Graphical abstract

Keywords: Plant natural products, Ureases, Human health, Helicobacter pylori, Canavalia ensiformis, Food production
Abstract
Ureases, enzymes that catalyze urea hydrolysis, have received considerable attention for their impact on living organisms’ health and life quality. On the one hand, the persistence of urease activity in human and animal cells can be the cause of some diseases and pathogen infections. On the other hand, food production can be negatively affected by ureases of soil microbiota that, in turn, lead to losses of nitrogenous nutrients in fields supplemented with urea as fertilizer. In this context, nature has proven to be a rich resource of natural products bearing a variety of scaffolds that decrease the ureolytic activity of ureases from different organisms. Therefore, this work compiles the state-of-the-art researches focused on the potential of plant natural products (present in extracts or as pure compounds) as urease inhibitors of clinical and/or agricultural interests. Emphasis is given to ureases of Helicobacter pylori, Canavalia ensiformis and soil microbiota although the active site of this class of hydrolases is conserved among living organisms.
Introduction
Urease (EC 3.5.1.5) is a key enzyme for the global nitrogen cycle, occurring in plants, fungi and bacteria. This type of hydrolase speeds up by one-hundred-trillion-fold the urea hydrolysis rate to ammonia (NH3) and carbon dioxide [1–3].
Since its discovery in plants [4], Canavalia ensiformis (Fabaceae) urease has been exhaustively investigated and became the milestone in Biochemistry science as the first enzyme to be crystallized [5] and also proven to be strictly dependent on nickel ions (Ni2+) [6]. The dependence on nickel ions for catalytic activity is a unique feature of urease among hydrolytic enzymes [1,2]. The first three-dimensional structure of a urease was fully reported by Jabri and coworkers in 1995 from Crystallography studies performed with urease from Klebsiella aerogenes [7]. Later on, other structures were disclosed for ureases from Bacillus pasteurii [8], Helicobacter pylori [9] and most recently C. ensiformis [10]. Indeed, the elucidation of the urease structure from a legume was crucial to better understand the requirements for ureolytic activity of this class of enzymes in different organisms [10]. The great similarity of amino acid sequence among ureases from multiple origins [11] suggests a common ancestral for this enzyme. Ureases share a basic trimeric array with 1, 2 or 3 subunits that can fuse forming hexameric or dodecameric architecture. Each active site contains two Ni2+ ions apart from each other in 3.5–3.7 Å, bridged by oxygen atoms of a lysine carbamate residue and a hydroxide ion [3,12]. Plants and fungi ureases exhibit a single polypeptide chain while bacteria have two or three different subunits (α, β and γ) [1,13]. The incorporation of Ni2+ in protein structure is assisted by accessory proteins, believed to be urease-specific chaperones [11].
Ureases in the context of Helicobacter pylori
The increase of medium pH by the accumulation of NH3 is a urease trait of tremendous medical importance [3]. Urine and/or gastrointestinal infections by ureolytic bacteria can cause health complications in humans and animals, which include kidney stone formation, pyelonephritis, hepatic encephalopathy and ultimately hepatic coma [3,12]. Therefore, major public health issues are related with H. pylori, gram-negative bacteria that are able to survive in an environment as acidic as that of the stomach (pH 2). As a consequence, H. pylori infection can induce gastric inflammation and increase the risk for the development of duodenal and gastric ulcers, gastric adenocarcinoma and gastric lymphoma [3,14]. About 50% of global population is committed by H. pylori. This bacteria species can persist in the stomach for the whole life of infected individuals without causing disease symptoms. The high prevalence of H. pylori in human population indicates that such microorganism has developed mechanisms for resistance against host defenses [14]. Urease enzyme in cytoplasm and/or bound to H. pylori surface is the main virulence factor of such human pathogen [15]. It is postulated that the lyses of some pathogen cells leads to the release of cytosolic ureases that bind to the surface of intact bacterial cells and cause the hydrolysis of urea present in human guts at a concentration of 3 mM. The NH3 formed increases the medium pH, which creates a friendly environment for H. pylori survival [15,16].
During the past 20 years, the recommended first-line therapy for H. pylori eradication consisted of the combination of the antibiotics amoxicillin and clarithromycin with omeprazole, a proton pump cell inhibitor. However, the increase of H. pylori resistance to these antibiotics (particularly to clarithromycin) made this therapy a non-attractive option in recent years [2,17,18]. Indeed, other treatment strategies have emerged to fight H. pylori infection, which include the use of bismuth salts combined with a proton pump cell inhibitor or the combination of other classes of antibiotics (e.g. fluoroquinolones, aminopenicillins, tetracyclines, etc.) [2,18,19].
Additionally, urease inhibitors may be effective therapies for the treatment of diseases caused by urease-dependent pathogenic microorganisms. However, the commercially available urease inhibitors, such as phosphorodiamidates, hydroxamic acid derivatives and imidazoles are toxic and of low stability, features that prevent their clinical use [20,21]. Then, the search for novel urease inhibitors with improved stability and low toxicity is mandatory to improve life quality of human beings and animals.
Ureases in the context of agriculture
Urea is used as a nitrogen fertilizer in agriculture worldwide. This organic compound exhibits some advantages over other nitrogen fertilizer, namely, high N content (46%), low price, water solubility and easy management [22]. However, under field conditions, urea efficiency is markedly reduced due to nitrogen losses (over 50%) caused, among other factors, by NH3 volatilization from the action of microorganisms ureases present in soil matrices [1,22,23].
The excessive emission of NH3 to atmosphere gradually will cause an unbalance in nitrogen cycle, which can imply in disastrous long-term environmental consequences [24–27]. Most of the NH3 generated from urea-based fertilizers may impact negatively natural ecosystems by inducing eutrophication processes and formation of nitrous oxide, a greenhouse gas [23]. On the other hand, once produced in the soil solution, NH3 is converted to ammonium ion (NH4+) that, in turn, can undergo nitrification by the action of Nitrosomona and/or Nitrobacter species, yielding nitrate (NO3−). The NO3− uptaken by plant root cells will contribute to the production of amino acids, nucleic acids and some secondary metabolites, while the remainder still in soil can easily be leached to aquifers, rivers and lakes. Aquatic environments enriched with NO3− may go to eutrophication, resulting in algae blooms, reduction of fish and animal populations and threat to human health [23,28].
