Abstract
We demonstrated a role for the Mg2+ transporter TRPM7, a bifunctional protein with channel and α-kinase domains, in aldosterone signaling. Molecular mechanisms underlying this are elusive. Here we investigated the function of TRPM7 and its α-kinase domain on Mg2+ and pro-inflammatory signaling by aldosterone. Kidney cells (HEK-293) expressing wild-type human TRPM7 (WThTRPM7) or constructs in which the α-kinase domain was deleted (ΔKinase) or rendered inactive with a point mutation in the ATP binding site of the α-kinase domain (K1648R) were studied. Aldosterone rapidly increased [Mg2+]i and stimulated NADPH oxidase-derived generation of reactive oxygen species (ROS) in WT hTRPM7 and TRPM7 kinase dead mutant cells. Translocation of annexin-1 and calpain-II and spectrin cleavage (calpain target) were increased by aldosterone in WT hTRPM7 cells but not in α-kinase-deficient cells. Aldosterone stimulated phosphorylation of MAP kinases and increased expression of proinflammatory mediators ICAM-1, Cox-2 and PAI-1 in Δkinase and K1648R cells, effects that were inhibited by eplerenone (mineralocorticoid receptor (MR) blocker). 2-APB, a TRPM7 channel inhibitor, abrogated aldosterone-induced Mg2+ responses in WT hTRPM7 and mutant cells. In 2-APB-treated ΔKinase and K1648R cells, aldosterone-stimulated inflammatory responses were unchanged. These data indicate that aldosterone stimulates Mg2+` influx and ROS production in a TRPM7-sensitive, kinase-insensitive manner, whereas activation of annexin-1 requires the TRPM7 kinase domain. Moreover TRPM7 α-kinase modulates inflammatory signaling by aldosterone in a TRPM7 channel/Mg2+-independent manner. Our findings identify novel mechanisms for non-genomic actions of aldosterone involving differential signaling through MR-activated TRPM7 channel and α-kinase.
Keywords: TRPM7, aldosterone, signal transduction, Mg2+
1. Introduction
Aldosterone, a steroid hormone with mineralocorticoid activity, is typically associated with volume homeostasis and blood pressure regulation through its effects on renal Na+ reabsorption and K+ secretion (1). Aldosterone also controls renal handling of other ions, including Mg2+ (2). Hyperaldosteronism leads to renal K+ and Mg2+ wasting with associated cardiovascular injury and fibrosis. In addition to regulating renal ion transport through mineralocorticoid receptor (MR) genomic signaling, aldosterone influences non-classical including tissue remodeling, inflammation and fibrosis (3-5). Recent evidence suggests that these effects are mediated by MR and decreased [Mg2+]i since spironolactone and eplerenone (MR antagonists) as well as Mg2+ administration, ameliorate these processes (6,7). At the sub-cellular level Mg2+ regulates protein phosphorylation, modulates ion transport and is a cofactor for many enzymes (8). Moreover, through Mg2+-sensitive mitogen-activated protein (MAP) kinases, tyrosine kinases and reactive oxygen species (ROS), Mg2+ regulates signaling pathways associated with inflammation and fibrosis (8,9).
Molecular mechanisms whereby aldosterone controls cellular Mg2+ and its associated signaling pathways are unclear, but transient receptor potential melastatin cation channel 7 (TRPM7), has been implicated (10,11). TRPM7 belongs to the TRP ion channel superfamily and has a distinctive ion permeability profile, allowing Mg2+ and other divalent cations to comprise its inward current (12,13). Similar to its homologue TRPM6, it has the unique feature of an α-kinase domain at its carboxy-terminal and has channel-enzyme bifunctionality activity (10-14), hence referred to as a “chanzyme” (15). A number of downstream effector targets of TRPM7 α-kinase have been identified including: annexin-1, m-calpain, myosin IIA heavy chain and elongation factor 2 (eEF2) (16-18). In addition, PLCγ2 is phosphorylated by the Ser/Thr kinase domain of TRPM7 (19). TRPM7 has an essential and non-redundant function in cell growth and development because TRPM7-deficient cells die and Trpm7−/− mouse embryos do not survive past day 7 of embryogenesis (20,21).
The C-terminal kinase is homologous to α-kinases, atypical serine-threonine kinases, and structurally resembles protein kinase A. The functional relationship between the channel and kinase domains is unclear and there is conflicting data whether TRPM7 kinase signaling is essential for TRPM7 channel activity. Early studies suggested that the channel function depends on the α-kinase domain (22), although more recent data suggest that TRPM7 α-kinase is not essential for activation of the channel (12). TRPM7 channel regulation involves phosphorylation of at least 14 sites in the cytoplasmic domain, as demonstrated in a stable cell line expressing mouse TRPM7 (24). Although many factors, including aldosterone, influence TRPM7 function (25-27), the exact molecular processes remain unknown and it is unclear whether TRPM7 channel function and/or TRPM7 α-kinase activity is involved in aldosterone signaling.
To better understand these processes, we investigated the role of TRPM7 and its α-kinase domain in non-genomic signaling by aldosterone, focusing on Mg2+ transport and proinflammatory responses, by studying kidney cells (HEK-293) expressing wild-type human TRPM7 (WT hTRPM7) or constructs in which the α-kinase domain has been deleted (ΔKinase) or rendered inactive with a point mutation in the ATP binding site of the α-kinase domain (K1648R).
2. Methods
Please see supplemental data for expanded Methods section
2.1 Expression of wild-type human TRPM7 and mutant constructs in HEK-293 cells
WT hTRPM7, Δkinase and K1648R cDNA cloning and expression in HEK-293 T-Rex cells (Invitrogen) have been previously described (28). HEK-293 cells were transfected with a pcDNA4/TO plasmid that allowed tetracycline-inducible protein expression of WT hTRPM7 and hTRPM7 mutants for the α-kinase deletion or lacking of phosphotransferase activity. Protein expression was induced using tetracycline-controlled transcription (1 μg/ml).
2.2 Cell Stimulation Protocols
HEK-293 cells were induced for 48 hr, and rendered quiescent in serum-free DMEM supplemented with tetracycline for 24 hr. Growth-arrested cells were stimulated with 100 nmol/L aldosterone for short (1 to 60 min) or long (4 to 24 hrs) periods of time to examine non-genomic (acute) and genomic effects respectively. In some experiments cells were pre-exposed for 30 minutes to 50 μM 2-Aminoethoxydiphenyl borate (2-APB) (TRPM7 inhibitor), 10 μM eplerenone (MR antagonist), or 10 μM mifepristone (GR antagonsit). Concentrations of inhibitors used were based on previously published data (5,27).
2.3 Fluorescence Measurement of Mg2+
Mg2+ influx was assessed with Mag-Fura-2AM fluorescence dual excitation wavelength as previously described (29). Basal measurements were recorded in non-stimulated cells, and following 100 nM aldosterone stimulation using the Stallion Digital Hi-Speed Multi-Channel Imaging System (Zeiss, Germany). The emission wavelength was 520 nm, with alternating excitatory wavelengths of 340 and 380 nm. Results were expressed as the ratio of fluorescence acquired with excitation at 340 and 380 nm.
