 and 5). It is possible that the young woody shoots used in the transport experiments mentioned above retained the response mechanisms for geotropic bending present in shoot tips and therefore favored IAA transport toward the lower side. However, no such mechanism appears to be required for the induction of reaction wood. Recent analysis of agravitropic Arabidopsis mutants suggested different transduction pathways in the gravitropic responses in roots, shoots, and hypocotyls (Tasaka et al., 1999). In analogy to this, mechanisms involved in mature woody stems' responses to gravistimuli may differ from those involved in the tropical movements of roots and shoots.
Although reaction wood differentiates under similar endogenous IAA concentrations as normal wood in both poplar and pine, modifying the auxin balance by applying auxin or auxin transport inhibitors can induce reaction wood (for reviews, see Timell, 1986; Little and Savidge, 1987). In angiosperm trees, application of auxin on the upper side of stems prevents the formation of tension wood on that side. Moreover, ringing upright stems with IAA transport inhibitors caused tension wood to form below the ring, whereas adding auxin together with the transport inhibitor prevented the formation of tension wood. In gymnosperms, auxin applications at supra-optimal concentrations have been repeatedly observed to induce compression wood. Predicting the function of IAA from these experiments is, however, problematic because it is interpreted from assumed, and not measured, internal auxin levels that result from the applications. The observed induction of reaction wood may be a result of auxin concentrations that never occur in the intact plant, or the resulting IAA levels may be different from the assumed levels (see Sundberg et al., 1994). Nevertheless, application experiments suggest that auxin signaling cross-communicates with the signaling system inducing reaction wood. The possibility cannot be excluded that auxin response genes are involved and that the gravistimuli affect auxin sensitivity. It was recently shown that the expression of some Aux/IAA genes was altered after gravistimulation of poplar stems (Moyle et al., 2002).
Following detailed analyses of IAA in wood-forming tissues in recent years, it has been postulated that the major functions of auxin in these tissues include maintenance of the cambial meristem, provision of positional signals for the ordered pattern of xylem and phloem differentiation, and the coordination of stem growth with leaf biomass development (Sundberg et al., 2000). The results from the work presented here, together with data of Uggla et al. (2001), suggest that environmental modification of cambial growth patterns, such as the induction of reaction wood by gravistimuli and the photoperiodic induction of growth cessation, is not mediated by changes in the IAA level in the cambial tissues. Identification of the key components regulating these wood-specific traits is still awaited. But progress will be facilitated by expressed sequence tag sequencing and microarray analysis of tension wood forming tissues from poplar, which is ongoing in our laboratory as well as elsewhere. Combined with information from recent poplar genome sequencing and functional genomics approaches this data will shed new light on the molecular mechanisms of reaction wood formation.
MATERIALS AND METHODS
Plant Materials
Field-grown aspen tree (Populus tremula, denoted poplar) and Scots pine trees (Pinus sylvestris, denoted pine) of uniform size were selected from natural stands near Umeå, Sweden (63°50′ N, 20°20′ E). The poplar trees were 4.5 to 5.5 m high, 3 cm in diameter at breast height, and about 15 years old. The pine trees were 2.5 to 3 m high, 3 cm in diameter at breast height, and 15 years old. In late June, during the most active period of cambial growth, trees were bent and fixed with a string so that the midpoint of the stem was at an angle of about 45°. Stem pieces were sampled from the midpoint of the stems. Samples for hormone analysis were immediately frozen in liquid nitrogen, transported to the laboratory on dry ice, and stored at −80°C. Samples for microscopy were frozen on dry ice and stored at −20°C. Plant hormones were measured in stem pieces obtained from the upper and the lower quarter of the circumference. Stem pieces for IAA analysis across cambial region tissues were obtained from trees about 7 m high and 7 cm in diameter (breast height) after 15 d of bending.
Sample Preparation and Anatomy
Cambial region tissues were obtained by peeling the bark and scraping the exposed xylem and bark surfaces with a scalpel. The fractions were combined, so each sample consisted of differentiating and mature phloem elements, cambial zone cells, and radially expanding and maturing xylem elements.