There are current some alternatives to minimize nitrogen losses from urea fertilizers and improve its uptake by crops. Slow-release nitrogen fertilizers comprise agricultural inputs that consist on the fertilizer surface covered by hydrophobic chemicals to provide a physical barrier against water. This promotes the gradual release of urea to soil solution [29]. Another strategy is the use of nitrification inhibitors that are able to delay NH4+ oxidation by nitrifying bacteria, preventing NO3− formation and nitrogen leaching from the soil [29]. Urease inhibitors are some of the most used approaches to overcome nitrogen losses in field, as they delay urea hydrolysis, increasing the chances of urea incorporation in soil by rain, irrigation or mechanical operations [22].
Among the known soil urease inhibitors, N-(butyl) thiophosphoric triamide (NBPT) is currently the most efficient compound. In the presence of soil microbiota, NBPT is converted to the respective oxo-analogue called N-(butyl) phosphoric triamide (oxo-NBPT) that exhibit high capacity of inhibiting urease [30]. Many other substances have been investigated with respect to the potential to inhibit urease activity in soil, but very few were found to be promising for further studies. In this sense, the great challenge is to find good candidates that are eco-friendly, nontoxic and of low toxicity to plants, chemically stable, efficient at low concentrations, compatible with urea and of competitive costs.
Where to start digging up for new urease inhibitors?
There is no doubt that nature is a vast source of natural products of that exhibit a plethora of biological activities. The diversity of chemical structure makes natural products very valuable to pharmaceutical industries and agricultural segments as well. Natural products from plants, in particular, have been a great source of inspiration for improving human and animal life quality as disease therapeutics and also for increasing food resources [31–36].
In this context, the investigation of the potential of plant-derived natural products as urease inhibitors can be valuable for the development of therapeutics for diseases associated with intense urease activity and improved nitrogen fertilizer formulations to increase food production. This work brings an overview on the state-of-the-art research performed with plant crude extracts and/or pure plant-derived natural products were used as ureases inhibitors of pharmacological and agricultural interest.
Potential of plant extracts as urease inhibitors
Studies with focus on urease of clinical interest
The ethnomedicinal use of plants to treat chronic gastritis, ulcers and related gastroduodenal disorders, diseases that can be caused by H. pylori, is widely reported [37–39]. Studies carried out with several plant extracts allowed for the identification of urease inhibitors that may be useful for the control of H. pylori strains growth [40–43].
Alk(en)yl thiosulfinates (TS) are the main constituents of many foodstuffs, for example diallyl thiosulfinate (allicin) corresponds to around 70% of TS content in fresh aqueous garlic extract [44,45]. Commonly used as a flavoring, garlic (Allium sativum; Liliaceae) is recognized as an antimicrobial and anti-urease food due to allicin levels [44,46,47]. The urease inhibition by garlic extract is an irreversible time- and TS-concentration dependent; 18-min incubation of urease with garlic extract is sufficient to cause total loss of enzyme activity [44]. The inhibitory effect of TS-enriched garlic extract was attributed to the ability of TS to oxidize the –SH group of a cysteine residue present in the enzyme active site [44].
Plant juices obtained from A. sativum (garlic), Allium cepa (yellow and white onions), Allium porrum (leek), Brassica oleraceae var. capitata (cabbage; Brassicaceae) and Brassica oleraceae var. gemmifera (Brussels sprouts) were also effective urease inhibitors [45]. It was found that the higher the TS content, the better the juice was concerning the inhibition of ureolytic activity of urease. Thus, the best inhibitory effects were achieved when garlic juice was used, followed by the employment of Brussels sprouts one. With exception of cabbage juice, all foodstuffs juice tested lost the effect after heating at 95 °C [45]. Therefore, authors recommend the ingestion of raw garlic, onion, cabbage and Brussels sprout so that the urease inhibitory properties can be preserved and still work in the treatment of H. pylori infection [45]. The in vitro anti-H. pylori activity of methanolic leaf extracts (50 mg/mL) of Allium ascalonicum (Liliaceae) was found to be due to the ability of such extract to decrease urease activity [48]. The methanolic extracts were determined to contain alkaloids, cardiac glycosides, saponins and traces of flavonoids.
The antibacterial effect of alcoholic extract or essential oil of Cuminum cyminum (cumin; Apiaceae) on Klebsiella pneumonia (Gram-negative bacteria) was shown to be as result of the inhibition of urease activity [49]. Based on active site similarities shared by ureases, chemical constituents of cumin could also be effective against H. pylori, a hypothesis that should be further investigated.
To investigate the scientific basis for the traditional use of plants for the treatment of ulcers, an in vitro study was conducted with shoot extracts of Artemisia scoparia (Asteraceae) [50]. The concentration of methanolic crude extract necessary to inhibit C. ensiformis urease activity by 50% (IC50) was 4.1 mg/mL. Notably, the flavonoid fraction was shown to be even more effective as attested by the IC50 value of 2.1 mg/mL.
