Abstract
Idiopathic normal pressure hydrocephalus (iNPH) is a syndrome characterized by gait disturbance, cognitive deterioration and urinary incontinence in elderly individuals. These symptoms can be improved by shunt operation in some but not all patients. Therefore, discovering predictive factors for the surgical outcome is of great clinical importance. We used normalized power variance (NPV) of electroencephalography (EEG) waves, a sensitive measure of the instability of cortical electrical activity, and found significantly higher NPV in beta frequency band at the right fronto-temporo-occipital electrodes (Fp2, T4 and O2) in shunt responders compared to non-responders. By utilizing these differences, we were able to correctly identify responders and non-responders to shunt operation with a positive predictive value of 80% and a negative predictive value of 88%. Our findings indicate that NPV can be useful in noninvasively predicting the clinical outcome of shunt operation in patients with iNPH.
Idiopathic normal pressure hydrocephalus (iNPH) is a neuropsychiatric disorder characterized by excessive accumulation of cerebrospinal fluid (CSF) in the cerebral ventricles and symptoms of gait disturbance, cognitive deterioration and urinary incontinence. Despite the typical ventricular enlargement, CSF pressure remains normal and there is no history of antecedent cause, such as severe head trauma, subarachnoid hemorrhage, meningitis or tumor. The prevalence of iNPH has become apparent by community or population-based studies. These studies have indicated the existence of many iNPH patients who are not treated. For example, community-based studies in Japan showed that the prevalence of possible iNPH was 0.37% at age 70 years, 1.42% at age 80 years1 and 1.1% in the population older than 65 years, which suggests approximately 310,000 people may suffer from iNPH in Japan today2,3,4,5. Also, a population-based study in Sweden has shown that the prevalence of possible iNPH is 2.1% in the population older than 70 years, and currently approximately 2 million people in Europe may suffer from iNPH6.
An intriguing aspect of this syndrome is that it is potentially treatable, unlike other types of dementia. In particular, symptoms can improve after CSF shunt operation. However, some patients do not show improvement after shunt operation. Therefore, it is an urgent issue to find predictive factors for the surgical outcome. In a previous study, in order to investigate the mechanisms of functional recovery, we performed normalized power variance (NPV) analysis, which is a sensitive measure of the instability of cortical electrical activity7, on electroencephalography (EEG) data before and after CSF tapping, a procedure that drains a small volume of CSF (30 ml) by lumbar puncture. A striking finding was that cortical functional recovery induced by CSF tapping was associated with NPV changes. More specifically, improvement of walking time attributed to CSF tapping correlated with an alpha NPV decrease in the supplementary motor area (SMA) and alpha and beta NPV decreases in the prefrontal cortices (PFC), especially in the right anterior PFC. In addition, gait status recovery correlated with delta and alpha NPV decreases in the left dorsal premotor area (PMA), and attention recovery correlated with an alpha NPV decrease in the right dorsolateral PFC (DLPFC)8. These findings demonstrated the important involvement of the right anterior PFC and premotor cortex to gait improvement induced by CSF tapping in iNPH patients. In support to this notion, Miyoshi et al. revealed that frontal lobe dysfunction has significant correlation with gait disturbance in iNPH patients9. In a previous study, we also suggested a way to distinguish shunt responders from non-responders using a combination of NPV analysis and CSF tapping. However, CSF tapping is an invasive procedure and has risks of infection, post-procedural headache and bleeding. To make things worse, iNPH patients often have suffered myocardial or cerebral infarction in their past and take anti-platelet drugs, which increases the risk of bleeding by CSF tapping. In addition, the CSF tap test, a diagnostic procedure for predicting the shunt operation outcome using CSF tapping and clinical assessments, has a low negative predictive value10.
EEG has become a useful tool in neuroscience to investigate cortical functions8, and also in clinical practice for objective clinical assessment of neurological and neuropsychiatric disorders, such as epilepsy and Alzheimer's disease11. This usefulness mainly arises from the fact that EEG can directly measure brain electrical activity12,13. In specific, EEG time-series signals relate to dynamic postsynaptic potentials of the cerebral cortex, with millisecond temporal resolution11,14.
In the present study, we aimed to distinguish shunt responders from non-responders using only pre-CSF tapping EEG data, that is to say without performing CSF tapping, something that would significantly benefit iNPH patients as an alternative to tapping procedures.
