Abstract
To delay evolution of pest resistance to transgenic crops producing insecticidal proteins from Bacillus thuringiensis (Bt), the "pyramid" strategy uses plants that produce two or more toxins that kill the same pest. We conducted laboratory diet experiments with the cotton bollworm, Helicoverpa armigera, to evaluate cross-resistance and interactions between two toxins in pyramided Bt cotton (Cry1Ac and Cry2Ab). Selection with Cry1Ac for 125 generations produced 1000-fold resistance to Cry1Ac and 6.8-fold cross-resistance to Cry2Ab. Selection with Cry2Ab for 29 generations caused 5.6-fold resistance to Cry2Ab and 61-fold cross-resistance to Cry1Ac. Without exposure to Bt toxins, resistance to both toxins decreased. For each of the four resistant strains examined, 67 to 100% of the combinations of Cry1Ac and Cry2Ab tested yielded higher than expected mortality, reflecting synergism between these two toxins. Results showing minor cross-resistance to Cry2Ab caused by selection with Cry1Ac and synergism between these two toxins against resistant insects suggest that plants producing both toxins could prolong the efficacy of Bt cotton against this pest in China. Including toxins against which no cross-resistance occurs and integrating Bt cotton with other control tactics could also increase the sustainability of management strategies.
Transgenic crops that produce insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) effectively suppress some pests while causing little or no harm to most non-target organisms, including people1,2,3,4,5,6,7. In some cases, Bt crops have reduced insecticide use and increased yield and farmer profits8,9,10,11,12,13. These benefits have spurred an increase in the hectares planted to Bt crops from 1 million in 1996 to 76 million in 201314. However, rapid evolution of resistance to Bt crops in several pests has reduced these benefits15.
To delay pest adaptation, farmers in many countries now grow Bt crop “pyramids” that produce two or more toxins that kill the same pest, rather than first generation Bt crops that each produce a single toxin15. The rationale for such pyramids is that insects resistant to one toxin will be killed by the other toxin in the pyramid15. For example, transgenic cotton producing Bt toxins Cry1Ac and Cry2Ab is the only type of Bt cotton grown in Australia, and the predominant type of Bt cotton grown in India and the United States16,17. However, only first generation Bt cotton producing one toxin (Cry1Ac) is grown in China18, the world's leading producer of cotton.
Although two-toxin Bt cotton is generally expected to be more durable than Bt cotton producing only Cry1Ac, the extent of this advantage depends on several factors15,19,20. Two factors that can diminish the durability and efficacy of two-toxin cotton are cross-resistance and antagonism between toxins. Cross-resistance occurs when selection with one toxin produces resistance to the other toxin in a pyramid21. Antagonism occurs when the mortality caused by a combination of toxins is less than the mortality expected based on results with each of the toxins tested separately22.
Here we used laboratory experiments to evaluate cross-resistance and antagonism between Cry1Ac and Cry2Ab in cotton bollworm, Helicoverpa armigera, a major pest of cotton and other crops in Asia, Africa, and Australia8. Although Bt cotton producing Cry1Ac has remained effective against this pest in China, several studies have reported a low but significantly increasing frequency of resistance to Cry1Ac in field populations, providing an early warning of potentially more serious problems18,23,24. Because Bt cotton producing Cry1Ac and Cry2Ab could help to counter or delay resistance in China, we conducted this study to better understand responses of H. armigera to these toxins. Whereas many previous studies have examined the effects of selection with Cry1Ac on cross-resistance to Cry2Ab in H. armigera18, little is known about cross-resistance of this pest to Cry1Ac caused by selection with Cry2Ab. As far as we know, this is the first study to assess the effects of interactions between Cry1Ac and Cry2Ab on their toxicity to resistant and susceptible strains of H. armigera. We found that antagonism occurred infrequently between these toxins, but we discovered some cross-resistance, particularly to Cry1Ac caused by selection with Cry2Ab.
Results
Effects of selection with Cry1Ac, Cry2Ab, and a mixture of toxins
We calculated the resistance ratio as the concentration of toxin killing 50% (LC50) of larvae for a strain divided by the LC50 of the same toxin for the susceptible strain 96S, which was the parent strain for all of the selected strains. Selection of the 96-1Ac strain with Cry1Ac for 102 and 125 generations, respectively, yielded resistance ratios of 3000 and 1000 for Cry1Ac, but only 1.6 and 6.8 for Cry2Ab (Table 1). The difference in LC50 values of Cry2Ab between the 96-1Ac strain and its unselected parent strain 96S was significant after 125 generations, but not after 102 generations (Table 1). Selection of the 96-2Ab strain with Cry2Ab for 29 generations yielded weak resistance to Cry2Ab (resistance ratio = 5.6) and strong cross-resistance to Cry1Ac (resistance ratio = 61) (Table 1).
