Abstract
Formation of glycation products is major factor responsible in complications of diabetes. Worldwide trend is toward the use of natural additives in reducing the complications of diseases. Therefore, there is a growing interest in natural antiglycation found in plants. Herbs and spices are one of the most important targets to search for natural antiglycation from the point of view of safety. This study investigated the ability of some of the spices to inhibit glycation process in a hemoglobin/glucose model system and compared their potency with each other. For this subject the best concentration and time to incubate glucose with hemoglobin was investigated. Then the glycosylation degree of hemoglobin in the presence of extracts by the three concentrations 0.25, 0.5 and 1 μg/ml was measured colorimetrically at 520 nm. Results represent that some of extracts such as wild caraway, turmeric, cardamom and black pepper have inhibitory effects on hemoglobin glycation. But some of the extracts such as anise and saffron have not only inhibitory effects but also aggravated this event and have proglycation properties. In accordance with the results obtained we can conclude that wild caraway, turmeric, cardamom and black pepper especially wild caraway extracts are potent antiglycation agents, which can be of great value in the preventive glycation-associated complications in diabetes.
Keywords: Glycosylation, hemoglobin, wild caraway, turmeric diabetes
Recently, there is strong evidence to show that diabetes is associated with increased oxidative stress and diabetic patients are more susceptible to oxidative attack than normal subjects[1,2]. Oxidative stress occurred during hyperglycemia produces glycated proteins especially glycated hemoglobin and advanced glycated-end products. The accumulation of these products in living organisms leads to structural and functional modifications of proteins[3].
Animal studies show that glycation inhibitors such as aminoguanidine attenuate vascular complications of diabetic patients[4]. Therefore, using antioxidant supplements to suppress the glycation pathways may be beneficial for preventing diabetic complications. Results of some studies indicated that intake of antioxidants can reduce development of type 2 diabetes which support the hypothesis[5,6].
Spices have been used as flavoring, coloring and preservatives agents for thousands of years. Also spices have shown medicinal properties and influence on various body systems such as gastrointestinal, cardiovascular and reproductive and nervous systems[7]. Researchers found antioxidants effects for some spices like Pimpinella anisum, curcuma longa, Crocus sativus l and Piper nigrum, Ellitaria cardamum[8,9,10,11], but antiglycation properties of these spices haven’t demonstrated.
So, as oxidation occurred during protein glycation, it seems that the spices with antioxidant properties have antiglycation effects. Therefore, we examined the inhibitory effects of hydroalcoholic extract of some spices on glycation and oxidation-dependent damages to hemoglobin induced by glucose.
Anise, cinnamon, clove, wild caraway, tanners sumach, turmeric, saffron, cardamom, black pepper were purchased at a local market in Isfahan City, Iran. These spicesses were authenticated at faculty of Pharmacy, Isfahan University of Medical Science, Isfahan, Iran. The voucher specimen of them was deposited in this place. After grounding with electronic blender, they were soaked in methanol 70° for 72 h. Then extracts were filtered with Whatman no. 1 filter paper and dried. The dried extracts were stored at 4° until used[12].
The red blood cells washed thrice with 0.14 M NaCl solution and were lysed with CCl4 in phosphate buffer (0.01 M, pH=7.4). After lysing, the hemolysate was freed from debris by centrifugation. The supernatant was separated and its hemoglobin concentration was measured by the Drabkin method.
To find the best concentration of glucose, 5 g hemoglobin/100 ml phosphate buffer (0.01 M, pH 7.4) was incubated in the presence of different concentrations of glucose and the amount of glycosylation was measured by colorimetric method. Then, the hemoglobin was incubated with the best concentration of glucose at different times and the best time for glycosylation was determined.
After that, hemoglobin was incubated with the best concentration of glucose (10 mg/ml) and gentamycin (100 μg/ml) in the presence of different concentration of extracts (0, 0.25, 0.5 and 1 μg/ml) for 72 h (the best time for glycosylation). Then, the protein was precipitated with 20% trichloroacetic acid and treated with 0.5 ml oxalic acid in 1 ml phosphate buffer (0.01 M, pH 7.4) for 1 h at 100°. After cooling, trichloroacetic acid 40% was added and the solution centrifuged. Free sugars and hydroxymethyl furfural in the supernatant were treated with thiobarbituric acid for 30 min at 40° and a colored product with a maximum absorbance at 443 nm was yield. Comparing the colored product absorbance with control sample (a sample without extract), glycation inhibition percent of each extract was calculated according to the formula Percent inhibition of glycation=100×(AA−AB)/AA, where AA and AB are the absorbance values of the blank and of the tested samples[13].
Data were analysed using SPSS software (version 15.0). Glycation inhibition percent values were expressed as means±standard deviations of triplicate determinations. One-way Analysis of Variance (ANOVA) and Tukey as Post Hoc test were used to do comparisons between different extract. Student T test were used to do Comparisons between each extract and control. Probability values of less than 0.05 were considered to be significant.