2.4 Immunoblotting
Proteins from cell homogenates were separated by electrophoresis on a polyacrylamide gel and transferred onto a nitrocellulose membrane as previously described (11). Membranes were then incubated with specific antibodies overnight at 4°C. Antibodies were as follows: anti-p38MAPK [Thr180/Tyr182], anti-ERK1/2MAPK [Thr202/Tyr204], anti-SAPK/JNK [Thr183/Tyr185] (Cell Signaling), anti- intercellular adhesion molecule 1 (ICAM-1), anti- plasminogen activator inhibitor 1 (PAI-1), anti-mineralocorticoid receptor (MR), anti- glucocorticoid receptor (GR), anti-spectrin (Santa Cruz Biotechnology, Inc), anti- cyclooxygenase 2 (Cox-2, Cayman). Anti-GAPDH and antibodies to non phosphoproteins were used as loading controls and were carried out on the same membranes for phosphorylated proteins. After incubation with secondary antibodies, signals were revealed with chemiluminescence, visualized by autoradiography and quantified densitometrically.
2.5 Cytosol-membrane fractionation
Translocation of annexin-1, calpain-II and p47phox (cytosloc subunit of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase) from the cytosol to the membrane was assessed in HEK-293 cells expressing WT hTRPM7 and the mutants. Cells were lysed and partitioned to obtain cytosol- and membrane-enriched fractions. Western blotting was performed as described using anti-annexin-1, anti-calpain-II and anti-p47phox antibodies (Santa Cruz Biotechnology, Inc). Translocation was determined as the ratio of protein expression in membrane to cytosolic fractions.
2.6 Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity
NADPH-mediated ROS generation was measured in cell homogenates by lucigenin-derived chemiluminescence.
2.7 Measurement of ROS with dihydroethidium (DHE) staining
Intracellular generation of ROS was evaluated using the superoxide anion-sensitive dye DHE.
2.8 Statistical Analysis
Values are shown as means ± S.E. Group differences were evaluated by one-way analysis of variance (ANOVA) followed by the Dunnett post-test. Differences between mean values were considered statistically significant at p<0.05.
3. Results
3.1 Expression of TRPM7 WT and of TRPM7 kinase dead mutant in HEK cells
HEK-293 cells possess endogenous TRPM7, which is expressed at low levels (Fig 1A). To amplify TRPM7 expression, we expressed TRPM7 and TRPM7 mutants using tetracycline-controlled transcription. After the addition of tetracycline, optimal protein expression levels of WT hTRPM7 and the kinase dead mutants, ΔKinase and K1648R, were observed after 48 hr and sustained for 72 hr (Fig. 1A).
Figure 1. Tetracycline-controlled protein expression of WT hTRPM7, ΔKinase or K1648R does not influence MR or GR protein content in HEK-293 cells.
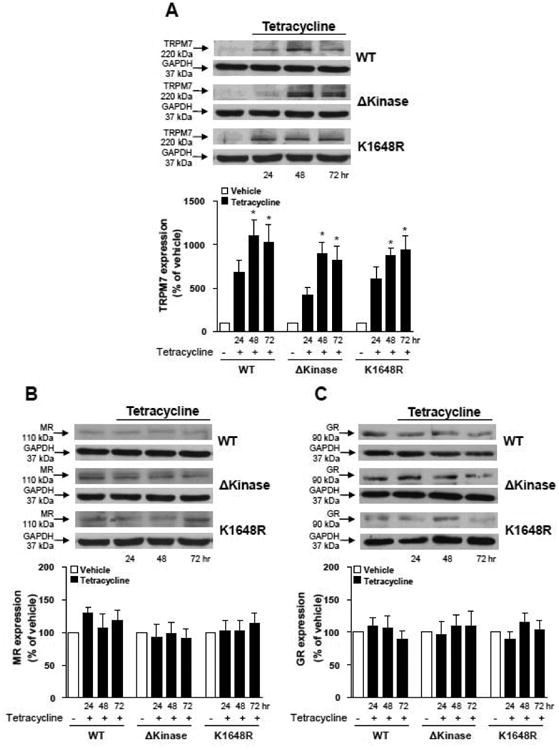
Stable inducible protein expression of WT hTRPM7 or the ΔKinase (deleted kinase) and K1648R (inactive kinase) hTRPM7 in HEK-293 cells was performed with a Tet repressor based system (protein expression induced by adding tetracycline to the growth media). Protein expression of (A) TRPM7, (B) MR, and (C) GR was evaluated in transfected HEK-293 cells untreated and treated with tetracycline for 24, 48 and 72 hr. GAPDH was used as loading control. Top panels, representative immunoblots of TRPM7, MR, GR, and GAPDH. Results are expressed as mean ± S.E of 5 independent experiments. *p<0.05 versus absence of tetracycline.
3.2 Endogenous expression of mineralocorticoid and glucocorticoid receptors in HEK cells
The presence of endogenous MR was detected in HEK-293 cells, with no changes in the receptor protein expression after tetracycline treatment (Fig. 1B). Aldosterone has also been shown to bind to glucocorticoid receptor (GR), albeit with much lower affinity. In our experimental conditions, GR was also detected in HEK-293 cells and its protein content was not altered by tetracycline (Fig. 1C).
3.3 Aldosterone-induced Mg2+ influx is similar in cells expressing human TRPM7 and TRPM7 α-kinase mutant
Figure 2 shows that WT hTRPM7 and the mutants display similar basal [Mg2+]i. Aldosterone induced an increase in Mag-FURA-2 fluorescence ratio with similar magnitude in HEK-293 cells expressing WT hTRPM7, Δkinase, and K1648R channels (Fig. 2A). Eplerenone reduced aldosterone-induced Mg2+ influx in HEK-293 cells expressing WT hTRPM7, Δkinase and K1648R channels (Fig. 2B). 2-APB inhibited aldosterone -mediated [Mg2+]i effects (supplemental Fig. S1). Aldosterone-stimulated rise in [Mg2+]i was lower in non-induced versus induced HEK-293 cells (supplemental Fig. S2).
Figure 2. Aldosterone induces TRPM7-dependent Mg2+ influx in HEK-293 cells.
