Graphical abstract
Keywords: Multidrug resistance (MDR), Multidrug resistance-associated protein 1 (MRP1), Natural products, P-gp (P-glycoprotein)
Abstract
Resistance of solid tumors to treatment is significantly attributed to pharmacokinetic reasons at both cellular and multi-cellular levels. Anticancer agent must be bio-available at the site of action in a cytotoxic concentration to exert its proposed activity. P-glycoprotein (P-gp) is a member of the ATP-dependent membrane transport proteins; it is known to pump substrates out of cells in ATP-dependent mechanism. The over-expression of P-gp in tumor cells reduces the intracellular drug concentrations, which decreases the cytotoxicity of a broad spectrum of antitumor drugs. Accordingly, P-gp inhibitors/blockers are potential enhancer for the cellular bioavailability of several clinically important anticancer drugs such as, anthracyclines, taxanes, vinca alkaloids, and podophyllotoxins. Besides several chemically synthesized P-gp inhibitors/blockers, some naturally occurring compounds and plant extracts were reported for their modulation of multidrug resistance; however, this review will focus only on major classes of naturally occurring inhibitors viz., flavonoids, coumarins, terpenoids, alkaloids and saponins.
Introduction
Definition and molecular background
Multidrug resistance (MDR) is the ability of drug resistant tumors to exhibit simultaneous resistance to a number of structurally and functionally unrelated chemotherapeutic agents.
P-glycoprotein (P-gp), the very famous MDR family member protein, was first characterized in multidrug resistant Chinese hamster ovary (CHO) cells by Ling and co-workers [1]. P-gp transports in a unidirectional fashion any xenobiotic as a substrate outward via an ATP-dependent mechanism. In tumor cells, expression of P-gp results in reduction of intracellular drug concentrations with consequent decrease in the cytotoxicity of a broad spectrum of antitumor drugs including anthracyclines (e.g. doxorubicin; DOX), vinca alkaloids (e.g. vincristine), podophyllotoxins (e.g. etoposide) and taxanes (e.g. taxol). Gene sequence analysis in different species revealed two human P-gp genes, three mouse P-gp genes and one P-gp gene in hamster cells [2]. Structure of human P-gp protein comprises 1280 amino acid in 12 transmembrane segments and one ATP-binding motif with three characteristic glycosylation sites [3].
Three different P-gp isoforms were identified (P-gp class I, II and III); only P-gp class I and III were characterized in various normal human tissues with potential role in the normal physiology of these tissues [4]. P-gp class III is expressed in liver hepatocytes; and mice lacking its expression fail biliary phopholipid secretion. P-gp is expressed as well in a wide range of epithelia with potential transport function, such as colon, small intestine, liver, pancreas, kidney, uterus and placenta. In addition, P-gp was found expressed in highly specialized capillary transport endothelia such as brain and testis [5–8].
Other MDR-related proteins were discovered within different types of malignancies [9] such as multidrug resistance related proteins (MRP’s) [10,11] and breast cancer resistance protein (BCRP-1) [12–14]. Compounds inhibiting these P-gp related efflux proteins are supposed to increase the intracellular concentration of chemotherapeutic agents in similar way to inhibiting P-gp molecule itself. [15–18].
Despite the role of P-gp transporter in normal physiology; the overexpression of P-gp (and related proteins) on tumor cells results in significant decrease in the intracellular concentration of a wide range of anticancer drugs nonetheless of natural origin. Early evidence for the role of P-gp in the efflux of anticancer drugs outward and abolishing their cytotoxicity was observed before more than two decades. Purified membrane vesicles from resistant tumor cells significantly bind more radiolabeled vincristine [19,20]. P-gp showed significant role in the transport of anthracyclines in Madin–Darby canine cells as well [20]. In addition, radiolabeled colchicines transport was found to be mediated by purified P-gp particles [21].
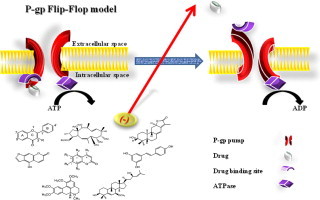
Several molecular mechanisms have been postulated for P-gp mode of action such as increasing the intracellular pH, depolarizing plasma membrane electric potential, proton and chloride ion pumps [22,23]. The leaflet flip model of Higgins and Gottesman appears to be the most descriptive molecular explanation to the mode of P-gp action [24].
P-gp receptor modulation
The P-gp inhibitor may act as a competitive blocker via occupying the drug binding sites or as a non-competitive antagonist by binding chemosensetizer sites [25]. Example for competitive binding of two drugs on the same binding site of P-gp molecule was found for competition between radiolabeled vinblasine and azidopine on purified P-gp molecules [26]. Similarly, binding of radiolabeled vinblasine was inhibited by co-incubation with vincristine and daunorubicin [27]. On the other hand, colchicines, actinomycin-D and calcium channel blockers do not compete for vinblastine-binding site within P-gp molecules; yet inhibiting the binding of radiolabeled vinblasine or azidopine would suggest multiple binding domains on P-gp molecules [28]. ATPase activity of P-gp molecule was first identified since the very early discovery of the P-gp molecule itself [29]. Yet several agents are designed and tested to inhibit particularly the ATPase function of P-gp molecules and related proteins [30,31]. That might explain the discrepancies in binding sites within P-gp molecules for different P-gp blockers.
Modulation of P-gp and chemosensetization
The process of chemosensetization involves the co-administration of a P-gp inhibitor with an anticancer drug in order to enhance intracellular anticancer drug accumulation via impairing the P-gp efflux function. Numerous compounds have been shown to inhibit the drug efflux function of P-gp and therefore, increase the intracellular concentration of chemotherapies with ultimate decrease in cellular resistance. Several chemical classes are known as P-gp inhibitors such as, cyclosporines, calmodulin inhibitors, indole alkaloids, coronary vasodilators, quinolines, hormones, calcium channel blockers, and several surfactants [32]. Considerable number of cancers exhibit either intrinsic or treatment induced acquired resistance. Over-expression of P-gp molecules correlates positively with poor response of tumor cells to chemotherapy [33] at both stages of first diagnosis (intrinsic resistance) and during disease relapse (acquired resistance) of several neoplastic disorders such as leukemias [34]; lymphomas [35]; adult and childhood sarcomas, and neuroblastomas [36].
The two major limitations for P-gp inhibitors to be used clinically are their unwanted immunosuppressive and cardiovascular effects. According to these two major hurdles, P-gp blockers are classified into first, second or third generation.
First generation P-gp blockers
Verapamil (VRP) is the prototype P-gp blocker [37] and was found to enhance intracellular accumulation of many anticancer drugs, including DOX in numerous cancer cell lines [22,38,39]. Further studies found that the P-gp inhibiting activity is shared by many other calcium channel blockers such as, diltiazem [38], bepridil [40], nicardipine, nifedipine [38], felodipine, and isradipine [41]. In addition, non-calcium channel blockers such as calmodulin antagonists (trifluorperazine, clopenthixol, trifluopromazine, and flupenthixol)[38], chlorpromazine and prochlorperazine, indole alkaloids, the anti-malarial quinine and the anti-arrhythmic quinidine [42] demonstrated P-gp inhibiting activity. P-gp inhibiting agents are pharmacologically active in vitro in concentration range from (1 to 50 μM). Similar serum-concentration range of these P-gp inhibiting agents is known to cause serious and devastating immunosuppressive and cardiovascular effects [25,43]. Cyclosporin-A (the commonly used immunosuppressive agent) remained one of the golden first generation P-gp inhibitors for several years [44–46].
Second generation P-gp blockers
The vast majority of all first generation P-gp inhibitors is therapeutic agents and reverses P-gp efflux activity in vitro at concentrations range higher than their individual clinical therapeutic windows. The search for non-toxic second generation P-gp blockers resulted in newer analogs of the first generation agents with more potent P-gp inhibition and considerably less toxicity. Several structural analogs for VRP were synthesized for better P-gp intrinsic activity and less cardiovascular effects. Among these analogues, dexverapamil (R-enantiomer of VRP), emopamil, gallopamil, and Ro11-2933 inhibit the P-gp activity in vitro equipotent to more potent than VRP, but with marginal toxicity in many animal models [47–50]. PSC 833 is a non-immunosuppressive analog of cyclosporine-A and more potent P-gp inhibitor in vitro [51,52].
Third generation P-gp blockers
Combinatorial chemistry and structure–activity relationships approaches were used to bring up the third generation P-gp blockers with antagonism function at nanomolar range (20–100 nM). Several of these compounds possess only P-gp blocking effect such as zosuquidar (LY335979) [53], elacridar (GF120918) [54], XR9051 [55], OC144-093 [44]. Others have dual P-gp and MRP blocking activity such as biricodar (VX-710), and timcodar (VX-853) [56] or dual P-gp and BCRP tariquidar (XR9576) [57]. These agents appear to possess acceptable toxicity profile; however their success in combination with anticancer agents is yet to be determined in clinical settings.
P-gp blockers of natural origin
Several naturally occurring compounds and plant extracts were reported for their modulation of multidrug resistance; however, this review will focus only on major classes viz., flavonoids, stilbenes, coumarins, terpenoids, alkaloids and saponins. In addition the review will cover the literature regarding natural P-gp inhibitors in the period between 1989 and 2015. Search was conducted on common databases such as ISI web of Knowledge, Scopus and using the Saudi digital library (SDL) for getting the full text.
Flavonoids and stilbenes
It was reported that plant derived polyphenolic compounds, mainly flavonoids and stilbenes or their synthetic derivatives, can modulate the main ABC transporters responsible for cancer drug resistance, including P-glycoprotein, multidrug resistance-associated protein 1 (MRP1) and breast cancer resistance protein (BCRP) [58]. Flavonoids and stilbenes represent the third generation of P-gp inhibitors and they produced a comparable effect to those of the well-known P-gp inhibitors verapamil and cyclosporine [59].
Several flavonoids and stilbenes (Fig. 1) have been reported to inhibit BCRP encoded by the ABCG2 gene. Thus, the consumption of flavonoids with high inhibitory activity could change pharmacokinetics and drug levels of drugs that are effluxed by BCRP. The following flavonoid structural features were found to contribute positively to BCRP inhibition; (A) A hydroxyl group in position 5, double bond between position 2 and 3, and a methoxyl group in position 3. (B) The exchange of a 3-methoxy group by an OH-group resulted in decrease in activity [60]. The following compounds are some examples of flavonoids reported as P-gp inhibitors; (−)-Epigallocatechin-3-gallate (EGCG) downregulates P-gp and BCRP but did not affect MRP1 in a tamoxifen resistant MCF-7 (breast cancer) cell line [61]. Flavonoids with hydrophobic group such as prenyl substituents might constitute the promising candidates for MDR reversal agents. 8-prenylnaringenin (8-isopentenylnaringenin) (Fig. 1), a potent phytoestrogen isolated from common hops (Humulus lupulus), strongly inhibited MRP1 transport activity in human erythrocytes. It was not able to modulate MDR in human adenocarcinoma cell line in spite of its ability to inhibit both P-glycoprotein and MRP1 activities [62].
