Abstract
Intravenous immunoglobulin (IVIG) has shown limited promise so far in human clinical studies on Alzheimer’s disease (AD), yet overwhelmingly positive preclinical work in animals and human brain cultures support the notion that the therapy remains potentially efficacious. Here, we elaborate on IVIG neuropreservation by demonstrating that IVIG protects human primary neurons against oxidative stress in vitro and that IVIG preserves antioxidant defense mechanism in vivo. Based on these results, we propose the following translational impact: If the dosage and treatment conditions are adequately optimized, then IVIG treatment could play a significant role in preventing and/or delaying the progression of neurodegenerative diseases, such as AD. We suggest that IVIG warrants further investigation to fully exploit its potential as an anti-oxidant, neuroprotective and synapto-protecting agent.
Introduction
Alzheimer’s disease (AD) is an age-dependent neurodegenerative disorder with no curative treatment options. According to the latest data, nearly 44 million people worldwide are suffering from AD [1]. Despite the vast amount of research in the neurochemistry and pharmacology of AD, there is no effective treatment available to modify the underlying pathology of this devastating disease; therefore, vigorous research at various fronts is needed [2–3]. Since AD is a multifactorial disease with genetic and environmental etiologies, including lifestyle, diet, stress and toxic exposures [4–5], there is no consensus of the causes of selective neuronal vulnerability in AD. Neurochemical dysfunctions associated with AD include the accumulation of inflammatory factors and reactive oxygen species (ROS), particularly within the hippocampal complex that mediates memory function [6–7]. These factors contribute to pathological cascades leading to neurodegeneration and synaptic loss and AD.
There are five FDA drugs currently approved for use in AD patients. However, they provide only slight symptomatic improvements and do not appear to modify disease progression. Therefore, a central focus of AD research is to test and develop agents that should prevent selective neuronal vulnerability and eventually the onset of symptomatic disease. Our laboratory is testing novel functions of important therapeutic agents in animal and cell culture models [8–10] as well as important strategies such as phytochemicals [11] and immunotherapy [12].
The most prevalent hypothesis is that AD results from the accumulation of potentially toxic amyloid-β (Aβ) peptides as extracellular senile plaques [7]. Aβ is cleaved from the amyloid-β precursor protein (APP) sequentially by β-secretase (or BACE1) and γ–secretase activity. Unfortunately, immunotherapeutic strategies targeting Aβ peptides have been unable to improve cognition in people with mild AD [13–14]. In addition, these clinical trials caused severe side effects [15]. For these reasons, other alternative targets and treatment options are being tested. It is reasoned that intravenous immunoglobulin (IVIG) therapy might be a novel alternative immunotherapy with significant advantages over amyloid-based strategies [16–17]. In a recent multicenter, placebo-controlled, double-blinded phase III trial of 390 subjects, the Gammaglobulin Alzheimer’s Partnership (GAP) study did not meet primary endpoints of slowing cognitive and functional decline after 18 months of treatment of Gammagard [18]. However, two useful positive results emerged from the GAP study results: IVIG’s positive safety profile and IVIG’s beneficial effects for pre-specified moderate AD and apoEε4 carrier subgroups [18].
Based on these results from the GAP trial, several studies are exploring the phenomenon of IVIG neuroprotection in tissue culture and animal models of AD pathology. Some animal studies have shown encouraging results in a variety of biochemical and behavioral parameters [19–21]. IVIG treatment protects mouse neurons from Aβ toxicity and, importantly, IVIG passes via the blood-brain barrier and binds to Aβ deposits in brain parenchyma [19]. Using the neuronal cell culture and transgenic (Tg) mouse models of AD, IVIG treatment has been shown to reduce hallmark AD pathology, including amyloid plaques, neurofibrillary tangles, inflammation, glial activation and oxidative stress [21–24]. IVIG administration has also shown beneficial effects on inflammation and synaptic function AD transgenic mice [21,22]. At the tissue culture front, initial experiments suggest that IVIG-treatment increased levels of synaptic proteins in primary rat hippocampal neurons versus vehicle-treated cells [23–24]. Furthermore, when the neuronal cells were challenged with oxidative stress agents, the IVIG treatment protected against neurotoxic insults. Therefore, IVIG treatment displays significant translational value for unveiling AD pathogenic mechanisms and potential preventive and therapeutic strategies for AD.