Table 1. Resistance and cross-resistance to Cry1Ac and Cry2Ab in H. armigera.
| Strain | Gen.a | Cry1Ac LC50 (95% FL)b | RRc | Cry2Ab LC50 (95% FL) | RRc |
|---|---|---|---|---|---|
| March 2008 | |||||
| 96S | 132 | 0.0330 (0.016–0.057) | 1.0 | 0.180 (0.094–0.31) | 1.0 |
| 96-1Ac | 102 | 97.9 (48–170)* | 3000 | 0.290 (0.16–0.50) | 1.6 |
| 96-Mix | 102 | 79.5 (35–150)* | 2400 | 2.83 (1.1–39)* | 16 |
| Aug. 2010 | |||||
| 96S | 160 | 0.0280 (0.010–0.061) | 1.0 | 0.386 (0.23–0.57) | 1.0 |
| 96-1Ac | 125 | 29.4d | 1000 | 2.64 (1.7–4.1)* | 6.8 |
| 96-Mix | 125 | 11.4 (5.8–89)* | 410 | 8.03 (4.4–75)* | 21 |
| Nov. 2010 | |||||
| 96-2Ab | 29 | 1.72 (0.97–2.8)* | 61 | 2.15 (1.3–3.5)* | 5.6 |
| 96-1Ac/2Ab | 29 | 0.793 (0.52–1.2)* | 28 | 13.1 (5.5–85)* | 34 |
| 96-1Ac/U | 29 | 0.572 (0.055–0.15)* | 20 | 0.477 (0.18–1.2) | 1.2 |
aGeneration.
bConcentration killing 50% with 95% fiducial limits in parentheses, units are μg toxin per cm2 diet.
cResistance ratio, the LC50 for a strain divided by the LC50 for 96S for the same toxin in the same year.
dThe highest concentration tested (4.76 μg Cry1Ac per cm2 diet) caused a mean of 27.8% mortality and the probit analysis did not yield 95% fiducial limits for the LC50.
*Significantly different from 96S tested in the same year based on non-overlap of 95% fiducial limits of the LC50 values.
Selection of the 96-Mix strain with a mixture containing Cry1Ac, a Cry2 toxin, Cry1C, and Vip3A caused significant resistance to both Cry1Ac and Cry2Ab (Table 1). After selection for 102 and 125 generations, respectively, the 96-Mix strain had resistance ratios of 2400 and 410 for Cry1Ac, as well as 16 and 21 for Cry2Ab (Table 1).
After the 96-1Ac strain had been selected for 102 generations, it was split into two sub-strains that were reared for an additional 29 generations as follows: 96-1Ac/2Ab was selected with Cry2Ab and 96-1Ac/U was reared without exposure to toxins. During the 29 generations of selection with Cry2Ab, the resistance ratio of 96-1Ac/2Ab increased for Cry2Ab from 6.8 to 34 (5-fold) and decreased for Cry1Ac from 1000 to 28 (36-fold) (Table 1). During the 29 generations without exposure to toxins, the resistance ratios of 96-1Ac/U decreased from 1000 to 20 (50-fold) for Cry1Ac and from 6.8 to 1.2 (6-fold) for Cry2Ab (Table 1). These decreases imply that, in the absence of exposure to Bt toxins, fitness was lower for resistant insects than susceptible insects.
Toxicity of mixtures of Cry1Ac and Cry2Ab to susceptible and resistant strains
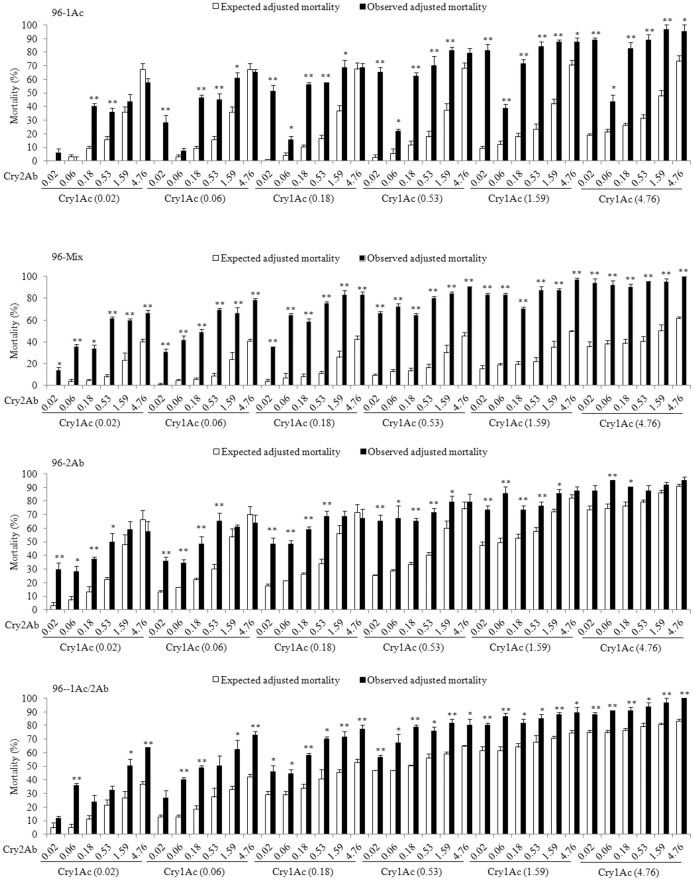
For the susceptible parent strain 96S and each of the four resistant strains (96-1Ac, 96-Mix, 96-2Ab, and 96-1Ac/2Ab), we evaluated observed versus expected mortality for 36 combinations of Cry1Ac and Cry2Ab (six concentrations of each toxin by six concentrations of the other toxin) (Figures 1 and 2). For the susceptible 96S strain, no significant deviation from the expected independent action of the toxins occurred in 30 of 36 (83%) combinations tested (Figures 1 and 3). Four combinations (11%) had lower than expected mortality, reflecting antagonism between Cry1Ac and Cry2Ab, and only two combinations (6%) produced higher than expected mortality, indicative of synergism (Figures 1 and 3). The six combinations in which antagonism or synergism occurred all involved one of the three lowest concentrations of Cry1Ac (0.02, 0.06 or 0.18 μg toxin per cm2 diet).