Results
Demographic and clinical results
We classified responders and non-responders according to shunt operation outcome as mentioned in the Materials and Methods section. One responder showed improvement in gait and cognitive domains and the other responders showed improvement only in the gait domain. Demographic and clinical data are shown in Table 1. There were no differences in age, gender, and clinical scores between groups. The rate of responders was relatively low because we adopted stricter classification criteria of shunt response than the Japanese Clinical Guidelines in order to select patients who showed significant improvements in iNPH symptoms as responders.
Table 1. Cognitive and gait function test scores of responders and non-responders.
| Test | Responders | Non-responders |
|---|---|---|
| Age | 76 ± 5.2 | 74 ± 7.0 |
| Gender (F/M) | 4/5 | 3/6 |
| WT | 24.6 ± 6.8 | 19.7 ± 5.9 |
| TUG | 15.6 ± 5.1 | 12.0 ± 2.9 |
| GSS | 6.3 ± 3.5 | 5.0 ± 1.8 |
| MMSE | 22.7 ± 3.6 | 23.8 ± 3.6 |
| FAB | 11.2 ± 3.3 | 11.5 ± 2.8 |
| TMT-A | 138 ± 95 | 97 ± 48 |
| WMS-R_Attention/Concentration index | 77.4 ± 15.8 | 80.6 ± 13.6 |
| WAIS-III-Digit Symbol-Coding | 5.6 ± 2.6 | 6.7 ± 1.9 |
| WAIS-III-Block Design | 6.5 ± 3.6 | 7.1 ± 2.1 |
Data are mean ± SD. WT: 10-meter reciprocating walking test, TUG: 3 m Timed Up and Go, GSS: Gait Status Scale, MMSE: Mini-Mental State Examination, FAB: Frontal Assessment Battery, TMT-A: Trail Making Test Part A, WMS-R: Wechsler Memory Scale-Revised, WAIS-III: Wechsler Adult Intelligence Scale-III.
NPV analysis results
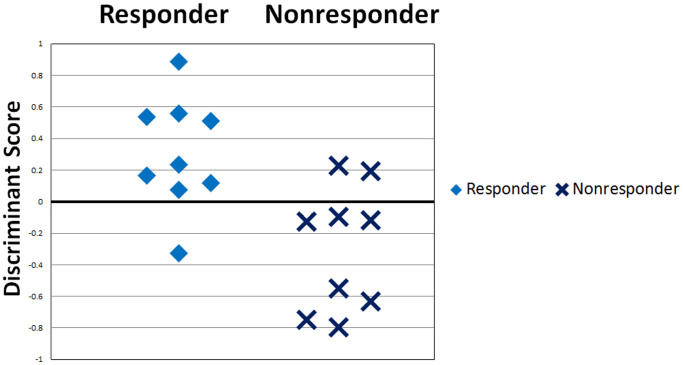
Statistically significant NPV differences between responders and non-responders in each frequency band are listed in Table 2. Responders had higher beta NPV values at the right anterior prefrontal electrode (Fp2), the right temporal electrode (T4) and the right occipital electrode (O2) compared to non-responders. Maps of beta NPV results of responders and non-responders and the t-values between them are shown in Fig. 1 (left, center and right). Using beta NPVs at Fp2, T4 and O2 electrodes simultaneously as discriminating variables, linear discriminant analysis yielded a discriminant score that most accurately distinguishes shunt responders from non-responders. The equation was: Discriminant score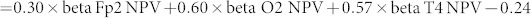 . The cut-off value of the score is zero, and positive or negative scores indicate responders or non-responders, respectively. This score could correctly identify responders and non-responders with a positive predictive value of 80% (8/10) and a negative predictive value of 88% (7/8) (Fig. 2).
. The cut-off value of the score is zero, and positive or negative scores indicate responders or non-responders, respectively. This score could correctly identify responders and non-responders with a positive predictive value of 80% (8/10) and a negative predictive value of 88% (7/8) (Fig. 2).
Table 2. NPV differences between responders and non-responders.
| NPV at band-electrode | t-value | p-value |
|---|---|---|
| Beta-Fp2 | 2.1 | 0.047 |
| Beta-O2 | 2.1 | 0.047 |
| Beta-T4 | 2.6 | 0.020 |
Figure 1. Beta band NPV in Responders and Non-responders and t-value between them.