A screening performed with over one hundred traditional Iranian herbal medicines revealed that 37 extracts inhibited urease activity by at least 70% when employed at 10 mg/mL. Urease inhibition near to 100% was achieved using methanolic (50%) extracts of Areca catechu (Arecaceae; fruit extract), Capsicum annuum (Solanaceae; fruit extract), Citrus aurantifolia (Rutaceae; fruit extract), Hibiscus gossypifolius (Malvaceae; herb extract), Hypericum perforatum (Hypericaceae; herb extract), Nymphea alba (Nymphaeaceae; flower extract), Papaver rhoeas (Papaveraceae; flower extract), Perlagonium graveolens (Geraniaceae; flower extract), Pistacia vera (Anacardiaceae; rind extract), Punica granatum (Lythraceae; flower and rind extracts), Quercus infectoria (Fagaceae; rind extract), Rheum ribes (Polygonaceae; root extract), Rosa centifolia (Rosaceae; flower extract), Sambucus ebulus (Adoxaceae; fruit extract) and Veratrum album (Melanthiaceae; leaf extract). Among these plant species, S. ebulus and R. ribes were the most potent exhibiting IC50 values of 57 and 92 μg/mL, respectively [51]. Inhibition of urease activity was observed for methanolic (50%) extracts of Camelia sinensis (Theaceae; IC50 for leaf extract = 35 μg/mL), C. aurantifolia (Rutaceae; IC50 for fruit extract = 28 μg/mL), Nasturtium officinale (Brassicaceae; IC50 for leaf extract = 18 μg/mL), P. granatum (IC50 for flower extract = 30 μg/mL) and Matricaria recutita (Asteraceae; IC50 for flower extract = 37 μg/mL) [42]. Moreover, the methanolic (50%) extract of a commercial green tea containing 70.6% epigallocatechin derivatives, 9.9% gallocatechin derivatives, 4.1% (−)-epicatechin and 1.1% catechin exhibited an IC50 of 13 μM against H. pylori urease [40]. The ingestion of drinking water containing green tea extract in the range of 500–2000 ppm by H. pylori-challenged Mongolian gerbil animals for 6 weeks suppressed both gastritis and bacterial infection prevalence [40].
Glycyrrhiza glabra (Leguminosae; licorice) is a common Mediterranean herb known by the antioxidant properties and ability to inhibit urease activity. The ethyl acetate root extract (2.5 mg/mL) of such plant species inhibited C. ensiformis urease by 72% while methanolic root extract negatively affected urease activity by 64% [52].
Whole-plant acetone extracts of the traditional Pakistan herb Fagonia arabica (Zygophyllaceae), were reported to be more potent than the metronidazole (reference drug) against H. pylori [43].
Aqueous extract of commercial powder of Origanum vulgare (oregano; Lamiaceae) and Vaccinium macrocarpon (cranberry; Ericaceae) were very efficient in controlling the growth and urease activity of H. pylori [41]. Such effect was attributed to the phenolic contents in both plant extracts. Methanolic (50%) extracts of Eucalyptus grandis (Myrtaceae) stem bark inhibited the activity of clinical isolated strains of H. pylori (UCH97001, UCH97009 and UCH98026) in a concentration-dependent manner (6.5–50.0 mg/mL) [53]. The authors attributed the anti-H. pylori effect of E. grandis extracts to the presence of tannins and triterpene saponins, based on other works published elsewhere [53 and cited Refs.]. The use of Paeonia emodi (Paeoniaceae) roots in Asia for medicinal purposes is ancient due to the inhibition of urease and α-chymotrypsin activities [54]. Ethanolic crude extracts of P. emodi shoots (12.5 μg/mL) inhibited C. ensiformis and B. pasteurii ureases by over 70% [54].
Two commercial samples of red wine with different resveratrol contents (1.3 or 10.5 μg/mL) were shown to inhibit ureases of H. pylori 26695, 1692/05 and 553A/02 strains [38]. Samples containing higher amounts of resveratrol were more potent although the effect of other constituents in the red wine studied cannot be ruled out.
Studies with focus on urease of agricultural interest
Polyphenolics-containing extracts obtained from the bark of Acacia decurrens (green wattle; Fabaceae) or seed coat of Terminalia chebula (inknut; Combretaceae) inhibited both pure urease (urease tablets-BDH) and soil ureases to the same extent that did mercuric chloride and catechol, known urease inhibitors [55]. Indeed, NH3 volatilization from soil surface was decreased upon soil fertilization with urea–polyphenol mixtures. These results highlight the potential of tannin-like polyphenols from green wattle and inknut as potent urease inhibitors [55]. Interestingly, addition of C. sinensis (black tea) waste to soil surface (50 g/kg soil) right before urease activity tests substantially affected enzyme activity [55].
Seed kernel powder of Azadirachta indica (neem; Meliaceae) was demonstrated to decrease the rate of urea hydrolysis in acidic soils, contributing to urea incorporation into soil to be hydrolyzed in the rhizosphere and then provide nitrogen for uptake by plant roots [56].
Another study has used several extracts from four plant species native to Mediterranean zone of Chile [57]. Ethanolic extracts from the bark of Acacia caven (Fabaceae) and Pinus radiate (Pinaceae) inhibited urease activity in soil as a result of phenolic contents in a concentration dependent manner. No direct correlation could be made with respect to the condensed tannins present in both plant species extracts [57].
Overall, the bodies of evidence about the inhibitory action of various plant extracts on urease of clinical and agricultural interest provide subsidies to further investigate which constituents mostly contribute for their biological profiles.
Isolated plant natural products as urease inhibitors
Polyphenols, specially flavonoids, have been pointed out as notable H. pylori urease inhibitors [58–60]. Therefore, genistein, an isoflavone widely produced by plants of Fabaceae family, was found to inhibit H. pylori urease by 50% when used at 430 μg/mL while its 7-O-glucoside derivative exhibited no effect on the enzyme activity (Fig. 1) [58].
Fig. 1.

Structure of flavonoids notable by the ability to inhibit ureases activity.