Figure 1. Observed versus expected mortality caused by combinations of Cry1Ac and Cry2Ab against the susceptible 96S strain of H. armigera.
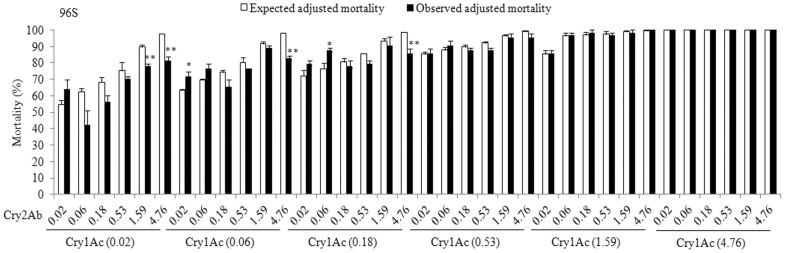
The concentrations (μg/cm2) of Cry1Ac and Cry2Ab in each combination are indicated below the x-axis. Black bars show observed mortality and white bars show expected mortality. Significant differences between observed and expected mortality are indicated with asterisks (*: P < 0.05 and **: P < 0.01 based on t-tests).
Figure 2. Observed versus expected mortality caused by combinations of Cry1Ac and Cry2Ab against four resistant strains of H. armigera (96-Cry1Ac, 96-Mix, 96-Cry2Ab, and 96-Cry1Ac/Cry2Ab).
The concentrations of Cry1Ac and Cry2Ab in each combination are indicated below the x-axis in μg protoxin per cm2 diet. Black bars show observed mortality and white bars show expected mortality. Significant differences between observed and expected mortality are indicated with asterisks (*: P < 0.05 and **: P < 0.01 based on t-tests).
Figure 3. Interactions between Cry1Ac and Cry2Ab against susceptible and resistant strains of H. armigera.
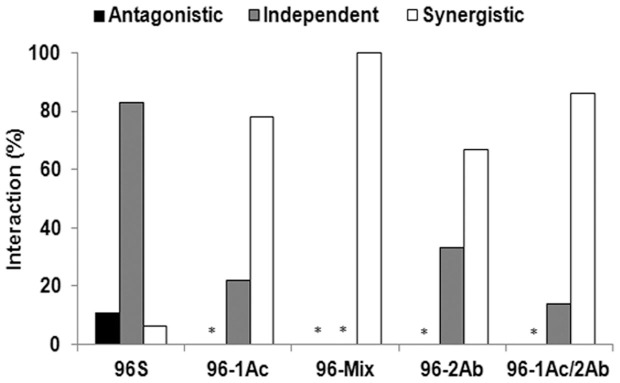
We tested 36 combinations of Cry1Ac and Cry2Ab against each of the five strains. The height of each bar indicates the percentage of the 36 combinations that yielded a particular type of interaction (antagonistic, independent, or synergistic). Asterisks indicate 0%.
By contrast, for each of the four resistant strains, 67 to 100% of the combinations tested showed higher than expected mortality, reflecting synergism between Cry1Ac and Cry2Ab, while all of the remaining combinations showed independent interactions (no synergism or antagonism) between the two toxins (Figures 2 and 3).
Discussion
The results show that against a susceptible strain of H. armigera, antagonism between Cry1Ac and Cry2Ab occurred in only 11% (4 of 36) of combinations tested, and only at relatively low concentrations of Cry1Ac (Figures 1 and 3). Moreover, against the four resistant strains studied (96-1Ac, 96-Mix, 96-2Ab, and 96-1Ac/2Ab), no antagonism occurred and synergism was seen in 67 to 100% of the combinations tested of these two toxins (Figures 1 and 3). These results suggest that antagonism would have little or no negative effect on the efficacy of a pyramid of Cry1Ac and Cry2Ab against susceptible or resistant H. armigera, particularly at relatively high concentrations of Cry1Ac. Field experiments with Bt cotton plants producing Cry1Ac alone, Cry2Ab alone, and both toxins would be needed to determine if the interactions between these toxins seen in our diet experiments also occur on plants in the field.