Fig. 1.
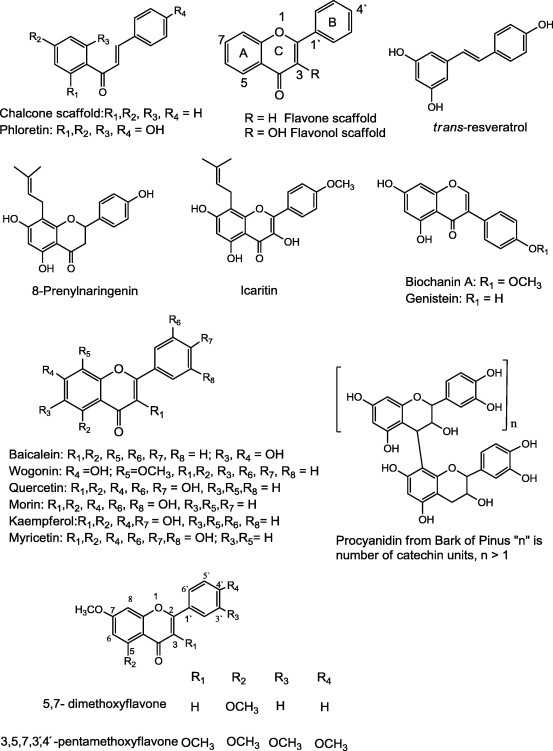
Structures of some flavonoids and stilbenes reported as P-gp inhibitors.
Icaritin (Fig. 1), isolated from Herba Epimedium, significantly increased the intracellular accumulation of ADR and decreased the expression of the MDR1 gene in a multiple drug-resistant HepG2 (liver cancer cell line)/adriamycin (HepG2/ADR) cell line compared with drug-sensitive HepG2 cells. In addition, icaritin significantly downregulates the expression of P-gp [63].
Baicalein (Fig. 1), the major flavonoid in Scutellariae radix, significantly enhanced the oral bioavailability of tamoxifen, which might be mainly due to inhibition of the CYP3A (Cytochrome P450, family 3, subfamily A) – mediated metabolism of tamoxifen in the small intestine and/or in the liver and inhibition of the P-gp efflux pump in the small intestine [64]. Silymarin can inhibit P-gp mediated efflux in Caco-2 cells (colon cancer cell line), suggesting they could potentially increase the absorption/bioavailability of co-administered drugs that are effluxed P-gp [65].
Biochanin A (Fig. 1) and silymarin were reported to potentiate DOX cytotoxicity in P-gp positive cells. The underlying mechanism(s) may involve direct interaction with P-gp as evidenced by flavonoid modulation of P-gp ATPase activity [66].
Nguyen et al. studied the effect of large number of flavonoids on P-gp inhibition. Biochanin-A, genistein, quercetin, chalcone, silymarin, phloretin, morin, and kaempferol (Fig. 1), significantly increased the accumulation of both daunomycin (DNM) and vinblastine (VBL) in human pancreatic adenocarcinoma Panc-1 cells. The study concluded that; the aforementioned compounds can inhibit MRP1-mediated drug transport through binding interactions with MRP1, as well as modulation of GSH (glutathione) concentrations [67].
Flavonoids from grape fruit juice such as kaempferol and naringenin caused a decrease in P-gp levels and MDR-1 transcript levels as well in the human immortalized tubular cell line (HK-2) [68].
In EPG85-257RDB cells (P-gp-positive gastric carcinoma cell line) quercetin (Fig. 1) acted as a chemosensitizer through decreasing of P-gp expression, inhibition of drug transport and downregulation of ABCB1 (ATP-binding cassette sub-family B member 1 ) gene expression [69]. Therefore, concurrent use of quercetin provides a therapeutic benefit by increasing the bioavailability of doxorubicin administered orally [70].
Procyanidine isolated from bark of Pinus massoniana (Fig. 1) markedly increased the accumulation of Rh123 (rhodamine 123) within cells by inhibiting its efflux in a dose-dependent manner. Procyanidine was a potent inhibitor of P-gp on BBB (blood brain barrier) and could improve the therapeutic effects on cerebral tumors of some drugs which are difficult to accumulate in the brain [71].
3,5,7,3′,4′-pentamethoxyflavone (Fig. 1) from Kaempferia parviflora rhizome increased the accumulation of Rh123 and daunorubicin in LLC-GA5-COL150 cells (a transfectant cell line of a porcine kidney epithelial cell line (LLC-PK1) with human MDR1 cDNA) in a concentration dependent manner. In addition, 5,7-dimethoxyflavone (Fig. 1) to a lesser degree increased Rh 123 accumulation in LLC-GA5-COL150 cells [72], and exhibited a stimulatory effect on the accumulation of doxorubicin in A549 cells (adenocarcinoma human alveolar basal epithelial cells) [73].
Myricetin (Fig. 1) significantly enhanced the cellular accumulation of Rh123 in MCF-7/ADR cells overexpressing P-gp. It increases the oral bioavailability of DOX due to the enhancement of its absorption in the gastro-intestinal tract via the inhibition of P-gp and reduction of first-pass metabolism of DOX due to inhibition of CYP3A in the small intestine and/or in the liver [70].
Wogonin (Fig. 1), a flavone, significantly potentiated etoposide-induced apoptosis in HL-60 (Human promyelocytic leukemia) cells. It impaired the function of P-gp and thus increased cellular content of etoposide in the cells. Moreover, wogonin is likely to act as an inhibitor of P-gp and potentiate the apoptotic action of etoposide. On the other hand, wogonin inhibited etoposide-induced apoptosis in thymocytes, one of the normal cells. The potentiation by wogonin is likely to be a specific action for cancer cells but not normal cells. Therefore, this flavone may be used to reduce the excretion of the anticancer agents via P-glycoprotein and increase the pharmacological action of it in cancer cells [74].
Resveratrol, a well-known stilbene, was reported to enhance the cytotoxic profile of both docetaxel and doxorubicin in solid tumors through inhibition of P-gp efflux and downregulation of MDR1 gene [75].
The effect of amino acid conjugation with flavonoid was studied on MDR. Conjugation of quercetin with glutamic acid moiety attached at 7-O position was potent as verapamil in reversing MDR and sensitized MDR MES-SA/Dx5 cells to various anticancer drugs. Analysis on Rh-123 accumulation confirmed that this conjugate inhibits drug efflux by P-gp, in addition, P-gp ATPase assay showed that this compound interacts with the drug-binding site of P-gp to stimulate its ATPase activity.[76]
Coumarins
Several naturally occurring and synthetic coumarins, furanocoumarin, pyranocoumarin and sesquiterpenoid coumarins were investigated for their ability to reverse multi drug resistance by inhibiting P-gp activity.
In a study carried out by Raad et al. [77], a set of 32 natural and synthetic coumarins were tested in order to evaluate their activity on human leukemic cells (K562/R7) overexpressing P-gp. They proved that coumarins substituted by a common α-(hydroxyisopropyl) dihydrofuran moiety, exhibited a significant inhibitory effect on P-gp when compared to the positive control cyclosporin A. The presence of phenyl group at position C4 in coumarin was found to be essential for activity. In addition, the [α-(hydroxyisopropyl-dihydrofuran) group, especially at positions C7–C8, also showed some interest for activity compared to other additional groups [77].
GUT-70 (Fig. 2), a tricyclic coumarin, isolated from the stem bark of Calophyllum brasiliense collected in Brazil, inhibited human leukemic cell lines, including the P-glycoprotein overexpressing cell lines, in a concentration and time-dependent manner with IC50 values from 2–5 μM [78].
Fig. 2.
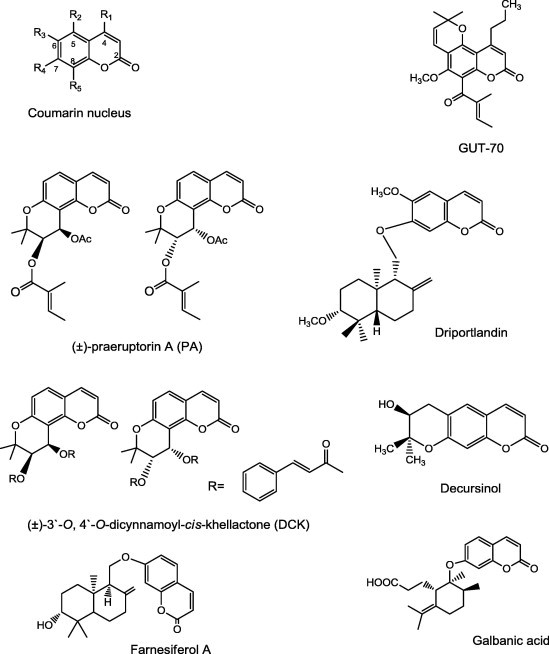
Structures of coumarins reported as P-gp inhibitors.
(±)-Praeruptorin A (PA) (Fig. 2), a naturally existing pyranocumarin isolated from the dried root of Peucedanum praeruptorum Dunn., re-sensitizes P-gp mediated MDR (P-gp-MDR) cancer cells to anticancer drugs. The PA derivative (±)-3′-O,4′-O-dicynnamoyl-cis-khellactone (DCK), was more potent than PA or verapamil in the reversal of P-gp-MDR. In P-gp-MDR cells DCK increased cellular accumulation of DOX without affecting the expression level of P-gp. DCK could bind simultaneously with substrates to P-gp through an allosteric site and thus affecting P-gp–substrate interactions [79].
Decursinol (Fig. 2), a major coumarin derived from the roots of Angelica gigas, showed high permeability in Caco-2 cell monolayers in the absorptive direction. Secretion increased in a concentration-dependent manner, with an efflux ratio of more than 2 at 50 μM, indicating that it could be transported through an active efflux transporter such as P-gp, multidrug resistance protein 2 or BCRP [80].
