Abstract
Land plants orient their growth relative to light and gravity through complex mechanisms that require auxin redistribution. Embryos of brown algae use similar environmental stimuli to orient their developmental polarity. These studies of the brown algae Fucus distichus examined whether auxin and auxin transport are also required during polarization in early embryos and to orient growth in already developed tissues. These embryos polarize with the gravity vector in the absence of a light cue. The auxin, indole-3-acetic acid (IAA), and auxin efflux inhibitors, such as naphthylphthalamic acid (NPA), reduced environmental polarization in response to gravity and light vectors. Young rhizoids are negatively phototropic, and NPA also inhibits rhizoid phototropism. The effect of IAA and NPA on gravity and photopolarization is maximal within 2.5 to 4.5 h after fertilization (AF). Over the first 6 h AF, auxin transport is relatively constant, suggesting that developmentally controlled sensitivity to auxin determines the narrow window during which NPA and IAA reduce environmental polarization. Actin patches were formed during the first hour AF and began to photolocalize within 3 h, coinciding with the time of NPA and IAA action. Treatment with NPA reduced the polar localization of actin patches but not patch formation. Latrunculin B prevented environmental polarization in a time frame that overlaps the formation of actin patches and IAA and NPA action. Latrunculin B also altered auxin transport. Together, these results indicate a role for auxin in the orientation of developmental polarity and suggest interactions between the actin cytoskeleton and auxin transport in F. distichus embryos.
Plants orient their growth in response to environmental stimuli, including light and gravity. In land plants, differential growth in response to unilateral light or the gravity vector is a well-characterized process that requires appropriate distribution of the plant hormone auxin (Muday, 2001; Boonsirichai et al., 2002). Auxin is transported from cell to cell in a polar fashion (Muday and DeLong, 2001; Friml, 2003). Asymmetric distribution of transport proteins has been suggested to control both normal polar transport and lateral redistribution of auxin when plants are reoriented relative to the light or gravity vectors (Muday, 2001; Friml et al., 2002). Identification of mutations in genes that encode putative auxin transport proteins that abolish phototropism or gravitropism supports this model (Bennett et al., 1996; Chen et al., 1998; Friml et al., 2002). Similarly, treatment with auxin efflux inhibitors also prevents phototropic and gravitropic curvature (Harper et al., 2000; Rashotte et al., 2000). Gradients of auxin across gravitropically stimulated plants have been directly measured (Philippar et al., 1999; Long et al., 2002) and indirectly identified through measurement of auxin-induced gene expression (Li et al., 1991; Rashotte et al., 2001; Ottenschlager et al., 2003). The proteins encoded by the PIN gene family each have unique localization in different planes of the plasma membrane, which are consistent with a role for these proteins in facilitating auxin transport with different polarities in different tissues (Friml, 2003). Changes in the localization of PIN3, a putative auxin efflux carrier, in response to changes in light or gravity, have been observed (Friml et al., 2002), further indicating that changes in auxin transport may be essential for environmentally controlled, differential growth.
Embryonic polarity in brown algae is also controlled by environmental gradients, with light being the best characterized signal (Belanger and Quatrano, 2000). Initial asymmetric cell division leads to formation of apical and basal daughter cells that are precursors to thallus and rhizoid tissues, respectively. The rhizoid forms on the shaded side of embryos (Kropf et al., 1999). This initial algal division resembles the first asymmetric division in land plant embryos that leads to the formation of apical and basal cells (Jurgens, 2001). In addition to light, a variety of other chemical, mechanical, and electrical signals have been shown to orient brown algal embryo polarity (Jaffe, 1968). Although polarized by centrifugation at 3,000g, a 1g stimulation has not been reported to polarize these embryos (Whitaker, 1937; Whitaker, 1940). In contrast, a 1g stimulation is sufficient to polarize single-celled fern spores of Ceratopteris richardii (Edwards and Roux, 1998). Whether and how embryos of brown algae are polarized by gravity remains unknown.
Numerous studies have explored the cellular events necessary for photopolarization of brown algal embryos (Kropf et al., 1999; Belanger and Quatrano, 2000). One of the earliest detectable events is the formation of an actin patch on the shaded side of Silvetia compressa embryos, which is the future site of rhizoid formation (Alessa and Kropf, 1999). Similar patches have been identified in Fucus distichus embryos later in development (Kropf et al., 1989). An intact and functional actin cytoskeleton is required for embryo polar development in both F. distichus and S. compressa, as cytochalasin or latrunculin B (LatB) renders the light-induced axis labile to another light pulse from an alternative orientation (Nelson and Jaffe, 1973; Quatrano, 1973; Hable and Kropf, 1998; Alessa and Kropf, 1999). Changes in actin organization are followed by local increases in cytosolic calcium (Pu and Robinson, 1998) and polarized vesicle secretion at the same site (Hable and Kropf, 1998). It was hypothesized that the actin patch recruits calcium channel proteins to its membrane position by both lateral movement of channels and local insertion of channel-containing vesicles (Kropf et al., 1999). As vesicle secretion in plants depends on calcium, a positive amplification process may occur between calcium and vesicle secretion at the rhizoid tip (Kropf et al., 1999).
The plant hormone auxin may play a role in embryo development (Geldner et al., 2000; Souter and Lindsey, 2000). Several Arabidopsis mutants with altered auxin responses have defects in embryo development, including auxin resistant6 (axr6), bodenlos (bdl), and monopteros (mp) (Berleth and Jurgens, 1993; Hamann et al., 1999; Hobbie et al., 2000). The mutations in the MP and BDL genes alter the division plane of the apical daughter cell and affect both the central and basal cell lineages (Berleth and Jurgens, 1993; Hamann et al., 1999), while mutations in AXR6, the suspensor divisions are abnormal (Hobbie et al., 2000). The proteins encoded by the BDL and MP genes so far indicate a role for auxin-regulated gene expression in these development processes (Jurgens, 2003). Finally, treatment of embryos with exogenous auxin alters developmental patterns in both land plants and algal embryos (Schiavone and Cooke, 1987; Hadfi et al., 1998; Basu et al., 2002).
Additional studies suggest that embryo development in both land plants and algae may be directly tied to proper auxin transport. Treatment of embryos with indole-3-acetic acid (IAA) efflux inhibitors led to altered developmental patterns in a number of species (Schiavone and Cooke, 1987; Hadfi et al., 1998; Geldner et al., 2000), including F. distichus (Basu et al., 2002). The altered morphology resembles the defects found in plants with a quadruple mutation in four PIN genes (Friml et al., 2003), which encode proteins that are likely to be part of the IAA efflux carrier complex (Friml, 2003). A recent report identified asymmetries in PIN gene expression in the earliest stages of embryo development (Friml et al., 2003). PIN7 is expressed at the earliest embryo stages, and the localization of PIN7 and resulting gradients in IAA-induced gene expression are consistent with the establishment of cell polarity, polar auxin efflux, and local auxin response resulting in apical-basal axis formation in the embryo (Friml et al., 2003).
F. distichus embryos are an ideal system to examine developmental polarity in response to light and gravity because of the exquisite sensitivity of these embryos to external gradients and the reproducible developmental response of large numbers of embryos. What is less clear is the function of auxin in these embryos that are evolutionarily distant from land plants (Baldauf et al., 2000). Although IAA has been found in a range of algae and other plants (Cooke et al., 2002), including F. distichus (Basu et al., 2002), the physiological function and mechanism of movement of this molecule in algae is not yet clear (Cooke et al., 2002). A previous report also indicates that in F. distichus embryos IAA movements are regulated by IAA efflux inhibitors, and that both IAA and IAA efflux inhibitor treatments lead to altered embryo structures, consistent with a role of auxin in F. distichus embryo development (Basu et al., 2002). Very few similar experiments have been performed in other multicellular algae, with the exception of Chara. Two studies examined whether auxin transport was regulated in this algae (Dibb-Fuller and Morris, 1992; Klämbt et al., 1992), which is generally considered to be the algal lineage most closely related to land plants (Baldauf et al., 2000). When only thallus tissues are examined, naphthylphthalamic acid (NPA) did not regulate IAA movements (Dibb-Fuller and Morris, 1992), but when tissue segments included rhizoids, IAA movements were modulated by IAA efflux inhibitors (Klämbt et al., 1992). A complete assessment of evolution of proteins and mechanisms that control IAA transport awaits additional studies.