The results here show some cross-resistance between Cry1Ac and Cry2Ab in H. armigera that could reduce the durability of a pyramid. Although the initial 102 generations of selection of the 96-1Ac strain with Cry1Ac did not produce significant cross-resistance to Cry2Ab, an additional 23 generations of selection with Cry1Ac yielded statistically significant 6.8-fold cross-resistance to Cry2Ab (Table 1). This 6.8-fold cross-resistance to Cry2Ab is numerically higher than any cross-resistance to Cry2Ab caused by selection with Cry1Ac in previous studies of 12 field- and laboratory-selected strains of H. armigera from Australia, China and India (Table 2). Four of the 12 Cry1Ac-selected strains showed significant cross-resistance based on the conservative criterion of non-overlap of the 95% fiducial limits of their LC50 values relative to those of a susceptible strain tested in the same study (resistance ratios for Cry2Ab = 2.5, 4.2, 5.8 and 6.8, Table 2). Considering all 14 estimates of cross-resistance to Cry2Ab from the 12 strains, the mean resistance ratio for Cry2Ab is 1.3, which is significantly greater than the mean resistance ratio of 1.0 expected in the absence of cross-resistance (paired t-test, df = 13, t = 2.5, P = 0.025). These results indicate that on average, selection with Cry1Ac caused minor cross-resistance to Cry2Ab in H. armigera.
Table 2. Resistance to Cry1Ac and cross-resistance to Cry2Ab in Cry1Ac-selected strains of H. armigera.
| Country | Location | Yeara | Strain | Cry1Ac RRb | Cry2Ab RRb | Reference |
|---|---|---|---|---|---|---|
| Lab-selected | ||||||
| Australia | Multiple | NAc | BX | 44 | 1.4 | 46 |
| China | Anyang | 2011 | AY2 | 1200 | 5.8* | 18 |
| China | Gaoyangd | 2001 | SCD-r1 | 440 | 1.2 | 47 |
| China | Gaoyang | 2001 | GYBT | 560 | 1.4e | 48 |
| China | Langfang | 2000 | LFR10 | 250f | 1.0f | 49 |
| China | Qiuxian | 2011 | QX7 | 450 | 4.2* | 18 |
| China | Xiajin | 2009 | XJ-r15 | 140 | 1.4 | 50 |
| China | Xinxiang | 1996 | 96-1Acg | 3000f | 1.1 (F75)f | 49 |
| China | Xinxiang | 1996 | 96-1Ac | 3000 | 1.6 (F102) | This paper |
| China | Xinxiang | 1996 | 96-1Ac | 1000 | 6.8* (F125) | This paper |
| India | Akola | NA | Cry1Ac-r | 72 | 1.1 | 33 |
| Field-selectedh | ||||||
| China | Anyang | 2010 | Ay | 16 | 2.5* | 23 |
| China | Nanyang | 2010 | Ny | 6.0 | 0.9 | 23 |
| China | Xiajin | 2010 | Xj | 8.7 | 1.7 | 23 |
*Significant cross-resistance to Cry2Ab based on no overlap between the 95% fiducial limits for the LC50 of the Cry1Ac-selected strain and a susceptible strain.
aThe year when insects were sampled from the field to start the strain.
bResistance ratio, LC50 of Cry1Ac (or Cry2Ab) for the resistant strain divided by LC50 of Cry1Ac (or Cry2Ab) for a susceptible strain.
cNot available.
dThe r1 allele from GYBT was introduced by repeated crossing and selection into the susceptible SCD strain.
eBased on Cry2Aa, which is similar to Cry2Ab.
fBased on concentration of toxin causing 50% weight loss (WLC50) of the resistant strain divided by WLC50 of susceptible strain 96S.
gBtR (the strain name used in the reference cited) is the same strain as 96-1Ac, data are from generation F75.
hThree strains from northern China (Ay, Ny, Xj) had been exposed extensively to Bt cotton producing Cry1Ac and had significant resistance to both Cry1Ac protoxin and activated toxin relative to the susceptible field strain from Shawan in northwest China that had little exposure to Bt cotton; RR values are for protoxin.
Selection of the 96-2Ab strain (derived from the susceptible 96S strain) with Cry2Ab caused 61-fold cross-resistance to Cry1Ac (Table 1). Thus, the results here provide evidence of asymmetrical cross-resistance between Cry1Ac and Cry2Ab in H. armigera, similar to the pattern observed in laboratory strains of pink bollworm (Pectinophora gossypiella), in which selection with Cry1Ac did not cause strong cross-resistance to Cry2Ab, but selection with Cry2Ab caused strong cross-resistance to Cry1Ac25. By contrast, a strain of H. armigera from Australia with 9600-fold resistance to Cry2Aa did not have significant cross-resistance to Cry1Ac26.
In addition, the results here show that resistance to Cry1Ac decreased by a factor of 36 (from 1000-fold to 28-fold) during 29 generations of selection with Cry2Ab (Table 1) in the 96-1Ac/2Ab strain, which was derived from 96S and selected with Cry1Ac followed by Cry2Ab. By comparison, resistance in the 96-1Ac/U strain decreased by a factor of 50 (from 1000-fold to 20-fold) during 29 generations without exposure to any Bt toxin (Table 1). After these 29 generations, the LC50 of Cry1Ac did not differ significantly between 96-1Ac/Cry2Ab and 96-1Ac/U, indicating that in this case, selection with Cry2Ab did not significantly increase resistance to Cry1Ac.