Sesquiterpene coumarins
Farnesiferol A (Fig. 2) (from the roots of Ferula persica) and galbanic acid (Fig. 2) (from the roots of Ferula szowitsiana) significantly inhibited the P-gp activity compared to verapamil in a doxorubicin resistant breast cancer cell line (MCF7/ADR) [81]. Similarly, driportlandin (Fig. 2), isolated from Euphorbia portlandica, was more active on the reversal of multidrug resistance (MDR) of mouse lymphoma cells than verapamil [82].
Furanocoumarin
Dihydroxybergamotin and other furanocoumarins contained in grapefruit juice, such as bergamotin, FC726, bergaptol and bergapten (Fig. 3), increased the steady-state uptake of [3H]-vinblastine by Caco-2 cells due to inhibition of drug efflux transporters, such as P-gp [83].
Fig. 3.
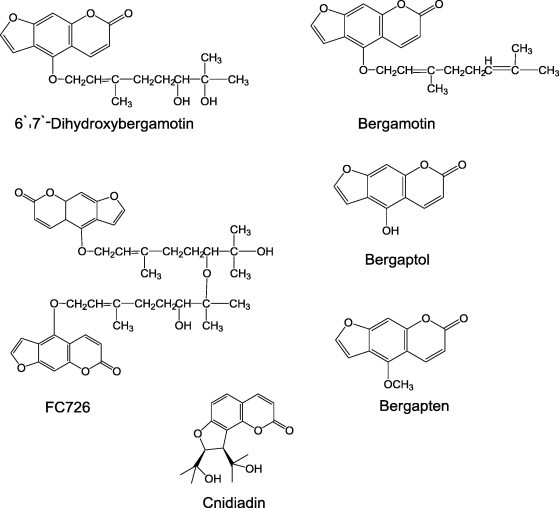
Furanocoumarins with P-gp inhibitory effect.
Moreover, cnidiadin isolated from Tordylium apulum (Apiaceae) (Fig. 3) is a cytotoxic agent found to be capable of competitively inhibiting the binding and efflux of drug by P-gp and of enhancing the cell toxicity of vinca alkaloids in Madin–Darby canine kidney (MDCK-MDR1) cells and mutant human carcinoma (KB/VCR) overexpressing P-gp [84].
Terpenoids
Sesquiterpenes
Celastraceae plants represent highly effective and specific modulators of the MDR phenotype in Leishmania, due to their dihydro-β-agarofuran sesquiterpenes (Fig. 4). Some of them could be considered as lead compounds for further development [85]. In a study by Cortes et al. (2005), the inhibitory activity of a series of 76 dihydro-β-agarofuran sesquiterpenes was tested on NIH-3T3 (mouse embryo tissue fibroblast) cells expressing the human P-gp multidrug transporter, to establish quantitative comparisons of their respective abilities to block the drug efflux from target cells. The most important pharmacophoric features of these compounds were in the region of the substituents at the C-2, C-3 and C-8 positions, which seem to be critical for determination of the overall effectiveness of sesquiterpenes as P-gp inhibitors [86]. A 3D QSAR (Quantitative structure–activity relationship) study concluded that, the esterification level of the compounds, the presence of two aromatic ester moieties and the size of the molecule are important factors for the reversal activity. Most of the analyzed compounds showed low intrinsic toxicity and many of them were able to efficiently overcome the MDR phenotype in the resistant line by modulating drug accumulation. Compounds MAMA 7, MAMA10, MACHU 4, MACU 5, MACU 7 and MACU 8 (Fig. 4) were the most active compounds.
Fig. 4.
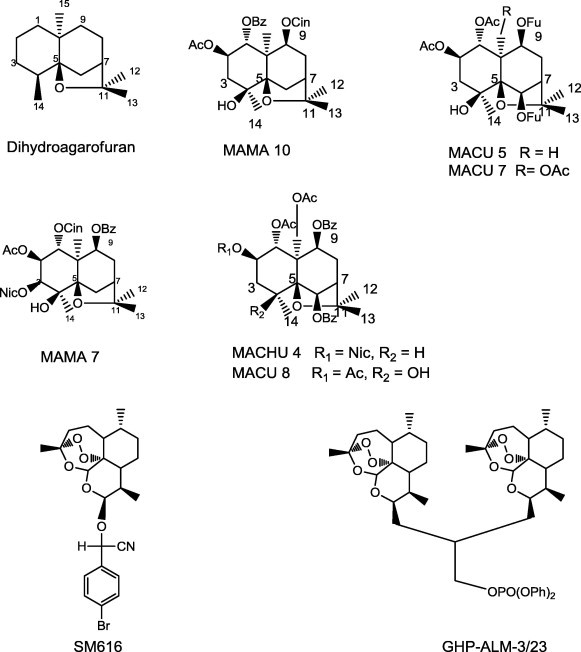
Structures of the most active sesquiterpenes as reversal agents of MDR phenotype of Leishmania.
Two derivatives of the anti-malarial artemisinin, SM616 and GHP-AJM-3/23 (Fig. 4) inhibited P-gp activity in sensitive CCRF-CEM leukemia cells and P-gp over-expressing multidrug-resistant CEM/ADR5000 leukemia cells as well as in porcine brain capillary endothelial cells (PBCEC) [87].
A non-cytotoxic concentration of β-caryophyllene significantly increased the anticancer activity of α-humulene and isocaryophyllene on MCF-7 cells. Moreover, β-caryophyllene potentiated the anticancer activity of paclitaxel on MCF-7, DLD-1 (colon adenocarcinoma) and L-929 (murine fibroblast) cell lines [88].
Limonoid
Obacunone, a limonoids isolated from Phellodendron amurense (Rutaceae), showed significant P-gp MDR inhibition activity in MES-SA/DX5 (human MDR uterine sarcoma cell line) and HCT15 cells (human colorectal cancer cell line) with an ED50 value of 0.028 pg/mL and 0.0011 pg/mL, respectively [89].
Diterpenes
Different skeletones of diterpenes including jatrophanes, lathyranes, uphoractine, pepluane and paraliane that were isolated from Euphorbia species were assayed for P-gp inhibitory activity in mouse lymphoma cells by using the Rh 123 exclusion test (Fig. 5). The effect on drug accumulation in drug-resistant cells is proportional to the hydrophobicity of diterpenes. Highly active compounds can be found among the jatrophanes, lathyranes and also among the tetracyclic diterpenes [90].
Fig. 5.
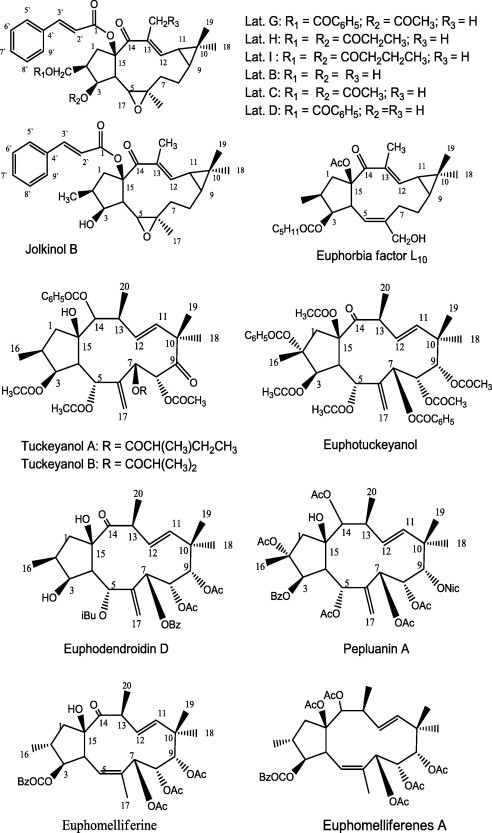
Macrocyclic lathyrane and jatrophanene diterpenes with P-gp inhibitory effects.
Macrocyclic lathyrane, and jatrophane diterpenes may be valuable as lead compounds for the development of P-gp modulators in different multidrug-resistant cancer cells. The macrocyclic lathyrane diterpene latilagascene B (lat. b, Fig. 5), previously isolated from Euphorbia lagascae, its acylated derivatives, latilagascenes G, H, and I and the macrocyclic diterpenes of the jatrophane-type, tuckeyanols A and B, and euphotuckeyanol (Fig. 5), isolated from Euphorbia tuckeyana, displayed very strong modulating activity against P-gp on human MDR1 gene-transfected and parental L5178 mouse lymphoma cell lines [91].
The macrocyclic lathyrane diterpenes, latilagascenes D–F and jolkinol B (Fig. 5), isolated from the methanol extract of Euphorbia lagascae, displayed potent activity on mouse lymphoma cells compared with that of the positive control, verapamil [92].
The macrocyclic lathyrane polyester Euphorbia factor L10 (Fig. 5) has been obtained from the seeds of the caper spurge (E. lathyris) as a novel chemotype for P-gp inhibitors [93]. Euphodendroidin D and pepluanin A were the most powerful inhibitors of daunomycin-efflux activity within the class of jatrophane diterpenes. Their efficiency was found to be at least twofold higher than the conventional modulator cyclosporin A. The analysis of euphodendroidin’s activities has evidenced the involvement of ring A in P-gp binding, highlighting the relevance of a free hydroxyl group at position 3, together with the negative effect of this group at position 2 (Fig. 6). In addition, substitution at the proximal C-5 with a large group also decreased the activity. The biological evaluation of modified jatrophanes has shown effect of substitution at positions 3, 6 and 15 and the importance of relative configuration of hydroxyl groups. In addition, substitution at position 6 affects the inhibitory ability in a way that dramatically depends on the location of the free hydroxyl group. Euphocharacin’s activities highlighted the positive roles of benzoyl and propyl at C-9 and C-3, respectively, and confirmed the negative role of hydroxyl at C-2 and the positive effect of the same group at C-15. The results of biological assays on pepluanins have been used to extend the structure activity relationships to the other oxygenated carbon atoms of the medium-sized ring (C-7/C-15). Within the set of compounds investigated, pepluanin A with acetoyl at C-8 and nicotinyl (Nic) at C-9 has been reported as the most powerful inhibitor, outperforming cyclosporine A by a factor of at least two in the inhibition of P-gp-mediated daunomycin transport. Taken together, these observations suggest that jatrophanes and modified jatrophanes share a common gross pharmacophore, which is dramatically affected by changes of the oxygenation pattern, but surprisingly tolerant in terms of modifications of connectivity as summarized in Fig. 6 [94].
Fig. 6.
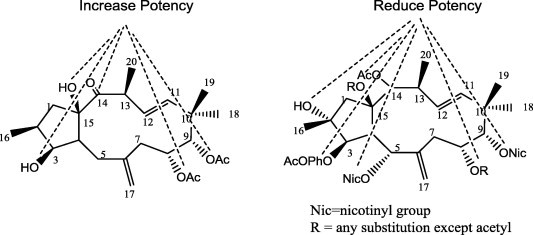
Pharmacophoric elements for the anti-MDR activity of P-gp within the class of jatrophane diterpenes.