The therapeutic potential of Lonicera japonica (Caprifoliaceae) against H. pylori is well known [61]. A pool of flavonoids extracted from flowers of this plant exhibited an IC50 value of 946 μM on H. pylori urease [62]. By testing pure compounds, the flavonols quercetin, rutin, myricetin and myricitrin and the flavones luteolin and luteolin 7-O-glucoside were found the most potent against H. pylori urease, presenting IC50 values of 11.2 μM, 67.6 μM, 77.2 μM, 98.7 μM, 35.5 μM, and 55.8 μM, respectively [62]. Quercetin-4′-O-d-glucoside (Fig. 1) isolated from A. cepa (Liliaceae) showed an IC50 of 190 μM against C. ensiformis urease [63]. Other, quercetin glucoderivatives (Fig. 1) isolated from Psidium guajava fruits (guava; Myrtaceae) negatively affected the activity of C. ensiformis urease, such as isoquercitrin (IC50 = 160 μM), quercitrin (IC50 = 200 μM), avicularin (IC50 = 140 μM) and guaijaverin (IC50 = 120 μM). The IC50 for quercetin aglycone toward C. ensiformis urease was determined to be 80 μM [63].
A study carried out with seven natural products isolated from a butanolic subfraction of the ethanolic extract of Celtis africana (Celtidaceae) revealed the remarkable antiureolitic property of four flavone C-glucosides with IC50 lower than 50 μM (Table 1) [64].
Table 1.
Concentration (μM) of C-glycosylflavonoids necessary to inhibit the activity of Canavalia ensiformis urease by 50%.
| Compounds | IC50 (μM) |
|---|---|
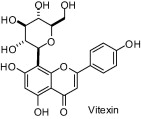 |
35 |
 |
28 |
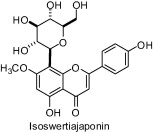 |
38 |
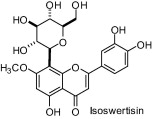 |
43 |
Baicalin (Fig. 2), a flavone glucuronide and main constituent of dried roots of Scutellariae baicalensis (Lamiaceae), was able to inhibit C. ensiformis urease (IC50 = 2.7 mM), exhibiting an inhibition constant (Ki) of 3.89 × 10−3 mM [65]. Another flavone C-glucuronide (scutellarin; Fig. 2) isolated from Erigeron breviscapus (Asteraceae) was shown to be twice as potent (IC50 = 1.4 mM) as baicalin with respect to the inhibition of C. ensiformis urease [66]. The inhibitory effect o scutellarin was attributed to its ability to bind the sulfhydryl group of l-cysteine residue present in the enzyme active site [66].
Fig. 2.

Structures of polyphenols with remarkable inhibitory effect on ureases.
Methyl gallate and 1,2,3,4,6-penta-O-galloyl-d-glucoside (PGG) (Fig. 2), widely produced by Paeonia lactiflora (Paeoniaceae) roots, were tested as pure compounds against H. pylori urease [67]. It was observed that PGG (IC50 = 72 μM) is roughly as potent as the reference inhibitor acetohydroxamic acid. Methyl gallate presented an IC50 of 1.3 mM [67].
Coumarins are phenylpropanoid compounds produced by various plant families. Ten pure coumarins out of 24 tested by Jadhav and coworkers [68] against H. pylori urease were shown to be very promising enzyme inhibitors. The IC50 for such natural products were lower than 75 μM (Table 2).
Table 2.
Concentration (μM) of some coumarins necessary to inhibit the activity of Helicobacter pylori urease by 50%.
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | IC50 (μM) |
| 1 | H | OH | H | H | H | H | 58.9 |
| 2 | H | CH3 | H | H | OH | OH | 54.6 |
| 3 | H | H | H | CH3 | OH | H | 68.9 |
| 4 | H | CH3 | H | H | OH | H | 72.6 |
| 5 | H | OH | H | CH3 | H | H | 70.7 |
| 6 | H | CH3 | H | OH | H | H | 61.9 |
| 7 | H | C6H5 | H | H | OH | H | 48.9 |
| 8 | H | C6H5 | OH | H | OH | H | 53.9 |
| 9 | H | C6H5 | H | H | OH | OH | 55.3 |
| 10 | C6H5 | OH | OH | H | OH | H | 47.8 |
Vernonione (Fig. 3), a terpene isolated from methanolic extracts of Vernonia cinerascens (Asteraceae) roots, is another example of plant natural product capable of inhibiting C. ensiformis urease (IC50 = 227.6 μM) [69]. Sulforaphane [CH3S(O)(CH2)4NCS], an isothiocyanate derivative abundant in cruciferous vegetables, were proven to inactivate H. pylori urease by covalently binding to thiol group of one or more l-cysteine residues to form dithiocarbamates [70]. Atranorin (Fig. 3) was the most effective urease inhibitor out of the 21 natural products isolated from stem bark of Stereospermum acuminatissimum (Bignoniaceae) [71]. Atranorin (IC50 of 18.2 μM) was as potent as thiourea (IC50 = 21.0 μM), a known urease inhibitor [71]. A myrsinol-type diterpene ester purified from Euphorbia decipiens (Euphorbiaceae; whole plant) exhibited an IC50 of 81.4 μM toward C. ensiformis urease [72]. The novel sphingolipids named ophiamide A and ophiamide B (Fig. 3), isolated from methanolic extracts of Heliotropium ophioglossum (Boraginaceae), inhibited C. ensiformis urease activity with IC50 values of 23.1 μM and 12.6 μM, respectively [73].
Fig. 3.
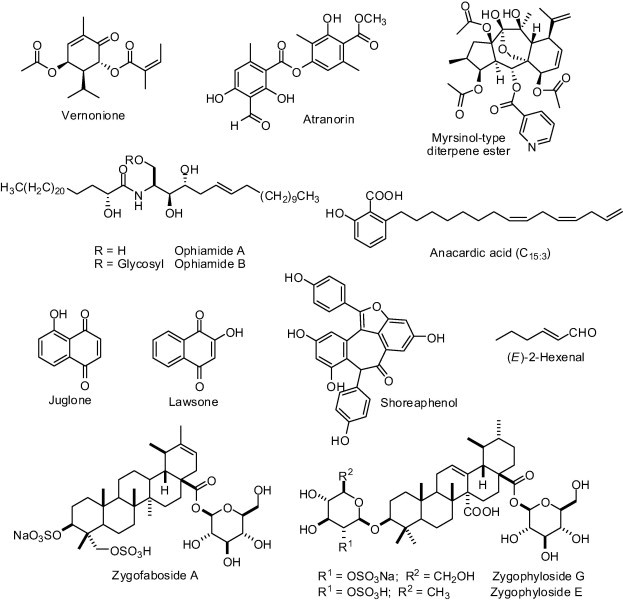
Structure of natural products from different classes that exhibit activity against ureases.
