Abstract
Cardiovascular disease has been associated with 40% of deaths in high-income countries and 28% in lower-income countries. The relationship between periodontitis and acute myocardial infarction is well documented, but it has not been established whether the extent and severity of periodontitis influence the infarct size. This cross-sectional and analytic study was designed to investigate the association of chronic periodontitis extent and severity with acute myocardial infarct size as indicated by serum cardiac troponin I and myoglobin levels. Sociodemographic, periodontal, cardiologic, and hematologic variables were gathered in 112 consecutive patients with myocardial infarction. The extent (Arbes Index) and severity (Periodontal Inflammatory Severity Index) of the chronic periodontitis were significantly associated with troponin I levels after controlling for sociodemographic and clinical confounders (change in R2 = .041, p < .02, and R2 = .031, p = .04). However, only the extent index accounted for levels of myoglobin (change in R2 = .030, p < .05), total leukocytes (change in R2 = .041 p < .02), and neutrophils (change in R2 = .059, p < .01). Mediated regression analysis showed that leukocytes and neutrophils may underlie these observed relationships of chronic periodontitis with troponin I and myoglobin. To our knowledge, this study contributes the first research data demonstrating that the extent and severity of periodontitis is positively associated with acute myocardial infarct size as measured by serum troponin I and myoglobin levels.
Keywords: myocardial infarction, troponin I, troponin T, myoglobin, neutrophils, chronic periodontitis
Introduction
Two prevalent and related health problems—chronic periodontitis (CP) and acute myocardial infarction (AMI)—were evaluated in this cross-sectional and analytic study. Cardiovascular disease (CVD) has been associated with 40% of deaths in high-income countries and 28% of those in low- to medium-income countries. It has been estimated that by 2030, 32.5% of deaths will be caused by CVD, especially AMI (Gaziano and Gaziano, 2012). According to a U.S. survey, CP affects around 35% of the adult population, and moderate to advanced forms of the disease are present in 13% to 15% of adults (Albandar et al., 1999), with an even higher prevalence among the elderly (Eke et al., 2012).
The size of the myocardial infarction is key to the final outcome of the patient (Antman et al., 1996), and the gold standard biomarkers for its estimation are troponins (cTnI and cTnT; Thygesen et al., 2010). cTnI is considered more reliable than cTnT as a risk stratification index (Keller et al., 2009), and a worse mortality rate was reported in patients with higher serum cTnI levels after adjusting for ST segment depression and age (Antman et al., 1996). Myoglobin (Myo) is a nonspecific myocardial necrosis marker that appears in the first 1 to 3 hr postinfarction. The determination of serum Myo and cTnI levels helps to predict an adverse AMI outcome and may be useful for an early AMI diagnosis (Sanchis et al., 2003).
Periodontitis is an infectious disease that affects the tooth-supporting tissues and exhibits a wide range of clinical, microbiological, and immunologic manifestations. It is associated with and probably caused by a multifaceted dynamic interaction of specific infectious agents, host immune response, harmful environmental exposure, and genetic susceptibility factors (Slots, 2013). A moderate but significant association has been found between CP and CVD (Mattila et al., 1989; Beck et al., 1996), while cross-sectional and case-control studies have reported that periodontitis is epidemiologically associated with acute coronary syndrome and AMI (Arbes et al., 1999; Cueto et al., 2005; Bahekar et al., 2007). However, the underlying mechanisms have not been elucidated, and no data are available on the relationship between CP extent or severity and AMI size.
An elevated peripheral blood leukocyte (Lk) count is linked to a higher incidence of coronary heart disease and mortality in the general population. During the first few hours after an AMI—with ST segment elevation (STEMI) or without (NSTEMI)—the total Lk count is an independent predictor of long-term mortality (Núñez et al., 2005). The Lk count has been associated with the extent of atherosclerosis (Kostis et al., 1984), while the migration of Lk to atheromata may cause plaque instability (Libby et al., 2002). The relationship of Lk count and periodontitis with the presence of AMI is well documented (Renvert et al., 2010).