In addition, macrocyclic jatrophane diterpenes, named euphomelliferine and euphomelliferine A (Fig. 5) isolated from the methanolic extract of E. mellifera displayed a significant MDR reversing activity, in a dose-dependent manner, on human MDR1-gene-transferred mouse cells (L5178Y MDR) and on human colon adenocarcinoma cells (COLO 320). They did not induce apoptosis in the COLO 320 cells [95].
The ent-abietane lactones, helioscopinolides A, B, E and F (Fig. 7), isolated from Euphorbia species were able to reverse the MDR of the tested human MDR1 gene-transfected mouse lymphoma cells, in a concentration-dependent manner in sub-cytotoxic concentration [96].
Fig. 7.
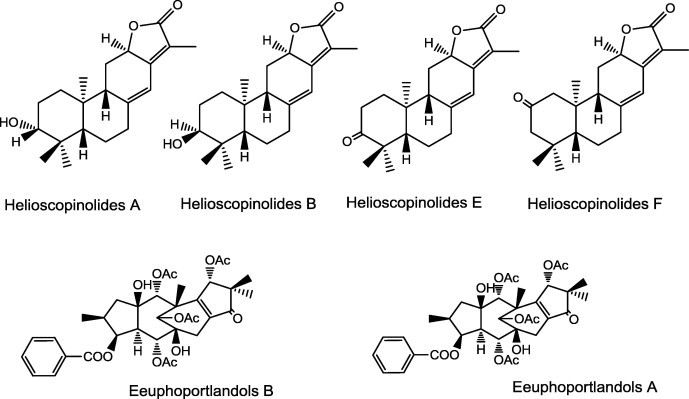
Ent-abietane lactones from Euphorbia pubescens, E.tuckeyana and E. porlandica with P-gp inhibitory effects.
The tetracyclic diterpene polyesters, euphoportlandols A and B (Fig. 7), isolated from Euphorbia portlandica, revealed significant inhibition of P-gp activity [97].
Alkaloids
Alkaloids are basic nitrogenous compounds derived from plant source and are classified into different groups based on the amino acid they are derived from. Many reports revealed the ability of alkaloids to inhibit P-gp. There are two substructural features present in compounds that modulate P-gp-associated MDR: (A) a basic nitrogen atom and (B) two planar aromatic rings. Glaucine (Fig. 8), an alkaloid component of Chinese herbal plant Yanhusuo (Corydalis yanhusuo W.T. Wang, YHS) inhibits P-gp and MRP1-mediated efflux and activates ATPase activities of the transporters. Furthermore, glaucine suppresses expression of ABC transporter genes. It reverses the resistance of MCF-7/ADR to adriamycin and mitoxantrone effectively [98].
Fig. 8.
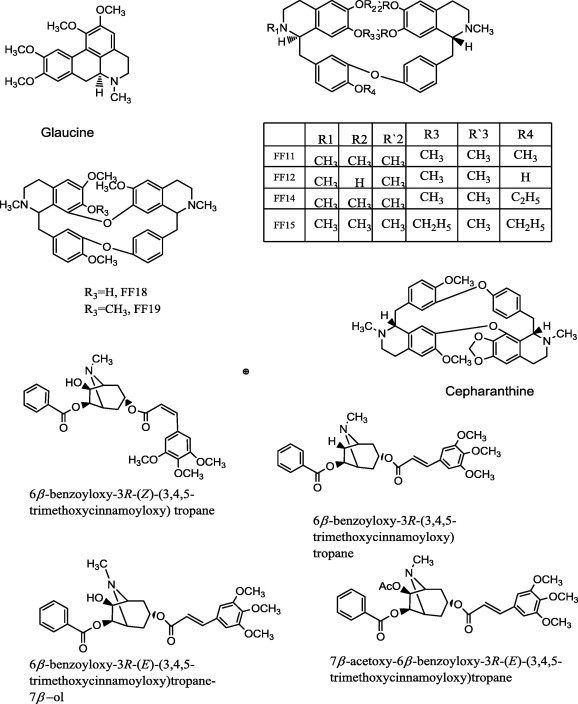
Examples of alkaloids reported as P-gp inhibitors.
Lobeline, a piperidine alkaloid from Lobelia inflata and several other Lobelia species, inhibited P-gp activity. MDR reversal potential of lobeline could be demonstrated in cells treated with DOX in that lobeline can sensitize resistant tumor cells at non-toxic concentrations. However, lobeline cannot block BCRP (Breast Cancer Resistance Protein) dependent mitoxantrone efflux [99].
Reserpine is an effective “modulator” of P-gp-associated multidrug resistance (MDR) in multidrug-resistant human leukemia cell line, CEM/VLB100. Reserpine derivatives with pendant benzoyl function in an appropriate spatial orientation can modulate MDR. It was found that the relative disposition of aromatic rings and basic nitrogen atom is important for modulators of P-gp-associated MDR, and they suggest a ligand-receptor relationship for these agents [100].
Benzylisoquinoline alkaloids (Fig. 8) have a strong history in modulation of MDR. Naturally occurring bisbenzylisoquinoline alkaloids that were isolated from natural plants were tested in vitro as MDR modifiers. Six of these natural compounds (FF19, FF18, FF15, FF14, FF11 and FF12) showed potent activities to restore sensitivity of resistant tumor cells, such as MCF-7/ADR and (MDR nasopharyngeal carcinoma) KBv200 cells, to many antitumor drugs including doxorubicin and vincristine [101].
Cepharanthine, a bisbenzylisoquinoline (biscoclaurine) alkaloid, completely overcomes resistance of a multidrug-resistant subline, ChR-24, derived from human KB carcinoma cells, to vincristine, actinomycin D, and daunomycin, and partially overcomes resistance to adriamycin. Moreover, this compound enhanced sensitivity to (ADM) and vincristine (VCR), and enhanced apoptosis induced by ADM and VCR of P-gp negative K562 cells (human chronic myelogenous leukemia). Cepharanthine changed the distribution of ADM from cytoplasmic vesicles to nucleoplasm in K562 cells by inhibiting the acidification of cytoplasm organelles [102].
Another class of bioactive alkaloids is tropane esters (Fig. 8). Some of these compounds were isolated from Erythroxylum rotundifolium and evaluated against a panel of human cancer cells.
6β-benzoyloxy-3R-(Z)-(3,4,5-trimethoxycinnamoyloxy)tropane and 6β-benzoyloxy-3α-(3,4,5-trimethoxycinnamoyloxy)tropane, 6β-benzoyloxy-3α-(E)-(3,4,5-trimethoxycinnamoyloxy)tropane-7β-ol, and 7β-acetoxy-6β-benzoyloxy-3α-(E)-(3,4,5-trimethoxycinnamoyloxy)tropane, demonstrated greatest activity against MDR oral epidermoid carcinoma (KB-V1) cells incubated in the presence of vinblastine. Thus tropane esters of this type can reverse the MDR phenotype, presumably by interacting with P-gp [103,104].
Pervilleines B and C, obtained from a chloroform extract of the roots of Erythroxylum pervillei were found to restore the vinblastine (VLB) sensitivity of cultured multidrug-resistant KB-V1 cells PB through inhibiton of P-gp [105–107]. Veralosinine (Veratrum lobelianum) and veranigrine (V. nigrum) (Fig. 9) modify transport activity of MDR1 in multidrug-resistant human MDR1-gene-transfected mouse lymphoma cells (L5178Y). They enhanced the antiproliferative effects of doxorubicin on MDR cells [108].
Fig. 9.
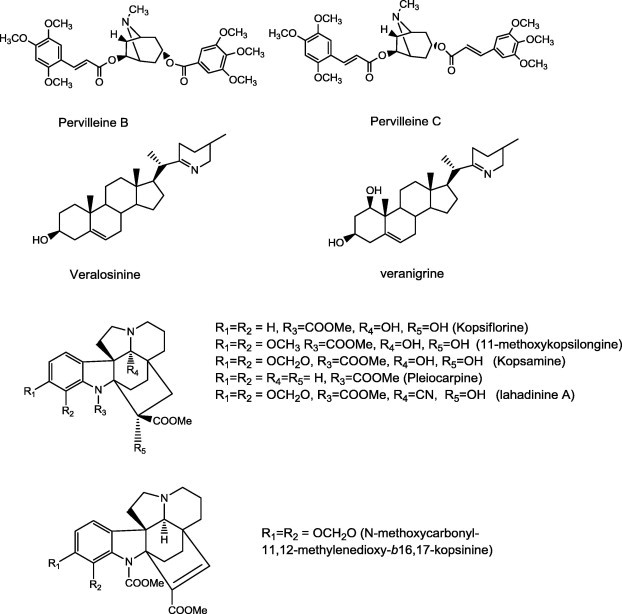
Examples of alkaloids reported as P-gp inhibitors.
A series of indole alkaloids of the aspidofractinine-type was assessed for their potential in reversing MDR in vincristine-resistant KB cells. Of the compounds tested, kopsiflorine, kopsamine, pleiocarpine, 11-methoxykopsilongine, lahadinine A and N-methoxycarbonyl-11,12-methylenedioxy-Δ16,17-kopsinine were found to show appreciable activity [109]. Kopsiflorine (Fig. 9) that was isolated from Kopsia dasyrachis, enhanced cytotoxicity of vincristine in drug-resistant KB cells (VJ-300) in a concentration-dependent manner. It was found that, kopsiflorine interacts directly with P-glycoprotein and inhibits the efflux of antitumor agents in drug-resistant cells [110].
Steroidal saponins
Saponins are a class of compounds present in some plant families and classified into steroidal and triterpenoidal. Steroidal saponins isolated from Paris polyphylla (Trilliaceae) (Fig. 10), 3-O-Rha(1 → 2)[Ara(1 → 4)]Glc-pennogenine, gracillin and polyphyllin D, and ecdysteroids 20-hydroxyecdysone and pinnatasterone showed inhibition of P-gp-mediated daunorubicin efflux in K562/R7 (human leukemic) cell line [111]. The cucurbitacins, balsaminagenin B, balsaminoside A, karavelagenin C (Fig. 10), reversed multidrug resistance on human MDR1 gene transfected mouse lymphoma cells compared with that of verapamil. Moreover, in the checkerboard model of combination chemotherapy, the interaction between doxorubicin and compounds balsaminagenin B, balsaminoside A, karavelagenin C synergistically enhanced the effect of the anticancer drug using daunorubicin and doxorubicin-resistant acute myelogenous leukemia sublines (AML-2/D100 and AML-2/DX100), which overexpress P-gp and MRP, respectively [112].