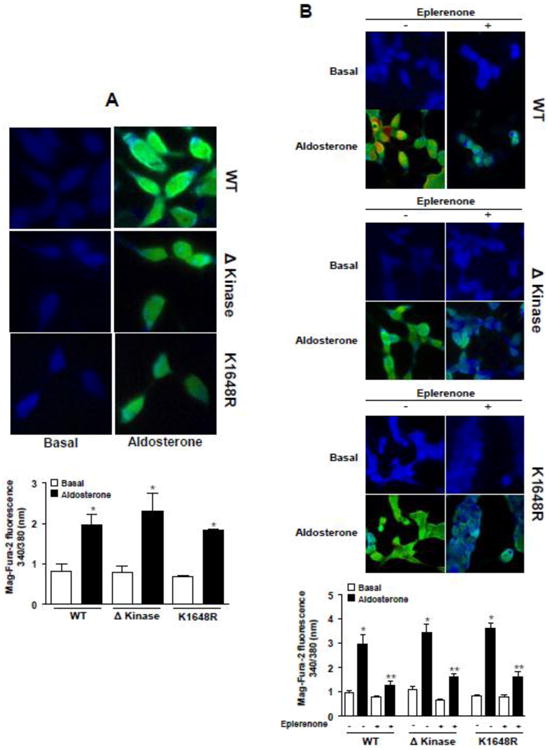
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were loaded with Mag-FURA-2 and were stimulated with 100 nM aldosterone in the absence or presence of the MR antagonist eplerenone (10 μM). Changes in Mag-FURA-2 fluorescence were recorded (excitation ratio: 340/380 nm). (A) Bar graph, Mag-FURA-2 fluorescence ratio peak obtained after aldosterone stimulation. (B) Bar graph, Mag-FURA-2 fluorescence ratio peak obtained after aldosterone stimulation in the absence and in presence of eplerenone. Top panels, representative images of aldosterone effect on Mag-FURA-2 fluorescence intensity. Results are expressed as mean ± SE of 6-8 independent experiments.*p<0.05, versus corresponding basal levels; **p <0.05, aldosterone in the absence versus presence of eplerenone.
3.4 Deletion or mutation in the TRPM7 α-kinase domain does not affect aldosterone-induced NADPH oxidase activation and ROS generation
In WT hTRPM7, Δkinase and K1648R cells aldosterone increased ROS generation as assessed by DHE fluorescence and lucigenin chemiluminescence with a similar magnitude of change (Figs. 3A, 3B). These effects were associated with an increase in p47phox translocation, an index of NADPH oxidase activity, in aldosterone-stimulated HEK-293 cells overexpressing WT hTRPM7, Δkinase or K1648R mutants (Fig. 3C). Aldosterone failed to induce a significant ROS response in cells that were not exposed to tetracycline (supplemental Figs. S3A-S3C).
Figure 3. Deletion or a single point mutation in the ATP binding site of the TRPM7 kinase domain does not affect aldosterone-induced generation of reactive oxygen species (ROS) and NADPH oxidase activation.
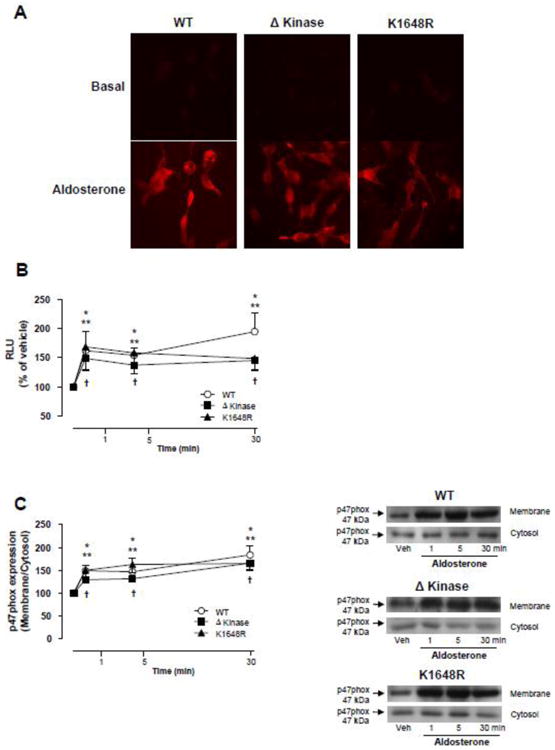
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were stimulated with 100 nM aldosterone (1 to 30 min). (A) Representative images of intracellular DHE fluorescence in aldosterone stimulated cells (30 min). (B) Line graph, NADPH-derived ROS was measured by lucigenin chemiluminescence in homogenates from aldosterone-stimulated cells. The values were normalized by protein concentration in each sample and expressed as relative luminescence units (RLU). (C) NADPH oxidase p47phox cytosolic subunit translocates to the cell membrane upon aldosterone stimulation. Cell membrane and cytosol were fractionated and immunoblotted using antibody to p47phox. Line graph, effect of aldosterone on p47phox translocation from the cytosol to the cell membrane. Translocation was determined by the protein expression ratio in membrane to cytosol fractions. Side panels, representative immunoblots of p47 phox. Results are means ± S.E. (% of vehicle) of 7-10 independent experiments. *p<0.05 WT hTRPM7 versus vehicle; **p<0.05 ΔKinase versus vehicle;†p<0.05 K1648R versus vehicle.
3.5 Aldosterone-induced annexin-1 and calpain-II translocation to the cell membrane is blunted in cells expressing hTRPM7 α-kinase mutants
To explore the function of TRPM7 α-kinase domain, we investigated whether aldosterone induces activation of its well known downstream targets, annexin-1 and calpain-II. These proteins are predominantly located in the cytosol and upon activation translocate to the plasma membrane. To monitor the dynamic changes in the cellular distribution of annexin-1 and calpain-II after aldosterone stimulation, cell lysates were partitioned into membrane and cytosol-enriched fractions. Figure 4A shows that aldosterone induces an increase of annexin-1 content in the membrane fraction of WT hTRPM7 cells, an effect that is rapid and transient, since the translocation peak was obtained within 5 min returning to basal levels after 30 min. In Δkinase and K1648R mutants, aldosterone failed to stimulate annexin-1 translocation. Long-term aldosterone stimulation did not elicit annexin-1 translocation in WT hTRPM7, Δkinase or K1648R cells (Fig. 4B). Unlike annexin-1, calpain-II did not translocate to the membrane upon aldosterone short term stimulation (Fig. 4C) in WT hTRPM7, whereas with long-term stimulation (Fig. 4D) the calpain-II content was increased in membrane fractions of these cells. Aldosterone had no effect on calpain-II translocation in Δkinase and K1648R hTRPM7 expressing cells at any stimulation time point. Aldosterone-induced annexin-1 and calpain-II translocation to the cell membrane was associated with WT hTRPM7 expression, since no effects were observed in non-induced cells (supplemental Figs. S4A, S4B). Spectrin, a cytoskeletal protein, exhibits high sensitivity to calpain proteolytic activity (30) and hence spectrin cleaved fragment is used as an index of calpain activity. We found that aldosterone induced a progressive increase in the cleaved spectrin fragment in HEK-293 cells expressing WT hTRPM7 but not in those expressing Δkinase and K1648R mutants (Fig. 5A). This effect was inhibited by eplerenone in WT hTRPM7 cells (Fig. 5B). Spectrin cleavage by aldosterone was not observed in tetracycline untreated cells (supplemental Fig. S4C).