Neutrophils, a key protective cell type in noninflamed periodontal tissues, have been described as a 2-edged sword, because their overactivity can produce tissue damage and prolong the extent and severity of inflammatory periodontal disease (Scott and Krauss, 2012). Elastase and cathepsin G are the main microbiocidal enzymes of neutrophils, and their activity was significantly correlated with AMI in CP patients. Salivary elastase activity was significantly associated with periodontitis (Mantyla et al., 2012), and both enzymes can trigger the coagulation cascade, activate platelets, and enhance vascular thrombus growth (Massberg et al., 2010). In this regard, increased platelet activation has been reported in CP patients (Papapanagiotou et al., 2009).
The contribution of periodontitis-associated systemic inflammation to other classic CVD risk factors is not fully known. Thus, no published study has addressed the relationship of the extent and severity of CP with AMI size. The objective of this investigation was to determine the association of CP extent and severity with AMI size as indicated by cardiac cTnI and Myo levels.
Materials & Methods
This cross-sectional and analytic study included 112 consecutive AMI patients who underwent diagnostic coronary angiography at the “Virgen de las Nieves” University Hospital of Granada (Spain) between July and December 2012. AMI was defined by cardiac necrosis markers (cTnI and Myo), typical ECG changes (STEMI and NSTEMI), and characteristic clinical symptoms. The inclusion criterion was the presence of AMI. Exclusion criteria were as follows: the presence of cancer, AIDS, or infectious or autoimmune disease; a history of chronic antibiotic therapy; recent treatment with corticoids or nonsteroidal anti-inflammatory drugs; renal failure (creatinine > 1.5 mg/dL); a history of trauma; and current receipt of periodontal treatment. Data were gathered on age, sex, marital status, personal medical history (diabetes, hypertension, dyslipidemia, and body mass index), and smoking habit. The study was performed according to the STROBE guidelines for cross-sectional studies. Approval was obtained from the ethics committee of our hospital, and informed consent was signed by each patient. Patients were treated in accordance with the Helsinki Declaration of 2008.
Periodontal Examination
Bedridden patients underwent dental examination on the fourth day after discharge from the intensive care unit. All oral clinical examinations were performed by a single periodontal specialist (R.M.) blinded to the clinical, cardiology study, and hematologic data of the patients. Intra- and interexaminer reliability (F.M.) was assessed by the kappa statistic, which was 0.78 and 0.82, respectively, demonstrating a high degree of conformity in the observations. A calibrated PCPUNC15 periodontal probe (Hu-Friedy, Chicago, IL, USA) was used to measure attachment loss at 6 sites per tooth, excluding third molars. The extent of periodontal disease was measured by a slight modification of the Arbes index (Arbes et al., 1999), considering the percentage of sites with attachment loss of 4 mm or more and categorizing patients as follows: 0% = no periodontitis, >0%–33% = mild periodontitis, >33%–67% = moderate periodontitis, and >67% = severe periodontitis.
We also calculated a novel index derived from periodontal variables designated the Periodontal Inflammatory Severity Index (PISI), a modification of the Periodontal Index for Risk of Infectiousness (PIRI; Geerts et al., 2004), to quantify the severity of the periodontal disease. The PISI was designed to overcome the fact that the PIRI does not consider pockets with probing depth of 4 mm, even when the number of these pockets is of clinical relevance. PISI was defined as the sum of the product of the number of sites and the clinical attachment loss at each site: PISI = 1/(t + 1)∑ dini, where i is the site, d is the attachment loss in millimetres, t is the number of remaining teeth, and n is the absolute frequency of the sites. We also measured the plaque index (O’Leary et al., 1972) and bleeding index (Ainamo and Bay, 1975).