Fig. 10.
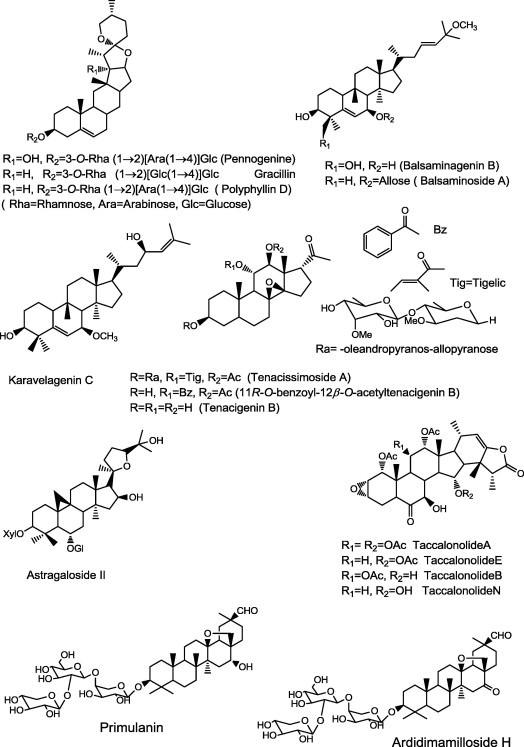
Steroidal saponins that reported as P-gp inhibitors.
Protopanaxatriol ginsenosides (PTG) has a chemosensitizing effect on P-gp-mediated MDR cells by increasing the intracellular accumulation of drugs through direct interaction with P-gp at the azidopine site. In addition, PTG may have a beneficial effect on cancer chemotherapy with respect to the possibility of long-term use without the concern of P-gp activation [113]. Tenacissimoside A (Fig. 10) and 11R-O-benzoyl-12-O-acetyltenacigenin B, two derivatives of tenacigenin B from the plant Marsdenia tenacissima, reversed multidrug resistance in P-gp-overexpressing multidrug-resistant cancer cells. They increased sensitivity of HepG2/Dox cells to the antitumor drugs doxorubicin, vinblastine, puromycin, and paclitaxel [114].
Astragaloside II (Fig. 10) is a saponin widely used in traditional Chinese medicine. It has been reported that astragaloside has antitumor effects on hepatocellular carcinoma Bel-7402 cells in vitro and in vivo. Astragaloside II showed strong potency to increase 5-fluorouracil cytotoxicity toward 5-fluorouracil-resistant human hepatic cancer cells Bel-7402/FU. The mechanism of astragaloside II on P-gp-mediated MDR demonstrated that astragaloside II significantly increased the intracellular accumulation of Rh 123 via inhibition of P-gp transport function. Astragaloside II could down regulate the expression of the P-gp and MDR-1 gene. In addition, astragaloside II suppressed phosphorylation of extracellular signal regulated kinase 1/2, p38 and c-Jun N-terminal kinase [115].
The taccalonolides (Fig. 10) are a class of structurally and mechanistically distinct microtubule-stabilizing agents isolated from Tacca chantrieri. Taccalonolides A, E, B, and N were effective in vitro against cell lines that overexpress P-gp and MRP7. In addition, taccalonolides A and E were highly active in vivo against a doxorubicin and paclitaxel-resistant P-gp-expressing tumor, Mam17/ADR. Taccalonolides have advantages over the taxanes (microtubule stabilizers) in their ability to circumvent multiple drug resistance mechanisms [116].
Using in vitro assays saponins; primulanin and ardisimamilloside that were isolated from Labisia pumila showed significant modulation of P-gp, CYPs (cytochrome P450s), and PXR (Pregnane X receptor) suggesting a potential to alter the pharmacokinetic and pharmacodynamic properties of conventional drugs if used concomitantly [117].
Finally, the literature found regarding the P-gp inhibition by natural products was too much to be covered in one review article, therefore the current review focused only on major classes of naturally occurring compounds. However, several reports were found concerning the activity of various plant extracts [118] and their constituents as P-gp inhibitors; viz., anthraquinones [119], phenylbutanoids [120], phytosterols [121], cannabinoids [122], carotenoids [123], curcumenoids [124], volatile oils [125], sulfur compounds [126], and polyacetylenes. [127] In addition, some vitamins such as vitamin D revealed significant reversal of multi-drug resistance in many resistant cells [128]. Moreover, several marine and marine derived endophytes, a major source of natural products, revealed significant P-gp inhibition such as lamellarine O and shornephine A [129,130]. These previously mentioned classes, among others, could be the focus of another separate review article.
Conclusion and future prospectives
Natural products are some of the major sources for drug discovery. There is a considerably growing body of evidence concerning the reversal of multidrug resistance though p-gp inhibition and other related proteins, using naturally occurring compounds. A large number of compounds were found to be P-gp blocking through the inhibition of the pumb ATPase activity. However, some classes of natural products, especially polyphenols, were reported to be non-specific enzyme inhibitors which could affect other human enzymatic targets such as cytochrome system thus affecting the whole pharmacokinetic and toxicity profile of anticancer drugs. Therefore, it is necessary not only to investigate the P-gp blockade rather than studying the mechanism and selectivity of such blockade in terms of ATPase inhibition. In addition, P-gp blockade might possess significant role in affecting the cellular pharmacokinetics of anticancer drugs; however since its discovery (since more than four decades), no significant clinical output for any P-gp inhibitor could be declared. On the other hand, intratumoral distribution and tissue pharmacokinetics of anticancer drugs constitutes robust pharmacokinetic barrier that hinders their bioavailability within intratumoral micromillieu. The influence of P-gp inhibition on tissue pharmacokinetics and intratumoral distribution of anticancer drugs is rarely studied. Yet, it is pretty complicated to assess the avascular intratumoral distribution of P-gp substrates attributed to P-gp blockade. This might be attributed to the heterogenous avascular distribution of the P-gp blocker per se. Finally, it is strongly recommended to study the influence of P-gp blockade in enhancing the avascular distribution of P-gp substrates using suitable three dimensional avascular solid tumor model to facilitate clinical interpretation.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Hossam M. Abdallah, PhD, received his M. S. degree in Pharmacognosy and PhD in Chemistry of Natural Products from Faculty of Pharmacy, Cairo University. In 2013, he was promoted to Associate Professor of Pharmacognosy, Faculty of Pharmacy, Cairo University. Currently, he is working at Natural Products and alternative medicine Department, Faculty of Pharmacy, King Abdulaziz University. He has 29 peer-reviewed publications, a reviewer in several peer-reviewed journals in the field of natural products, a PI and CO-I in more than ten projects. He has a scientific communication with two international institutes.

Ahmed M. Al-Abd has graduated (2000) from Faculty of Pharmacy, Ain Shams University. He obtained his PhD degree (Pharmacology and Toxicology) from Beni-Suif University, Egypt in 2011. Currently, he is an Assistant Professor at King Abdulaziz University and visiting scholar in Bouvé College of Health Sciences, Northeastern University, USA. He is an author and co-author for more than 25 peer-reviewed publications; Co-inventor in 2 patents. Ahmed Al-Abd has a broad international scientific communication network (collaborator with more than 15 research institutes in more than 5 different countries worldwide).

Dr. Riham Salah El Dine was graduated at 1998 from Faculty of Pharmacy, Cairo University. Afterward, she worked as teaching assistant in Department of Pharmacognosy, Cairo University from 1998 to 2003. In 2004, she was enrolled as a PhD candidate in Institute of Natural Medicine, Toyama, Japan. She got her PhD under the supervision of Dr. Masao Hattori. After PhD, she was awarded a postdoctoral fellowship in the same laboratory for 6 months. From 2008 up to date, she is appointed as an Assistant Professor, Faculty of Pharmacy, Cairo University. She is a co-author of more than 17 peer-reviewed publications; and a reviewer for several peer-reviewed journals in the field of natural products.