One potential explanation for the results with the 96-1Ac/Cry2Ab and 96-1Ac/U strains is that the 1000-fold resistance to Cry1Ac in the 96-1Ac strain was associated with a large fitness cost that was not overcome by subsequent selection with Cry2Ab. On the other hand, exposure of the 96-2Ab strain to Cry2Ab (which was initially susceptible to Cry1Ac), caused sufficient selection for cross-resistance to Cry1Ac to increase its LC50 of Cry1Ac by 61-fold (Table 1). This suggests that the fitness cost was low or nil for this relatively low level of resistance to Cry1Ac. In general, mutations conferring high levels of resistance to Bt toxins are most likely to cause fitness costs27.
Additional work is needed to determine the genetic basis and mechanism of asymmetrical cross-resistance between Cry1Ac and Cry2Ab in H. armigera and P. gossypiella. In both of these insects, cross-resistance was detected between these toxins despite the finding that Cry1A toxins do not share binding sites with Cry2A toxins28,29,30. These results imply that lack of shared binding sites is necessary, but not sufficient, to infer that cross-resistance does not occur between toxins. In general, mechanisms of resistance other than reduced binding to midgut receptors can confer cross-resistance between toxins that do not share binding sites31,32.
The increasing frequency of resistance to Cry1Ac in populations of H. armigera from China and cross-resistance between Cry1Ac and Cry2Ab in some strains of H. armigera raise concerns about potential resistance of field populations to two-toxin Bt cotton producing Cry1Ac and Cry2Ab18,23. In bioassays with Bt cotton leaves containing Cry1Ac and Cry2Ab, survival was 13 times higher for the Cry1Ac-selected Res-Bt strain of H. armigera (32%) relative to a susceptible strain (2.4%), even though this strain had only 72-fold resistance to Cry1Ac and no cross-resistance to Cry2Ab33. Similar results with Helicoverpa zea show that survival from neonate to adult on Bt cotton producing Cry1Ac and Cry2Ab was 11 times higher for the Cry1Ac-selected GA-R strain (6.7%) relative to its unselected parent strain (0.6%), even though resistance of GA-R relative to GA was only 10-fold to Cry1Ac and 2-fold to Cry2Ab15.
Although Cry1Ac is the only Bt toxin produced by transgenic cotton grown in China, two-toxin Bt cotton producing Cry1Ac and Cry2Ab has become the predominant type of Bt cotton grown in India and the United States, and the only type of Bt cotton grown in Australia16. In China, a shift now to Bt cotton producing both Cry1Ac and Cry2Ab would probably delay evolution of resistance in H. armigera and in P. gossypiella34. However, considering the increasing frequency of resistance of H. armigera in China to Cry1Ac and the risk of an associated increase in survival on Bt cotton producing Cry1Ac and Cry2Ab discussed above, switching to Bt cotton producing a toxin other than Cry1Ac or Cry2Ab could be particularly useful in China23. For example, three-toxin Bt cotton producing Vip3Aa, Cry1Ac and Cry2Ab is expected to be available commercially in a few years35. Vip3Aa could be especially valuable in China because of the lack of cross-resistance in H. armigera between this toxin and either Cry1Ac or Cry2Ab35,36. However, H. armigera can adapt to Vip3A34. In China, the high proportion of non-Bt host plants for H. armigera other than cotton, including corn and soybean, provides a “natural refuge” for susceptible insects and helps to slow evolution of resistance8,37. In addition to increasing the number and diversity of toxins in Bt cotton, integration of Bt cotton with other control tactics could help to delay resistance and provide a more sustainable pest management system10,18.
Methods
Bt Toxins
We obtained Cry1Ac protoxin from the native Bacillus thuringiensis (Bt) HD73 strain and Cry2Ab protoxin from the engineered HD73− strain containing the cry2Ab gene. Both strains were kindly supplied by Biotechnology Research Laboratory, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The Bt strains were grown at 30°C in peptone-beef extract (PB) medium (0.5% peptone and 0.3% beef extract)38 until 50% of the crystal was released. Protoxins were extracted according to previously published methods39. Because Cry2Ab protoxin was produced as an inclusion rather than a pure crystal, we added 5 min of sonication (Noise Isolating Tamber, Ningbo Scientz Biotechnology Co., LTD) after the inclusion was dissolved in 1 M NaCl. Activated toxins were prepared and purified as described previously40. The concentrations of Cry1Ac and Cry2Ab protoxins were estimated on SDS–PAGE with a set of known BSA solutions as standards41.
Insect Strains
All strains were reared on an artificial diet at 27 ± 1°C, 60 ± 10% relative humidity, and a photoperiod of 14 light:10 dark. To promote the long-term success of the strains, we reared at least 10,000 neonates and 500 pupae for each strain in most generations.
We used one susceptible strain (96S) and five resistant strains of H. armigera derived from 96S: 96-1Ac, 96-Mix, 96-2Ab, 96-1Ac/2Ab, and 96-1Ac/U. The name of each of the five resistant strains begins with 96- to emphasize that 96S was their parent strain, while the characters that follow indicate the selection regime. 96S was started with 20 pairs of adults collected in 1996 in Xinxiang County, Henan Province of China from conventional cotton that had been treated a few times yearly with Bt sprays42. 96S was reared in the laboratory for 160 generations without exposure to Bt toxins.