Figure 4. Annexin-1 and Calpain-II, downstream targets of TRPM7 kinase domain, translocate to the cell membrane upon aldosterone stimulation in HEK-293 cells expressing WT hTRPM7 but not in ΔKinase and K1648R hTRPM7 mutants.
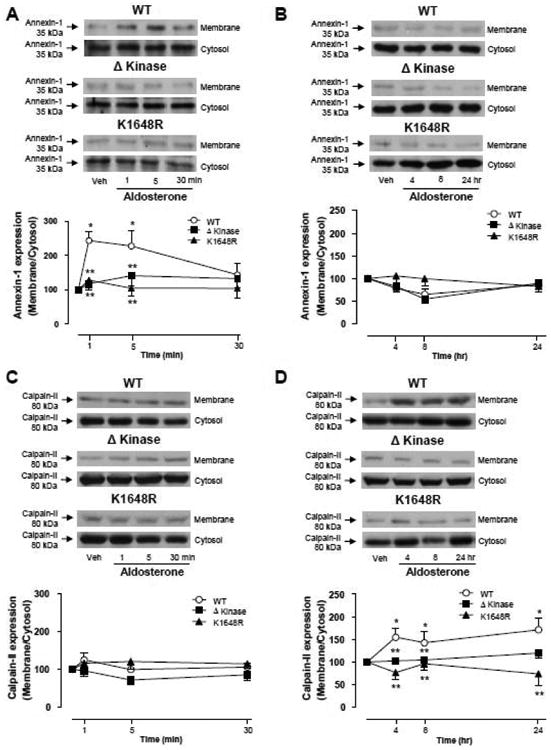
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were stimulated with 100 nM aldosterone for short (1, 5, and 30 minutes) or long term (4, 8, and 24 hr). Cell membrane and cytosol were fractionated. Line graphs, effects of aldosterone on annexin-1 (A, B) and calpain-II (C, D) translocation from the cytosol to the cell membrane. Translocation was determined by the protein expression ratio in membrane to cytosol fractions. Top panels, representative immunoblots of annexin-1 and calpain-II. Results are expressed as mean ± S.E. (% of vehicle,) of 5 independent experiments. *p<0.05 versus vehicle; ** p<0.05 versus corresponding stimulation time in HEK-293 cells expressing WT hTRPM7.
Figure 5. Aldosterone-induced spectrin cleavage, a specific downstream target of calpain-II, is blunted in HEK-293 cells expressing ΔKinase and K1648R hTRPM7 mutants.
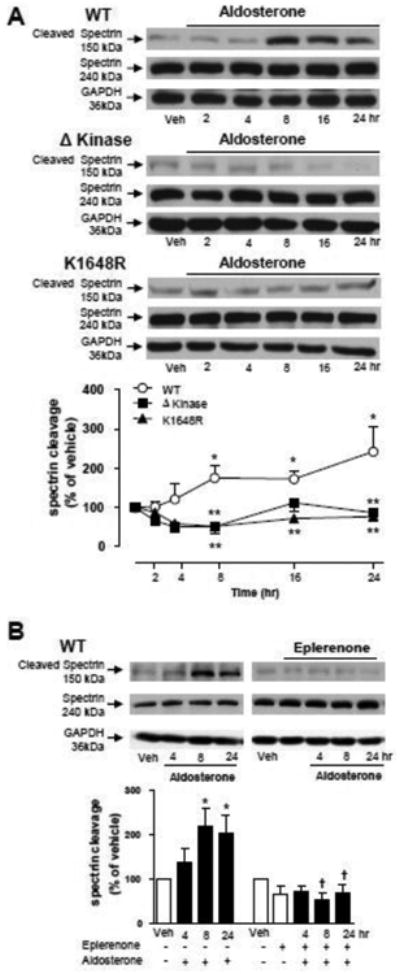
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were stimulated with 100 nM aldosterone (4, 8 and 24 hr) in the absence or presence of the MR antagonist eplerenone (10 μM). (A) Line graph, effect of aldosterone on spectrin cleavage, defined as the ratio of cleaved spectrin to intact spectrin. (B) Bar graph, effect of eplerenone on aldosterone-induced spectrin cleavage in WT hTRPM7. GAPDH was used as loading control. Results are expressed as % of vehicle (mean ± S.E.) of 5 independent experiments. Top panels, representative immunoblots of spectrin, cleaved spectrin, and GAPDH. *p<0.05 versus vehicle; ** p<0.05 versus corresponding stimulation time in HEK-293 cells expressing WT hTRPM7; †p<0.05 versus corresponding stimulation time in the absence of eplerenone.
3.6 Aldosterone-induced MAPK phosphorylation in cells expressing TRPM7 α-kinase mutants
To investigate the potential TRPM7 α-kinase-dependent mechanisms by which aldosterone mediates proinflammatory events, the phosphorylation status of p38MAPK, SAPK/JNK, and ERK1/2 was studied in HEK-293 cells overexpressing WT hTRPM7, Δkinase or K1648R mutants. As shown in Figure 6A, aldosterone stimulation resulted in a rapid and sustained increase of p38MAPK phosphorylation in cells expressing Δkinase and K1648R mutants. Similar effects were observed for SAPK/JNK (Fig. 6B) and ERK1/2 (Fig. 6C). No significant effects of aldosterone on MAPK phosphorylation were observed in WT hTRPM7 cells. In the presence of eplerenone, aldosterone failed to induce p38MAPK, SAPK/JNK and ERK1/2 phosphorylation in mutant cells (supplemental Fig. S5A-S5C). Aldosterone did not increase MAPK phosphorylation in cells that were not exposed to tetracycline (supplemental Fig. S6).
Figure 6. Aldosterone induces MAPKs phosphorylation in ΔKinase and K1648R but not in WT hTRPM7 cells.
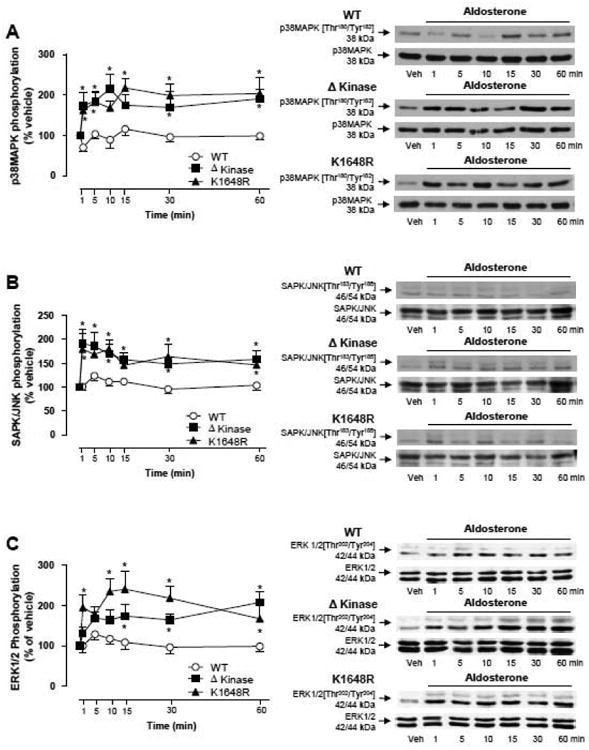
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were stimulated with 100 nM aldosterone (1- 60 min). Line graphs, effect of aldosterone on (A) p38MAPK, (B) SAPK/JNK MAPK, and (C) ERK1/2 MAPK phosphorylation. Results are expressed as % of vehicle (mean ± S.E.) of 7 independent experiments. Side panels, representative immunoblots of p38MAPK [Thr180/Tyr182], p38MAPK, SAPK/JNK MAPK [Thr183/Tyr185], SAPK/JNK MAPK, ERK1/2 MAPK [Thr202/Tyr204], ERK1/2 MAPK. *p<0.05 versus corresponding stimulation time in HEK-293 cells expressing WT hTRPM7.