Ali El Halawany, PhD, was graduated from Faculty of Pharmacy, Cairo University in 1996. He obtained his master degree in Pharmacognosy in 2002. In 2004, he was selected for MEXT scholarship by the Japanese government to be enrolled as PhD Candidate in Institute of Natural Medicine, Toyama, Japan. After finishing the PhD in 2007, he was awarded a COE scholarship in the same laboratory for 6 months followed by another 6 months as a visiting researcher. In 2009, he worked at Institute of Natural Medicine as a foreign researcher for 1 year till 2010. From April 2014 up to date, he is working as an Associate Professor, Department of Pharmacognosy, Cairo University. He is an author/co-author for more than 28 peer-reviewed publications; Co-inventor in 1 US patent, a member in the editorial board of BFOPCU journal and a reviewer for several peer-reviewed journals in the field of natural products.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ling V., Thompson L.H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- 2.Krishna R., Mayer L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4):265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen C.J., Chin J.E., Ueda K., Clark D.P., Pastan I., Gottesman M.M. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.H., Bradley G., Zhang J.T., Ling V. Differential expression of P-glycoprotein genes in primary rat hepatocyte culture. J Cell Physiol. 1993;157(2):392–402. doi: 10.1002/jcp.1041570223. [DOI] [PubMed] [Google Scholar]
- 5.Thiebaut F., Tsuruo T., Hamada H., Gottesman M.M., Pastan I., Willingham M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawara I., Nakahama M., Hamada H., Tsuruo T., Mori S. Apparent stronger expression in the human adrenal cortex than in the human adrenal medulla of Mr 170,000–180,000 P-glycoprotein. Cancer Res. 1988;48(16):4611–4614. [PubMed] [Google Scholar]
- 7.Cordon-Cardo C., O’Brien J.P., Casals D., Rittman-Grauer L., Biedler J.L., Melamed M.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arceci R.J., Croop J.M., Horwitz S.B., Housman D. The gene encoding multidrug resistance is induced and expressed at high levels during pregnancy in the secretory epithelium of the uterus. Proc Natl Acad Sci USA. 1988;85(12):4350–4354. doi: 10.1073/pnas.85.12.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai K., Kusano I., Ido M., Sakurai M., Shiraishi T., Yatani R. Identification of a P-glycoprotein-related protein (mini-P-glycoprotein) which is overexpressed in multidrug resistant cells. Biochem Biophys Res Commun. 1994;198(2):804–810. doi: 10.1006/bbrc.1994.1115. [DOI] [PubMed] [Google Scholar]
- 10.Young L.C., Campling B.G., Cole S.P., Deeley R.G., Gerlach J.H. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7(6):1798–1804. [PubMed] [Google Scholar]
- 11.Zeng H., Bain L.J., Belinsky M.G., Kruh G.D. Expression of multidrug resistance protein-3 (multispecific organic anion transporter-D) in human embryonic kidney 293 cells confers resistance to anticancer agents. Cancer Res. 1999;59(23):5964–5967. [PubMed] [Google Scholar]
- 12.Jonker J.W., Smit J.W., Brinkhuis R.F., Maliepaard M., Beijnen J.H., Schellens J.H. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92(20):1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 13.Ross D.D., Karp J.E., Chen T.T., Doyle L.A. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96(1):365–368. [PubMed] [Google Scholar]
- 14.Maliepaard M., van Gastelen M.A., de Jong L.A., Pluim D., van Waardenburg R.C., Ruevekamp-Helmers M.C. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59(18):4559–4563. [PubMed] [Google Scholar]
- 15.Gollapudi S., Thadepalli F., Kim C.H., Gupta S. Difloxacin reverses multidrug resistance in HL-60/AR cells that overexpress the multidrug resistance-related protein (MRP) gene. Oncol Res. 1995;7(5):213–225. [PubMed] [Google Scholar]
- 16.Louisa M., Soediro T.M., Suyatna F.D. In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac J Cancer Prev. 2014;15(4):1639–1642. doi: 10.7314/apjcp.2014.15.4.1639. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata S., Oka M., Shiozawa K., Tsukamoto K., Nakatomi K., Soda H. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280(5):1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 18.Xie N., Mou L., Yuan J., Liu W., Deng T., Li Z. Modulating drug resistance by targeting BCRP/ABCG2 using retrovirus-mediated RNA interference. PLoS ONE. 2014;9(7):e103463. doi: 10.1371/journal.pone.0103463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horio M., Lovelace E., Pastan I., Gottesman M.M. Agents which reverse multidrug-resistance are inhibitors of [3H]vinblastine transport by isolated vesicles. Biochim Biophys Acta. 1991;1061(1):106–110. doi: 10.1016/0005-2736(91)90274-c. [DOI] [PubMed] [Google Scholar]
- 20.Horio M., Chin K.V., Currier S.J., Goldenberg S., Williams C., Pastan I. Transepithelial transport of drugs by the multidrug transporter in cultured Madin-Darby canine kidney cell epithelia. J Biol Chem. 1989;264(25):14880–14884. [PubMed] [Google Scholar]
- 21.Sharom F.J., Yu X., Doige C.A. Functional reconstitution of drug transport and ATPase activity in proteoliposomes containing partially purified P-glycoprotein. J Biol Chem. 1993;268(32):24197–24202. [PubMed] [Google Scholar]
- 22.Roepe P.D. Analysis of the steady-state and initial rate of doxorubicin efflux from a series of multidrug-resistant cells expressing different levels of P-glycoprotein. Biochemistry. 1992;31(50):12555–12564. doi: 10.1021/bi00165a003. [DOI] [PubMed] [Google Scholar]
- 23.Roepe P.D., Wei L.Y., Cruz J., Carlson D. Lower electrical membrane potential and altered pHi homeostasis in multidrug-resistant (MDR) cells: further characterization of a series of MDR cell lines expressing different levels of P-glycoprotein. Biochemistry. 1993;32(41):11042–11056. doi: 10.1021/bi00092a014. [DOI] [PubMed] [Google Scholar]
- 24.Higgins C.F., Gottesman M.M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 25.Ford J.M. Modulators of multidrug resistance. Preclinical studies. Hematol Oncol Clin North Am. 1995;9(2):337–361. [PubMed] [Google Scholar]
- 26.Gottesman M.M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 27.Cornwell M.M., Safa A.R., Felsted R.L., Gottesman M.M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci USA. 1986;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safa A.R., Glover C.J., Meyers M.B., Biedler J.L., Felsted R.L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986;261(14):6137–6140. [PubMed] [Google Scholar]
- 29.Urbatsch I.L., al-Shawi M.K., Senior A.E. Characterization of the ATPase activity of purified Chinese hamster P-glycoprotein. Biochemistry. 1994;33(23):7069–7076. doi: 10.1021/bi00189a008. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Q.F., Zhang D.M., Wang J.N., Zhang H.W., Zheng Z.Y., Yu D.C. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int. 2014 doi: 10.1111/liv.12524. [DOI] [PubMed] [Google Scholar]
- 31.Sen R., Natarajan K., Bhullar J., Shukla S., Fang H.B., Cai L. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol Cancer Ther. 2012;11(9):2033–2044. doi: 10.1158/1535-7163.MCT-12-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lum B.L., Gosland M.P. MDR expression in normal tissues. Pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematol Oncol Clin North Am. 1995;9(2):319–336. [PubMed] [Google Scholar]
- 33.Bradley G., Ling V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994;13(2):223–233. doi: 10.1007/BF00689638. [DOI] [PubMed] [Google Scholar]
- 34.Campos L., Guyotat D., Archimbaud E., Calmard-Oriol P., Tsuruo T., Troncy J. Clinical significance of multidrug resistance P-glycoprotein expression on acute nonlymphoblastic leukemia cells at diagnosis. Blood. 1992;79(2):473–476. [PubMed] [Google Scholar]
- 35.Niehans G.A., Jaszcz W., Brunetto V., Perri R.T., Gajl-Peczalska K., Wick M.R. Immunohistochemical identification of P-glycoprotein in previously untreated, diffuse large cell and immunoblastic lymphomas. Cancer Res. 1992;52(13):3768–3775. [PubMed] [Google Scholar]
- 36.Fojo A.T., Ueda K., Slamon D.J., Poplack D.G., Gottesman M.M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41(5):1967–1972. [PubMed] [Google Scholar]
- 38.Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982;42(11):4730–4733. [PubMed] [Google Scholar]
- 39.Al-Abd A.M., Mahmoud A.M., El-Sherbiny G.A., El-Moselhy M.A., Nofal S.M., El-Latif H.A. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Prolif. 2011;44(6):591–601. doi: 10.1111/j.1365-2184.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuurhuis G.J., Broxterman H.J., van der Hoeven J.J., Pinedo H.M., Lankelma J. Potentiation of doxorubicin cytotoxicity by the calcium antagonist bepridil in anthracycline-resistant and -sensitive cell lines. A comparison with verapamil. Cancer Chemother Pharmacol. 1987;20(4):285–290. doi: 10.1007/BF00262578. [DOI] [PubMed] [Google Scholar]
- 41.Hollt V., Kouba M., Dietel M., Vogt G. Stereoisomers of calcium antagonists which differ markedly in their potencies as calcium blockers are equally effective in modulating drug transport by P-glycoprotein. Biochem Pharmacol. 1992;43(12):2601–2608. doi: 10.1016/0006-2952(92)90149-d. [DOI] [PubMed] [Google Scholar]
- 42.Sehested M., Jensen P.B., Skovsgaard T., Bindslev N., Demant E.J., Friche E. Inhibition of vincristine binding to plasma membrane vesicles from daunorubicin-resistant Ehrlich ascites cells by multidrug resistance modulators. Br J Cancer. 1989;60(6):809–814. doi: 10.1038/bjc.1989.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lampidis T.J., Krishan A., Planas L., Tapiero H. Reversal of intrinsic resistance to adriamycin in normal cells by verapamil. Cancer Drug Deliv. 1986;3(4):251–259. doi: 10.1089/cdd.1986.3.251. [DOI] [PubMed] [Google Scholar]
- 44.Newman M.J., Rodarte J.C., Benbatoul K.D., Romano S.J., Zhang C., Krane S. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000;60(11):2964–2972. [PubMed] [Google Scholar]
- 45.Spoelstra E.C., Dekker H., Schuurhuis G.J., Broxterman H.J., Lankelma J. P-glycoprotein drug efflux pump involved in the mechanisms of intrinsic drug resistance in various colon cancer cell lines. Evidence for a saturation of active daunorubicin transport. Biochem Pharmacol. 1991;41(3):349–359. doi: 10.1016/0006-2952(91)90531-9. [DOI] [PubMed] [Google Scholar]
- 46.Chao N.J., Aihara M., Blume K.G., Sikic B.I. Modulation of etoposide (VP-16) cytotoxicity by verapamil or cyclosporine in multidrug-resistant human leukemic cell lines and normal bone marrow. Exp Hematol. 1990;18(11):1193–1198. [PubMed] [Google Scholar]