Each resistant strain was selected at progressively increasing toxin concentrations incorporated in diet so that about 20% of the selected neonates pupated in each selected generation42. The 96-1Ac strain (previously called BtR42,43) was selected for the first 60 generations with solubilized Cry1Ac protoxin42 and in subsequent generations with MVPII, a liquid formulation containing Cry1Ac protoxin encapsulated in Pseudomonas fluorescens44. To minimize differences between 96S and 96-1Ac that are unrelated to resistance, these two strains were crossed in generations 27, 49, 69 and 8742. Based on growth inhibition bioassays in which Cry1Ac was incorporated in diet, the resistance ratio for 96-1Ac relative to 96S was reported previously as 170 in generation 16, 210 in generation 34, and 2900 in generation 8742. The 96-Mix strain (previously called BtI43) was selected with a mixture of Bt toxins (Cry1Ac, Cry2, Cry1C, and Vip3A) in technical powder (32,000 IU per mg, Wuhan Kernel Bio-tech Co., Ltd. Wuhan, China).
Starting in March 2008, three strains were initiated and reared for 29 generations as follows: The 96-2Ab strain was derived from 96S and selected with Cry2Ab. After 96-1Ac had been selected with Cry1Ac for 125 generations, two strains were derived from 96-1Ac: 96-1Ac/2Ab was selected with Cry2Ab and 96-1Ac/U was reared without exposure to Cry1Ac.
Bioassays
We used diet overlay bioassays45 to evaluate larval responses to Bt toxins singly and in combinations. We dispensed 1.0 mL of diet in each well of 24-well plates (TianJin Xiangyushun Co., TianJin, China). After the diet solidified, 60 µL of a dilution containing the desired concentration of one or more protoxins in 50 mM pH 10.0 Na2CO3 was overlaid on the diet surface of each well. For controls, 60 µL of 50 mM pH 10.0 Na2CO3 was overlaid on the diet surface of each well. After the diet was air-dried, one neonate that had hatched within 6 h was transferred onto the diet surface of each well. The bioassay plates were held at 27 ± 1°C, 60 ± 10% RH, and a photoperiod of 14L:10D. For each concentration of each treatment (single toxin, combination, or control), we conducted three replicates (24 neonates per plate X 3 replicates = 72 neonates total per concentration for each treatment). After 7 days, dead insects and those that were first instars were scored as dead.
We used at least five toxin concentrations to evaluate the LC50 values of Cry1Ac and Cry2Ab for each of the five strains (Table 1). To evaluate interactions between Cry1Ac and Cry2Ab, we tested each of the five strains at six concentrations (0.02, 0.06, 0.18, 0.53, 1.59, and 4.76 μg protoxin per cm2) of Cry1Ac and Cry2Ab alone as well as all 36 combinations of the six concentrations of the two toxins (6 concentrations of Cry1Ac X 6 concentrations of Cry2Ab, Figure 1). Experiments were done in August 2010 for 96S, 96-Mix and 96-1Ac; and in November 2010 for 96-2Ab and 96-1Ac/2Ab.
Statistical Analysis
We used probit analysis (Polo-Plus, LeOra Software) to calculate the toxin concentration causing 50% mortality (LC50) and its 95% fiducial limits (FL), We calculated resistance ratios as the LC50 of a strain divided by the LC50 of the susceptible (96S) strain.
To evaluate interactions between Cry1Ac and Cry2Ab, we first calculated the expected mortality for each combination of the two toxins using the following formula: 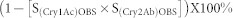 22where S(Cry1Ac)OBS is the observed proportion of larvae that survived exposure to Cry1Ac, S(Cry2Ab)OBS is the observed proportion of larvae that survived exposure to Cry2Ab, and S(Cry1Ac)OBS × S(Cry2Ab)OBS is the proportion of larvae expected to survive exposure to a combination of Cry1Ac and Cry2Ab. To calculate excepted mortalities, we first calculated the observed adjusted survival for each toxin test singly as survival on treated diet divided by survival on untreated diet (control). All of the results reported for treated diet are based on adjusted survival. The differences of expected and observed mortalities of each combination were compared with Student's t-test.
22where S(Cry1Ac)OBS is the observed proportion of larvae that survived exposure to Cry1Ac, S(Cry2Ab)OBS is the observed proportion of larvae that survived exposure to Cry2Ab, and S(Cry1Ac)OBS × S(Cry2Ab)OBS is the proportion of larvae expected to survive exposure to a combination of Cry1Ac and Cry2Ab. To calculate excepted mortalities, we first calculated the observed adjusted survival for each toxin test singly as survival on treated diet divided by survival on untreated diet (control). All of the results reported for treated diet are based on adjusted survival. The differences of expected and observed mortalities of each combination were compared with Student's t-test.
Author Contributions
G.L., J.W. and Y.G. designed the study. J.W. performed the experiments. J.W., X.L. and B.T. analyzed data and wrote the manuscript. G.L. and K.W. provided the insects. J.Z. provided the Bt strains that produce either Cry1Ac or Cry2Ab. All authors have read and approved the manuscript for publication.