To characterize the role of IVIG in preclinical models, we have performed a series of studies on the effects of IVIG (Gammagard, 10%, Baxter Healthcare) in both primary human fetal brain (HFB) cultures [25] and in the 3xTg mouse model of AD [21]. Here we report that IVIG preserves HFB viability and protects these cultures from oxidative stress. Moreover, studies in the 3xTg mouse reveal that IVIG preserves the expression levels of key anti-oxidants in the face of mounting AD-like pathology. These results suggest that IVIG plays a neuropreservative role by reducing the toxic effects of ROS and support a growing list of potential pro-survival properties of this well-tolerated immunotherapy.
Experimental model and design
Primary Human Fetal Brain Cultures and Treatment Conditions
The HFB cultures were prepared [26] and treated at DIV16 with different doses (5–20 mg/ml) of IVIG (Gammagard, 10%; Baxter Healthcare) (n=4) for 72 hr, as indicated in the figures. The HFB cultures were also separately treated with glycine and Buminate (Baxter Healthcare) to serve as vehicle and placebo, respectively. For the ROS experiments, the cell culture contained ‘antioxidant-free B27’ media for each respective treatment. Following IVIG treatments, media was aspirated off from the plate, and the HFB cells were washed with PBS. The HFB cells were then treated with fresh media containing either IVIG alone or 50μM H2O2 (representing ROS) without the addition of IVIG. The HFB cells were harvested after 24 hr of H2O2 exposure, and morphology was recorded by imaging and immunocytochemistry (ICC). Effects of IVIG alone on neurons were assessed for the cellular viability assay by Cell Titer Glo (CTG) and for toxicity by the lactate dehydrogenase (LDH) assay; details of these procedures have been previously described [26].
3xTg Mouse Treatments and Analysis
3xTg mice, a well-established mouse model for AD bearing human mutant APPswe, PS1M146V, and TauP301L transgenes, were randomized into experimental and placebo groups at three months of age and treated for either three or six months [21]. The experimental groups (n = 15) received 400 mg/kg bodyweight of Gammagard, 10% (Baxter Healthcare) intravenously via the retro-orbital sinus every two weeks under anesthesia. This dosage was consistent with dosage levels tested in the GAP trial. The placebo group received injections of 10% heat-inactivated bovine serum albumin (Sigma) in saline. A baseline cohort (three months old, no treatment, n = 8) was also evaluated. Mice were transcardially perfused with saline and brains from each group were hemisected. One hemisphere was immersion-fixed in 4% paraformaldehyde/0.1% glutaraldehyde for 24 hr and stored in cryoprotectant. From the other hemisphere, hippocampus, frontoparietal cortex, and cerebellum were rapidly dissected and snap-frozen [12–14].
Plasma collection
Cardiac puncture of the right ventricle was performed to draw ~300 μl blood prior to perfusion of each animal. Animals were fasted overnight prior to sacrifice to control for metabolic fluctuations. Blood was drawn into EDTA-treated tubes and immediately spun at 1500 rpm for 10 min at 4 °C to separate the plasma, buffy coat, and erythrocyte fractions. Plasma was snap-frozen and stored at −80 °C.
Plasma mRNA amplification and custom microarray analysis
mRNA from collected plasma fractions was extracted in Trizol (Life Tech) and amplified using terminal continuation RNA amplification methodology, as previously reported [21]. A detailed description of this method can be found at http://cdr.rfmh.org/Labgoups/ginsberg_protocol.html. The last step of the process uses neuronal cDNA as a template to synthesize radiolabeled hybridization probes by in vitro transcription. Probes are then hybridized to custom-designed microarrays with 576 cDNAs encoding functional gene classes mediating critical cellular pathways [27,28]. Each array was analyzed on a Storm phosphor imager with ImageQuant software (GE, Piscataway, NJ). RNA bound to each cDNA was expressed as a ratio of the total hybridization signal intensity of the array (i.e., global normalization) [27,28], thereby minimizing variations due to differences in the specific activity of the probe and the absolute quantity of probe present [27,28]. Data analysis generated expression profiles of the relative changes in mRNA levels for quantitative comparison among baseline, IVIG- and placebo-treated animals.
Quantitative PCR (qPCR)
qPCR was performed on frozen hippocampal tissue from placebo and IVIG-treated mice (n = 6). Samples were assayed on a real-time PCR cycler (7900HT, Applied Biosystems) in 96-well optical plates as described previously [27,28]. The ddCT method was employed to determine relative SYP, APP, SYN1, and SYT1 gene level differences with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) qPCR products used as a control [27,28].