Cardiology Study and Hematologic Measurements
Coronary angiography (Buhlin et al., 2011) was used to determine the presence of coronary artery disease. The diagnostic coronary angiography and echocardiogram for each patient were analyzed by an experienced interventional cardiologist (J.A.R.-H.) blinded to the clinical, biological, and dental data. A peripheral blood sample was drawn into EDTA-containing tubes from each patient upon arrival in the emergency department and was analyzed in the clinical chemistry laboratory for standard Lk and neutrophil counts and peak cTnI and Myo values. All parameters were evaluated upon the patient’s arrival at the emergency unit and at 6, 8, 12, and 18 hr, recording the maximum cTnI peak. cTnI elevation was defined by an increase of >1 ng/mL (upper normal limit). cTnI was determined with a chemiluminescent immunoassay supplied by Beckman Coulter Inc. (Brea, CA, USA), which establishes a sensitivity of 96% and a specificity of 94% for a cutoff value of 0.50 ng/mL. Myo elevation was defined by an increase of >70 ng/mL at any measurement time point.
Statistical Analyses
In this cross-sectional study, we used linear regression and mediated linear regression to disentangle the associations between indicators of AMI size (cTnI and Myo) and indices of CP extent (Arbes) and severity (PISI). Lk and neutrophil counts were potential mediators of the association between CP and AMI size. Age, sex, hypertension, diabetes mellitus, dyslipidemia, body mass index, and smoking habit were considered as potential confounders. The effect of these confounders on the association between the periodontal indices and outcomes was analyzed by hierarchical linear regression, introducing the confounders in the first regression model and the predictors (Arbes or PISI index) in the second. R2 change served to indicate the improvement in AMI size prediction when the periodontal index was introduced as a predictor in the second linear model. The adjusted R2 was used to evaluate the predictive ability of the first model (confounders alone) and the second (confounders + periodontal index), separately assessing each periodontal index for each outcome and mediator. Next, the direct and indirect effects of the CP on AMI size were estimated by mediated multiple regression (MacKinnon et al., 2007; Preacher and Hayes, 2008). Regression residuals were analyzed to examine normality and outliers. Mediated regression modeling was performed with the bias-corrected and accelerated (BCa) bootstrapping procedure implemented by Hayes’s PROCESS macro tool (http://www.afhayes.com). We ran 5,000 bootstrapping samples to estimate the statistical significance of the effects. When necessary, variables were log-transformed to improve normality fitting. The chi-square exact test was applied to determine the significance of differences among the categories of categorical variables. SPSS 20.0 was used for the statistical analysis.
Results
Sociodemographic, clinical, and biochemical results for the 112 AMI patients in the study are exhibited in Table 1. The sample largely comprised overweight married males around 60 yr of age with primary schooling and hypertension. STEMI was the most frequent AMI type. cTnI and Myo levels were higher in STEMI patients versus NSTEMI patients (51.54 vs. 23.15, p < .001; 840.64 vs. 492.20, p = .001, respectively) and in males versus females (43.170 vs. 28.95, p = .045; 806.98 vs. 203.40, p = .003, respectively). Myo levels were higher in patients with a history of hypertension versus those without (829.69 vs. 494.48, p = .045). No other significant effects were observed. Of the 112 patients studied, 73 (65.18%) had periodontitis; 59 (80.82%) had a probing depth of 4 to 6 mm; and 14 (19.18%) had pocket depths of 7 to 9 mm. As expected, because of the PIRI limitation mentioned above, only 48% of patients showed periodontitis according to this index. PISI correlated with PIRI (r = .72, p < .05). Bleeding index scores were related to cTnI and Myo levels (r = .21, p < .025; r = .19, p < .05, respectively) but not to Lk or neutrophil levels (r = .09, p = .34; r = .13, p = .18, respectively) and were therefore excluded from further analysis.
Table 1.