This study examined the role of polar auxin transport in the development of F. distichus embryo polarity in response to environmental gradients, such as gravity and light. It also examined the effect of IAA and IAA efflux inhibitors on embryo polarization by gravity and light and on the phototropism of rhizoids. The temporal developmental sensitivity to IAA and IAA efflux inhibitors was compared to the timing of changes in the actin cytoskeleton. Formation and photolocalization of actin patches were directly visualized in the presence and absence of an IAA efflux inhibitor. The timing of developmental sensitivity to LatB, an actin-depolymerizing drug, was compared to actin patch formation. Together, these results suggest that auxin plays an important role in developing polarity in response to environmental gradients.
RESULTS
Gravity Polarizes F. distichus Embryos
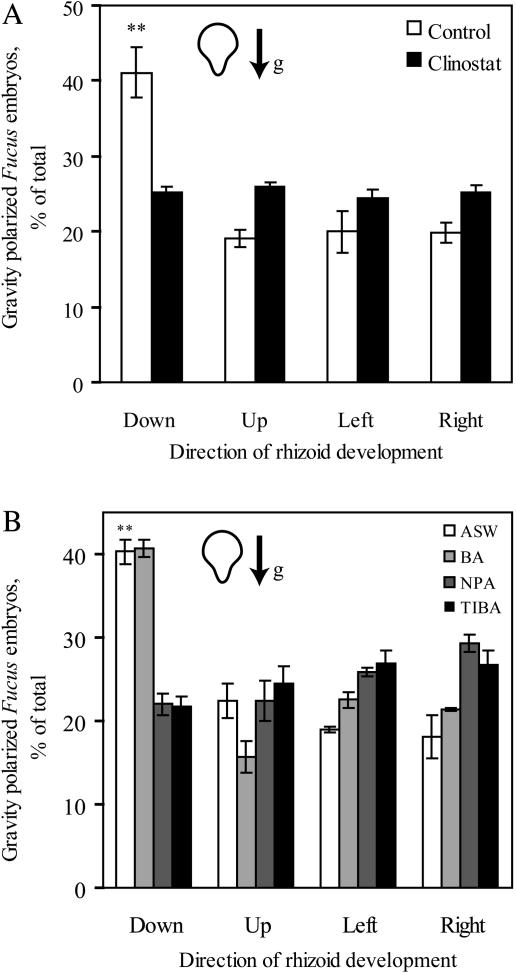
Gravity's effect on developmental polarity was examined by growing F. distichus embryos on vertically oriented slides in the dark. The orientation of rhizoid formation relative to the gravity vector was measured from the percentage of embryos polarized in each of four directions by examining multiple embryos in several fields of the microscope. The average growth orientation relative to gravity is reported in Figure 1A. Forty percent of the embryos polarized in the same direction as the gravity vector. Comparison of this 40% polarization with a random distribution (25% in any direction) is significantly different, as judged by chi-square test (P < 0.005).
Figure 1.
F. distichus embryos are polarized by gravity, and the polarization is randomized by clinostat rotation. A, F. distichus embryos were grown in the dark in ASW either with a continuous gravity vector or with clinostat rotation. B, Gravity polarization of F. distichus embryos is randomized by auxin efflux inhibitors. The effect of BA, NPA, or TIBA at 50 μm, on the percentage of germinating embryos that are gravity polarized in the dark in ASW. The average and se of three separate experiments are reported, with n > 100 embryos per experiment. The ** represent P values <0.005 using a chi-square test.
To verify that polarization was due to the gravity vector and not another environmental gradient, zygotes were placed in vertically oriented petri dishes and mounted on a clinostat with vertically oriented and stationary petri dishes as the control. Through continuous and slow rotation, the clinostat randomizes the gravity vector and is an appropriate negative control for unidirectional gravity stimulation. When the zygotes were clinorotated, polarization was not significantly different from random (25% in each quadrant) as judged by chi-square test, in contrast to vertical stationary controls, which exhibit 40% gravity polarization, as shown in Figure 1A.
Auxin Efflux Inhibitors Reduce Environmental Polarization
To determine if auxin transport is important for gravity- and photopolarization in F. distichus embryos, the effect of IAA efflux inhibitors, NPA and triiodobenzoic acid (TIBA), were examined. The percentage of embryos germinating in each of four directions, with gravity polarization resulting in downward growth, is reported in Figure 1B. NPA and TIBA (50 μm) resulted in a random distribution of polarization relative to gravity, as judged by chi-square test (P > 0.4). Controls with artificial seawater (ASW) and added benzoic acid (BA), a weak acid control, also at 50 μm, showed gravity polarization that was statistically different from random, as judged by chi-square test (P < 5 × 10−4). Ethanol or dimethyl sulfoxide (DMSO) (0.5% v/v) in ASW had no effect (data not shown). BA, TIBA, or NPA treatments did not affect the percent germination at this concentration.
The relative strength of light and gravity's polarizing signals was compared as well as the effect of auxin efflux inhibitor concentrations on photopolarization. Embryos were grown in the presence of indicated NPA, TIBA, or BA concentrations, and the percentage of total embryos photopolarized is plotted as a function of concentration of added compound in Figure 2A. For the BA control, more than 80% of the embryos are photopolarized, which is consistently greater than the 40% to 50% gravity polarization. Additionally, when gravity and light polarize embryos in two different directions, the light polarization is dominant (data not shown).
Figure 2.
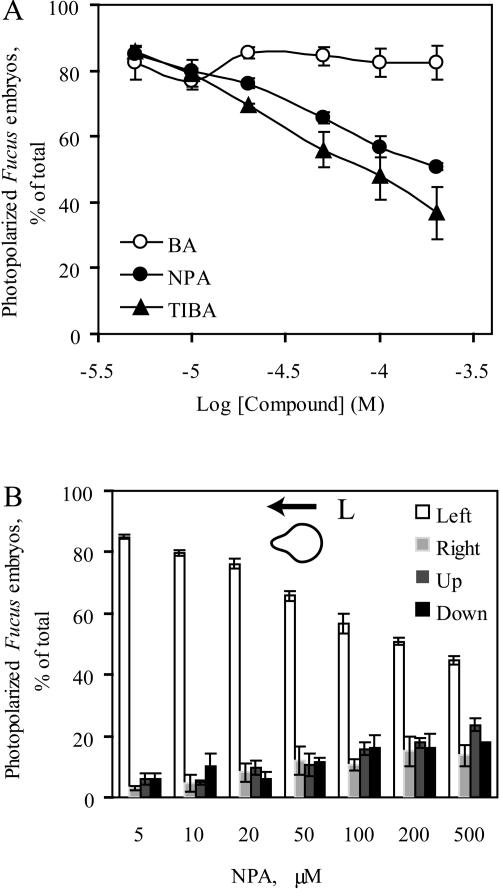
Inhibition of photopolarization of F. distichus embryos increases with auxin efflux inhibitor concentration. The percent photopolarization is normalized relative to all the germinating embryos. A, Percentage of embryos germinating in the dark quadrant is plotted as a function of the inhibitor concentration. B, The percent photopolarization is normalized relative to all the germinating embryos and is reported in all four quadrants as a function of the NPA concentration. For both graphs, the average and se of three separate experiments are reported, with n > 100 embryos per experiment.
Increasing concentrations of NPA or TIBA led to a dose-dependent decrease in photopolarization, as shown in Figure 2A. The significance of the NPA effect was analyzed by one-way ANOVA after assumptions of normality and homogeneity of variances were met. The one-way ANOVA revealed a significant effect of NPA treatment: F7,16 = 77.16, P < 10−6. Duncan's multiple-range post hoc test showed that the degree of photopolarization was significantly reduced compared to the control with NPA at 20 μm (P < 0.05) and very significantly at all concentrations greater than 50 μm (P < 10−4). As variances were not homogeneous for TIBA treatment, an ANOVA could not be performed on these data. A nonparametric equivalent of one-way ANOVA, the Kruskal-Wallis ANOVA, was performed and detected a significant effect of TIBA concentration: chi-square = 18.33, df = 6, P = 0.006. In a post-hoc comparison of treatments, a Mann-Whitney U test showed that TIBA concentrations above 50 μm differed significantly from the control: (U = 0.0, Z = 1.96, P = 0.05). The dose response curve for IAA efflux inhibition of gravity-induced polarization showed similar concentration dependence (data not shown). In contrast, analysis of BA treatments by a one-way ANOVA showed no significant effect of BA on photopolarization (F7,16 = 0.48, P = 0.83).
A more complete examination of the distribution of embryos in all four quadrants in the presence of increasing concentrations of NPA is shown in Figure 2B. The percentage of embryos photopolarized decreased with increasing NPA concentrations. Additionally, as the NPA concentration increased, the embryos that did not photopolarize increased, with a random distribution in the other three quadrants. Similar results were seen with TIBA treatment (data not shown). Solvent controls containing ethanol in ASW or 0.5% (v/v) DMSO had no effect. BA or NPA treatments did not affect the germination at any of these concentrations, although at the highest TIBA concentration (200 μm), germination after 48 h was reduced to 25% of control values.