- 47.Nawrath H., Raschack M. Effects of (−)-desmethoxyverapamil on heart and vascular smooth muscle. J Pharmacol Exp Ther. 1987;242(3):1090–1097. [PubMed] [Google Scholar]
- 48.Pirker R., Keilhauer G., Raschack M., Lechner C., Ludwig H. Reversal of multi-drug resistance in human KB cell lines by structural analogs of verapamil. Int J Cancer. 1990;45(5):916–919. doi: 10.1002/ijc.2910450523. [DOI] [PubMed] [Google Scholar]
- 49.Kessel D., Wilberding C. Promotion of daunorubicin uptake and toxicity by the calcium antagonist tiapamil and its analogs. Cancer Treat Rep. 1985;69(6):673–676. [PubMed] [Google Scholar]
- 50.Alaoui-Jamali M.A., Schecter R.L., Rustum Y.M., Centurioni M.G., Lehnert S., Batist G. In vivo reversal of doxorubicin resistance by a new tiapamil analog Ro11-2933. J Pharmacol Exp Ther. 1993;264(3):1299–1304. [PubMed] [Google Scholar]
- 51.Boesch D., Gaveriaux C., Jachez B., Pourtier-Manzanedo A., Bollinger P., Loor F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991;51(16):4226–4233. [PubMed] [Google Scholar]
- 52.Jonsson B., Nilsson K., Nygren P., Larsson R. SDZ PSC-833 – a novel potent in vitro chemosensitizer in multiple myeloma. Anticancer Drugs. 1992;3(6):641–646. doi: 10.1097/00001813-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Dantzig A.H., Shepard R.L., Cao J., Law K.L., Ehlhardt W.J., Baughman T.M. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996;56(18):4171–4179. [PubMed] [Google Scholar]
- 54.Hyafil F., Vergely C., Du Vignaud P., Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53(19):4595–4602. [PubMed] [Google Scholar]
- 55.Dale I.L., Tuffley W., Callaghan R., Holmes J.A., Martin K., Luscombe M. Reversal of P-glycoprotein-mediated multidrug resistance by XR9051, a novel diketopiperazine derivative. Br J Cancer. 1998;78(7):885–892. doi: 10.1038/bjc.1998.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullin S., Mani N., Grossman T.H. Inhibition of antibiotic efflux in bacteria by the novel multidrug resistance inhibitors biricodar (VX-710) and timcodar (VX-853) Antimicrob Agents Chemother. 2004;48(11):4171–4176. doi: 10.1128/AAC.48.11.4171-4176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kannan P., Telu S., Shukla S., Ambudkar S.V., Pike V.W., Halldin C. The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2) ACS Chem Neurosci. 2011;2(2):82–89. doi: 10.1021/cn100078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michalak K., Wesołowska O. Polyphenols counteract tumor cell chemoresistance conferred by multidrug resistance proteins. Anti-Cancer Agents Med Chem. 2012;12(8):880–890. doi: 10.2174/187152012802650011. [DOI] [PubMed] [Google Scholar]
- 59.Bansal T., Jaggi M., Khar R., Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharmaceut Sci. 2009;12(1):46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 60.Pick A., Müller H., Mayer R., Haenisch B., Pajeva I.K., Weigt M. Structure–activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP) Bioorg Med Chem. 2011;19(6):2090–2102. doi: 10.1016/j.bmc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 61.Farabegoli F., Papi A., Bartolini G., Ostan R., Orlandi M. (-)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17(5):356–362. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Wesołowska O., Wiśniewski J., Środa K., Krawczenko A., Bielawska-Pohl A., Paprocka M. 8-Prenylnaringenin is an inhibitor of multidrug resistance-associated transporters, P-glycoprotein and MRP1. Eur J Pharmacol. 2010;644(1):32–40. doi: 10.1016/j.ejphar.2010.06.069. [DOI] [PubMed] [Google Scholar]
- 63.Sun L., Chen W., Qu L., Wu J., Si J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and P-glycoprotein expression. Mol Med Rep. 2013;8(6):1883–1887. doi: 10.3892/mmr.2013.1742. [DOI] [PubMed] [Google Scholar]
- 64.Li C., Kim M., Choi H., Choi J. Effects of baicalein on the pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats: possible role of cytochrome p450 3A4 and P-glycoprotein inhibition by baicalein. Arch Pharm Res. 2011;34(11):1965–1972. doi: 10.1007/s12272-011-1117-9. [DOI] [PubMed] [Google Scholar]
- 65.Zhang S., Morris M.E. Effect of the flavonoids biochanin A and silymarin on the P-glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm Res. 2003;20(8):1184–1191. doi: 10.1023/a:1025044913766. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S., Morris M.E. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exper Therap. 2003;304(3):1258–1267. doi: 10.1124/jpet.102.044412. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen H., Zhang S., Morris M.E. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci. 2003;92(2):250–257. doi: 10.1002/jps.10283. [DOI] [PubMed] [Google Scholar]
- 68.Romiti N., Tramonti G., Donati A., Chieli E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004;76(3):293–302. doi: 10.1016/j.lfs.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 69.Borska S., Chmielewska M., Wysocka T., Drag-Zalesinska M., Zabel M., Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDR. Food Chem Toxicol. 2012;50(9):3375–3383. doi: 10.1016/j.fct.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 70.Choi J.-S., Piao Y.-J., Kang K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: role of CYP3A4 and P-gp inhibition by quercetin. Arch Pharm Res. 2011;34(4):607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- 71.He L., Zhao C., Yan M., Zhang L.Y., Xia Y.Z. Inhibition of P-glycoprotein function by procyanidine on blood–brain barrier. Phytother Res. 2009;23(7):933–937. doi: 10.1002/ptr.2781. [DOI] [PubMed] [Google Scholar]
- 72.Patanasethanont D., Nagai J., Yumoto R., Murakami T., Sutthanut K., Sripanidkulchai B.O. Effects of Kaempferia parviflora extracts and their flavone constituents on p-glycoprotein function. J Pharm Sci. 2007;96(1):223–233. doi: 10.1002/jps.20769. [DOI] [PubMed] [Google Scholar]
- 73.Patanasethanont D., Nagai J., Matsuura C., Fukui K., Sutthanut K., Sripanidkulchai Bo. Modulation of function of multidrug resistance associated-proteins by Kaempferia parviflora extracts and their components. Eur J Pharmacol. 2007;566(1-3):67–74. doi: 10.1016/j.ejphar.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Lee E., Enomoto R., Koshiba C., Hirano H. Inhibition of P-glycoprotein by wogonin is involved with the potentiation of etoposide-induced apoptosis in cancer cells. Ann N Y Acad Sci. 2009;1171:132–136. doi: 10.1111/j.1749-6632.2009.04722.x. [DOI] [PubMed] [Google Scholar]
- 75.Al-Abd A., Mahmoud A., El-Sherbiny G., El-Moselhy M., Nofal S., El-Latif H. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Prolif. 2011;44(6):591–601. doi: 10.1111/j.1365-2184.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim M.K., Choo H., Chong Y. Water-soluble and cleavable quercetin-amino Acid conjugates as safe modulators for p-glycoprotein-based multidrug resistance. J Med Chem. 2014;57(17):7216–7233. doi: 10.1021/jm500290c. [DOI] [PubMed] [Google Scholar]
- 77.Raad I., Terreux R., Richomme P., Matera E.-L., Dumontet C., Raynaud J. Structure–activity relationship of natural and synthetic coumarins inhibiting the multidrug transporter P-glycoprotein. Bioorg Med Chem. 2006;14(20):6979–6987. doi: 10.1016/j.bmc.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 78.Kimura S., Ito C., Jyoko N., Segawa H., Kuroda J., Okada M. Inhibition of leukemic cell growth by a novel anti-cancer drug (GUT-70) from Calophyllum brasiliense that acts by induction of apoptosis. Int J Cancer. 2005;113(1):158–165. doi: 10.1002/ijc.20505. [DOI] [PubMed] [Google Scholar]
- 79.Shen X., Chen G., Zhu G., Fong W.F. (±)-3′-O, 4′-O-dicynnamoyl-cis-khellactone, a derivative of (±)-praeruptorin A, reverses P-glycoprotein mediated multidrug resistance in cancer cells. Bioorg Med Chem. 2006;14(21):7138–7145. doi: 10.1016/j.bmc.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 80.Song J.S., Chae J.W., Lee K.R., Lee B.H., Choi E.J., Ahn S.H. Pharmacokinetic characterization of decursinol derived from Angelica gigas Nakai in rats. Xenobiotica. 2011;41(10):895–902. doi: 10.3109/00498254.2011.587551. [DOI] [PubMed] [Google Scholar]
- 81.Hanafi-Bojd M.Y., Iranshahi M., Mosaffa F., Tehrani S.O., Kalalinia F., Behravan J. Farnesiferol A from Ferula persica and galbanic acid from Ferula szowitsiana inhibit P-glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011;77(14):1590–1593. doi: 10.1055/s-0030-1270987. [DOI] [PubMed] [Google Scholar]
- 82.Ferreira M.-J.U. A new sesquiterpene-coumarin ether and a new abietane diterpene and their effects as inhibitors of P-glycoprotein. Planta Med. 2004;70:828–833. doi: 10.1055/s-2004-827231. [DOI] [PubMed] [Google Scholar]
- 83.Ohnishi A., Matsuo H., Yamada S., Takanaga H., Morimoto S., Shoyama Y. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. British J Pharmacol. 2000;130(6):1369–1377. doi: 10.1038/sj.bjp.0703433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barthomeuf C., Grassi J., Demeule M., Fournier C., Boivin D., Beliveau R. Inhibition of P-glycoprotein transport function and reversion of MDR1 multidrug resistance by cnidiadin. Cancer Chem Pharmacol. 2005;56(2):173–181. doi: 10.1007/s00280-004-0914-y. [DOI] [PubMed] [Google Scholar]
- 85.Cortés-Selva F., Jiménez I.A., Muñoz-Martínez F., Campillo M., Bazzocchi I.L., Pardo L. Dihidro-β-agarofuran sesquiterpenes: a new class of reversal agents of the multidrug resistance phenotype mediated by P-glycoprotein in the protozoan parasite Leishmania. Curr Pharm Des. 2005;11(24):3125–3159. doi: 10.2174/1381612054864920. [DOI] [PubMed] [Google Scholar]
- 86.Reyes C.P., Muñoz-Martínez F., Torrecillas I.R., Mendoza C.R., Gamarro F., Bazzocchi I.L. Biological evaluation, structure-activity relationships, and three-dimensional quantitative structure-activity relationship studies of dihydro-β-agarofuran sesquiterpenes as modulators of P-glycoprotein-dependent multidrug resistance. J Med Chem. 2007;50(20):4808–4817. doi: 10.1021/jm070290v. [DOI] [PubMed] [Google Scholar]
- 87.Steglich B., Mahringer A., Li Y., Posner G.H., Fricker G., Efferth T. Inhibition of P-glycoprotein by two artemisinin derivatives. Nat Prod Bioprosp. 2012;2(2):59–64. [Google Scholar]
- 88.Legault J., Pichette A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol. 2007;59(12):1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 89.Min Y.D., Kwon H.C., Yang M.C., Lee K.H., Choi S.U., Lee K.R. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Arch Pharm Res. 2007;30(1):58–63. doi: 10.1007/BF02977779. [DOI] [PubMed] [Google Scholar]