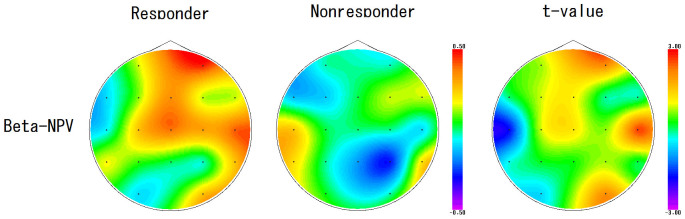
Beta band NPV in responders (left) and non-responders (center) and t-value between them (right). Responders had higher beta NPV values at the right anterior prefrontal electrode (Fp2), the right temporal electrode (T4) and the right occipital electrode (O2).
Figure 2. Distributions of the discriminant score in responders and non-responders.
Using beta NPVs at Fp2, T4 and O2 electrodes as discriminating variables, linear discriminant analysis yielded a discriminant score that separates responders and non-responders. With zero as the cut-off value, a discriminant score > 0 indicates shunt responders and a discriminant score < 0 indicates non-responders. This score could correctly identify responders and shunt non-responders with a positive predictive value of 80% (8/10) and a negative predictive value of 88% (7/8).
Power spectral analysis results
There were no significant power differences between responders and non-responders.
Discussion
In the present study, we employed NPV analysis, which sensitively detects the instability of cortical electrical activity, to find objective predictive EEG markers of shunt operation outcome in patients with iNPH. Our main findings were that: (1) responders had significantly higher values of beta NPV at the right fronto-temporo-occipital cortices [the right anterior prefrontal electrode (Fp2), the right temporal electrode (T4) and the right occipital electrode (O2)] compared to non-responders as shown in Figure 1, and (2) using these beta NPVs, we could correctly identify responders and non-responders with a positive predictive value of 80% (8/10) and a negative predictive value of 88% (7/8), as shown in Figure 2.
The regions indicated by our main findings, that is the right fronto-temporo-occipital cortices, may correspond to the area of the right ventral attention network (VAN). Right VAN was originally discovered as a visual recognition network, where visual information that has flowed from the occipital lobe is compared to visual/spatial memory in the right temporal cortex and then identified in the right temporal or frontal cortex15,16,17,18. In support to our data, accumulating results from previous studies have shown that recovery of gait induced by CSF drainage in iNPH patients may be attributed to recovery of right VAN, in addition to gait related motor areas. For example, Lenfeldt et al. and Mataró et al., using functional magnetic resonance imaging (fMRI) and single photon emission computed tomography (SPECT), have demonstrated that in addition to motor related areas, areas in the right temporoparietal junction (TPJ), which is a hub region of the right VAN, also enhanced their activity after external lumbar drainage and shunt operation in iNPH patients19,20. Allali et al., using the walking test in dual task conditions, have reported that after CSF tapping iNPH patients significantly improved allocation of attention to gait during dual task21, which is a function of right VAN17,22, compared to iNPH-like patients. Also, in a previous study, we found that alpha and beta NPV changes at the right anterior prefrontal electrode (Fp2) correlated with changes in walking time, which suggests a recruitment of the right VAN in gait control in iNPH patients8. This recruitment of VAN during gait has also been seen in patients with Parkinson's disease, and these authors discussed that an associated burn-out of VAN may lead to the freezing of gait23. Recruitment of the right VAN has also been found in healthy subjects during line tracing by hand24. These authors suggested that the role of the right VAN during motion is to modulate attention and adapt the movement using spatial information. Taking into account all of this information, our main finding (1) indicates that the area which showed a difference in cortical electrical activity corresponds to the right VAN, which supports gait function in iNPH patients. This is also consistent with our results of gait and cognitive assessment. Functional recovery induced by shunt operation occurs mainly in the gait domain but not in the cognitive domain25.