Pure juglone and lawsone (Fig. 3), constitutional plant naphthoquinone isomers, were tested against C. ensiformis urease, in which it was found that only the former is active, exhibiting an IC50 value of 4.8 μM in 40-min reactions [74].
Six congeners of shoreaphenol purified from stem bark of Hopea exalata (Dipterocapaceae) were tested against C. ensiformis urease revealing that shoreaphenol (Fig. 3) was the only oligostilbenoid capable of inhibiting the enzyme activity (IC50 = 126.8 μM) [75].
The anti-H. pylori properties of anacardic acid (C15:3) and (E)-2-hexenal (Fig. 3), both isolated from Anacardium occidentale (Anacardiaceae), was confirmed to be a result of urease inhibition [76]. Anacardic acid (IC50 = 125 μg/mL) and (E)-2-hexenal (IC50 = 50 μg/mL) were identified as competitive and non-competitive urease inhibitors, respectively [76].
The inhibitory effect on C. ensiformis urease of ursane-type sulfated saponin glycoderivatives was reported with zygofaboside A, zygophyloside E and zygophyloside G (Fig. 3) being able to inhibit in the range of 40–87% when used at 500 μM [77]. Such natural products were isolated from shoots of the plant species Zygophyllum fabago (Zygophyllaceae).
Example of alkaloids with expressive inhibitory effect on the ureolytic activity of C. ensiformis urease is also reported in the literature. Govaniadine, caseadine, caseamine and protopine (Fig. 4), all isolated from whole plant powder of Corydalis govaniana (Fumariaceae), presented IC50 values of 20.2 μM, 38.9 μM, 66.7 μM and 54.1 μM, respectively, thus having the potential to urease-associated physiological complications [78].
Fig. 4.

Structure of plant alkaloids that exhibit activity against ureases.
Concluding remarks
The body of evidence presented in this overview clearly demonstrates the great potential of plant secondary metabolites of different classes to negatively affect the activity of ureases. The use of this knowledge can contribute for the design of novel, safe and less costing urease inhibitors with the aim to improve human and animals life quality either by fighting urease-related diseases or by increasing the quality and food production. Although the environmental aspects were not the primary scope of this review, the use of urease inhibitors in agricultural practices can surely be valuable for the reduction of greenhouse gas emissions. Scientists engaged in the search for natural sources of urease inhibitors have some challenges to overcome, namely (i) plant-family-guided expansion of the number of explored extracts, (ii) identification and isolation of the major constituents of promising plant extracts, (iii) stablishment of structure–activity relationships accompanied by in silico (docking) studies, (iv) evaluation of the mechanism of action of the pure natural compounds and (v) production of the promising compounds in large scale when the availability is limited in nature.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This work was financially supported, in part, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). LVM and AdF are recipients of research fellowships from CNPq.
Biographies

Luzia V. Modolo received her PhD degree in Functional and Molecular Biology in 2004 from the State University of Campinas (SP, Brazil). She is currently the Head of the Department of Botany at the Federal University of Minas Gerais (MG, Brazil). Dr. Modolo is also the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and Group o Studies on Plant Biochemistry (www.gebioplan.com). Her research interests include the signalling processes coordinated in plant tissues in response to environmental stress, plant nutrition and plant secondary metabolism.

Aline X. de Souza was born in 1987. She earned her Lic. degree in Biology Sciences at the Federal University of Minas Gerais (MG, Brazil) in 2013 when she also started her Master studies in Plant Biology under the mentoring of Dr. Luzia V. Modolo. Her primary interest includes the development of novel urease inhibitors for improving plant nitrogen nutrition.

Lívia P. Horta received her Master degree in Plant Biology in 2012 at the Federal University of Minas Gerais (MG, Brazil). She is currently PhD student at the same institution under the mentoring of Dr. Luzia V. Modolo. Her research interest is in Plant Nutrition with focus on urease inhibitors as well as plant responses to environmental stresses.

Débora P. Araujo was born in 1982. She earned her BSc. degree in Chemistry in 2008 at the Federal University of Juiz de Fora (MG, Brazil). She received her MSc. degree in Chemistry from the Federal University of Minas Gerais (MG, Brazil) in 2011 when she also started her PhD studies in Chemistry under the mentoring of Dr. Ângelo de Fátima. Her research interests are in the field of Organic and Medicinal Chemistry.

Ângelo de Fátima received his PhD degree in Science in 2005 from the State University of Campinas (SP, Brazil). He is currently Associate Professor of the Department of Chemistry at the Federal University of Minas Gerais (MG, Brazil). Dr. de Fátima is the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and Group o Studies on Organic and Biological Chemistry. His research interests include the synthesis of molecules with biological, functional profile and the evaluation of their activities against cancer cells, fungi, bacteria and virus of clinical interest.
Footnotes
Peer review under responsibility of Cairo University.
![]()
This work was made possible partly by the Network for the Development of Novel Urease Inhibitors (www.redniu.org).
Contributor Information
Luzia V. Modolo, Email: lvmodolo@icb.ufmg.br.
Ângelo de Fátima, Email: adefatima@qui.ufmg.br.