- 90.Molnár J., Gyémánt N., Tanaka M., Hohmann J., Bergmann-Leitner E., Molnár P. Inhibition of multidrug resistance of cancer cells by natural diterpenes, triterpenes and carotenoids. Curr Pharm Des. 2006;12(3):287–311. doi: 10.2174/138161206775201893. [DOI] [PubMed] [Google Scholar]
- 91.Duarte N., Járdánházy A., Molnár J., Hilgeroth A., Ferreira M.-J.U. Synergistic interaction between p-glycoprotein modulators and epirubicine on resistant cancer cells. Bioorg Med Chem. 2008;16(20):9323–9330. doi: 10.1016/j.bmc.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 92.Duarte N., Varga A., Cherepnev G., Radics R., Molnár J., Ferreira M.-J.U. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg Med Chem. 2007;15(1):546–554. doi: 10.1016/j.bmc.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 93.Appendino G., Porta C.D., Conseil G., Sterner O., Mercalli E., Dumontet C. A new P-glycoprotein inhibitor from the caper spurge (Euphorbia lathyris) J Nat Prod. 2003;66(1):140–142. doi: 10.1021/np0203537. [DOI] [PubMed] [Google Scholar]
- 94.Corea G., Di Pietro A., Dumontet C., Fattorusso E., Lanzotti V. Jatrophane diterpenes from Euphorbia spp. as modulators of multidrug resistance in cancer therapy. Phytochem Rev. 2009;8(2):431–447. [Google Scholar]
- 95.Valente I., Reis M., Duarte N., Serly J., Molnár J., Ferreira M.J.U. Jatrophane diterpenes from Euphorbia mellifera and their activity as P-glycoprotein modulators on multidrug-resistant mouse lymphoma and human colon adenocarcinoma cells. J Nat Prod. 2012;75(11):1915–1921. doi: 10.1021/np3004003. [DOI] [PubMed] [Google Scholar]
- 96.Ferreira M.J.U., Gyémánt N., Madureira A.M., Molnár J. Inhibition of P-glycoprotein transport activity in a resistant mouse lymphoma cell line by diterpenic lactones. Anticancer Res. 2005;25(5):3259–3262. [PubMed] [Google Scholar]
- 97.Madureira A.M., Gyémánt N., Ascenso J.R., Abreu P.M., Molnár J., Ferreira M.J.U. Euphoportlandols A and B, tetracylic diterpene polyesters from Euphorbia portlandica and their anti-MDR effects in cancer cells. J Nat Prod. 2006;69(6):950–953. doi: 10.1021/np060046r. [DOI] [PubMed] [Google Scholar]
- 98.Lei Y., Tan J., Wink M., Ma Y., Li N., Su G. An isoquinoline alkaloid from the Chinese herbal plant Corydalis yanhusuo WT Wang inhibits P-glycoprotein and multidrug resistance-associate protein 1. Food Chem. 2013;136(3):1117–1121. doi: 10.1016/j.foodchem.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 99.Ma Y., Wink M. Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine. 2008;15(9):754–758. doi: 10.1016/j.phymed.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 100.Pearce H., Safa A.R., Bach N., Winter M., Cirtain M.C., Beck W.T. Essential features of the P-glycoprotein pharmacophore as defined by a series of reserpine analogs that modulate multidrug resistance. Proc Natl Acad Sci. 1989;86(13):5128–5132. doi: 10.1073/pnas.86.13.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fu L.W., Deng Z.A., Pan Q.C., Fan W. Screening and discovery of novel MDR modifiers from naturally occurring bisbenzylisoquinoline alkaloids. Anticancer Res. 2001;21(4 A):2273–2280. [PubMed] [Google Scholar]
- 102.Ikeda R., Che X.F., Yamaguchi T., Ushiyama M., Zheng C.L., Okumura H. Cepharanthine potently enhances the sensitivity of anticancer agents in K562 cells. Cancer Sci. 2005;96(6):372–376. doi: 10.1111/j.1349-7006.2005.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chávez D., Cui B., Chai H.B., García R., Mejía M., Farnsworth N.R. Reversal of multidrug resistance by tropane alkaloids from the stems of Erythroxylum rotundifolium. J Nat Prod. 2002;65(4):606–610. doi: 10.1021/np0104774. [DOI] [PubMed] [Google Scholar]
- 104.Mi Q., Cui B., Chávez D., Chai H., Zhu H., Cordell G.A. Characterization of tropane alkaloid aromatic esters that reverse the multidrug-resistance phenotype. Anticancer Res. 2002;22(3):1385–1398. [PubMed] [Google Scholar]
- 105.Mi Q., Cui B., Silva G.L., Lantvit D., Lim E., Chai H. Pervilleines B and C, new tropane alkaloid aromatic esters that reverse the multidrug-resistance in the hollow fiber assay. Cancer Lett. 2002;184(1):13–20. doi: 10.1016/s0304-3835(02)00202-1. [DOI] [PubMed] [Google Scholar]
- 106.Mi Q., Cui B., Lantvit D., Reyes-Lim E., Chai H., Pezzuto J.M. Pervilleine F, a new tropane alkaloid aromatic ester that reverses multidrug resistance. Anticancer Res. 2003;23(5 A):3607–3615. [PubMed] [Google Scholar]
- 107.Silva G.L., Cui B., Chávez D., You M., Chai H.B., Rasoanaivo P. Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from Erythroxylum pervillei. J Nat Prod. 2001;64(12):1514–1520. doi: 10.1021/np010295+. [DOI] [PubMed] [Google Scholar]
- 108.Ivanova A., Serly J., Christov V., Stamboliyska B., Molnar J. Alkaloids derived from genus Veratrum and Peganum of Mongolian origin as multidrug resistance inhibitors of cancer cells. Fitoterapia. 2011;82(4):570–575. doi: 10.1016/j.fitote.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 109.Kam T.-S., Subramaniam G., Sim K.-M., Yoganathan K., Koyano T., Toyoshima M. Reversal of multidrug resistance (MDR) by aspidofractinine-type indole alkaloids. Bioorg Med Chem Lett. 1998;8(19):2769–2772. doi: 10.1016/s0960-894x(98)00486-7. [DOI] [PubMed] [Google Scholar]
- 110.Rho M.C., Toyoshima M., Hayashi M., Koyano T., Subramaniam G., Kam T.S. Reversal of multidrug resistance by kopsiflorine isolated from Kopsia dasyrachis. Planta Med. 1999;65(4):307–310. doi: 10.1055/s-1999-13991. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen V.T.B., Darbour N., Bayet C., Doreau A., Raad I., Phung B.H. Selective modulation of P-glycoprotein activity by steroidal saponines from Paris polyphylla. Fitoterapia. 2009;80(1):39–42. doi: 10.1016/j.fitote.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Ramalhete C., Molnár J., Mulhovo S., Rosário V.E., Ferreira M.-J.U. New potent P-glycoprotein modulators with the cucurbitane scaffold and their synergistic interaction with doxorubicin on resistant cancer cells. Bioorg Med Chem. 2009;17(19):6942–6951. doi: 10.1016/j.bmc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 113.Choi C.H., Kang G., Min Y.D. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from Korean red ginseng. Planta Med. 2003;69(3):235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- 114.Hu Y.J., Shen X.L., Lu H.L., Zhang Y.H., Huang X.A., Fu L.C. Tenacigenin B derivatives reverse P-glycoprotein-mediated multidrug resistance in HepG2/Dox cells. J Nat Prod. 2008;71(6):1049–1051. doi: 10.1021/np070458f. [DOI] [PubMed] [Google Scholar]
- 115.Huang C., Xu D., Xia Q., Wang P., Rong C., Su Y. Reversal of P-glycoprotein-mediated multidrug resistance of human hepatic cancer cells by Astragaloside II. J Pharm Pharmacol. 2012;64(12):1741–1750. doi: 10.1111/j.2042-7158.2012.01549.x. [DOI] [PubMed] [Google Scholar]
- 116.Risinger A.L., Jackson E.M., Polin L.A., Helms G.L., LeBoeuf D.A., Joe P.A. The taccalonolides: microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008;68(21):8881–8888. doi: 10.1158/0008-5472.CAN-08-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manda V.K., Dale O.R., Awortwe C., Ali Z., Khan I.A., Walker L.A. Evaluation of drug interaction potential of Labisia pumila (Kacip Fatimah) and its constituents. Front Pharmacol. 2014;5:178. doi: 10.3389/fphar.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eichhorn T., Efferth T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbs. J Ethnopharmacol. 2012;141(2):557–570. doi: 10.1016/j.jep.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 119.Tran T.P., Kim H.G., Choi J.H., Na M.K., Jeong H.G. Reversal of P-glycoprotein-mediated multidrug resistance is induced by mollugin in MCF-7/adriamycin cells. Phytomedicine. 2013;20(7):622–631. doi: 10.1016/j.phymed.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 120.Chung S.Y., Han A.R., Sung M.K., Jung H.J., Nam J.W., Seo E.K. Potent modulation of p-glycoprotein activity by naturally occurring phenylbutenoids from Zingiber cassumunar. Phytother Res. 2009;23(4):472–476. doi: 10.1002/ptr.2650. [DOI] [PubMed] [Google Scholar]
- 121.Rubis B., Polrolniczak A., Knula H., Potapinska O., Kaczmarek M., Rybczynska M. Phytosterols in physiological concentrations target multidrug resistant cancer cells. Med Chem. 2010;6(4):184–190. doi: 10.2174/1573406411006040184. [DOI] [PubMed] [Google Scholar]
- 122.Holland M., Panetta J., Hoskins J., Bebawy M., Roufogalis B., Allen J. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol. 2006;71(8):1146–1154. doi: 10.1016/j.bcp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 123.Eid S.Y., El-Readi M.Z., Wink M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine. 2012;19(11):977–987. doi: 10.1016/j.phymed.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 124.Chearwae W., Anuchapreeda S., Nandigama K., Ambudkar S.V., Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68(10):2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 125.Saab A.M., Guerrini A., Sacchetti G., Maietti S., Zeino M., Arend J. Phytochemical analysis and cytotoxicity towards multidrug-resistant leukemia cells of essential oils derived from lebanese medicinal plants. Planta Med. 2012;78(18):1927–1931. doi: 10.1055/s-0032-1327896. [DOI] [PubMed] [Google Scholar]
- 126.Lai K.C., Kuo C.L., Ho H.C., Yang J.S., Ma C.Y., Lu H.F. Diallyl sulfide, diallyl disulfide and diallyl trisulfide affect drug resistant gene expression in colo 205 human colon cancer cells in vitro and in vivo. Phytomedicine. 2012;19(7):625–630. doi: 10.1016/j.phymed.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 127.Romiti N., Pellati F., Nieri P., Benvenuti S., Adinolfi B., Chieli E. P-glycoprotein inhibitory activity of lipophilic constituents of Echinacea pallida roots in a human proximal tubular cell line. Planta Med. 2008;74(3):264–266. doi: 10.1055/s-2008-1034308. [DOI] [PubMed] [Google Scholar]
- 128.Yan M., Nuriding H. Reversal effect of vitamin D on different multidrug-resistant cells. Genet Mol Res. 2014;13(3):6239–6247. doi: 10.4238/2014.August.15.6. [DOI] [PubMed] [Google Scholar]
- 129.Khalil Z.G., Huang X.C., Raju R., Piggott A.M., Capon R.J., Shornephine A. Structure, chemical stability, and P-glycoprotein inhibitory properties of a rare diketomorpholine from an Australian marine-derived Aspergillus sp. J Org Chem. 2014;79(18):8700–8705. doi: 10.1021/jo501501z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang X.C., Xiao X., Zhang Y.K., Talele T.T., Salim A.A., Chen Z.S. Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar Drugs. 2014;12(7):3818–3837. doi: 10.3390/md12073818. [DOI] [PMC free article] [PubMed] [Google Scholar]