Since responders and non-responders exhibit different cortical electrical activities in the beta band, it is worthwhile to comment on the role and spatial distribution of the beta frequency band in cortical electrical activity. Beta oscillation is activated during isometric contraction, suppressed by voluntary movements, and greatest after movement termination, something that is described as post movement beta rebound (PMBR). This characteristic modification of beta is observed in motor related areas (sensorimotor and premotor areas, the basal ganglia and the cerebellum). Beta oscillation in motor related areas is thought to maintain the ongoing motor set and disorders of beta oscillation are closely related to motor impairment diseases26. For example, motor impaired patients with Parkinson's disease show abnormal enhancement of contralateral beta power in primary sensorimotor areas during isometric contraction, related to the severity of motor impairment27. These patients also have disturbance of the PMBR28. In addition, in a previous study, we found that beta oscillation in right anterior PFC, which may be an anterior part of motor supporting areas (right VAN), was implicated in gait control in iNPH patients8. However, iNPH “responder” patients showed significant difference of beta activity in motor supporting areas (right VAN) and not in motor and premotor areas, compared to non-responders. This may be explained by the fact that almost all non-responders improved their gait status, without accompanying improvements of the other walking tests. Gait status is closely associated with the left dorsal PMA8,24 and the higher-order motor areas including PMA control the cortical electrical activity of the primary motor cortex29. Therefore, we can presume that non-responders also recovered activity in motor and premotor areas to some extent, thus showing no difference compared to responders.
In a previous study, we found a correlation of NPV with brain function: an NPV increase, which describes the destabilization of cortical electrical activity, reflects functional worsening, while an NPV decrease, describing the stabilization of cortical electrical activity, reflects functional improvement8. Thus, our present study result that responders had higher beta NPV values in the right VAN is consistent with the symptom of gait disturbance in iNPH patients. However, non-responders had lower beta NPV values in the right VAN, something that contradicts gait disturbance status. This paradoxical stabilization of beta cortical electrical activity might indicate irreversible cortical impairment.
Altogether, NPV analysis can sensitively detect the difference of right VAN activity between responders and non-responders even in pre-CSF tapping EEG data, unlike other neuroimaging methods, such as fMRI, SPECT and EEG power analysis. This may rise from the fact that EEG directly detects cortical electrical activity with high temporal resolution and that NPV analysis is highly sensitive to instability of cortical electrical activity. Our findings indicate that NPV in the beta frequency band can be an electrophysiological hallmark for the prediction of shunt operation outcome without the need of performing CSF tapping. An important therapeutic implication of our results is that NPV analysis could be a useful non-invasive method to discriminate shunt responders from non-responders among the many untreated iNPH patients of the general population.
Our results should be interpreted with caution because of the following limitations: First, the sample size of iNPH patients was small and our results were not corrected for multiple comparisons. Therefore, our findings were preliminary and their validity should be further tested in notably larger size of patients. However, our main findings of NPV differences between responders and non-responders are consistent with the neuroimaging findings of iNPH and the role and spatial distribution of relative cortical functional networks. Therefore, we can assume that NPV is a reliable measure of cortical electrical activity. Second, we did not directly detect an involvement of the right VAN in gait recovery before and after shunt operation. Therefore, further studies are needed to ascertain the correlation between the right VAN activity and gait recovery.
In conclusion, our research showed that NPV reliably detects the differences of cortical electrical activity between shunt responders and non-responders in the beta frequency band, at right VAN areas of patients with iNPH. This could be done without the need of performing CSF drainage, when no other neuroimaging method has so far been able to do so. Based on our results, we suggest that NPV in the beta frequency band at right VAN areas can correctly distinguish responders from non-responders to shunt operation before the operation itself. Therefore, NPV in beta frequency band could be a powerful non-invasive tool in predicting shunt operation outcome in patients suffering from iNPH, a conclusion of important therapeutic implications.
Methods
Subjects
iNPH patients who underwent shunt operation between April 2010 and December 2013 were consecutively recruited from the Neuropsychological Clinic of the Department of Neuropsychiatry of Osaka University Hospital.