Acknowledgments
This research was supported by the Key Project for Breeding Genetically Modified Organisms (2014ZX08011-002) and the National Natural Science Foundation of China (No. 30971921, 31321004).
Footnotes
B.E.T. is coauthor of a patent on modified Bt toxins, "Suppression of Resistance in Insects to Bacillus thuringiensis Cry Toxins, Using Toxins that do not Require the Cadherin Receptor" (patent numbers: CA2690188A1, CN101730712A, EP2184293A2,EP2184293A4, EP2184293B1, WO2008150150A2, WO2008150150A3). Pioneer, Dow AgroSciences, Monsanto, Syngenta and Bayer CropScience did not provide funding to support this work, but may be affected financially by publication of this paper and have funded other work by B.E.T. and X.L.
References
- Mendelsohn M., Kough J., Vaituzis Z. & Matthews K. Are Bt crops safe? Nat. Biotechnol. 21, 1003–1009 (2003). [DOI] [PubMed] [Google Scholar]
- Duan J. J., Marvier M., Huesing J., Dively G. & Young Z. Y. 2008. A meta-analysis of effects of Bt crops on honey bees (Hymentoptera: Apidae). PLoS One 3, e1415. 10.1371/journal.pone.0001415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanahuja G., Banakar R., Twyman R., Capell T. & Christou P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol. J. 9, 283–300 (2011). [DOI] [PubMed] [Google Scholar]
- Lu Y. H., Wu K. M., Jiang Y. Y., Guo Y. Y. & Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365 (2012). [DOI] [PubMed] [Google Scholar]
- Hendriksma H. P. et al. Effect of stacked insecticidal Cry proteins from maize pollen on nurse bees (Apis mellifera carnica) and their gut bacteria. PLoS One 8, e59589. 10.1371/journal.pone.0059589 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas C., Lumbierres B., Pons X. & Albajes R. No effects of Bacillus thuringiensis maize on nontarget organisms in the field in southern Europe: a meta-analysis of 26 arthropod taxa. Transgenic Res. 23, 135–143 (2014). [DOI] [PubMed] [Google Scholar]
- Wolfenbarger L. L. R., Carrière Y. & Owen M. 29 environmental effects. Handbook on Agriculture, Biotechnology and Development, 10.4337/9780857938350 (2014). [DOI] [Google Scholar]
- Wu K. M., Lu Y. H., Feng H. Q., Jiang Y. Y. & Zhao J. Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 321, 1676–1678 (2008). [DOI] [PubMed] [Google Scholar]
- Carpenter J. E. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat. Biotechnol. 28, 319–321 (2010). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E. et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat. Biotechnol. 28, 1304–1307 (2010). [DOI] [PubMed] [Google Scholar]
- Edgerton M. D. et al. Transgenic insect resistance traits increase corn yield and yield stability. Nat. Biotechnol. 30, 493–496 (2012). [DOI] [PubMed] [Google Scholar]
- Kathage J. & Qaim M. Economic impacts and impact dynamics of Bt (Bacillus thuringiensis) cotton in India. Proc. Natl. Acad. Sci. USA 109, 11652–11656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Chavas J. P. & Lauer J. Commercialized transgenic traits, maize productivity and yield risk. Nat. Biotechnol. 31, 111–114 (2013). [DOI] [PubMed] [Google Scholar]
- James C. ISAAA Briefs No. 46: Global status of commercialized biotech/GM crops: 2013. Ithaca, NY: ISAAA (2013). [Google Scholar]
- Brévault T. et al. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. USA 15, 5806–5811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E., Brévault T. & Carrière Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521 (2013). [DOI] [PubMed] [Google Scholar]
- Fabrick J. A. et al. Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to Bt cotton in India. PLoS One 9, e97900. 10.1371/journal.pone.0097900 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. et al. Dominant resistance to Bt cotton and minor cross-resistance to Bt toxin Cry2Ab in cotton bollworm from China. Evol. Appl. 6, 1222–1235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Z. et al. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol. 21, 1493–1497 (2003). [DOI] [PubMed] [Google Scholar]
- Zhao J. Z. et al. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc. Natl. Acad. Sci. USA 24, 8426–8430 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E., Mota-Sanchez D., Whalon M. E., Hollingworth R. M. & Carrière Y. Defining terms for proactive management of resistance to Bt crops and pesticides. J Econ Entomol. 107, 497–507 (2014). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E. et al. Efficacy of genetically modified Bt toxins alone and in combinations against pink bollworm resistant to Cry1Ac and Cry2Ab. PLoS One 8, e80496. 10.1371/journal.pone.0080496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. N. et al. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS One 6, e22874. 10.1371/journal.pone.0022874 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. N. et al. Diverse genetic basis of field-evolved resistance to Bt cotton in cotton bollworm from China. Proc. Natl. Acad. Sci. USA 109, 10275–10280 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E. et al. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc. Natl. Acad. Sci. USA 29, 11889–11894 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon R. J., Olsen K. M., Downes S. & Addison S. Frequency of alleles conferring resistance to the Bt toxins Cry1Ac and Cry2Ab in Australian Populations of Helicoverpa armigera (Lepidoptera: Noctuidae). J Econ Entomol. 100, 1844–1853 (2007). [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Carriere Y. & Tabashnik B. E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 54, 147–163 (2009). [DOI] [PubMed] [Google Scholar]