3.7 Expression of inflammatory markers in cells expressing TRPM7 α-kinase mutants
Aldosterone stimulation increased expression of ICAM-1(Fig. 7A), Cox-2 (Fig. 7B), and PAI-1 (Fig. 7C) in Δkinase and K1648R mutants but not in WT hTRPM-7 cells. Eplerenone prevented these effects (supplemental Figs. S7A-S7C). Aldosterone had no significant effect on ICAM-1,Cox-2 or PAI-1 in tetracycline-untreated cells (supplemental Fig. S8).
Figure 7. Aldosterone induces increase of ICAM-1, Cox-2 and PAI-1 protein expression in ΔKinase and K1648R but not in WT hTRPM7 cells.
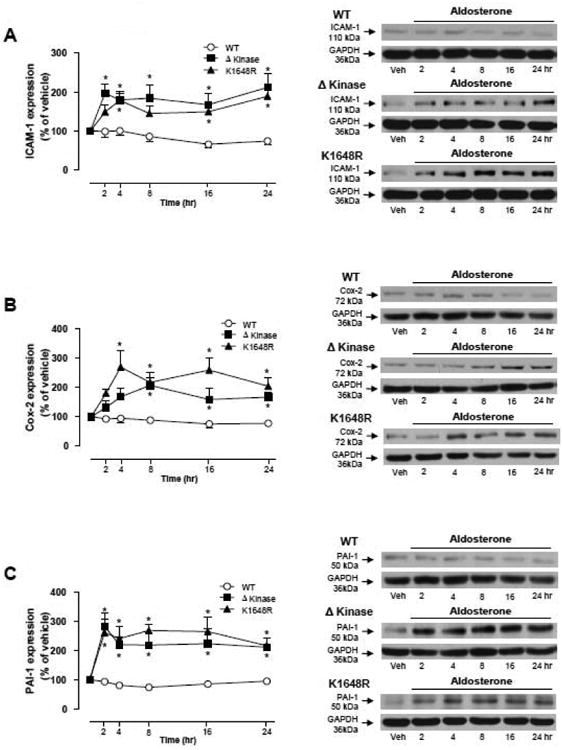
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were stimulated with 100 nM aldosterone (4, 8 and 24 hr). Line graphs, effect of aldosterone on (A) ICAM-1, (B) Cox-2, and (C) PAI-1 protein expression. GAPDH was used as loading control. Results are expressed as % of vehicle (mean ± S.E.) of 7 independent experiments. Side panels, representative immunoblots of ICAM-1, Cox-2, PAI-1, and GAPDH. *p<0.05 versus corresponding stimulation time in HEK-293 cells expressing WT hTRPM7.
3.8 2-APB effects on aldosterone-induced pro-inflammatory responses
To evaluate whether inhibition of TRPM7 channel influences aldosterone-induced inflammatory responses, cells were pre-exposed to 2-APB, which we and others have shown to inhibit TRPM7 channel activity (27,30). As shown in figures 8 and 9, in 2-APB-treated WT hTRPM-7 cells, aldosterone-induced activation of p38MAP kinase and JNK was augmented, responses that were associated with upregulation of pro-inflammatory proteins Cox-2 and ICAM-1. In Δkinase and K1648R mutants, in which aldosterone stimulated activation of MAP kinases and increased expression of pro-inflammatory proteins, 2-APB pre-treatment did not alter aldosterone-induced responses (figures 8B-F, 9B-F)).
Figure 8. Effects of 2-APB on aldosterone-induced activation of pro-inflammatory MAP kinases, p38MAP kinase and JNK, in WT hTRPM7, ΔKinase and K1648R cells.
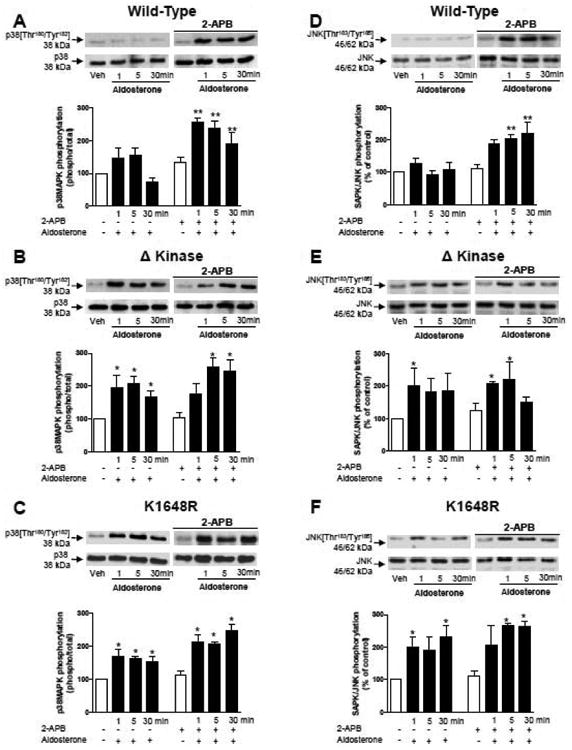
HEK-293 cells expressing WT hTRPM7 channels (A, D) or the mutants ΔKinase (deleted kinase) (B, E) and K1648R (inactive kinase) (C, F) were stimulated with 100 nM aldosterone (1-30 mins) in the absence and presence of 2-APB (50 μM). Top panels are representative immunoblots of p38MAPK [Thr180/Tyr182] and JNK MAPK [Thr183/ Tyr185]. Results are expressed as the phosphorylated:total protein content relative to control (white bars) taken as 100%. Bar graphs are means ± SE of 5 to 6 independent experiments. *p<0.05; **p<0.01 versus control.
Figure 9. Effects of 2-APB on aldosterone-induced expression of pro-inflammatory PROTEINS Cox-2 and ICAM-1 in WT hTRPM7, ΔKinase and K1648R cells.