Sociodemographic, Clinical, and Biochemical Variables, n = 112
| Variable | Percentage | p Value |
|---|---|---|
| Sex: male | 83.9 | <.001 |
| Education: primary school | 78 | <.001 |
| Marital status: married | 92 | <.001 |
| Smoking: smokers | 47 | .128 |
| Hypertension: hypertensives | 64.3 | .002 |
| Diabetes: diabetics | 31.3 | <.001 |
| Dyslipidemia: dyslipidemics | 49.1 | .148 |
| ST elevation | 62.5 | .005 |
| Variable | Mean | SEM |
| Age | 60.42 | 0.88 |
| Smoking | 13.1 | 1.68 |
| Body mass index | 28.91 | 0.41 |
| Arbes index | 21.3 | 2.46 |
| PISI | 1.99 | 0.33 |
| Bleeding index | 11.87 | 1.64 |
| Plaque index | 8.12 | 1.02 |
| Troponin I | 40.89 | 3.64 |
| Myoglobin | 709.97 | 88.12 |
| Total leukocyte count | 10.73 | 0.39 |
| Neutrophils | 71.09 | 1.13 |
PISI, Periodontal Inflammatory Severity Index; STEMI, ST segment elevation myocardial infarction.
Given that the confounders were in general associated with the outcomes (cTnI and Myo) rather than with the predictors or potential mediators, a mediation regression model was tested in which confounders were linked to the 2 outcomes.
Hierarchical linear regression (Table 2) showed that after the removal of confounding effects (Model I), the addition of the Arbes index (Model II) improved the prediction of cTnI levels by 4.1% (R2 change = .041, β = 0.005, p = .018, whole-model adjusted R2 = .21, F[11, 100] = 3.65, p < .001). Similar results were observed for Myo levels (R2 change = .03, β = 0.004, p < .05, whole-model adjusted R2 = .176, F[11, 100] = 3.16, p = .001), total Lk counts (R2 change = .047, β = 0.001, p = .018), and neutrophil counts (R2 change = 0.059, β = 0.001, p < .01, whole-model adjusted R2 = .098). ST elevation and sex significantly contributed to the predictions of the levels of cTnI (β = 0.491, p < .001; β = −0.277, p = .051, respectively) and Myo (β = 0.363, p = .001; β = −0.43, p = .003). Hypertension also contributed to the prediction of Myo levels (β = 0.215, p = .047). However, these factors made no significant contribution to the prediction of Lk or neutrophil counts. Figure 1 depicts the changes in the prediction of biochemical variables with the introduction of the CP extent and severity indices as predictors.
Table 2.
Hierarchical Multiple Linear Regression Analysis for Troponin I and Myoglobin Levels
| Troponin I |
Myoglobin |
Total Leukocyte Count |
Neutrophils |
|||||
|---|---|---|---|---|---|---|---|---|
| B | p | B | p | B | p | B | p | |
| Age | −.003 | .610 | −.004 | .497 | −.002 | .351 | .001 | .510 |
| Sex | −.277 | .051 | −.430 | .003 | −.075 | .061 | −.035 | .088 |
| Marital status | .164 | .101 | .134 | .177 | .019 | .499 | −.016 | .281 |
| Education | −.061 | .438 | −.002 | .976 | −.027 | .221 | −.004 | .739 |
| ST elevation | .491 | .000 | .363 | .001 | .051 | .082 | .029 | .056 |
| Body mass index | −.017 | .156 | −.013 | .277 | .004 | .292 | .001 | .725 |
| Smoking | −.003 | .310 | −.005 | .082 | .001 | .226 | .000 | .674 |
| Hypertension | .019 | .859 | .215 | .045 | −.008 | .792 | −.018 | .260 |
| Diabetes | −.112 | .324 | −.045 | .687 | .035 | .267 | −.005 | .764 |
| Dyslipidemia | .032 | .753 | .094 | .351 | −.011 | .710 | −.025 | .086 |
| Model I: Adj. R2 | .170 | .001 | .152 | .002 | .049 | .126 | .042 | .154 |
| Model II: Change in R2 | ||||||||
| 1: CP extension | .041 | .018 | .03 | .048 | .047 | .018 | .059 | .008 |
| 2: CP severity | .031 | .040 | .023 | .083 | .028 | .07 | .025 | .087 |
B, unstandardized regression coefficient; CP, chronic periodontitis.