- Karim S., Riazuddin S., Gould F. & Dean D. H. Determination of receptor binding properties of Bacillus thuringiensis delta-endotoxins to cotton bollworm (Helicoverpa zea) and pink bollworm (Pectinophora gossypiella) midgut brush border membrane vesicles. Pest. Biochem. Physiol. 67, 198–216 (2000). [Google Scholar]
- González-Cabrera J., Escriche B., Tabashnik B. E. & Ferré J. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectinophora gossypiella). Insect Biochem. Mol. Biol. 33, 929–935 (2003). [DOI] [PubMed] [Google Scholar]
- Estela A., Escriche B. & Ferré J. Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae). Appl. Environ. Microb. 70, 1378–1384 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F. et al. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl. Acad. Sci. USA 89, 7986–7990 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré J. & Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47, 501–533 (2002). [DOI] [PubMed] [Google Scholar]
- Rajagopal R. et al. Resistance of Helicoverpa armigera to Cry1Ac toxin from Bacillus thuringiensis is due to improper processing of the protoxin. Biochem J 419, 309–316 (2009). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E., Wu K. M. & Wu Y. D. Early detection of field-evolved resistance to Bt cotton in China: Cotton bollworm and pink bollworm. J. Invertebr. Pathol. 110, 301–306 (2012). [DOI] [PubMed] [Google Scholar]
- Mahon R. J., Downes S. J. & James B. Vip3A resistance alleles exist at high levels in Australian targets before release of cotton expressing this toxin. PLoS One 7, e39192. 10.1371/journal.pone.0039192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. J. et al. Vip3Aa tolerance response of Helicoverpa armigera populations from a Cry1Ac cotton planting region. J Econ Entomol. 103, 2169–2173 (2010). [DOI] [PubMed] [Google Scholar]
- Jin L. et al. Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nature Biotechnol. 10.1038/nbt.3100 (2014). [DOI] [PubMed] [Google Scholar]
- Wang G. J. et al. Engineered Bacillus thuringiensis G033A with broad insecticidal activity against lepidopteran and coleopteran pests. Appl. Microbiol. Biotechnol. 72, 924–930 (2006). [DOI] [PubMed] [Google Scholar]
- Luo K., Banks D. & Adang M. J. Toxicity, binding, and permeability analyses of four Bacillus thuringiensis Cry1 δ-endotoxins by use of brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl. Environ. Microbiol. 65, 457–464 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J. et al. Cloning and characterization of a novel Cry1A toxin from Bacillus thuringiensis with high toxicity to the Asian corn borer and other lepidopteran insects. FEMS Microbiol. Lett. 280, 95–101(2008). [DOI] [PubMed] [Google Scholar]
- Ma G., Rahman M. M., Grant W., Schmidt O. & Asgari S. Insect tolerance to the crystal toxins Cry1Ac and Cry2Ab is mediated by the binding of monomeric toxin to lipophorin glycolipids causing oligomerization and sequestration reactions. Dev. Compar. Immunol. 37, 184–192(2012). [DOI] [PubMed] [Google Scholar]
- Liang G. M. et al. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J. Invertebr. Pathol. 97, 142–149 (2008). [DOI] [PubMed] [Google Scholar]
- Cao G. C., Zhang L. L., Liang G. M., Li X. C. & Wu K. M. Involvement of nonbinding site proteinases in the development of resistance of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry1Ac. J Econ Entomol 106, 2514–2521 (2013). [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E. et al. Inheritance of resistance to Bt toxin Cry1Ac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae). J Econ Entomol 95, 1018–1026 (2002). [DOI] [PubMed] [Google Scholar]
- Hernández-Rodríguez C. S., Van Vliet A., Bautsoens N., Van Rie J. & Ferré J. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl Environ Microbiol 74, 7654–7659 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhurst R. J., James W., Bird L. J. & Beard C. Resistance to the Cry1Ac δ-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 96, 1290–1299 (2003). [DOI] [PubMed] [Google Scholar]
- Yang Y. H. et al. Introgression of a disrupted cadherin gene enables susceptible Helicoverpa armigera to obtain resistance to Bacillus thuringiensis toxin Cry1Ac. Bull. Entomol. Res. 99, 175–181 (2009). [DOI] [PubMed] [Google Scholar]
- Xu X. J., Yu L. Y. & Wu Y. D. Disruption of a cadherin gene associated with resistance to Cry1Ac δ-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 71, 948–954 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. et al. Cross-resistance studies of Cry1Ac-resistant strains of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry2Ab. J Econ Entomol 100, 909–915 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang H. N., Wu S. W., Yang Y. H., Tabashnik B. E. & Wu Y. D. Non-recessive Bt toxin resistance conferred by an intracellular cadherin mutation in field-selected populations of cotton bollworm. PLoS One 7, e53418. 10.1371/journal.pone.0053418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]