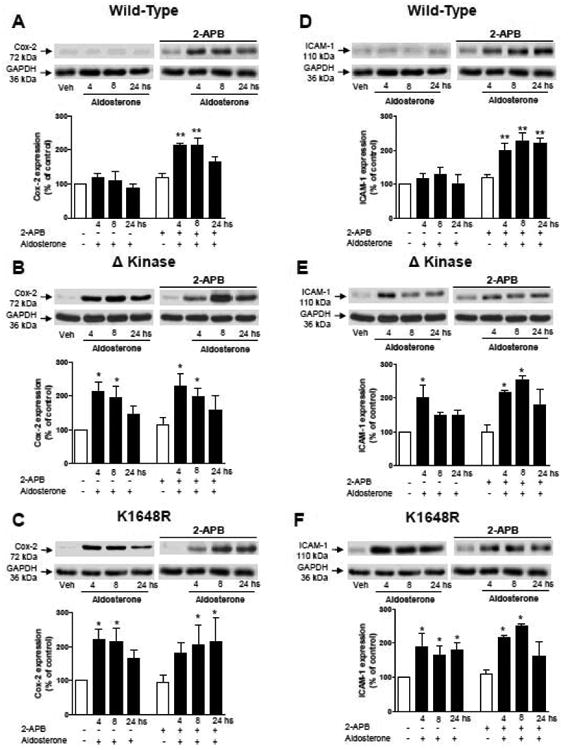
HEK-293 cells expressing WT hTRPM7 channels (A, D) or the mutants ΔKinase (deleted kinase) (B, E) and K1648R (inactive kinase) (C, F) were stimulated with 100 nM aldosterone (1-30 mins) in the absence and presence of 2-APB (50 μM). Top panels are representative immunoblots of Cox-2 and ICAM-1. Results are Cox-2 or ICAM-1 expression normalised to the housekeeping protein GAPDH. Data are presented as expression relative to control conditions (white bars) taken as 100%. Bar graphs are means ± SE of 5 to 6 independent experiments. *p<0.05; **p<0.01 versus control.
4. Discussion
In the present study we have uncovered novel TRPM7-mediated aldosterone signaling pathways in renal cells. Major findings demonstrate that: 1) aldosterone, through MR-dependent mechanisms, rapidly stimulates TRPM7-mediated Mg2+ influx; 2) Mg2+ transport and activation of NADPH oxidase by aldosterone are TRPM7 α-kinase-independent, whereas calpain and annexin-1 signaling are TRPM7 α-kinase-dependent, and 3) deficiency of TRPM7 phosphotransferase activity or absence of TRPM7 α-kinase domain is associated with MAP kinase activation and pro-inflammatory responses by aldosterone. Our findings indicate that aldosterone signaling is differentially regulated by TRPM7 and TRPM7 α-kinase. We identify new discrete signaling pathways through TRPM7/TRPM7 α-kinase by aldosterone/MR and indicate a disconnect between TRPM7 channel and α-kinase. Such processes involve, in part, non-genomic rapid signaling events.
Renal cells represent an excellent model to interrogate aldosterone and TRPM7 signaling because they endogenously express MRs and TRPM7 and associated signaling machinery. Moreover the kidney is the major physiological target organ for aldosterone. Through MR, aldosterone stimulated Mg2+ influx in cells containing an intact WT hTRPM7 system as well as in cells deficient in TRPM7 α-kinase activity. These data indicate that aldosterone/MR regulates Mg2+ transport and [Mg2+]i in a TRPM7 channel-dependent, kinase-independent manner, since inactivation or deletion of the kinase domain did not influence aldosterone-induced Mg2+ responses. While some studies suggested that a functioning α-kinase domain is necessary for TRPM7-mediated Mg2+ influx (15), others have shown that the α-kinase domain is not required, (12, reviewed in 33) a finding that we confirm here. The aldosterone concentration that we studied is higher than that in plasma, but may reflect the elevated concentration of local aldosterone at the tissue level, especially in pathological conditions, such as in hypertension and kidney disease (35,36). Accordingly, the doses examined in our study may have pathophysiological significance. Moreover, most in vitro studies examine aldosterone in the nanomolar range (2-4).
Associated with the rapid Mg2+ effect by aldosterone, was an increase in ROS generation, due in large part to activation of NADPH oxidase as evidenced by increased cytosol-to-membrane translocation of p47phox, and enhanced NADPH oxidase-driven ROS formation. Aldosterone-induced activation of NADPH oxidase has previously been demonstrated in kidney and vascular cells (37-40). Functionally aldosterone-induced ROS generation is associated with cellular growth, migration and secretion, through pathways that involve Ca2+, MAPK and activation of transcription factors, processes that are Mg2+-sensitive. Here we show that NADPH oxidase regulation by aldosterone involves TRPM7, but not TRPM7 α-kinase, a phenomenon similar to that for [Mg2+]i regulation. TRPM7 kinase has been shown to modulates the Mg2+ sensitivity of the channel (12). Accordingly, the rapid aldosterone-induced Mg2+ and ROS responses may rely primarily on TRPM7 channel activation. This is further supported by the findings that 2-APB abrogated [Mg2+]i effects of aldosterone. Such dissociation of the kinase from the ion-conducting pore has been shown to be important in Fas-induced apoptosis, cell survival and cell stress, phenomena that involve Mg2+ and ROS (41). Although we can not establish the interdependencies of TRPM7-regulated Mg2+ and ROS in our paradigm, we have previously shown that Mg2+ influences ROS generation (10,11) and there is growing evidence that Mg2+ modulates ROS levels via NADPH oxidase as well as through mitochondria and increased glutathione transferase activity (42,43). These phenomena, through TRPM7 channel, may link divalent cations and ROS signaling, important in cell regulation.
Among the best characterized downstream signaling targets for TRPM7 are annexin-1 and calpain–II, which have diverse cellular functions (44). Annexin-1, implicated in cell proliferation and differentiation, is characteristically associated with anti-inflammatory responses (45), mediated in part through inhibition of MAP kinases (46). In WT hTRPM7 cells, activation of annexin-1 as assessed by membrane translocation, was significantly increased by aldsoterone. This response, which was rapid, occurring within minutes, was abrogated by deletion of the α-kinase domain or a point mutation that renders the α-kinase catalytically inactive. Since annexin-1 regulates anti-inflammatory signaling, in part through interference with MAP kinases, decreased activation in the context of down-regulated α-kinase may promote inflammation (47).
α-kinase was also involved in calpain-II signaling by aldosterone, because calpain translocation and spectrin cleavage were inhibited in TRPM7 α-kinase-deficient/inactivated cells. Calpain-II, a Ca2+-dependent protease, has been implicated in apoptosis and cell growth (48) and is influenced by aldosterone, since spironolactone, a MR blocker, inhibited cardiac remodeling in a model of atrial fibrillation (49). In line with the effects on cell growth, TRPM7 α-kinase-regulated calpain-II activation by aldosterone was not acute, but occurred over hours, suggesting probable genomic signaling and de novo protein synthesis. These differential kinetic responses indicate that aldosterone/MR signaling via TRPM7 is highly regulated through, as yet, unknown intermediaries.