Statistical analysis
Array and qPCR-based expression levels among the baseline, placebo, and IVIG groups were compared using one-way ANOVA with Bonferroni’s post hoc test for multiple comparisons. Expression levels on the custom arrays were analyzed using GeneLinker bioinformatics software (IOS, Kingston, ON). P-values were adjusted using a false discovery rate controlling procedure to reduce type I error due to the large number of genes analyzed simultaneously. The level of significance was set at α = 0.01 (two-tailed).
Results
1. Effect of IVIG on Cellular Toxicity by LDH Assay
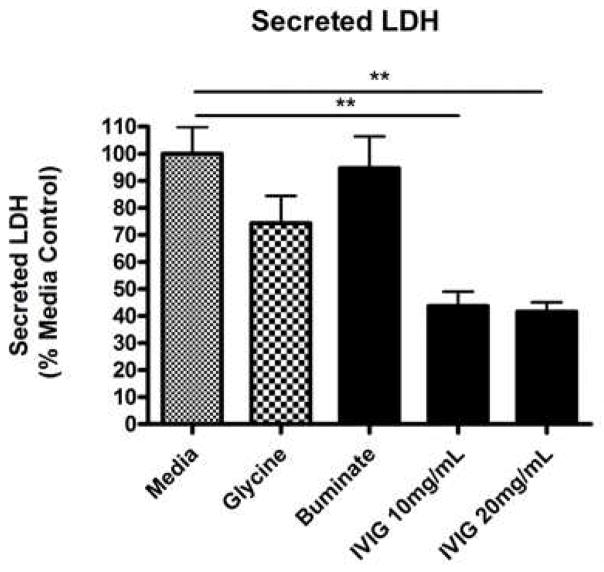
An LDH based assay of cell integrity was performed on conditioned medium samples and lysates of the adherent cells. Increased LDH release is directly proportional to increased membrane damage and cell lysis. The data are shown as the percentages of total LDH in the conditioned medium of the cells from each treatment vs. the control set to 100% (Fig. 1). There was a significant decrease in LDH release with IVIG treatmnt vs the control media (Fig. 1) (p < 0.05). This suggests that the integrity of the cell membrane is preserved and enhanced with IVIG relative to control treatment.
Fig 1. IVIG preserves and promotes membrane integrity in human primary neurons.
HFB were prepared and seeded (2–3×105 cells/well) onto 24-well plates as described previously [26]. At DIV16, cells were treated for four days with either media, vehicle (glycine buffer), negative control (Buminate, Baxter), IVIG (Gammagard 10%, Baxter) 10 mg/mL or IVIG 20 mg/mL. At the end of the treatments, the conditioned medium (CM) samples were collected from each well without disturbing the cells. An equal volume of CM was subjected to the LDH assay as described previously. The data are shown as the percentage of total LDH in the CM from each treatment vs percent mean media control (set to 100). A significant decrease in LDH release with IVIG treatment vs the control media (ANOVA, p < 0.05) suggests the preservation and enhancement of the cell membrane integrity with IVIG treatments.
2. Effect of IVIG Treatments on Neuronal Viability in HFB Neuronal Cultures
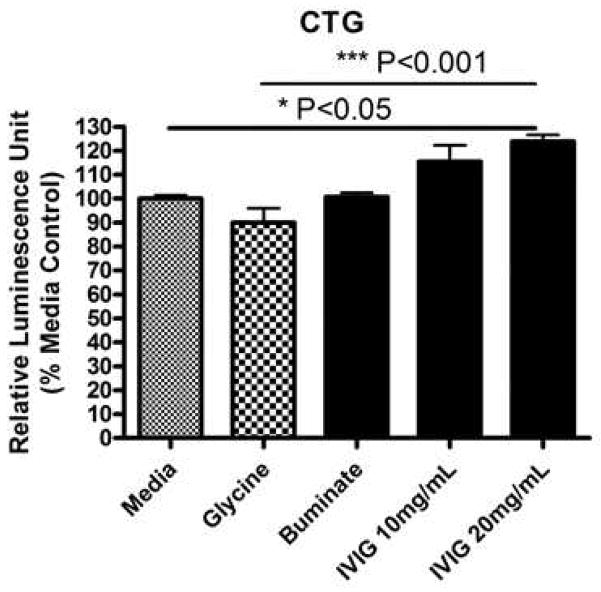
Neuronal viability was assayed by the sensitive CTG measurements. CTG assay showed a significant increase in cell viability with high dose of IVIG (20 mg/mL) treatment (p < 0.05). CTG results suggest that the range of IVIG doses tested was well-tolerated by the primary neuron-enriched human fetal brain cultures (at DIV16) (Fig. 2). Cellular morphology did not show any change at any concentration of IVIG tested (data not shown). Thus, IVIG treatments were well tolerated and able to promote neurite extension.