The effects of auxin transport inhibitors on germination and rhizoid elongation were carefully examined to test the possibility that their effects were simply on delaying germination and polarity initiation. At 48 h, germination was between 80% and 100% of the control value for NPA and BA at the inhibitor doses used in Figures 1 and 2. If the number of germinating embryos in the presence and absence of NPA are plotted as a function of time after fertilization (AF), the slopes are parallel and reach a maximum at equal times (data not shown). This result suggests that NPA does not alter the kinetics of rhizoid initiation. Additionally, plotting rhizoid elongation versus NPA concentrations showed no changes (data not shown). Therefore, it seems unlikely that auxin transport inhibitors only act by delaying rhizoid formation and/or elongation.
Photopolarization was more strongly inhibited by TIBA than NPA, as in other comparisons of these inhibitors (Rubery and Jacobs, 1990). Although neither BA nor NPA affected germination at any of the tested concentrations when germination was scored at 18 h AF or later, TIBA treatments led to a dose-dependent decrease in percent germination at concentrations above 10 μm (data not shown), as found in previous studies (Novotny and Forman, 1974). More samples were then analyzed at the higher concentrations to maintain similar numbers of embryos in all samples in which this inhibitor was used. The similarities between the effects of NPA and TIBA on photopolarization suggest that TIBA's effect on germination is not relevant to its inhibition of photopolarization. Although TIBA and NPA concentrations needed to block photopolarization are relatively high, they are similar to those required to block stem phototropism in land plants (Li et al., 1991; Haga and Iino, 1998).
Phototropic Reorientation of Rhizoids Is Blocked by Auxin Efflux Inhibitors
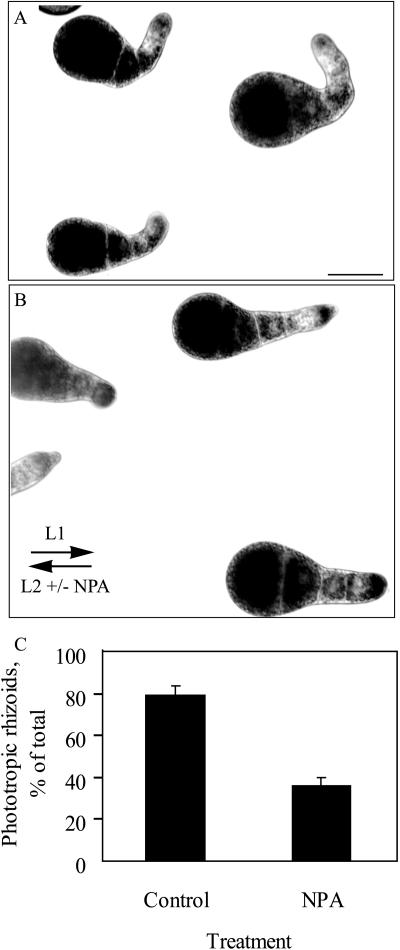
To determine if rhizoids exhibited phototropic curvature, embryos were reoriented relative to unilateral light and rhizoid orientation was examined. Embryos were photopolarized by unilateral light beginning 1 h AF and, at 18 h AF, reoriented by 180°. The resulting changes in growth orientation were documented at 48 h AF (Fig. 3A). Most rhizoids turned 180° and grew away from the direction of the new light vector. In contrast, embryos reoriented in the presence of NPA had significantly reduced phototropic curvature as judged by Student's t test (P < 0.001) (Fig. 3, B and C).
Figure 3.
F. distichus rhizoids are negatively phototropic, and phototropism is blocked by NPA. A, Embryos were polarized by 18 h of unilateral light and then reoriented 180°. The initial (L1) and final (L2) light vectors are indicated. B, Addition of NPA at the time of reorientation in response to light prevents the rhizoid phototropism. C, The effect of NPA treatment on phototropism of multiple embryos is quantified for more than 100 embryos. The size bar = 50 microns.
Excess Auxin Can Block Environmental Polarization
Initial studies examined embryo orientation in four directions. This method is more complex and the results more subjective; thus, further experiments examined only two directions, as is standard (Haga and Iino, 1998; Alessa and Kropf, 1999). With two sectors, the polarization range is between 50% (random) to 100% for complete polarization. To prevent confusion with earlier plots in which random is 25% polarization, these data are presented as normalized percent polarization, according to the procedure used by Alessa and Kropf (1999). A random value (50% polarization) can be converted to a value of 0% polarization using the equation: normalized percentage of polarization = (polarized embryos − nonpolarized embryos)/(polarized embryos + nonpolarized embryos) × 100%. For comparison, Table I reports NPA's effect on gravity and light polarization using this method. Again, NPA significantly decreased polarization. The effect of incubating embryos in 50 μm exogenous IAA was also examined (Table I). Excess IAA reduced the degree of polarization by both light and gravity with a magnitude of effect similar to NPA.
Table I.
Effect of IAA and NPA on polarization of F. distichus embryos
| Treatment
|
Normalized Percentage Polarizationa
|
|
|---|---|---|
| Gravity | Light | |
| Control | 44.5 ± 3.3 | 76.4 ± 2.4 |
| NPA | 8.8 ± 2.7*** | 4.2 ± 3.0*** |
| Control | 39.4 ± 4.4 | 74.3 ± 2.5 |
| IAA | 7.5 ± 2.6*** | 7.9 ± 5.2*** |
Embryo germination in each of two sectors was measured, and the normalized percentage of polarization was calculated and is equal to (polarized embryos − nonpolarized embryos)/(polarized embryos + nonpolarized embryos) × 100%. The reported values are the average and se of three separate experiments with n > 100 embryos per experiment.
Polarization in treatments was compared to controls by Student's t test and these values were statistically significant with P values < 0.001.
NPA and IAA Reduce F. distichus Polarization before the First Cell Division
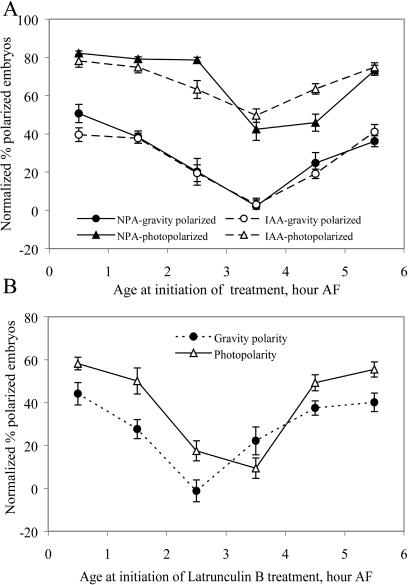
To define the window during which NPA and IAA exert their effect, embryos were placed in ASW, then moved to ASW containing NPA or IAA at the indicated times AF, and then returned to ASW after a 1-h incubation. For gravity polarization, the embryos remained in the dark for the entire experiment and, for photopolarization, received unilateral light only during the first 7 h AF, including the 1-h treatment. The results indicate that NPA and IAA affect photopolarization and gravitropism between 2.5 and 4.5 h AF, with a maximal effect at 3.5 h AF as shown in Figure 4A. The timing of IAA and NPA effects were similar to the previously reported time of 3 to 5 h AF required for embryo selection of a light-dependent axis (Kropf et al., 1989) and well before the 10 to 12 h AF required to commit to developmental polarity and the 24 h needed for rhizoid germination (Kropf et al., 1989). Additionally, the ability of NPA or IAA treatment to alter embryo polarization as a result of a 1-h treatment during the initial hours of exposure to unilateral light suggested that the action of NPA is not simply to delay germination and/or polarization until after the embryos are no longer responsive to the signal.
Figure 4.
Maximal response to NPA, IAA, and LatB occurs before 5 h AF. A, The number of embryos that are photopolarized or gravity polarized in response to uniform light or gravity were determined after 1-h treatments with NPA at the indicated ages AF. B, The number of embryos that are photopolarized or gravity polarized in response to uniform light or gravity were determined after 1-h treatments with LatB at the indicated ages AF. For both graphs, the average and se of three separate experiments are reported, with n > 100 embryos per experiment.