The inclusion criteria were: (1) age > 60 years; (2) at least one of the following three symptoms: gait disturbance, cognitive impairment and urinary incontinence; (3) dilated cerebral ventricles and narrowed subarachnoid space at the high convexity without severe cortical atrophy on MRI; (4) absence of diseases or conditions that might cause relative clinical symptoms or imaging findings; (5) no history of severe head trauma, subarachnoid hemorrhage, meningitis, tumor and aqueduct stenosis; (6) normal CSF pressure (<200 mm H2O) and contents at lumbar puncture, (7) right handedness and (8) a positive response to CSF tap test based on the Japanese Clinical Guidelines for iNPH5,30 and undergoing a shunt operation. Exclusion criteria were: (1) comorbidities of motor or psychiatric disorders, such as Parkinson's disease, Alzheimer's disease or schizophrenia (Alzheimer's disease was defined by severe episodic memory impairment31 and a higher Alzheimer index (>3438), evaluated using the measures of amyloid beta and tau protein in CSF32) and (2) a lack of 500-seconds (500-s) artifact free epochs in the EEG recordings. A total of 23 patients met the inclusion criteria, of which one patient was excluded due to comorbidities and four patients due to lack of artifact free epochs. In the end, 18 patients were included in this study. 13 patients were followed up for one-year and five patients for six months after shunt operation.
This study was approved by the Ethics Committee of Osaka University Hospital and written informed consent was obtained from the participants or their families. CSF tap test and shunt operation were carried out in accordance with the Japanese Clinical Guidelines for iNPH.
Gait assessment
Assessments of gait and cognition have already been described previously; for further details, please refer to our previous study8. Gait disturbance was assessed by the 10-meter reciprocating walking test (WT), the 3 m Timed Up and Go (TUG) test33 and the Gait Status Scale (GSS)34. The improvement thresholds were set at 10% improvement in the WT and TUG, and 1 point improvement in the GSS, based on standard deviations of gait function tests in iNPH patients in previous studies34,35. Improvement in WT, TUG and GSS was identified as clinical improvement in gait domain.
Cognitive assessment
Cognitive impairment was assessed by the Frontal Assessment Battery (FAB), Mini-Mental State Examination (MMSE), Wechsler Memory Scale-Revised (WMS-R)-Attention/Concentration Index, Wechsler Adult Intelligence Scale-III (WAIS-III)-Block Design/-Digit Symbol Coding, and Trail Making Test Part A (TMT-A). The improvement thresholds of the cognitive tests were set at 2, 4, 15, 3, 3 points and 30% respectively, based on previous studies31,36,37,38. Improvement in more than half of these cognitive tests was identified as clinical improvement in cognitive domain.
Assessment of shunt operation outcome
Before shunt operation, WT and TUG were performed in the morning and afternoon for five consecutive days, in order to reach a plateau of improvement by practice, and then the fastest time was adopted as the time of WT and TUG before CSF tapping. Gait disturbance and cognitive impairment were assessed by the aforementioned tests. Urinary incontinence was excluded from assessment in our study because of low reliability, as the frequency of micturition was sometimes only self-reported. After shunt operation, gait disturbance and cognitive impairment were evaluated at post-operative visits of 1, 3, 6 months, and 1 year. Shunt operation was considered positive if at least one symptom showed clinical improvement at any time of assessment after operation; otherwise it was considered negative. Consequently, the patients were classified as responders and non-responders to shunt operation. This classification criteria is stricter than the Japanese Clinical Guidelines for iNPH5, because we selected patients who showed significant improvements in iNPH symptoms as responders.
EEG recording
EEG data acquisition and the procedure of power spectral and NPV analysis have already been described in detail elsewhere8. EEG data during awake, eyes-closed resting condition were recorded for about 20 minutes using a 19-electrode EEG system (EEG-1000/EEG-1200, Nihon Kohden Inc., Tokyo, Japan), and filtered through a band-pass filter of 0.53 to 120 Hz with a sampling rate of 500 Hz. Subjects were instructed to close their eyes, relax, but stay awake. During the EEG sessions, drowsiness was avoided by providing instructions once again. For each recording, 500-s artifact free epochs were selected off-line, and imported into NPV and Power analyses.
NPV analysis
In NPV analysis program, all electrodes were re-referenced to the average reference (i.e. mean electrical potential of the 19 electrodes). NPV was calculated for every 2.56-s EEG epoch, where NPV was defined as the variance of power divided by the square of mean power, in order to obtain relative values comparable among different subjects. The output of the NPV analysis program was a z-score spatial map, which displays how many units of the standard deviation the observed NPV values at each electrode site were above or below the mean NPV values of healthy controls39. The healthy controls used in the program (27 men and 25 women, age 71.5 ± 8.4 years) had normal results in cognitive tests and magnetic resonance imaging (MRI), without any history of psychiatric or neurological disorders.