Quantitative results of the isolated extracts from the analysed spices have been summarized in (Table 1). In each 100 g of the dried spices, dried extracts weights vary from 4.92 g (cinnamon) to 59.55 g (saffron). Inhibitory effects of extracts on glucose-mediated glycation have been presented in (Table 1). Despite of anise and saffron the extract of wild caraway, turmeric, cardamom and black pepper were effectively able to reduce the amount of end product of hemoglobin glycosylation. While for cinnamon, clove and sumach this effect was average.
TABLE 1.
INHIBITORY EFFECTS OF EXTRACTS ON HEMOGLOBIN GLYCATION
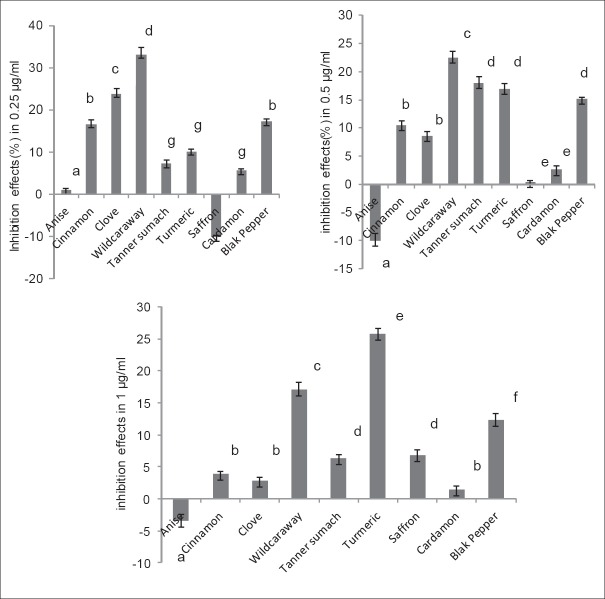
Inhibition effects of different concentrations of each extract on hemoglobin glycation have been compared separately (fig. 1), and except for black pepper and cardamom, significant difference was observed for other extracts.
Fig. 1.
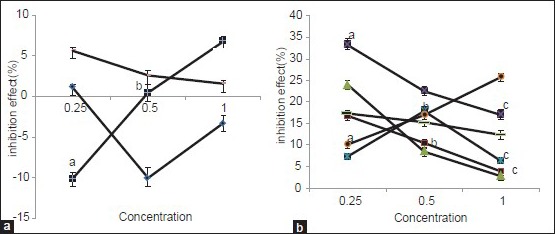
Comparison between inhibition effects on concentration variable.
(A) Spices with or low effect or without effects on hemoglobin glycation, anise (−♦−), saffron (−■−) and cardamom (−−). (B) Spices with required inhibition effects on hemoglobin glycation, cinnamon (−■−) clove (−σ−), wild caraway (−o−), tanner sumach (−π−), turmeric (−□−) and black pepper (−−). Similar characters: insignificant differences, no similar characters: significant differences.
According to the (fig. 2), in concentration 0.25 μg/ml wild caraway with 33.12% and anise with 1.17% showed the highest and the lowest inhibition effects, respectively. Saffron in this concentration had proglycation effect (−10.15%). The highest inhibition percent was observed in concentration of 0.5 μg/ml of wild caraway, turmeric and tanners sumach (33.19, 16.99 and 17.76%) and the same concentration of anise showed proglycation effect (−9.97±1.30). Turmeric (1 μg/ml) with 25.83% and Anise with −3.25% had the highest and the lowest inhibition effects on hemoglobin glycation, respectively.
Fig. 2.
Comparison between inhibition effects on extract variable.
Similar characters: insignificant differences, Different characters: significant differences.
According to our results some of the extracts have significantly inhibitory effects on hemoglobin glycation. The percent of inhibition vary from 33.12% (wild caraway in 0.25 μg/ml) to 0.42% (saffron 0.5 μg/ml). Some of the extracts have proglycation properties as 0.25 μg/ml of Saffron (−10.15%) and anise at 0.5 μg/ml (−9.97%). These different effects may be originated from the existence of various compounds in these extracts that have different effects.
Glycosylation of haemoglobin takes place under physiological condition by a reaction between glucose and N-terminal valine of haemoglobin molecule's beta chain[14]. The oxidative steps are also involved in glycation and the process can therefore be called glycoxidation[15]. Regarding the presence of free radicals and oxidative steps in the glycoxidation process, At first glance, it seems that the antiglycation activities of the extracts are due to the antioxidant properties[16].
In research studies, the presence of antioxidant compounds were proved in all these spices, as coumarin, cinnamaldehyde in cinnamon, curcumin in turmeric, eugenol in cardamom and sesquiterpenoid in black pepper. But some of these spices can’t inhibit hemoglobin glycation as anise[17].
In this study Anise couldn’t effectively reduce hemoglobin glycation however its antioxidant activity has been obtained. So that, this spice effectively scavenged nitric oxide, superoxide and DPPH radicals and it also decrease lipid peroxidation and protein oxidation in diabetics[8]. In β-carotene bleaching system antioxidant activity of the essential oil of anise was almost equal to butylated hydroxytoluene (BHT)[16]. These antonyms can be having different reasons. For example, the flavonoids as the most important antioxidant components, have invert-U effects and act with different intensity[19]. This means that the maximum activity of each flavonoid is at a particular dose and at higher and lower doses than this dose, the activity is descended.