References
- 1.Krajewska B., Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B Enzym. 2009;59(1):9–21. [Google Scholar]
- 2.Follmer C. Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol. 2010;63:424–430. doi: 10.1136/jcp.2009.072595. [DOI] [PubMed] [Google Scholar]
- 3.Maroney M.J., Ciurli S. Nonredox nickel enzymes. Chem Rev. 2014;114:4206–4228. doi: 10.1021/cr4004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi T. On the occurrence of urease in higher plants. J Coll Agric Tokyo Imp Univ. 1909;1:1–14. [Google Scholar]
- 5.Sumner J.B. The isolation and crystallization of the enzyme urease. J Biol Chem. 1926;69:435–441. [Google Scholar]
- 6.Dixon N.E., Gazzola C., Watters J.J., Blakeley R.L., Zerner B. Jack Bean Urease (EC 3.5.1.5) [letter]. A metalloenzyme. A simple biological role for nickel? J Am Chem Soc. 1975;97(14):4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- 7.Jabri E., Carr M.B., Hausinger R.P., Karplus P.A. The crystal structure of urease from Klebsiella aerogenes. Science. 1995;268:998–1004. [PubMed] [Google Scholar]
- 8.Benini S., Rypniewski W.R., Wilson K.S., Miletti S., Ciurli S., Mangani S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure. 1999;7(2):205–216. doi: 10.1016/S0969-2126(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 9.Ha N.C., Oh S.T., Sung J.Y., Cha K.A., Lee M.H., Oh B.H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Biol. 2001;8(6):505–509. doi: 10.1038/88563. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramanian A., Ponnuraj K. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J Mol Biol. 2010;400:274–283. doi: 10.1016/j.jmb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Sirko A., Brodzik R. Plant ureases: roles and regulation. Acta Biochim Pol. 2000;47:1189–1195. [PubMed] [Google Scholar]
- 12.Boer J.L., Mulrooney S.B., Hausinger R.P. Nickel-dependent metalloenzymes. Arch Biochem Biophys. 2014;544:142–152. doi: 10.1016/j.abb.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yathindra N. Structure of an enzyme revealed 80 years after it was crystallized – differential functional behaviour of plant and microbial ureases uncovered. Curr Sci. 2010;99:566–568. [Google Scholar]
- 14.Algood H.M.S., Cover T.L. Helicobacter pylori persistence. An overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19(4):597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs G., Weeks D.L., Wen Yi, Marcus E.A., Scott D.R. Acid acclimation by Helicobacter pylori. Physiology. 2005;20:429–438. doi: 10.1152/physiol.00032.2005. [DOI] [PubMed] [Google Scholar]
- 16.Stingl K., Altendorf K., Bakker E.P. Acid survival of Helicobacter pylori: how does urease activity trigger cytoplasmic pH homeostasis? Trends Microbiol. 2002;10(2):70–74. doi: 10.1016/s0966-842x(01)02287-9. [DOI] [PubMed] [Google Scholar]
- 17.Graham D.Y., Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 18.Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5(2):103–109. doi: 10.1177/1756283X11432492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfertheiner P., Megraud F., O’Morain C., Bazzoli F., El-Omar E., Graham D. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azizian H., Nabati F., Sharifi A., Siavoshi F., Mahdayi M., Amanlou M. Large-scale virtual screening for the identification of new Helicobacter pylori urease inhibitor scaffolds. J Mol Model. 2012;18:2917–2927. doi: 10.1007/s00894-011-1310-2. [DOI] [PubMed] [Google Scholar]
- 21.Ibrar A., Khan I., Abbas N. Structurally diversified heterocycles and related privileged scaffolds as potential urease inhibitors: a brief overview. Arch Pharm Chem Life Sci. 2013;346:423–446. doi: 10.1002/ardp.201300041. [DOI] [PubMed] [Google Scholar]
- 22.Artola E., Cruchaga S., Ariz I., Moran J.F., Garnica M., Houdusse F. Effect of N-(n-butyl) thiophosphoric triamide on urea metabolism and the assimilation of ammonium by Triticum aestivum L. Plant Growth Regul. 2011;63:73–79. [Google Scholar]
- 23.Cameron K.C., Di H.J., Moir J.L. Nitrogen losses from the soil/plant system: a review. Ann Appl Biol. 2013;162:145–173. [Google Scholar]
- 24.Lara Cabezas W.A.R., Korndörfer G.H., Motta S.A. Volatilização de N-NH3 na cultura de milho: II. Avaliação de fontes sólidas e fluidas em sistema de plantio direto e convencional. Rev Bras Cienc Solo. 1997;21(3):489–496. [Google Scholar]
- 25.Sangoi L., Ernani P.R., Lech V.A., Rampazzo C. Volatilização de N-NH3 em decorrência da forma de aplicação de ureia, manejo de resíduos e tipo de solo, em laboratório. Cienc Rural. 2003;33(4):687–692. [Google Scholar]
- 26.Jadoski S.O., Saito L.R., Prado C., Lopes E.C., Sales L.L.S.R. Characteristics of the nitrate leaching in intensive farming areas. Appl Res Agrotechnol. 2010;3(1):193–200. [Google Scholar]
- 27.Smart J.C.R., Hicks K., Morrissey T., Heinemeyer A., Sutton M.A., Ashmore M. Applying the ecosystem service concept to air quality management in the UK: a case study for ammonia. Environmetrics. 2011;22:649–661. [Google Scholar]
- 28.Canfield D.E., Glazer A.N., Falkowski P.G. The evolution and future of Earth’s nitrogen cycle. Science. 2010;330:192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- 29.Akiyama H., Yan X.Y., Yagi K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Glob Change Biol. 2010;16:1837–1846. [Google Scholar]
- 30.Kawakami E.M., Oosterhuis D.M., Snider J.L., Mozaffari M. Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD. Eur J Agron. 2012;43:147–154. [Google Scholar]