Fig 2. IVIG preserves cell viability in human primary neurons.
HFB cultures (DIV16) were prepared, cultured, seeded and treated as describe in Fig. 1. At the end of the treatments, the CTG-based cell viability assay was performed with an equal volume of cell suspension. The data are shown as the percentage of CTG from the percent mean control (100%). CTG assay shows a significant increase in cell viability with high dose of IVIG (20 mg/mL) treatment (ANOVA, p < 0.05). This range of IVIG treatments was well-tolerated by the neuronal cells. Cellular morphology was unaltered at the concentrations tested (not shown).
3. Effect of IVIG Treatments on Neuropreservation and Neuroprotection
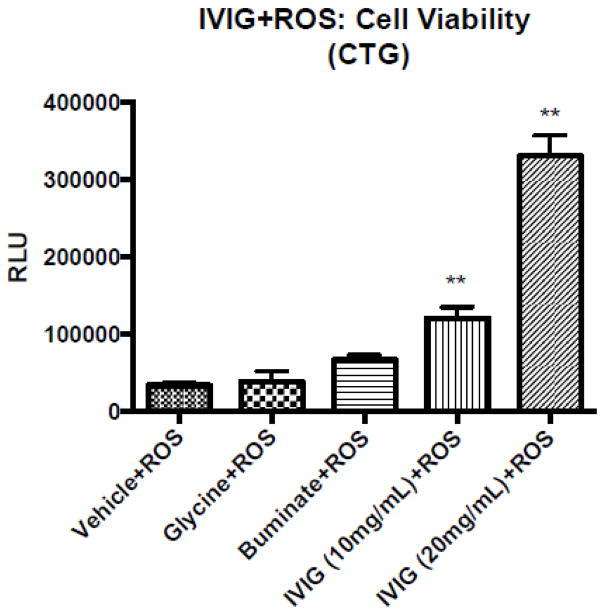
When human primary neuronal cultures were treated with different doses of IVIG, and concurrently challenged with oxidative stress-promoting agents (50 μM of ROS), we noted a significant neuropreservation with IVIG (10 to 20 mg/mL) from the placebo (data not shown). These results suggest that IVIG treatment is neuropreservative in human neurons. In another set of experiments, when human primary neurons were treated with different doses of IVIG, and cells were washed off excessive IVIG, and then subsequently challenged with 50 μM of ROS alone for 24 hr, cellular morphology revealed significant neuroprotection with IVIG relative to placebo (data not shown). Here, HFB cells were first exposed with H2O2 for 24 hr, and then cells were washed with PBS. The HFB cells were further treated with either medium, glycine, Buminate, IVIG 10 mg/ml or IVIG 20 mg/ml for 72 hr. At the end of the treatments, the CTG-based cell viability assay was performed with an equal volume of cell suspension. The CTG assay showed a significant decrease in cell viability when the cells were pretreated with ROS (vehicle + ROS). Brain cultures containing neurons were not rescued against ROS pre-exposure in the presence of either glycine or Buminate (Fig. 3). Notably, neuron containing cultures were gradually rescued against ROS pre-exposure in the presence of increasing dose of IVIG (Fig. 3). Thus, IVIG pre-treatment protects human neurons from ROS-mediated damage.
Fig 3. IVIG treatments protect human neurons and against ROS insults.