Moreover, other mechanisms beside the scavenging free radicals may be responsible for this inhibition like metal-chelating property. Moreover, the metal-chelating property of extracts has been proven to be responsible for alleviation of the Amadori reaction in glycation system. The rationale behind this speculation includes the previous report that, diethylenetriaminepentaacetic acid, a known metal-chelating activity, reduced albumin glycation[20]. Also, a strong relationship between glucose associated oxidative modifications and amount of transition metals like copper and iron was seen[21]. So, the desirable effects of some of the extracts like wild caraway may be related to the other mechanisms apart from scavenging free radicals.
In conclusion we can say that in addition of antioxidant activity, there are different mechanisms for inhibition of glycation and likely a mixture of these mechanisms participate in this effect.
Footnotes
Naderi, et al.: Antiglycation Properties of Spices
REFERENCES
- 1.Wen Y, Skidmore JC, Porter-Turner MM, Rea CA, Khokher MA, Singh BM. Relationship of glycation, antioxidant status and oxidative stress to vascular endothelial damage in diabetes. Diabetes Obes Metab. 2002;4:305–8. doi: 10.1046/j.1463-1326.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–8. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar S, Figarola JL. Novel inhibitors of advanced glycation end products. Arch Biochem Biophys. 2003;419:63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicki M, Oliveira MR, Pereira TV, Reginatto FH, Dal-Pizzol F, Moreira JC. Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem. 2007;100:719–24. doi: 10.1016/j.fct.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Lampe JW. Spicing up a vegetarian diet: Chemopreventive effects of phytochemicals. Am J Clin Nutr. 2003;78:57–83. doi: 10.1093/ajcn/78.3.579S. [DOI] [PubMed] [Google Scholar]
- 6.Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type II diabetes. Diabetes Care. 2004;27:362–6. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 7.Mohammad Y, Mohammad I, Nagwa MA, Mohammad Z. Role of spices in Diabetes Mellitus. Res J Pharm Biol Chem Sci. 2010;1:83–90. [Google Scholar]
- 8.Rajeshwari C, Abirami M, Andallu B. In Vivo and In Vitro antioxidant potential of aniseeds (Pimpinella anisum) Asian J Exp Biol Sci. 2011;2:80–9. [Google Scholar]
- 9.Bengmark V. Plant-derived Health-Effects of turmeric and curcumenoids. Kuwait Med J. 2006;38:267–75. [Google Scholar]
- 10.Bhargava K. Medicinal uses and pharmacological properties of Crocus sativus linn (saffron) Int J Pharm Pharm Sci. 2011;3:22–6. [Google Scholar]
- 11.Xinpeng B, Zhang W, Chen W, Guo Z, Liu X. Antihepatotoxic and anti-oxidant effects of extracts from Piper nigrum L. root. Afr J Biotech. 2011;10:267–72. [Google Scholar]
- 12.Asgary S, Dinani NJ, Madani H, Mahzouni P. Ethanolic extract of Artemisia aucheri induces regression of aorta wall fatty streaks in hypercholesterolemic rabbits. Pharmazie. 2008;63:394–7. [PubMed] [Google Scholar]
- 13.Asgary S, Naderi GH, Movahedian A, Sajjadian A, Kafil F, Fatehi Z. Inhibitory effects of Crataegus curvisepala, Salvia hydrangea and Betula pendula on in vitro protein glycosylation ARYA Atheroscler J. 2006;4:238. [Google Scholar]
- 14.Kareem I, Jaweed SA, Bardapurka JS, Patil VP. Study of magnesium, glycosylated hemoglobin and lipid profile in diabetic retinopathy. Indian J Clin Biochem. 2004;19:124–7. doi: 10.1007/BF02894270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortwerth B, James J, Simpson H, Linetsky M. The generation of superoxide anions in glycation reactions with sugars, osones, and 3-deoxyosones. Biochem Bioph Res Com. 1998;245:161–5. doi: 10.1006/bbrc.1998.8401. [DOI] [PubMed] [Google Scholar]
- 16.Shahsavari N, Barzegar M, Sahari M, Naghdibadi H. Antioxidant Activity and Chemical Characterization of Essential Oil of Bunium persicum. Plant Foods Hum Nutr. 2008;63:183–8. doi: 10.1007/s11130-008-0091-y. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res. 2006;50:1030–8. doi: 10.1002/mnfr.200600067. [DOI] [PubMed] [Google Scholar]
- 18.Sharififar F, Yassa N, Mozaffarian V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak J Pharm Sci. 2010;23:300–4. [PubMed] [Google Scholar]
- 19.Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid composition of citrus juices. Molecules. 2007;12:1641–73. doi: 10.3390/12081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff SP, Dean RT. Glucose autoxidation and protein modification: The potential role of autooxidative glycosylation in diabetes. Biochem J. 1987;245:243–50. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Moin M. Effects of a fixed dose of Melissa officinalis extract in Alzheimer's disease: A randomized, placebo controlled trial. Br J Clin Pharmaco. 2003;55:443–4. [Google Scholar]