Tangential sections for IAA measurements across the cambial region tissues were obtained by trimming frozen samples into 2 mm (tangential) × 10 mm (radial) × 15 mm (vertical) blocks consisting of phloem, cambium, and xylem. The blocks were cryosectioned at −20°C with an HM 505E microtome (Microm Laborgeräte, Walldorf, Germany) according to Uggla and Sundberg (2001). The radial position of the tangential sections was determined in cross sections sampled after every third tangential section. Endogenous IAA was measured separately in each tangential section. For each tree, IAA was expressed as IAAtot, calculated by summing the amounts of IAA/cm2 section for each section of the sampling series (missing values were interpolated). IAAtot thus expresses the total amount of IAA per tangential cm2 area.
Anatomical investigations were performed on transverse hand-cut sections obtained from fresh material by a cryomicrotome and stained with toluidine blue. The width of annual rings and the number of cells at different stages of development, as defined by Uggla et al. (1998), were determined under the microscope at five positions.
Quantification of IAA and IAA Conjugates
Endogenous IAA was quantified using an isotope dilution mass spectrometry technique, according to the method of Edlund et al. (1995). Briefly, plant tissue was homogenized in liquid nitrogen and extracted in 0.01 m phosphate buffer with [13C6]IAA (Cambridge Isotope Laboratories, Woburn, MA) added as an internal standard. Samples were purified by addition of XAD-7 ion-exchange resin (Serva, Heidelberg) and eluted in dichloromethane. The samples were methylated and trimethylsilylated before analysis by GC-MS-SRM using a JEOL MStation mass spectrometer (JEOL, Tokyo). All data were analyzed by the JEOL MS-MP 9021D data system. IAA conjugates were estimated by splitting the sample into two aliquots after homogenization. One of the aliquots was hydrolyzed with 7 n NaOH at 100°C for 3 h, neutralized with HCl, and purified as described before, giving the total amount of IAA (free IAA and hydrolyzable IAA). The other aliquot was analyzed for free IAA as described above. The amount of IAA conjugates (hydrolyzable IAA) was calculated as the difference between the total and the free IAA concentrations.
Acknowledgments
We thank Drs. Ewa Mellerowicz and Rishikesh Bhalerao for valuable comments on the manuscript and Erik Walfridsson for technical assistance.
This research was supported by Formas, the Swedish Research Council, and the Foundation for Strategic Research.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.038927.
References
- Cronshaw J, Morey PR (1968) The effect of plant growth substances on the development of tension wood in horizontally inclined stems of Acer Rubrum seedlings. Protoplasma 65: 379–391 [Google Scholar]
- Edlund A, Eklöf S, Sundberg B, Moritz T, Sandberg G (1995) A microscale technique for gas chromatography-mass spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissues. Plant Physiol 108: 1043–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkowa E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Funada R, Mizukami E, Kubo T, Fushitani M, Sugiyama T (1990) Distribution of indole-3-acetic acid and compression wood formation in the stems of inclined Cryptomerica japonica. Holzforschung 44: 331–334 [Google Scholar]
- Haygreen JG, Bowyer JL (1996) Forest Products and Wood Science, Ed 3. Iowa State University Press, Ames, IA, pp 108–120
- Jourez B, Riboux A, Leclercq A (2001) Anatomical characteristics of tension wood and opposite wood in young inclined stems of poplar (Populus euramericana CV “Ghoy”). IAWA J 22: 133–157 [Google Scholar]
- Lachaud S (1986) Xylogenésè chez les dicotédones arborescentes. V. Formation du bois de tension et transport de l'acide indole acétice tritié chez le Hêtre. Can J Bot 65: 1253–1258 [Google Scholar]
- Leach RWA, Wareing PF (1967) Distribution of auxin in horizontal woody stems in relation to gravimorphism. Nature 214: 1025–1027 [Google Scholar]
- Little CHA, Pharis RP (1995) Hormonal control of radial and longitudinal growth in the tree stem. In BL Gartner, ed, Plant Stems: Physiology and Functional Morphology. Academic Press, San Diego, pp 281–319
- Little CHA, Savidge RA (1987) The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul 6: 137–169 [Google Scholar]