To further investigate molecular processes associated with TRPM7 signaling by aldosterone, we focused on MAP kinases, master signaling molecules typically associated with inflammation and cell stress (50). Aldosterone induced a significant increase in activation of ERK1/2, p38MAPK and SAPK/JNK in cells in which TRPM7 α-kinase domain was absent or inactive, but not in cells in which WT hTRPM7 was intact. Similar patterns were observed for the proinflammatory proteins ICAM-1, PAI-1 and Cox2, which are downstream of MAP kinases.
To interrogate in greater detail the potential role of TRPM7 channel in inflammatory processes associated with TRPM7 α-kinase, we exposed wild-type and TRPM7 α-kinase-deficient cells 2-APB, a TRPM7 channel inhibitor. 2-APB augmented activation of pro-inflammatory MAP kinases (p38MAP kinase and JNK), effects that were associated with increased expression of pro-inflammatory mediators (Cox-2 and ICAM-1). Considering that 2-APB blocked aldosterone-induced Mg2+ influx without inhibiting pro-inflammatory signaling, it may be possible that TRPM7 channel-related changes in [Mg2+]i are dissociated from inflammatory responses induced by aldosterone, which might be regulated primarily by the α-kinase. In further support of this, 2-APB did not significantly alter aldosterone-induced activation of pro-inflammatory signaling pathways nor expression of Cox-2 and ICAM-1 in cells lacking functional α-kinase. These findings have a number of implications. Firstly TRPM7 channel and TRPM7 α-kinase have distinct molecular functions that may not be interdependent, and secondly, TRPM7 α-kinase downregulation promotes inflammation by aldosterone suggesting that TRPM7 α-kinase may negatively regulate inflammatory signaling. These findings are in line with our observations that activation of the anti-inflammatory protein annexin-1 was blunted in TRPM7 α-kinase-deficient cells. A link between anti-inflammatory effects of annexin-1 and decreased MAP kinase activation has previously been reported (47). We speculate that aldosterone may influence inflammation by inhibiting α-kinase activity. This however remains to be demonstrated.
5. Conclusions
Our study has identified novel mechanisms for aldosterone signaling through TRPM7 kinase-dependent and -independent pathways. Whereas aldosterone stimulates Mg2+ influx, NADPH oxidase activity and superoxide anion production in a TRPM7 channel sensitive, kinase-insensitive manner, activation of annexin-1 and calpain depend on TRPM7 α-kinase. Moreover, TRPM7 α-kinase may play an important role in modulating inflammatory responses by aldosterone. Our results identify new molecular mechanisms for aldosterone signaling that involve MR-activated TRPM7/TRPM7 α-kinase, mediated, in part, through non-genomic processes. Such phenomena could contribute to the pleiotropic actions of aldosterone, especially those associated with cellular divalent cation transport and pro-inflammatory signaling.
Supplementary Material
Highlights.
TRPM7 plays an important role in non-genomic signalling of aldsoterone.
TRPM7 channel and α-kinase domains have differential functions in aldosterone signalling
Aldosterone stimulates Mg2+ influx and reactive oxygen species production through TRPM7 channel, but not TRPM7 α-kinase.
TRPM7 α-kinase modulates inflammatory signaling by aldosterone in a channel/Mg2+-independent manner.
Differential signalling through TRPM7 channel and kinase domains may contribute to pleiotropic actions of aldosterone.
Acknowledgments
This study was funded by grants from the Canadian Institute of Health Research (CIHR) (to RMT and AS), the National Institutes of Health (R01GM068801 to ALP, R21AI0088421 (NIAID)), and R01GM90123 (includes funding from NIGMS and the Office of Dietary Supplements, ODS, to CS). RMT was supported through a Canada Research Chair/Canadian Foundation for Innovation award. AY was supported by a fellowship from the Heart and Stroke Foundation of Canada and ACM a fellowship from the CIHR.
Footnotes
Authors Contributions: A Y, GEC, SOC, TAA and ACM designed the protocols, conducted the experiments, and analysed the data. WV and PM. performed experiments, analyzed the data and contributed to the discussion. ALP and CS provided the HEK293 cells, and reviewed the article. AS supervised some experiments and contributed to the final draft of the manuscript. R.M.T is the guarantor of this work and has full access to the entire dataset and takes full responsibility for the integrity of the data and the accuracy of the data analysis. All authors have seen and approved the final version of the article.
Disclosures: No conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nguyen Dinh Cat A, Jaisser F. Extrarenal effects of aldosterone. Curr Opin Nephrol Hypertension. 2012;21:147–56. doi: 10.1097/MNH.0b013e32834fb25b. [DOI] [PubMed] [Google Scholar]
- 2.Queisser N, Schupp N, Stopper H, Schinzel R, Oteiza PI. Aldosterone increases kidney tubule cell oxidants through calcium-mediated activation of NADPH oxidase and nitric oxide synthase. Free Radic Biol Med. 2011;51:1996–2006. doi: 10.1016/j.freeradbiomed.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension. 2005;45:773–779. doi: 10.1161/01.HYP.0000154365.30593.d3. [DOI] [PubMed] [Google Scholar]
- 4.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–8. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen Dinh, Cat A, Briones AM, Callera GE, Yogi A, He Y, Montezano AC, Touyz RM. Adiposity-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension. 2011;58(3):479–88. doi: 10.1161/HYPERTENSIONAHA.110.168872. [DOI] [PubMed] [Google Scholar]
- 6.Runyan AL, Sun Y, Bhattacharya SK, Ahokas RA, Chhokar VS, Gerling IC, Weber KT. Responses in extracellular and intracellular calcium and magnesium in aldosteronism. J Lab Clin Med. 2005;146:76–84. doi: 10.1016/j.lab.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Wehling M. Effects of aldosterone and mineralocorticoid receptor blockade on intracellular electrolytes. Heart Fail Rev. 2005;10:39–46. doi: 10.1007/s10741-005-2347-z. [DOI] [PubMed] [Google Scholar]
- 8.Touyz RM, Yao G. Modulation of vascular smooth muscle cell growth by magnesium-role of mitogen-activated protein kinases. J Cell Physiol. 2003;197:326–35. doi: 10.1002/jcp.10393. [DOI] [PubMed] [Google Scholar]
- 9.Fortpied J, Maliekal P, Vertommen D, Van Schaftingen E. Magnesium-dependent phosphatase-1 is a protein-fructosamine-6-phosphatase potentially involved in glycation repair. J Biol Chem. 2006;281:18378–85. doi: 10.1074/jbc.M513208200. [DOI] [PubMed] [Google Scholar]
- 10.Sontia B, Montezano AC, Paravicini T, Tabet F, Touyz RM. Downregulation of renal TRPM7 and increased inflammation and fibrosis in aldosterone-infused mice: effects of magnesium. Hypertension. 2008;51:915–21. doi: 10.1161/HYPERTENSIONAHA.107.100339. [DOI] [PubMed] [Google Scholar]