Human fetal brain cultures (DIV16) were prepared, cultured and seeded as described in Fig. 1. For the ROS experiments, the cell culture contained ‘antioxidant-free B27’ media for each respective treatment. The HFB cells were first exposed with H2O2 on each well at 37C for 24 hr. Following H2O2 insult, media were aspirated off from each well and the HFB cells were washed with PBS. The HFB cells were then treated with either medium, glycine, Buminate, IVIG 10 mg/ml or IVIg 20 mg/ml for 72 hr. At the end of the treatments, the CTG-based cell viability assay was performed with an equal volume of cell suspension. CTG assay shows a significant decrease in cell viability when the cells were prior insulted with ROS (vehicle +ROS). Brain cultures containing neurons were not rescued against ROS pre-exposure in the presence of either glycine or Buminate. Notably, neuron containing cultures were gradually rescued against ROS pre-exposure in the presence of increasing dose of IVIG. Thus, IVIG pre-treatment protects human neurons from ROS-mediated damage.
4. IVIG preserves key antioxidant levels in the 3xTg mouse model of AD
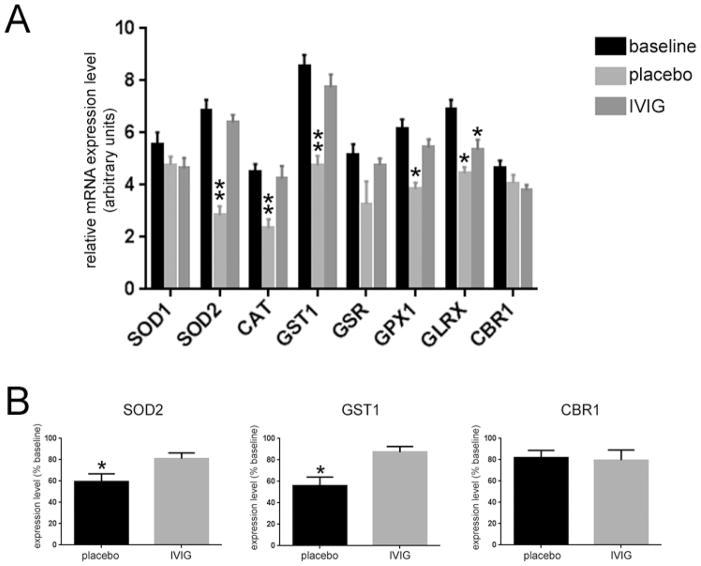
To understand potential mechanisms for IVIG-mediated neuroprotection and preservation of primary neuronal cultures against ROS, we looked to plasma gene expression profiling experiments that were performed on 3xTg mice treated with intravenous IVIG or placebo for six months. When compared to placebo, IVIG preserved plasma levels of several key antioxidant enzymes, including superoxide dismutase 2 (SOD2), catalase (CAT), glutathione S-transferase (GST1), and glutathione peroxidase (GPX1) (Fig. 4A). Notably, preservation of SOD2 and GST1 gene expression was also observed in hippocampal tissue from the same IVIG-treated mice relative to placebo (Fig. 4B), indicating that IVIG modifies AD-like disease progression in preclinical models by stimulating a strong antioxidant response. Moreover, these data suggest that antioxidant mRNA levels in plasma may be reliable prognostic biomarkers for IVIG therapeutic efficacy.
Fig 4. IVIG regulates plasma and hippocampal levels of antioxidant mRNAs in 3xTg mice.
A) IVIG increases plasma levels of select mRNAs regulating detoxifying antioxidant pathways. Bar graph shows that six months of placebo treatment resulted in a 40–60% reduction in the expression levels of transcripts encoding superoxide dismutase 2 (SOD2), catalase (CAT), glutathione S-transferase 1 (GST1), and glutathione peroxidase 1 (GPX1) compared to baseline, whereas six months of IVIG treatment preserved the levels of these antioxidant enzyme transcripts. n = 12/group *, p < 0.01 compared to baseline, **, p < 0.001 compared to baseline, via one-way ANOVA with Bonferroni’s post hoc test for multiple comparisons. B) Antioxidant transcript levels are also preserved in the hippocampus of IVIG-treated mice. Bar graphs show relative expression levels of SOD2, GST1, and carbonyl reductase 1 (CBR1) in placebo and IVIG-treated mice compared to baseline, as quantified by qPCR. SOD2 and GST1 levels were reduced by ~40% in placebo-treated mice, whereas levels of these transcripts were unchanged in IVIG-treated mice. n = 6/group *, p < 0.05 compared to baseline, via one-way ANOVA with Bonferroni’s post hoc test for multiple comparisons. Other abbreviations: SOD1 (superoxide dismutase 1), GSR (glutathione reductase), GLRX (glutaredoxin).