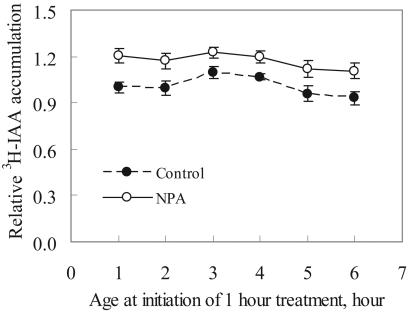
To determine if the window of developmental sensitivity to IAA and NPA is due to changes in auxin transport, the accumulation of tritiated IAA into embryos and its regulation by NPA were examined during the first 6 h AF. Embryos were treated for 1 h with NPA at the indicated ages, and then tritiated IAA added for another 1-h incubation, after which the accumulated IAA was measured (Fig. 5). IAA accumulation was similar at all these developmental ages, with a consistent increase by NPA over the range of 1 to 6 h. The significance of the NPA effect was analyzed by one-way ANOVA after assumptions of normality and homogeneity of variances were met. The one-way ANOVA revealed no significant differences in the IAA accumulation over time in the presence of NPA (F5,138 = 1.04, P = 0.4). Because variances were not homogeneous in the absence of NPA, a one-way ANOVA could not be performed on these data. A nonparametric equivalent of one-way ANOVA, the Kruskal-Wallis ANOVA, was performed and found a marginally significant variation: chi-square = 11.26, df = 5, P = 0.047. A more specific comparison of each time point to the starting time point by Mann-Whitney U test indicated no significant differences from the starting value (P ≥ 0.085).
Figure 5.
Effect of NPA on IAA accumulation during early development of F. distichus embryos. Embryos were treated with 50 μm NPA or 0.5% DMSO (v/v) for 1 h at the indicated age, and then 50 nm [3H]IAA was added. After incubation 1 h, the accumulated [3H]IAA in F. distichus embryos was determined. The values were normalized relative to the control value for the 1-h embryos. The reported values are the average and se of four separate experiments with n > 23.
These results contrast with NPA's and IAA's large differences in developmental effects during the same time. This suggests that the narrow window in which IAA and NPA reduce environmental polarization is not due to differences in auxin transport but is likely due to the specific developmental window in which embryo development is sensitive to auxin. We cannot rule out that this subtle difference in IAA accumulation reflects a larger change in transport of endogenous IAA.
NPA Modulates Formation and Localization of Actin Patches during Photopolarization
Actin patches form at the future site of rhizoid formation and have been observed as early as 3 h AF in S. compressa embryos (Alessa and Kropf, 1999) and 8 h AF in F. distichus embryos (Kropf et al., 1989). The first experimental question was whether actin patch formation could be observed in the first 5 h following fertilization in F. distichus embryos, when auxins and auxin efflux inhibitors act. Actin patch formation and polarization were observed using the F-actin-specific probe rhodamine phalloidin (RhPh) and immunocytochemical approaches using an actin monoclonal antibody in living or fixed embryos, respectively.
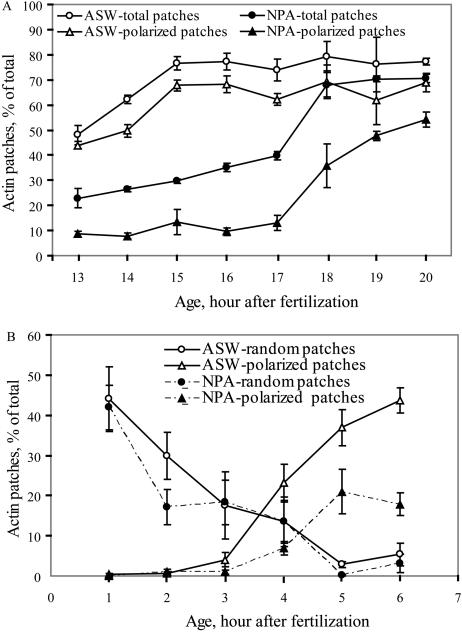
A few actin patches were first observed at 3 to 5 h AF using RhPh in living, saponin-permeabilized embryos. Patches detected at 5 or 6 and 15 h AF were visualized by both epifluorescence and laser scanning confocal microscopy (LSCM; Fig. 6, A–D). By LSCM, the patches are quite distinct and truly appear as patches, as seen in all embryos examined by this approach. In contrast, by epifluorescence microscopy, the patches are less distinct and are localized to a region that covers less than about one-fourth of the embryo but had sufficient polarity to determine their orientation relative to the light vector. These patches were consistently detected in hundreds of embryos by epifluorescence. Using RhPh and either form of microscopy, patches were not detected at high frequency until 8 h AF, as previously reported (Kropf et al., 1989). At 8 h, most patches were formed on the shaded side of the embryo in control samples. Figure 7A shows the frequency of patch formation and polarization as detected by epifluorescence microscopy. By 20 h AF, the number of actin patches formed in NPA-treated embryos was similar to the ASW controls, but the number of polarized patches only reached about 50%, which is equivalent to the number of polarized embryos in this experiment. Additionally, in this experiment, we quantified the timing of germination. Although NPA delayed rhizoid germination by several hours, both germination and actin patch formation reached the same levels as untreated embryos by 20 h AF. These results indicate that NPA treatment unlinks patch position from the direction of environmental polarization, presumably resulting in the random orientation of rhizoid outgrowth.
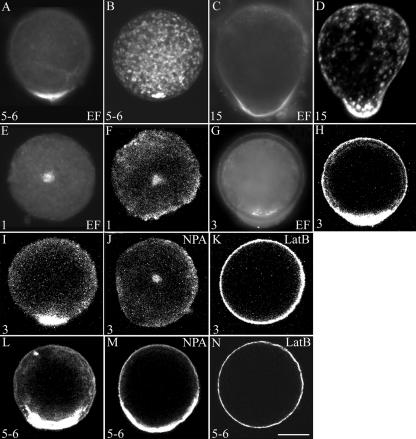
Figure 6.
Actin patches can be visualized in young embryos. Representative actin patches were visualized by RhPh (A–D) or actin antibodies (E–N). All pictures are ASW controls except where noted. NPA or LatB treatments are noted in the upper right corner of the images. Ages of embryos are indicated in the bottom left of each panel. All images are LSCM except where indicated as epifluorescence images (EF). The size bar = 25 microns.
Figure 7.
NPA delays formation and photolocalization of actin patches. A, Embryos were stained with RhPh at the indicated age, and the percentage of embryos with random and photopolarized actin patches were quantified. B, Actin patch formation and localization was also examined by antibody staining, and patches can be visualized at earlier time points, prior to photolocalization. For both graphs, the experiment was repeated three times, with 50 embryos per treatment and the average and se of the averaged experiments reported.
When actin antibodies were used to visualize actin in fixed cells, patches were consistently found much earlier, commonly between 1 and 2 h AF. Figure 6, E to J, L, and M, shows actin patches in both ASW- and NPA-treated samples, as visualized by epifluorescence and LSCM. These patches are not initially localized on the shaded side but rather in the center of the embryo in the controls. LSCM verified that the actin patch position is close to but below the surface (Fig. 6, E and F). Patches and actin rings around the site of rhizoid initiation were visualized in S. compressa (Alessa and Kropf, 1999), but no actin rings were found in any samples in F. distichus. As visualized by LSCM, NPA-treated embryo actin patches were similarly sized and shaped as the controls, but they differed in position. The effect of NPA on patch formation and position was examined at early time points using this method, to obtain higher temporal resolution during early development. The patches in ASW-treated samples localized to the shaded side of the embryo (Fig. 6, I and L), while NPA reduced the number of photolocalized patches, as shown in Figure 7B. At these early time points, the formation of patches is reduced by NPA. Consistent with the RhPh data, many of the patches that form do not photolocalize, contrasting with the ASW controls in which all of the patches at 7 h had photolocalized. Patches were found either in the center or on the shaded side of the embryo, consistent with the suggestion for S. compressa that patches do not move but reform during photopolarization (Alessa and Kropf, 1999).
The actin patch localization data obtained by both staining approaches indicated that NPA treatments reduced actin patch localization, yet the possibility that this is an indirect effect due to delayed patch formation should be considered. The actin antibodies showed with high temporal resolution that NPA delayed formation of polarized patches and also delayed formation of patches at early time points. Comparing the number of actin patches formed during RhPh staining to the number of germinating embryos indicated that actin patches do form in all the germinating embryos, but that the amount of detectable patches is maximal about 3 h later in the NPA-treated samples (at 18 rather than 15 h AF). Since embryos are treated with unilateral light during the first 7 h AF, it is possible that this delay in patch formation allows the directional light signal to be lost. One point that argues against this hypothesis is that gravity polarization (which is continual during 48 h AF) is also randomized by NPA treatment. This suggests that delays in actin patch formation would not be adequate to randomize polarity under these conditions. Finally, the ability of IAA and NPA treatments to act during a 1-h window in the first 5 h of unilateral light treatment also suggests that effect is not simply a delay in patch formation and/or germination until times later than those sensitive to unilateral light.