- 31.Rice M.J., Legg M., Powell K.A. Natural products in agriculture – a view from the industry. Pestic Sci. 1998;52(2):184–188. [Google Scholar]
- 32.de Fátima A., Modolo L.V., Sanches A.C., Porto R.R. Wound healing agents: the role of natural and non-natural products in drug development. Mini Rev Med Chem. 2008;8:879–888. doi: 10.2174/138955708785132738. [DOI] [PubMed] [Google Scholar]
- 33.Dayan F.E., Cantrell C.L., Duke S.O. Natural products in crop protection. Bioorg Med Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 34.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drugs leads. BBA-Gen Subjects. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva F.G., Horta L.P., Faria R.O., Stehmann J.R., Modolo L.V. Stressing conditions as tools to boost the biosynthesis of valuable plant natural products. Recent Pat Biotechnol. 2014;8:89–101. doi: 10.2174/1872208307666131218124836. [DOI] [PubMed] [Google Scholar]
- 36.de Fátima A., Terra B.S., da Silva C.M., da Silva D.L., Araujo D.P., Silva-Neto L. From nature to market: examples of natural products that became drugs. Recent Pat Biotechnol. 2014;8:76–88. doi: 10.2174/1872208307666131220163108. [DOI] [PubMed] [Google Scholar]
- 37.Cowan M.M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulo L., Oleastro M., Gallardo E., Queiroz J.A., Domingues F. Anti-Helicobacter pylori and urease inhibitory activities of resveratrol and red wine. Food Res Int. 2011;44(4):964–969. [Google Scholar]
- 39.Toyang N.J., Verpoorte R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae) J Ethnopharmacol. 2013;146(3):681–723. doi: 10.1016/j.jep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Matsubara S., Shibata H., Ishikawa F., Yokokura T., Takahashi M., Sugimura T. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003;310(3):715–719. doi: 10.1016/j.bbrc.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 41.Lin Y.T., Kwon Y.I., Labbe R.G., Shetty K. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl Environ Microbiol. 2005;71(12):8558–8564. doi: 10.1128/AEM.71.12.8558-8564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biglar M., Soltani K., Nabati F., Bazl R., Mojab F., Amanlou M. A preliminary investigation of the jack-bean urease inhibition by randomly selected traditionally used herbal medicine. Iran J Pharm Res. 2012;11(3):831. [PMC free article] [PubMed] [Google Scholar]
- 43.Amin M., Anwar F., Naz F., Mehmood T., Saari N. Anti-Helicobacter pylori and urease inhibition activities of some traditional medicinal plants. Molecules. 2013;18(2):2135–2149. doi: 10.3390/molecules18022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juszkiewicz A., Zaborska A., Łaptaś A., Olech Z. A study of the inhibition of jack bean urease by garlic extract. Food Chem. 2004;85(4):553–558. [Google Scholar]
- 45.Olech Z., Zaborska W., Kot M. Jack bean urease inhibition by crude juices of Allium and Brassica plants. Determination of thiosulfinates. Food Chem. 2014;145:154–160. doi: 10.1016/j.foodchem.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 46.Cavallito C.J., Bailey J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J Am Chem Soc. 1944;66(11):1950–1951. [Google Scholar]
- 47.Ankri S., Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1(2):125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 48.Adeniyi B.A., Anyiam F.M. In vitro anti-Helicobacter pylori potential of methanol extract of Allium ascalonicum Linn. (Liliaceae) leaf: susceptibility and effect on urease activity. Phytother Res. 2004;18(5):358–361. doi: 10.1002/ptr.1265. [DOI] [PubMed] [Google Scholar]
- 49.Derakhshan S., Sattari M., Bigdeli M. Effect of subinhibitory concentrations of cumin (Cuminum cyminum L.) seed essential oil and alcoholic extract on the morphology, capsule expression and urease activity of Klebsiella pneumoniae. Int J Antimicrob Agents. 2008;32(5):432–436. doi: 10.1016/j.ijantimicag.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Khan M.A., Khan H., Tariq S.A., Pervez S. Urease inhibitory activity of aerial parts of Artemisia scoparia: exploration in an in vitro study. Ulcers. 2014;184736:5. [Google Scholar]
- 51.Nabati F., Mojab F., Habibi-Rezaei M., Bagherzadeh K., Amanlou M., Yousefi B. Large scale screening of commonly used Iranian traditional medicinal plants against urease activity. DARU J Pharm Sci. 2012;20(1):72. doi: 10.1186/2008-2231-20-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lateef M., Iqbal L., Fatima N., Siddiqui K., Afza N., Zia-ul-Haq M. Evaluation of antioxidant and urease inhibition activities of roots of Glycyrrhiza glabra. Pak J Pharm Sci. 2012;25(1):99–102. [PubMed] [Google Scholar]
- 53.Adeniyi B.A., Onwubuche B.C., Anyiam F.M., Ekundayo O., Mahady G.B. Anti-Helicobacter pylori activities of Eucalyptus grandis: effects on susceptibility, urease activity and cell surface hydrophobicity. Pharm Biol. 2009;47(1):13–17. [Google Scholar]
- 54.Khan T., Ahmad M., Nisar M., Ahmad M., Lodhi M.A., Choudhary M.I. Enzyme inhibition and radical scavenging activities of aerial parts of Paeonia emodi Wall.(Paeoniaceae) J Enzyme Inhib Med Chem. 2005;20(3):245–249. doi: 10.1080/14756360400026220. [DOI] [PubMed] [Google Scholar]
- 55.Fernando V., Roberts G.R. The partial inhibition of soil urease by naturally occurring polyphenols. Plant Soil. 1976;44(1):81–86. [Google Scholar]