Discussion
We have previously reported a potent neuropreservatory effect of IVIG against ROS-mediated oxidative insults in primary human neurons [25]. Moreover, IVIG was also shown to prevent neurofibrillary degeneration of CA1 hippocampal neurons in vivo in the 3xTg mouse [21]. Plasma gene expression profiling experiments revealed that IVIG stabilizes cytoskeletal and calcium signaling plasticity and phosphorylation-based cellular homeostatic mechanisms in the face of mounting AD pathology in these mice. Here, we show that IVIG also preserves cellular antioxidant defense mechanisms that may serve as the basis for its ability to prevent ROS-mediated neuronal cell death. Taken together, these data show that IVIG treatment has great potential to preserve and protect neurons by abrogating AD-related oxidative insults (Fig. 5) and presents a powerful preclinical tool for understanding these mechanisms.
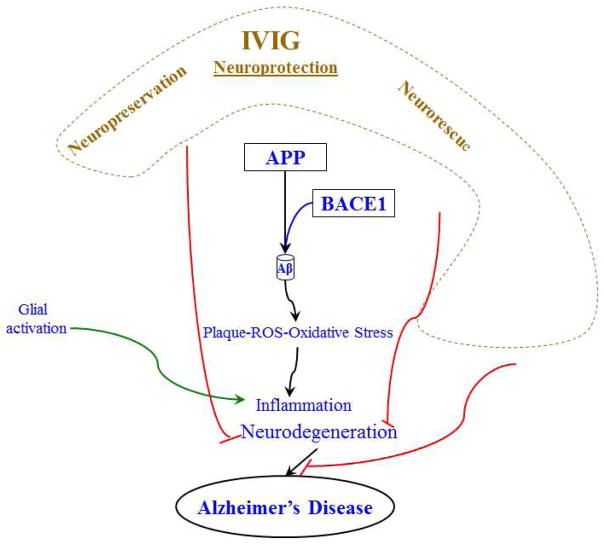
Figure 5. Schematic diagram highlighting the potential role of IVIG as an anti-oxidant, anti-inflammatory and neuroprotective agent.
Neurochemical dysfunctions associated with AD include the accumulation of inflammatory factors, glial activation, and reactive oxygen species (ROS). These factors are belived to contribute to pathological cascades leading to neurodegeneration and synaptic loss and AD. According to the amyloid hypothesis, AD results from the accumulation of potentially toxic Aβ peptides as extracellular senile plaques. Aβ is cleaved from the amyloid-β precursor protein (APP) sequentially by secretase enzymes. As discussed in the text, IVIG treatment has been shown to lower the hallmark AD pathology, including amyloid plaques, neurofibrillary tangles, inflammation, and oxidative stress using the neuronal cell culture and transgenic mouse models of AD. IVIG administration has produced significant effects on inflammation and synaptic function in the AD Tg mice model. At the tissue culture front, IVIG-treatment increased levels of synaptic proteins in primary rat hippocampal neurons. Furthermore, when the neuronal cells were challenged with oxidative stress agents, the IVIG treatment protected against neurotoxic insults. Therefore, IVIG treatment displays significant translational value for unveiling AD pathogenic mechanisms and potential preventive and therapeutic strategies for AD.
Our results also suggest that IVIG treatment even at high doses (20 mg/ml) was well tolerated by human brain cultures, including primary neurons, as there was neither cellular toxicity nor a loss of cell viability as assessed by several independent techniques, including morphology, CTG and LDH assays. Furthermore, IVIG treatment did not affect any behavioral phenotypes or mortality in 3xTg mice (not shown). These observations mimic the good safety profile for IVIG in previous clinical trials and underscore its importance as a promising therapeutic agent for further clinical trials. If the dosage, duration, and other treatment conditions are adequately optimized, then IVIG treatment may fulfill its promise to prevent and delay the onset of AD pathology and symptoms
Acknowledgments
We sincerely appreciate the grant support from the Alzheimer’s Association (Zenith award and IIRG; DKL), the NIH (DKL and SEC), Baxter Healthcare (DKL and SEC), and the St. Mary’s Foundation (SEC).