An Intact Actin Cytoskeleton Is Needed for Embryo Polarization and IAA Transport
To determine if formation of the polar axis induced by gravity and light also requires an intact actin cytoskeleton, F. distichus zygotes were exposed to LatB for 6 h, beginning 1 h AF. For gravity polarization, the embryos were in the dark for the entire experiment. For photopolarization, they were exposed to unilateral light during the first 7 h AF, and then the drug was removed, and the embryos were placed in the dark. LSCM verified that LatB altered the distribution and formation of actin patches, presumably by depolymerizing fine actin filaments in embryos treated for either 3 or 6 h (Fig. 6, K and N). No actin patches were detected in numerous embryos after LatB treatment, and actin networks are completely gone by 6 h. The percentage of embryos polarized after 48 h in the presence and absence of LatB was quantified, as reported in Table II. The number of polarized embryos between LatB-treated and control samples differed significantly as judged by Student's t test. For the LatB treatments, the normalized percent polarization is slightly negative, suggesting that more embryos polarized against the light and gravity vectors than with the vectors after LatB treatment. When the actual numbers of embryos that polarize in each direction are compared, they are not significantly different as judged by Student's t test, indicating that there is no meaningful polarization after LatB treatment. The decreased polarity of embryos in 30 nm LatB suggests that intact F-actin regulates not only photopolarization, as reported previously (Hable and Kropf, 1998), but also gravity polarization of F. distichus embryos.
Table II.
Effect of LatB on polarization of F. distichus embryos
| Control | LatB | |
|---|---|---|
| % Gravity polarized embryosa | 40.02 ± 3.09 | −4.75 ± 2.25*** |
| % Photopolarized embryosa | 73.69 ± 4.01 | −3.71 ± 3.64*** |
Percentage (%) of polarized embryo = (polarized embryos − nonpolarized embryos)/(polarized embryos + nonpolarized embryos) × 100%. The reported values are the average and se of three separate experiments with n > 100 embryos per experiment.
Represents P value < 0.001 as judged by Student's t test.
To examine the timing of sensitivity to LatB relative to IAA and IAA efflux inhibitors, zygotes were treated with LatB for 1-h windows during the first 6.5 h AF, and the effect on gravity and photopolarization of F. distichus embryos was quantified after 48 h. The embryos were in the dark for gravity polarization and, for photopolarization, exposed to light for the first 7 h AF, including the 1 h during LatB treatment, as shown in Figure 4B. LatB effects are observed between 1.5 and 3.5 h AF. The effects of LatB on gravity polarization are consistently found beginning at 2.5 h AF in three separate experiments (average of these experiments shown here), whereas the timing of the LatB effect on photopolarization is often slightly later in several, but not all, experiments at 3.5 h AF. The significance of this difference is not yet clear, but in both cases the timing of the LatB effect on polarization coincides with the timing of actin patch formation, and slightly precedes, but overlaps the timing of maximal NPA and IAA effects, and is also well before the first cell division of F. distichus zygotes.
The effect of LatB on [3H]IAA accumulation in F. distichus embryos was determined following treatment of 1-h-old embryos with 0 nm (0.05% DMSO control), 10 nm, 30 nm, and 100 nm LatB (Table III). [3H]IAA accumulation increased with the concentration of LatB, which was most effective at 100 nm (1.6-fold increase). The significance of the LatB effect was analyzed by one-way ANOVA after assumptions of normality and homogeneity of variances were met. The one-way ANOVA revealed a significant effect of treatment: F3,20 = 6.33, P = 0.003. Duncan's multiple-range post hoc test showed that LatB at 30 and 100 nm was significantly different from the control (P ≤ 0.005). The elevated IAA accumulation is consistent with a reduction of IAA efflux, and the magnitude of the LatB effect parallels the NPA effect on IAA accumulation in Figure 5 and as previously reported (Basu et al., 2002).
Table III.
Effect of LatB on 3[H]IAA accumulation in F. distichus zygotes
The reported values are the average and se of a representative experiment with six replicates.
These values were statistically different from the controls as judged by Duncan's multiple-range post hoc test with P ≤ 0.005.
DISCUSSION
Land plants and algae respond to environmental gradients with changes in developmental program or growth orientation. Auxins have been strongly implicated in controlling growth orientation in response to light and gravity (Muday, 2001; Boonsirichai et al., 2002), but their role in environmentally responsive morphological changes is less clear. Embryos of brown algae are a particularly interesting system in which to examine the potential role of auxin in formation of embryonic polarity. Embryos of brown algae are initially completely symmetrical, but after fertilization, they rapidly develop polarity in response to light and other environmental gradients (Kropf et al., 1999).
The gravity vector orients the polarity of F. distichus embryos. Growth on a clinostat randomizes their orientation, suggesting that gravity polarization is both statistically and physiologically relevant. However, low-intensity unilateral light gradients will completely overwhelm the gravity polarization. Embryos released in intertidal pools during low tide must quickly adhere to the substrate before they are washed away. Environmental polarization orients the site of rhizoid outgrowth and embryo attachment. The rhizoid develops into the holdfast that attaches the algae to the substrate. Since embryos are released and fertilized predominantly during midday (Pearson and Brawley, 1996), and commitment to a polar axis requires 12 h, gravity may act as a reinforcing cue during the dark hours, particularly during months with short days.
The requirement for changes in polar auxin transport to allow differential growth responses to light or gravity in land plants has been demonstrated by mutant selection and use of auxin transport inhibitors (Muday, 2001; Boonsirichai et al., 2002). As selection of individual brown algae with mutations is not possible, the most feasible approach to explore whether auxin transport is important in environmental control of developmental polarity in brown algal embryos is to examine the effect of IAA efflux inhibitors and exogenous auxin. Although the presence of IAA in algae so far appears to be universal, the function and transport of IAA have been tested in very few species (Cooke et al., 2002), and sometimes with contradictory findings (Dibb-Fuller and Morris, 1992; Klämbt et al., 1992). In F. distichus, previous evidence has demonstrated that IAA is present and that IAA accumulation is modulated by IAA efflux inhibitors (Basu et al., 2002). Finally, IAA and IAA efflux inhibitor treatment alter F. distichus embryo developmental patterns (Basu et al., 2002). These experiments are consistent with IAA transport mechanisms that parallel those in land plants and a role of IAA in F. distichus embryo development. A broader evolutionary context for these experiments awaits further studies in additional algal species (Cooke et al., 2002).
IAA efflux inhibitors and exogenous auxin randomize embryo orientation in response to light and gravity. Increasing concentrations of the IAA efflux inhibitors, NPA and TIBA, have a dose-dependent effect, while the weak acid control, BA, has no effect at similar concentrations. Treating embryos with IAA also randomizes their orientation relative to environmental factors. These results suggest that auxin and its appropriate distribution are important in environmental polarization. Additionally, once the rhizoid is formed, its growth orientation remains sensitive to light and can change growth orientation in response to a new light vector. IAA efflux inhibitors also inhibit this growth reorientation. Although these changes in rhizoid growth in response to changing light orientation do superficially resemble phototropic curvature, there are differences in mechanism. Phototropism requires differences in growth rates in two distinct sets of cells across a complex organ, whereas this light-regulated growth process is due to asymmetric growth at the tip of a single cell. Therefore, the mechanisms for this growth orientation change more closely resemble the mechanisms for initial formation of cellular polarity in response to light gradients, during which a single cell grows nonuniformly, using asymmetric targeting of vesicles containing membrane and wall components needed for asymmetric growth (Hable and Kropf, 1998). Growth orientation changes and formation of initial polarity are sensitive to IAA and IAA efflux inhibitors, consistent with a similar role for auxin in both processes.
If changes in auxin concentration or distribution mediate environmental polarization, they should act at a specific developmental time. To test this hypothesis, embryos were exposed to IAA and NPA for 1 h at various times AF, and photo- and gravity-polarization were measured. Both compounds had maximal effects at 3.5 h AF, well before brown algal embryos exhibit asymmetric growth and consistent with their earliest developmental changes (Kropf et al., 1999). This narrow window is likely due to a specific developmental sensitivity to these compounds, since IAA accumulation and its regulation by NPA do not change during the first 8 h of development.