- 56.Mohanty S., Patra A.K., Chhonkar P.K. Neem (Azadirachta indica) seed kernel powder retards urease and nitrification activities in different soils at contrasting moisture and temperature regimes. Bioresour Technol. 2008;99:894–899. doi: 10.1016/j.biortech.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Suescun F., Paulino L., Zagal E., Ovalle C., Muñoz C. Plant extracts from the Mediterranean zone of Chile potentially affect soil microbial activity related to N transformations: a laboratory experiment. Acta Agricult Scand Sect B – Soil Plant Sci. 2012;62:556–564. [Google Scholar]
- 58.Bae E., Han M.J., Kim D. In vitro anti-Helicobacter pylori activity of irisolidone isolated from the flowers and rhizomes of Pueraria thunbergiana. Planta Med. 2001;67:161–163. doi: 10.1055/s-2001-11499. [DOI] [PubMed] [Google Scholar]
- 59.Shin J.E., Kim J.M., Bae E.A., Hyun Y.J., Kim D.H. In vitro inhibitory effect of flavonoids on growth, infection and vacuolation of Helicobacter pylori. Planta Med. 2005;71:197–201. doi: 10.1055/s-2005-837816. [DOI] [PubMed] [Google Scholar]
- 60.Laghari A.H., Memon S., Nelofar A., Khan K.M., Yasmin A., Syed M.N. A new flavanenol with urease-inhibition activity isolated from roots of manna plant camelthorn Alhagi maurorum. J Mol Struct. 2010;965(1):65–67. [Google Scholar]
- 61.Ma F., Chen Y., Li J., Qing H., Wang J., Zhang Y. Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol. 2010;16:5629–5634. doi: 10.3748/wjg.v16.i44.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao Z., Wang X., Peng Z., Huang S., Yang P., Li Q. Molecular docking, kinetics study, and structure–activity relationship analysis of quercetin and its analogous as Helicobacter pylori urease inhibitors. Agric Food Chem. 2012;60:10572–10577. doi: 10.1021/jf303393n. [DOI] [PubMed] [Google Scholar]
- 63.Shabana S., Kawai A., Kai K., Akiyama K., Hayashi H. Inhibitory activity against urease of quercetin glycosides isolated from Allium cepa and Psidium guajava. Biosci Biotechnol Biochem. 2010;74:878–880. doi: 10.1271/bbb.90895. [DOI] [PubMed] [Google Scholar]
- 64.Perveen S., El-Shafae A.M., Al-Taweel A., Fawzy G.A., Malik A., Afza N. Antioxidant and urease inhibitory C-glycosylflavonoids from Celtis africana. J Asian Nat Prod Res. 2011;13:799–804. doi: 10.1080/10286020.2011.593171. [DOI] [PubMed] [Google Scholar]
- 65.Tan L., Su J., Wu D., Yu X., Su Z., He J. Kinetics and mechanism study of competitive inhibition of jack-bean urease by baicalin. Sci World J. 2013;879501:9. doi: 10.1155/2013/879501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D., Yu X., Xie J., Su Z., Su J., Tan L. Inactivation of jack bean urease by scutellarin: elucidation of inhibitory efficacy, kinetics and mechanism. Fitoterapia. 2013;91:60–67. doi: 10.1016/j.fitote.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Ngan L.T.M., Moon J.K., Shibamoto T., Ahn Y.J. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J Agric Food Chem. 2012;60:9062–9073. doi: 10.1021/jf3035034. [DOI] [PubMed] [Google Scholar]
- 68.Jadhav S.G., Meshram R.J., Gond D.S., Gacche R.N. Inhibition of growth of Helicobacter pylori and its urease by coumarin derivatives: molecular docking analysis. J Pharm Res. 2013;7:705–711. [Google Scholar]
- 69.Ahmad I., Chaudhary B.A., Ashraf M., Uzair M., Janbaz K.H. Vernonione, a new urease inhibitory carvotacetone derivative from Vernonia cinerascens. J Chem Soc Pakistan. 2012;34(3):639–642. [Google Scholar]
- 70.Fahey J.W., Stephenson K.K., Wade K.L., Talalay P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem Biophys Res Commun. 2013;435:1–7. doi: 10.1016/j.bbrc.2013.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramsay K.S.T., Wafo P., Ali Z., Khan A., Oluyemisi O.O., Marasini B.P. Chemical constituents of Stereospermum acuminatissimum and their urease and α-chymotrypsin inhibitions. Fitoterapia. 2012;83(1):204–208. doi: 10.1016/j.fitote.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 72.Ahmad V.U., Hussain J., Hussain H., Jassbi A.R., Ullah F., Lodhi M.A. First natural urease inhibitor from Euphorbia decipiens. Chem Pharm Bull. 2003;51:719–723. doi: 10.1248/cpb.51.719. [DOI] [PubMed] [Google Scholar]
- 73.Firdous S., Ansari N.H., Fatima I., Malik A., Afza N., Iqbal L. Ophiamides A–B, new potent urease inhibitory sphingolipids from Heliotropium ophioglossum. Arch Pharm Res. 2012;35(7):1133–1137. doi: 10.1007/s12272-012-0702-x. [DOI] [PubMed] [Google Scholar]
- 74.Kot N., Karcz W., Zaborska W. 5-Hydroxy-1,4-naphthoquinone (juglone) and 2-hydroxy-1,4-naphthoquinone (lawsone) influence on jack bean urease activity: elucidation of the difference in inhibition activity. Bioorg Chem. 2010;38:132–137. doi: 10.1016/j.bioorg.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Ge H.M., Huang B., Tan S.H., Shi D.H., Song Y.C., Tan R.X. Bioactive oligostilbenoids from the stem bark of Hopea exalata. J Nat Prod. 2006;69:1800–1802. doi: 10.1021/np060242y. [DOI] [PubMed] [Google Scholar]
- 76.Kubo J., Lee J.R., Kubo I. Anti-Helicobacter pylori agents from the cashew apple. J Agric Food Chem. 1999;47:533–537. doi: 10.1021/jf9808980. [DOI] [PubMed] [Google Scholar]
- 77.Khan S.S., Khan A., Khan A., Wadood A., Farroq U., Ahmed A. Urease inhibitory activity of ursane type sulfate saponins from the aerial parts of Zygophyllum fabago Linn. Phytomedicine. 2014;21:379–382. doi: 10.1016/j.phymed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Shrestha R.L.S., Adhikari A., Marasini B.P., Jha R.N., Choudhary M.I. Novel inhibitors of urease from Corydalis govaniana Wall. Phytochem Lett. 2013;6:228–231. [Google Scholar]