- Morey PR, Cronshaw J (1968. a) Induction of tension wood by 2,4-dinitrophenol and auxins. Protoplasma 65: 393–405 [Google Scholar]
- Morey PR, Cronshaw J (1968. b) Developmental changes in the secondary xylem of Acer Rubrum induced by various auxins and 2,3,5-tri-iodobenzoic acid. Protoplasma 65: 287–313 [Google Scholar]
- Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. Plant J 31: 675–685 [DOI] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M, et al. (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 96: 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philosoph-Hadas S, Friedman H, Meir S, Berkovitz-SimanTov R, Rosenberger I, Halevy AH, Kaufman PB, Balk P, Woltering EJ (2001) Gravitropism in cut flower stalks of snapdragon. Adv Space Res 27: 921–932 [DOI] [PubMed] [Google Scholar]
- Schrader J, Baba K, May ST, Palme K, Bennet M, Bahlerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA 100: 10096–10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurfield G (1973) Reaction wood: its structure and function. Science 179: 647–655 [DOI] [PubMed] [Google Scholar]
- Srivastava LM (2002) Plant Growth and Development. Academic Press, London, pp 329–339
- Starbuck CJ, Phelps JE (1986) Induction of compression wood in rooted cuttings of Pseudotsuga menziesii (Mirb.) Franco by indole-3-acetic acid. IAWA Bull 7: 13–16 [Google Scholar]
- Sundberg B, Little CHA, Cui K (1990) Distribution of indole-3-acetic acid and the occurrence of its alkali-labile conjugates in the extraxylary region of Pinus sylvestris stems. Plant Physiol 93: 1295–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg B, Tuominen H, Little CHA (1994) Effects of the indole-3-acetic acid (IAA) transport inhibitors N-1-naphtylphthalamic acid and morphactin on endogenous IAA dynamics in relation to compression wood formation in 1-year old Pinus sylvestris L. shoots. Plant Physiol 106: 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg B, Uggla C, Tuominen H (2000) Cambial growth and auxin gradients. In R Savidge, J Barnett, and R Napier, eds, Cell and Molecular Biology of Wood Formation. BIOS, Oxford, pp 169–188
- Tasaka M, Kato T, Fukaki H (1999) The endodermis and shoot gravitropism. Trends Plant Sci 4: 103–107 [DOI] [PubMed] [Google Scholar]
- Timell TE (1969) The chemical composition of tension wood. Sven papperstidn 72: 173–181 [Google Scholar]
- Timell TE (1986) Compression Wood in Gymnosperms, Vol 2. Springer-Verlag, Heidelberg, pp 983–1262
- Tuominen H, Puech L, Fink S, Sundberg B (1997) A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol 115: 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Puech L, Regan S, Fink S, Olsson O, Sundberg B (2000) Cambial-region-specific expression of the Agrobacterium iaa genes in transgenic aspen visualized by a linked uidA reporter gene. Plant Physiol 123: 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Sitbon F, Jacobsson C, Sandberg G, Olsson O, Sundberg B (1995) Altered growth and wood characteristics in transgenic hybrid aspen expressing Agrobacterium tumefaciens T-DNA indoleacetic acid-biosynthetic genes. Plant Physiol 109: 1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EJ, Sundberg B (1998) Indole-3-acetic acid controls cambial growth in Scots pine by positional signalling. Plant Physiol 117: 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Magel E, Moritz T, Sundberg B (2001) Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol 125: 2029–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B (1996) Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA 93: 9282–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Sundberg B (2001) Sampling of cambial region tissues for high resolution analysis. In NJ Chaffey, ed, Wood Formation in Trees. Taylor & Francis, London, pp 215–228
- Wareing PF, Hanney CEA, Digby J (1964) The role of endogenous hormones in cambial activity and xylem differentiation. In MH Zimmermann, ed, The Formation of Wood in Forest Trees. Academic Press, New York, pp 323–344
- Wilson BF, Archer RR (1977) Reaction wood: induction and mechanical action. Annu Rev Plant Physiol 28: 23–43 [Google Scholar]
- Wilson BF, Chien CT, Zaerr JB (1989) Distribution of endogenous indole-3-acetic acid and compression wood formation in reoriented branches of douglas-fir. Plant Physiol 91: 338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]