NPA and IAA have similar inhibitory effects on polarization, which can be explained by two hypotheses. First, since NPA blocks IAA efflux either by direct inhibition of an auxin transport protein (Rubery, 1990) or altering cycling of PIN1-type auxin transporters (Geldner et al., 2001; Muday et al., 2003), both treatments should elevate internal IAA concentrations, which could prevent formation or perception of light- or gravity-induced gradients of IAA. A second and perhaps better hypothesis is that localized IAA efflux and the resulting elevation of IAA concentration at one position on the embryo may be part of the polarization process. Inhibiting this local IAA efflux or overwhelming the local IAA concentration by IAA efflux inhibitors or exogenous IAA, respectively, would reduce environmental polarization. Conversely, embryos cultured in a gradient of IAA concentrations should orient toward the higher concentrations. Jaffe (1968) suggested that local IAA efflux mediated the positive group effect. In support of this hypothesis, embryo growth in the presence of uniform IAA or NPA reduced the group effect, while a gradient of IAA caused rhizoids to form toward the IAA source (H. Sun and G.K. Muday, unpublished data), consistent with a previous suggestion that IAA might polarize embryos (Olson and du Buy, 1937; Muday et al., 2003). These results suggest that external gradients of IAA are sufficient to polarize F. distichus embryos.
The mechanisms by which brown algal embryos develop polarity in response to light have been well summarized (Kropf et al., 1999; Belanger and Quatrano, 2000). The initial role of calcium currents and/or gradients was demonstrated by several investigators (Pu and Robinson, 1998; Robinson et al., 1999). Evidence also links the actin cytoskeleton to F. distichus polarity development (Fowler, 2000). In embryos of brown algae, a patch of F-actin localized at the presumptive rhizoid pole within 3 h AF in S. compressa embryos and 8 h AF in F. distichus embryos (Kropf et al., 1989; Alessa and Kropf, 1999). Treatment of F. distichus or S. compressa zygotes during the period of axis alignment with cytochalasin B/D or LatB prevented photopolarization by altering the organization or function of actin networks (Hable and Kropf, 1998). The role of the actin cytoskeleton in the initiation of F. distichus polarity has been questioned (Love et al., 1997; Robinson et al., 1999), but the results reported here support a role for actin, including treatments with the drug LatB. Changes in actin organization during development were verified by LSCM, and the LatB temporal maximum was found to coincide with or slightly precede IAA and NPA temporal maximum. The formation of an actin patch may initiate developmental polarity by targeting vesicles carrying transport proteins and building blocks for membranes to the point of future rhizoid outgrowth (Belanger and Quatrano, 2000; Fowler, 2000). The possibility that auxin efflux proteins are among these targeted proteins merits consideration. Increasing LatB concentrations increased IAA accumulation in F. distichus embryos, consistent with an inhibition of IAA efflux when actin is fragmented, and similar to reports that IAA transport is reduced by drugs that fragment the actin cytoskeleton (Butler et al., 1998). This effect might be mediated by altering the cycling of IAA efflux carriers to the plasma membrane, as suggested in Arabidopsis (Geldner et al., 2001).
IAA and the IAA efflux inhibitors act early in development, at about the same time that actin changes have been observed in S. compressa (Alessa and Kropf, 1999). As actin patches were previously not detectable until 8 h AF in F. distichus, we examined actin localization in response to unilateral light in F. distichus embryos using recently developed methods (Alessa and Kropf, 1999). Actin patches were detected in living embryos with RhPh and by immunolocalization with an actin antibody in fixed embryos. The actin-antibody approach proved more sensitive for detecting randomly distributed actin patches in the first hours AF and photolocalized patches beginning 3 h AF. LSCM failed to detect an actin ring with a hollow center as found in S. compressa embryos under similar conditions (Alessa and Kropf, 1999; Pu et al., 2000).
An important question was whether NPA randomized embryo alteration by altering the actin cytoskeleton's organization. Embryos were cultured in the presence and absence of NPA under unilateral light, and the number of embryos with actin patches and polarized actin patches were quantified using both RhPh and actin antibody localization. NPA reduced the polarization of actin patches using both methods. Of greatest significance were the effects observed with actin antibodies. There were significant reductions in the number of polarized patches in NPA-treated embryos at times between 3 and 6 h AF (Fig. 7B). These results suggest that NPA reduced photopolarization by randomizing the position of patch formation, thereby unlinking environmental stimulation from the positioning of the patch and presumably from controlling the direction of rhizoid formation.
Experimental evidence in land plants suggests that the actin cytoskeleton may also control the localization of the IAA efflux carrier protein complex. This IAA efflux complex likely consists of several proteins, including an integral membrane protein, which may be encoded by a PIN gene (Palme and Gälweiler, 1999) and/or an MDR-like gene (Noh et al., 2001), and a peripheral membrane regulatory protein, or NPA-binding protein (Muday, 2000). An NPA-binding protein has been reported to interact with the actin cytoskeleton (Butler et al., 1998; Hu et al., 2000), and this interaction may facilitate the asymmetric localization of the efflux carrier. The role of the actin interaction of this protein complex may be in modulation of the cycling of IAA efflux transporters from internal compartments to the membrane surface (Geldner et al., 2001, 2003). Treatment with cytochalasin prevents the cycling process (Geldner et al., 2001), consistent with this function. Finally, inhibition of vesicle movements to and from the endosome by brefeldin A (BFA) prevents PIN1 membrane localization, consistent with a carefully modulated vesicle-mediated membrane delivery and recycling process (Geldner et al., 2001).
NPA and TIBA have been shown to prevent polar IAA transport from cell to cell by inhibiting IAA efflux from individual plant cells (Rubery, 1990; Petrasek et al., 2003). Whether these compounds reduce IAA efflux by inhibiting IAA movement through a carrier protein or by a more complex action has not been demonstrated. TIBA in concert with BFA alters the localization of PIN1, which normally is found with a polar distribution on the basal membrane (Geldner et al., 2001). When roots are treated with TIBA alone, PIN1 localization is not affected, even though similar treatments completely prevent IAA transport (Geldner et al., 2001; Petrasek et al., 2003). BFA prevented the asymmetric membrane localization of PIN1, but effects are reversed in washout experiments, except when TIBA is present. Although TIBA had no effect on PIN1 localization when polarity had been previously established, it did appear to prevent polarity initiation when BFA randomized PIN1 protein distribution (Geldner et al., 2001). Higher doses of NPA have also been reported to have an effect similar to TIBA's in blocking restoration of PIN1 asymmetries (Geldner et al., 2001; Gil et al., 2001). F. distichus embryos are symmetrical at fertilization, and polarity is established during the first hours thereafter, perhaps like roots in which cellular polarity is randomized by BFA and then reformed upon its washout. NPA and TIBA could randomize embryo polarity by preventing proteins on the membrane from properly targeting the site of rhizoid outgrowth. Although the cellular mechanisms by which NPA reduces IAA transport are not yet clear, NPA does alter the localization of actin complexes formed in F. distichus embryos in response to environmental gradients.
These results implicate auxin and auxin transport in the polarization of embryos by environmental stimuli. IAA's and NPA's ability to act in a discrete time period that overlaps the photolocalization of actin patches and the timing of LatB action suggests that these compounds have specific developmental effects that are linked to the actin cytoskeleton. LatB's ability to alter auxin accumulation and NPA's alteration of actin patch polarization further suggest an interdependence of the actin cytoskeleton and auxin transport. These results suggest that the formation of cellular polarity and auxin transport polarity may be interwoven in embryo development.
MATERIAL AND METHODS
Chemicals
[3H]IAA was purchased from Amersham International (Arlington Heights, IL; 27.0 Ci/mmole). N-1-naphthylphthalamic acid (NPA) was purchased from Chemical Services (West Chester, PA). 2,3,5-triiodobenzoic acid (TIBA), indole-3-acetic acid (IAA), and all other chemicals were purchased from Sigma (St. Louis).
Fucus distichus Zygote Isolation
Reproductive fronds (receptacles) of sporophytes of F. distichus distichus (L.) were collected at South Beach, OR (South Jetty), transported on ice to Winston-Salem, NC, and stored in the dark at 4°C for 2 to 3 weeks. The gametes were released into ASW (450 mm NaCl, 10 mm KCl, 9 mm CaCl2, 30 mm MgCl2, 16 mm MgSO4, and 10 mm TES buffer, pH 7.5), and fertilization and development of zygotes were performed at 14 ± 1°C in constant illumination with cool-white fluorescent lights at 60 μmol m−2 s−1, as described (Quatrano, 1980). The germination rate for all samples independent of treatments was greater than 85%, unless indicated otherwise. Samples of zygotes were prepared at densities low enough to prevent surrounding embryos from altering embryo orientation.