References
- 1.http://www.brightfocus.org/alzheimers/about/understanding/facts.html
- 2.Schneider LS, Lahiri DK. The perils of Alzheimer’s drug development. Curr Alzheimer Res. 2009;6(1):77–78. doi: 10.2174/156720509787313871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahiri DK, Long JM, Maloney B, Greig NH. Lessons from a BACE1 inhibitor trial: Off-site but not off base. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2013.11.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci. 2005;25(4):823–9. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry (Nature Publishing Group) 2009;14(11):992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe DJ. SnapShot: pathobiology of Alzheimer’s disease. Cell. 2013;8;154(2):468–468.e1. doi: 10.1016/j.cell.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Perez FP, Bose D, Maloney B, Nho K, Shah K, Lahiri DK. Late-Onset Alzheimer’s Disease (LOAD) Heating up and Foxed by several proteins: Pathomolecular efects of the aging process. J Alzheimer Dis. 2014 doi: 10.3233/JAD-131544. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK. Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res. 2010;88(1):143–154. doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey JA, Lahiri DK. A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer’s disease. J Neurochem. 2010;112(4):843–853. doi: 10.1111/j.1471-4159.2009.06490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey JA, Ray B, Greig NH, Lahiri DK. Rivastigmine lowers Aβ and increases sAPPalpha levels, which parallel elevated synaptic markers and metabolic activity in degenerating primary rat neurons. PLoS ONE. 2011;6(7):e21954. doi: 10.1371/journal.pone.0021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by AGE and SAC treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011;117(3):388–402. doi: 10.1111/j.1471-4159.2010.07145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farlow MR, Brosch JR. Immunotherapy for Alzheimer’s disease. NeurolClin. 2013;31(3):869–78. doi: 10.1016/j.ncl.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects ofAbeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlledphase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 14.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 16.Dodel R, Neff F, Noelker C, Pul R, Du Y, Bacher M, et al. Intravenous immunoglobulins as atreatment for Alzheimer’s disease: rationale and current evidence. Drugs. 2010;70:513–28. doi: 10.2165/11533070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–36. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed 12/15/2013]; http://www.baxter.com/press_room/press_releases/2013/05_07_13_gap_study.html.
- 19.Magga J, Puli L, Pihlaja R, et al. Human intravenous immunoglobulin provides protection against Abeta toxicity by multiple mechanisms in a mouse model of Alzheimer’s disease. J Neuroinflammation. 2010;7:90. doi: 10.1186/1742-2094-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puli L, Pomeshchik Y, Olas K, Malm T, Koistinaho J, Tanila H. Effects of human intravenous immunoglobulin on amyloid pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflammation. 2012;29;9:105. doi: 10.1186/1742-2094-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Counts S, Perez S, He B, Mufson E. Intravenous Immunoglobulin Reduces Tau Pathology and Preserves Neuroplastic Gene Expression in the 3xTg Mouse Model of Alzheimer’s Disease. Current Alz Res. doi: 10.2174/1567205011666140812114037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudduth TL, Greenstein A, Wilcock DM. Intracranial injection of Gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Aβ in APP/PS1 mice along a different time course than anti-Aβ antibodies. J Neurosci. 2013;33(23):9684–92. doi: 10.1523/JNEUROSCI.1220-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey JA, Ray B, Lahiri DK. Intravenous immunoglobulin (IVIG) protects neuronal viability and synaptic markers in cultured degenerating primary hippocampal neurons. Societ Neurosci Abst 852.04. 2012 [Google Scholar]
- 24.Lahiri DK, Ray B. Effect of IVIG in preserving human primary neurons and protecting them against oxidative stress. Alzheimers Dement. 2013;9(4) Supplement:P800. [Google Scholar]
- 25.Lahiri DK, Ray B. Intravenous Immunoglobulin (IVIG) treatment exerts a protective effect against oxidative insults in primary human neuronal mixed cultures. Curr Alzheimer Res. 2014 doi: 10.2174/1567205011666140812113851. Under Revision. [DOI] [PubMed] [Google Scholar]
- 26.Long JM, Ray B, Lahiri DK. MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem. 2012;28(37):31298–10. doi: 10.1074/jbc.M112.366336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Counts SE, Che S, Ginsberg SD, Mufson EJ. Gender differences in neurotrophin and glutamate receptor expression in cholinergic nucleus basalis neurons during the progression of Alzheimer’s disease. J Chem Neuroanat. 2011;42:111–117. doi: 10.1016/j.jchemneu.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, Che S. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol Psychiatry. 2010;68:885–893. doi: 10.1016/j.biopsych.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]