In our study, NPVs of 2.56-s EEG epochs were collected at 0.64-s steps for the whole EEG epoch (moving average filter method). Then, collected NPVs were averaged and finally the stationary mean NPV value was obtained for each subject8. These analyses were executed for five frequency bands: delta (2–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–40 Hz).
Power analysis
Power was defined as the square of the amplitude of the EEG signal at each frequency band. Power spectral analysis was done by QP-220A Neuromap software (Nihon Kohden Inc., Tokyo, Japan) in which all electrodes were re-referenced to the average reference. In this study, the output of the power spectral analysis was also displayed as z-score spatial maps, using the same healthy controls as in NPV analysis.
Statistical group analysis and linear discriminant analysis
The differences in NPV or EEG power in each frequency band at each electrode site between responders and non-responders were assessed by the independent Student's t-test. The level of significance for these tests was set at p < 0.05. These results were not corrected for multiple comparisons, and the validity of each significant difference in NPV is discussed in the discussion section. In order to find an index that most accurately distinguishes responders from non-responders, we selected NPVs in beta frequency band that had significant differences between responders and non-responders as discriminating variables and performed linear discriminant analysis using the SPSS software version 12.0 (SPSS Japan Inc., and IBM Company Tokyo, Japan).
Author Contributions
Y.A., Hiroaki K. and R.I. designed the study. Y.A. and R.I. analyzed the EEG data and wrote the manuscript. T.T. provided data of Alzheimer index. T.M., H.M. and K.I. provided programs of NPV and power analyses. H.K., T.W., H.K., T.Y., K.N. and K.Y. recruited subjects, performed CSF tapping and assessed their symptoms. All authors (Y.A., H.K., T.T., R.I., L.C., T.W., S.I., M.H., T.K., H.K., T.Y., K.N., K.Y., M.I. and M.T.) reviewed the manuscript.
Acknowledgments
This study was supported by the Research Committee of Normal Pressure Hydrocephalus and Related Disorders; Studies on the Etiology, Pathogenesis and Therapy, of the Japanese Ministry of Health, Labour and Welfare (Tokyo, Japan).
References
- Iseki C. et al. Incidence of idiopathic normal pressure hydrocephalus (iNPH): A 10-year follow-up study of a rural community in Japan. J Neurol Sci. 15, 108–112 (2014). [DOI] [PubMed] [Google Scholar]
- Hiraoka K., Meguro K. & Mori E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol Med Chir. (Tokyo) 48, 197–199 (2008). [DOI] [PubMed] [Google Scholar]
- Iseki C. et al. Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI (AVIM) in the elderly: a prospective study in a Japanese population. J Neurol Sci. 277, 54–57 (2009). [DOI] [PubMed] [Google Scholar]
- Tanaka N., Yamaguchi S., Ishikawa H., Ishii H. & Meguro K. Prevalence of possible idiopathic normal-pressure hydrocephalus in Japan: the Osaki-Tajiri project. Neuroepidemiology 32, 171–175 (2009). [DOI] [PubMed] [Google Scholar]
- Mori E. et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir. (Tokyo) 52, 775–809 (2012). [DOI] [PubMed] [Google Scholar]
- Jaraj D. et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 22, 1449–1454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y. et al. Normalized power variance change between pre-ictal and ictal phase of an epilepsy patient using NAT analysis: A case study. Conf Proc IEEE Eng Med Biol Soc. 2013, 437–440 (2013). [DOI] [PubMed] [Google Scholar]
- Aoki Y. et al. EEG and Neuronal Activity Topography analysis can predict effectiveness of shunt operation in idiopathic normal pressure hydrocephalus patients. Neuroimage Clin. 19, 522–530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N. et al. Association between cognitive impairment and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 20, 71–76 (2005). [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Hashimoto M., Mori E., Kuwana N. & Kazui H. The value of the cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 9, 1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuet L. et al. Resting-state network disruption and APOE genotype in Alzheimer's disease: a lagged functional connectivity study. PLoS One 7, e46289 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R. et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 10, 675–679 (1999). [DOI] [PubMed] [Google Scholar]