Measurement of Gravity Polarization
Immediately AF, F. distichus zygotes in ASW at a density of 2,000 zygotes/mL, which is dilute enough to prevent the group effect, were added on the poly-l-lysine-coated slides, allowed to adhere for 1 min, and immediately placed in a vertical position in ASW. The slides were then placed at 14 ± 1°C in the dark for 48 h. The percent embryos polarized toward gravity was determined under a dissecting microscope. Their orientations were divided into four directions relative to gravity, with one centered on the gravity vector (down), the other opposite to the gravity vector (up), while the other two grew perpendicular to the vector (right and left). The percent gravity polarization was determined from the quadrant that included embryos that grew rhizoids with the gravity vector (down) and are reported as the percent of embryos that germinated in all four quadrants. Random orientation would yield 25% in any direction. At least 200 embryos per treatment were scored, and each experiment replicated at least three times. The average and ses of three separate experiments is reported. IAA efflux inhibitor treatments led to an increase in embryos with multiple or branched rhizoids when embryos were grown in the dark (Basu et al., 2002). Only embryos with a single rhizoid were quantified for this analysis. Chi-square tests determined if the percent of embryos germinating with the gravity vector was different from 25%, which is a random distribution.
For clinostat experiments, petri dishes were coated with poly-l-lysine, and aliquots of F. distichus zygotes (0.5 h AF) in ASW were allowed to stick to the petri dishes for 1 min. These petri dishes were filled with ASW, sealed with parafilm, immediately attached vertically to a clinostat rotating at 1 rpm, and placed in 14 ± 1°C in the dark for 48 h. Another set of petri dishes with zygotes was also placed vertically in a stationary mode at 14 ± 1°C in the dark for 48 h. Percent gravity polarization was determined under a dissecting scope, as described. Chi-square tests were performed as above.
Effects of Auxin Efflux Inhibitors, IAA, or LatB on Gravity Polarization, Photopolarization, and Phototropism
The effect of IAA efflux inhibitors, NPA, TIBA; the auxin, IAA; the weak acid control, BA; and the actin filament depolymerizing drug, LatB, on gravitropic polarity was examined. Embryos were placed in ASW supplemented with 50 μm of BA, NPA, TIBA, or IAA (or BA, NPA, and TIBA between 5 and 500 μm) or 30 nm of LatB in ASW or with ASW containing DMSO or ethanol for controls at the same concentrations as in the treatments (0.5%, v/v) and attached to poly-l-lysine-coated slides. The extent of gravity polarization was determined in two ways. For Figure 1B, the number of embryos in each of four directions was measured and reported directly. For Tables II and III, the number of embryos in two directions was determined, and the normalized percentage of polarized embryos calculated by the following equation. The normalized percentage of polarized embryos = (number of gravity polarized embryos − number of embryos oriented against gravity)/(number of gravity polarized embryos + number of embryos oriented against gravity) × 100%.
The effect of auxin efflux inhibitors, IAA, and LatB on photopolarization was also determined as described for gravity polarization. Zygotes were resuspended in ASW containing NPA, TIBA or BA or 50 μm IAA or 30 nm LatB. ASW containing 0.5% (v/v) DMSO or ethanol was used for controls. The slide was exposed to unilateral white fluorescent light at 60 μmol m−2 s−1 at 14 ± 1°C for 7 h. Following light exposure, the petri dish was placed in the dark at 14 ± 1°C. At the end of 48 h, the embryos' orientation relative to light was determined under a dissecting microscope. The extent of photopolarized embryos with the rhizoid germinated on the shaded side was determined in two ways, as described. For Figure 2, the number of embryos in each of four quadrants was measured and reported directly. Random orientation would yield 25% in each quadrant. For Table II, the number of embryos in two sectors was determined, and the normalized percentage of polarized embryos with random orientation yielding a value of 0 normalized percent photopolarization.
To examine the effect of auxin efflux inhibitors on phototropism, embryos were placed on horizontal coverslips and exposed to unilateral light from 1 to 18 h AF. The slides were then rotated 180° and percentage of cells with phototropic rhizoids determined at 48 h AF.
Timing of Effect of NPA, IAA, or LatB on Embryo Polarization
To determine when NPA, IAA, and LatB exert their maximal effect on polarization, F. distichus embryos were grown in a microfuge tube in untreated ASW for 0.5 h to 5.5 h AF. At the indicated times, embryos were placed on poly-l-lysine-coated slides and in ASW containing 50 μm of either NPA or IAA or 30 nm of LatB. The treatments were for 1 h, and for gravity polarization experiments, embryos were maintained in the dark for the duration. For photopolarization experiments, embryos were exposed to unilateral light for 7 h, including the 1-h treatment, and then slides were transferred to untreated ASW and to the dark for the remainder of 48 h at 14 ± 1°C. The number of gravity or photopolarized embryos was then quantified.
Effect of LatB and Developmental Age on [3H]IAA Accumulation in F. distichus Embryos
Two methods were used to measure [3H]IAA accumulation in F. distichus embryos. To examine the effect of LatB, a previously published procedure was used, removing excess radiolabeled IAA by filtration (Basu et al., 2002). To examine developmental changes in IAA accumulation, a new assay allowed easier analysis of samples at multiple times. The embryos were incubated in ASW, pH 6.0 for 1 h AF. Then the embryos were incubated, at 1, 2, 3, 4, 5, or 6 h AF in the absence and presence of NPA at concentrations of 50 μm in ASW with MES substituted for TES, pH 5.5 at 14 ± 1°C for 1 h, followed by incubation in 25 nm [3H]IAA, also in the presence and absence of NPA, in the dark for 1 h. The [3H]IAA-containing embryos were then recovered by centrifugation (Eppendorf Centrifuge 5415 C, 1,000 rpm, 2 min) to remove the excess [3H]IAA, and then washed twice with 1 mL cold ASW-MES. The tip of 1-mL microfuge tubes containing the pelleted embryos were cut, placed into scintillation tubes, and soaked for 30 min in 100 μL of methanol; 2.5 mL of scintillation fluid was added, and samples counted in a Beckman LS 6500 scintillation counter for 2 min. Background was determined by addition of 25 nm [3H]IAA to zygotes immediately before centrifugation and subtracted from all the values.
Visualization of Actin Patches in F. distichus Embryos in the Presence and Absence of NPA
The formation and localization of actin patches used two published procedures (Alessa and Kropf, 1999). For in vivo RhPh staining, poly-l-lysine-coated coverslips with attached embryos were placed into 0.5 mg mL−1 saponin in ASW for 15 to 20 min and then rinsed in ASW for 10 min followed by incubation in 25 μL of 1.2 × 10−6 M RhPh for 10 to 15 min. After labeling, the coverslips were rinsed again in ASW for 10 min and placed on slides to visualize immediately. Patches were defined as a bright section of staining in the center of very young (5–6 h) and along roughly one-third of the edge of older embryos. All of the above procedures were performed at 14 ± 1°C and stained in the dark.
For antibody staining, coverslips were transferred to fixative (3% paraformaldehyde (w/v) made fresh in actin stabilization buffer: 50 mm sodium PIPES, pH 6.9; 1.0 mm EGTA; 50 mm MgSO4; 0.05% Triton X-100 (v/v)) for 45 to 60 min. Coverslips were rinsed 3 times. Samples were blocked in 2.5% nonfat milk (w/v) dissolved in mPBS (mPBS: 137 mm NaCl, 2.7 mm KCL, 1.7 mm KH2PO4, 8 mm Na2HPO4, 5% glycerol (v/v), 0.1% sodium azide (w/v), 0.1% bovine serum albumin (w/v)) for 6 to 18 h. Actin was immunolocalized with a monoclonal antibody raised against chicken gizzard actin (Clone C4; ICN Biomedical, Aurora, OH) at a dilution of 1:100 in mPBS for 6 to 18 h at room temperature. Zygotes were rinsed in mPBS and incubated in Alex Fluor 564 conjugated goat anti-mouse antibody (Molecular Probes, Eugene, OR) at a dilution of 1:200 also for 6 to 18 h. Cells were rinsed and mounted in mPBS prior to observation.
Microscopy
Living, RhPh-labeled zygotes and actin immunofluorescence were observed with a Zeiss Axioplan microscope equipped with epifluorescence optics with a 546 ± 12 nm BP excitation filter, a 580-nm dicroic mirror, and a 590-nm long pass emission filter (Chroma Technology, Brattleboro, VT). Images were captured with a Hamamatsu Orca CCD camera. For LSCM, a Zeiss LSM510 was used with a 543-nm laser line with a 565- to 615-nm narrow bandpass emission filter or a 560-nm long pass filter. Z-series were collected at 1-μm steps, and collapsed images are shown. Images were processed in Adobe Photoshop and the levels, scale, and orientation of the embryos were corrected for consistency.