- 11.Yogi A, Callera GE, O'Connor SE, He Y, Correa JW, Tostes RC, Mazur A, Touyz RM. Dysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemia. J Hypertens. 2011;29:1400–10. doi: 10.1097/HJH.0b013e32834786d6. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 13.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz C, Perraud AL, Fleig A, Scharenberg AM. Dual-function ion channel/protein kinase: Novel components of vertebrate magnesium regulatory mechanisms. Pediatr Res. 2004;55:734–737. doi: 10.1203/01.PDR.0000117848.37520.A2. [DOI] [PubMed] [Google Scholar]
- 15.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 16.Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 17.Su LT, Chen HC, González-Pagán O, Overton JD, Xie J, Yue L, Runnels LW. TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J Mol Biol. 2010;396(4):858–69. doi: 10.1016/j.jmb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perraud AL, Zhao X, Ryazanov AG, Schmitz C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-K. Cellular Signal. 2011;23:586–593. doi: 10.1016/j.cellsig.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deason-Towne F, Perraud AL, Schmitz C. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C γ2 (PLCγ2) using TRPM7-kinase. Cell Signal. 2012;24:2070–2075. doi: 10.1016/j.cellsig.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–60. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryazanov LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat Commun. 2010;109:1–9. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlingmann KP, Gudermann T. A critical role of TRPM channel-kinase for human magnesium transport. J Physiol. 2005;566:301–308. doi: 10.1113/jphysiol.2004.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TY, Shin SK, Song MY, Lee JE, Park KS. Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem Biophys Res Commun. 2012;417:1030–4. doi: 10.1016/j.bbrc.2011.12.085. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 26.Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J. 2011;75:237–245. doi: 10.1253/circj.cj-10-1021. [DOI] [PubMed] [Google Scholar]
- 27.Yogi A, Callera GE, Tostes R, Touyz RM. Bradykinin regulates calpain and proinflammatory signaling through TRPM7-sensitive pathways in vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2009;296:R201–R207. doi: 10.1152/ajpregu.90602.2008. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 29.Touyz RM, Schiffrin EL. Angiotensin II and vasopressin modulate intracellular free magnesium in vascular smooth muscle cells through Na+-dependent protein kinase C pathways. J Biol Chem. 1996;271:24353–8. doi: 10.1074/jbc.271.40.24353. [DOI] [PubMed] [Google Scholar]
- 30.Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, van Leeuwen FN, Touyz RM. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56(3):453–62. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 31.Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem. 2004;279:3708–16. doi: 10.1074/jbc.M308820200. [DOI] [PubMed] [Google Scholar]
- 32.Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA. 2004;101:6009–14. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paravicini TM, Chubanov V, Gudermann T. TRPM7: A unique channel involved in magnesium homeostasis. Int J Biochem Cell Biol. 2012;44:1381–4. doi: 10.1016/j.biocel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Nadler MJS, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg-ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–5. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 35.Del Vecchio L, Procaccio M, Viganò S, Cusi D. Mechanisms of disease: The role of aldosterone in kidney damage and clinical benefits of its blockade. Nat Clin Pract Nephrol. 2007;3(1):42–9. doi: 10.1038/ncpneph0362. [DOI] [PubMed] [Google Scholar]
- 36.Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie IL. Alterations in aldosterone and angiotensin II levels in salt-induced hypertension. Clin Exp Hypertens. 2005;27(4):355–67. [PubMed] [Google Scholar]
- 37.Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E, van Leeuwen FN. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One. 2008;3:e1876. doi: 10.1371/journal.pone.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayorh MA, Rollins-Hairston A, Adiyiah J, Lyn D, Eatman D. Eplerenone suppresses aldosterone/salt-induced expression of NOX-4. J Ren Ang Ald Syst. 2011;12:195–201. doi: 10.1177/1470320310391330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi G, Fu Y, Jiang W, Yin A, Feng M, Wu Y, Kawai Y, Miyamori I, Fan C. Activation of Src-ATF1 pathway is involved in upregulation of Nox1, a catalytic subunit of NADPH oxidase, by aldosterone. Endocr J. 2011;58:491–9. doi: 10.1507/endocrj.k10e-383. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, Manning RD, Jr, Lu D, Gomez-Sanchez CE, Fu Y, Juncos LA, Liu R. (2011) Aldosterone stimulates superoxide production in macula densa cells. Am J Physiol Renal Physiol. 2011;301:F529–35. doi: 10.1152/ajprenal.00596.2010. Zhu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chien MM, Zahradka KE, Newell MK, Freed JH. Fas-induced B cell apoptosis requires an increase in free cytosolic magnesium as an early event. J Biol Chem. 1999;274:7059–66. doi: 10.1074/jbc.274.11.7059. [DOI] [PubMed] [Google Scholar]
- 42.Tamura M, Kanno M, Kai T. Destabilization of neutrophil NADPH oxidase by ATP and other trinucleotides and its prevention by Mg(2+) Biochim Biophys Acta. 2001;1510:270–7. doi: 10.1016/s0005-2736(00)00358-8. [DOI] [PubMed] [Google Scholar]
- 43.Shah NC, Liu JP, Iqbal J, Hussain M, Jiang XC, Li Z, Li Y, Zheng T, Li W, Sica AC, Perez-Albela JL, Altura BT, Altura BM. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: relevance to de novo synthesis of ceramide, serum Mg and atherogenesis. Int J Clin Exp Med. 2011;4:103–18. [PMC free article] [PubMed] [Google Scholar]
- 44.Dorovkov MV, Kostyukova AS, Ryazanov AG. Phosphorylation of annexin A1 by TRPM7 kinase: a switch regulating the induction of an α-helix. Biochemistry. 2011;50:2187–93. doi: 10.1021/bi101963h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SH, Kim DW, Kim HR, Woo SJ, Kim SM, Jo HS, Jeon SG, Cho SW, Park JH, Won MH, Park J, Eum WS, Choi SY. Anti-inflammatory effects of Tat-Annexin protein on ovalbumin-induced airway inflammation in a mouse model of asthma. Biochem Biophys Res Commun. 2012;417:1024–9. doi: 10.1016/j.bbrc.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 46.Araujo LP, Truzzi RR, Mendes GE, Luz MA, Burdmann EA, Oliani SM. Annexin A1 protein attenuates cyclosporine-induced renal hemodynamics changes and macrophage infiltration in rats. Inflamm Res. 2012;61:189–96. doi: 10.1007/s00011-011-0400-z. [DOI] [PubMed] [Google Scholar]
- 47.Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanismsof the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB J. 2012;26(12):4805–20. doi: 10.1096/fj.12-216382. [DOI] [PubMed] [Google Scholar]
- 48.Ono Y, Sorimachi H. Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824:224–36. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, Arnold JM, Abel ED, Peng T. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–94. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