- Kurimoto R. et al. Induced oscillatory responses during the Sternberg's visual memory task in patients with Alzheimer's disease and mild cognitive impairment. Neuroimage 59, 4132–4140 (2012). [DOI] [PubMed] [Google Scholar]
- Canuet L. et al. Resting-state EEG source localization and functional connectivity in schizophrenia-like psychosis of epilepsy. PLoS One 6, e27863 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall S. L. & Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex 17, 2400–2406 (2007). [DOI] [PubMed] [Google Scholar]
- Kravitz D. J., Saleem K. S., Baker C. I. & Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 12, 217–230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner A. D. Is visual processing in the dorsal stream accessible to consciousness? Proc Biol Sci. 22, 2289–2298 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz D. J., Saleem K. S., Baker C. I., Ungerleider L. G. & Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci. 17, 26–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfeldt N. et al. Idiopathic normal pressure hydrocephalus: increased supplementary motor activity accounts for improvement after CSF drainage. Brain 131, 2904–2912 (2008). [DOI] [PubMed] [Google Scholar]
- Mataró M. et al. Postsurgical cerebral perfusion changes in idiopathic normal pressure hydrocephalus: a statistical parametric mapping study of SPECT images. J Nucl Med. 44, 1884–1889 (2003). [PubMed] [Google Scholar]
- Allali G. et al. Dual-task related gait changes after CSF tapping: a new way to identify idiopathic normal pressure hydrocephalus. J Neuroeng Rehabil. 21, 10, 117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. & Shulman G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- Shine J. M. et al. Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 136, 3671–3681 (2013). [DOI] [PubMed] [Google Scholar]
- Callaert D. V. et al. Hemispheric asymmetries of motor versus nonmotor processes during (visuo)motor control. Hum Brain Mapp. 32, 1311–1329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto A. M. et al. Poor cognitive outcome in shunt-responsive idiopathic normal pressure hydrocephalus. Neurosurgery 72, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- Engel A. K. & Fries P. Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol. 20, 156–165 (2010). [DOI] [PubMed] [Google Scholar]
- Pollok B. et al. Motor-cortical oscillations in early stages of Parkinson's disease. J Physiol. 1, 3203–3212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D. et al. Effect of L-Dopa on the pattern of movement-related (de)synchronisation in advanced Parkinson's disease. Neurophysiol Clin. 33, 203–212 (2003). [DOI] [PubMed] [Google Scholar]
- Chistyakov A. V., Hafner H., Sinai A., Kaplan B. & Zaaroor M. Motor cortex disinhibition in normal-pressure hydrocephalus. J Neurosurg. 116, 453–459 (2012). [DOI] [PubMed] [Google Scholar]
- Kito Y. et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 21, 165–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino A. et al. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 21, 113–119 (2006). [DOI] [PubMed] [Google Scholar]
- Kanai M. et al. Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer's disease: a study in Japan. Ann Neurol. 44, 17–26 (1998). [DOI] [PubMed] [Google Scholar]
- Podsiadlo D. & Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 39,142–148 (1991). [DOI] [PubMed] [Google Scholar]
- Kubo Y. et al. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr Cogn Disord. 25, 37–45 (2008). [DOI] [PubMed] [Google Scholar]
- Agren-Wilsson A. et al. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol Scand. 116, 333–339 (2007). [DOI] [PubMed] [Google Scholar]
- Takagi R., Kajimoto Y., Kamiyoshi S., Miwa H. & Kondo T. The frontal assessment battery at bed side(FAB) in patients with Parkinson's disease. No To Shinkei 54, 897–902 (2002). [PubMed] [Google Scholar]
- Abe M. et al. Normative data on tests for frontal lobe functions: Trail Making Test, Verbal fluency, Wisconsin Card Sorting Test (Keio version). No To Shinkei 56, 567–574 (2004). [PubMed] [Google Scholar]
- Thomas G. et al. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 20, 163–168 (2005). [DOI] [PubMed] [Google Scholar]
- Musha T. et al. EEG markers for characterizing anomalous activities of cerebral neurons in NAT (neuronal activity topography) method. IEEE Trans Biomed Eng. 60, 2332–2338 (2013). [DOI] [PubMed] [Google Scholar]