Acknowledgments
We appreciate the advice of Darryl Kropf and Whitney Hable regarding actin staining procedures and helpful comments from members of the Muday laboratory during preparation of the manuscript. We also appreciate the assistance of Dr. Anita McCauley with the microscopy and Dr. Dave Anderson with the statistics.
This work was supported by the National Aeronautics and Space Administration (grant no. NAG2–1203 to G.K.M.) and the National Science Foundation (grant no. IBN–9318250 to G.K.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.034900.
References
- Alessa L, Kropf DL (1999) F-actin marks the rhizoid pole in living Pelvetia compressa zygotes. Development 126: 201–209 [DOI] [PubMed] [Google Scholar]
- Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF (2000) A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290: 972–977 [DOI] [PubMed] [Google Scholar]
- Basu S, Sun H, Brian L, Quatrano RL, Muday GK (2002) Early embryo development in Fucus distichus is auxin sensitive. Plant Physiol 130: 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger KD, Quatrano RS (2000) Polarity: the role of localized secretion. Curr Opin Plant Biol 3: 67–72 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G (1993) The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118: 575–587 [Google Scholar]
- Boonsirichai K, Guan C, Chen R, Masson PH (2002) Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Physiol Plant Mol Biol 53: 421–447 [DOI] [PubMed] [Google Scholar]
- Butler JH, Hu S, Brady SR, Dixon MW, Muday GK (1998) In vitro and in vivo evidence for actin association of the naphthylphthalamic acid-binding protein from zucchini hypocotyls. Plant J 13: 291–301 [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH (1998) The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA 95: 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke T, Poli D, Sztein A, Cohen J (2002) Evolutionary patterns in auxin action. Plant Mol Biol 49: 319–338 [PubMed] [Google Scholar]
- Dibb-Fuller JB, Morris DA (1992) Studies on the evolution of auxin carriers and phytotropin receptors: transmembrane auxin transport in unicellular and multicellular Chlorophyta. Planta 186: 219–226 [DOI] [PubMed] [Google Scholar]
- Edwards E, Roux S (1998) Influence of gravity and light on the developmental polarity of Ceratopteris richardii fern spores. Planta 205: 553–560 [DOI] [PubMed] [Google Scholar]
- Fowler J (2000) Cell polarity in algae and vascular plants. In D Drubin, ed, Cell Polarity: Frontiers in Molecular Biology, Vol 28. Oxford University Press, New York, pp 141–173
- Friml J (2003) Auxin transport - shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Geldner N, Hamann T, Jurgens G (2000) Is there a role for auxin in early embryogenesis? Plant Growth Regul 32: 187–191 [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hable WE, Kropf DL (1998) Roles of secretion and the cytoskeleton in cell adhesion and polarity establishment in Pelvetia compressa zygotes. Dev Biol 198: 45–56 [PubMed] [Google Scholar]
- Hadfi K, Speth V, Neuhaus G (1998) Auxin-induced developmental patterns in Brassica juncea embryos. Development 125: 879–887 [DOI] [PubMed] [Google Scholar]
- Haga K, Iino M (1998) Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol 117: 1473–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jurgens G (1999) The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126: 1387–1395 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M (2000) The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development 127: 23–32 [DOI] [PubMed] [Google Scholar]
- Hu S, Brady S, Kovar D, Staiger C, Clark G, Roux S, Muday G (2000) Identification of plant actin binding proteins by F-actin affinity chromatography. Plant J 24: 127–137 [DOI] [PubMed] [Google Scholar]
- Jaffe LF (1968) Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog 7: 295–328 [DOI] [PubMed] [Google Scholar]
- Jurgens G (2001) Apical-basal pattern formation in Arabidopsis embryogenesis. EMBO J 20: 3609–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens G (2003) Growing up green: cellular basis of plant development. Mech Dev 120: 1395–1406 [DOI] [PubMed] [Google Scholar]
- Klämbt D, Knauth B, Dittman I (1992) Auxin dependent growth of rhizoids of Chara globularis. Physiol Plant 85: 537–540 [Google Scholar]
- Kropf DL, Berge SK, Quatrano RS (1989) Actin localization during Fucus embryogenesis. Plant Cell 1: 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf DL, Bisgrove SR, Hable WE (1999) Establishing a growth axis in Fucoid algae. Trends Plant Sci 4: 490–494 [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ (1991) An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell 3: 1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Zhao W, Rashotte AM, Muday GK, Huber SC (2002) Gravity-stimulated changes in auxin and invertase gene expression in maize pulvinal cells. Plant Physiol 128: 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J, Brownlee C, Trewavas AJ (1997) Ca2+ and Calmodulin dynamics during photopolarization in Fucus serratus zygotes. Plant Physiol 115: 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday G (2000) Interactions between the actin cytoskeleton and an auxin transport protein. In CJ Staiger, F Baluska, D Volkmann, P Barlow, eds, Actin: A Dynamic Framework for Multiple Plant Cell Functions. Kluwer Academic Press, Dordrecht, The Netherlands, pp 541–556
- Muday GK (2001) Auxins and tropisms. Plant Growth Regul 20: 226–243 [DOI] [PubMed] [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6: 535–542 [DOI] [PubMed] [Google Scholar]
- Muday GK, Peer WA, Murphy AS (2003) Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci 8: 301–304 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Jaffe LF (1973) Cells without cytoplasmic movement respond to cytochalasin. Dev Biol 30: 206–208 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny AM, Forman M (1974) The relationship between changes in cell wall composition and the establishment of polarity in Fucus embryos. Dev Biol 40: 162–173 [DOI] [PubMed] [Google Scholar]
- Olson RA, du Buy HG (1937) The role of growth substance in the polarity and morphogenesis of Fucus. Am J Bot 24: 611–615 [Google Scholar]
- Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Gälweiler G (1999) PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol 2: 375–381 [DOI] [PubMed] [Google Scholar]
- Pearson G, Brawley S (1996) Reproductive ecology of Fucus distichus (Phaeophyceae): an intertidal alga with successful external fertilization. Mar Ecol Prog Ser 143: 211–223 [Google Scholar]
- Petrasek J, Cerna A, Schwarzerova K, Elckner M, Morris DA, Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131: 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M, et al. (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 96: 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu R, Robinson KR (1998) Cytoplasmic calcium gradients and calmodulin in the early development of the fucoid alga Pelvetia compressa. J Cell Sci 111: 3197–3207 [DOI] [PubMed] [Google Scholar]
- Pu R, Wozniak M, Robinson KR (2000) Cortical actin filaments form rapidly during photopolarization and are required for the development of calcium gradients in Pelvetia compressa zygotes. Dev Biol 222: 440–449 [DOI] [PubMed] [Google Scholar]
- Quatrano R (1980) Gamete release, fertilization and embryogenesis in the Fucales. In E Gantt, ed, Handbook of Phycological Methods: Developmental and Cytological Methods. Cambridge University Press, Cambridge, pp 59–68
- Quatrano RS (1973) Separation of processes associated with differentiation of two-celled Fucus embryos. Dev Biol 30: 209–213 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR, Wozniak M, Pu R, Messerli M (1999) Symmetry breaking in the zygotes of the Fucoid algae: controversies and recent progress. Curr Top Dev Biol 44: 101–125 [DOI] [PubMed] [Google Scholar]
- Rubery P, Jacobs M (1990) Auxin transport and its regulation by flavonoids. In R Pharis, S Rood, eds, Plant Growth Substances 1988. Springer Verlag, Berlin, pp 428–440
- Rubery PH (1990) Phytotropins: receptors and endogenous ligands. Symp Soc Exp Biol 44: 119–146 [PubMed] [Google Scholar]
- Schiavone FM, Cooke TJ (1987) Unusual patterns of somatic embryogenesis in the domesticated carrot: developmental effects of exogenous auxins and auxin transport inhibitors. Cell Differ 21: 53–62 [DOI] [PubMed] [Google Scholar]
- Souter M, Lindsey K (2000) Polarity and signaling in plant embryogenesis. J Exp Bot 51: 971–983 [DOI] [PubMed] [Google Scholar]
- Whitaker D (1937) Determination of polarity by centrifuging eggs of Fucus furcatus. Biol Bull 73: 249–260 [Google Scholar]
- Whitaker D (1940) The effects of ultra-centifuging and of pH on the development of Fucus eggs. J Cell Comp Physiol 15: 173–187 [Google Scholar]