Confounders were introduced in model I, and CP extension and severity were introduced afterward. Changes in R2 were used to evaluate the association of CP extension or severity with the indicators of the size of myocardial infarction.
Figure 1.
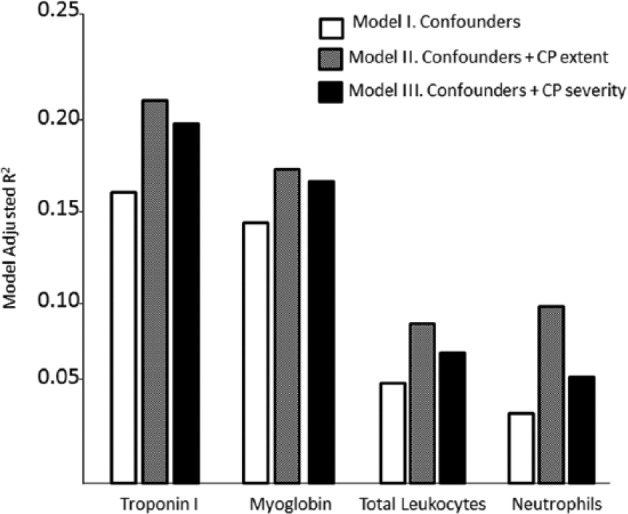
Graphic representation of increments in the prediction of biochemical indices with the introduction of chronic periodontitis (CP) extent and severity indices as predictors.
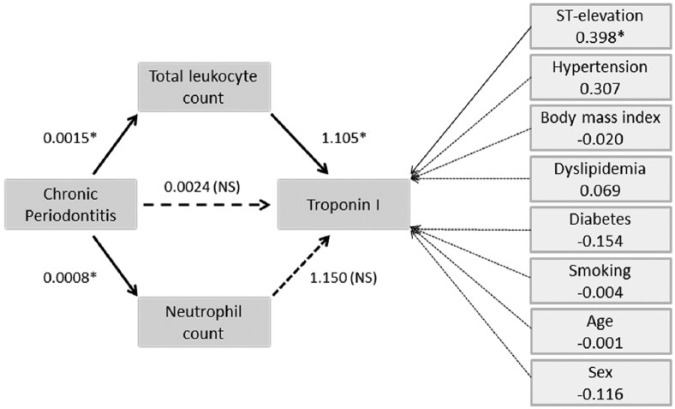
Given that only CP extent significantly accounted for variations in biochemical variables, the mediated regression analysis was restricted to the CP extent index, in which the significant CP-cTnI direct relationship became nonsignificant (c′ = 0.0024, p > .21), but the total indirect effect (mediated by Lk and neutrophil counts) was significant (estimated effect, 0.0026; BCa 95% confidence interval [CI]: 0.0011, 0.0045; Figure 2). Specifically, the indirect pathway was significant for the total Lk count (0.0016, BCa 95% CI: 0.0005, 0.0036) but not for the neutrophil count (0.0009, BCa 95% CI: –0.0001, 0.0028). The whole mediated model accounted for 40.88% of the cTnI variability (23.17% when ST elevation and the remaining covariates were removed, in which case neutrophil mediation also become significant).
Figure 2.
Mediated regression model for the chronic periodontitis–troponin I association. Arrows with dashed lines indicate nonsignificant relationships between the 2 connected variables. Arrows with solid lines show that leukocytes and neutrophils are significantly associated with an increase in troponin I levels and with chronic periodontitis. The model indicates that a higher severity of chronic periodontitis produces increased troponin I levels by augmenting the leukocyte and neutrophil counts. Troponin I levels are not affected by classical risk factors but are influenced by ST elevation. Regression coefficients are shown next to each arrow.
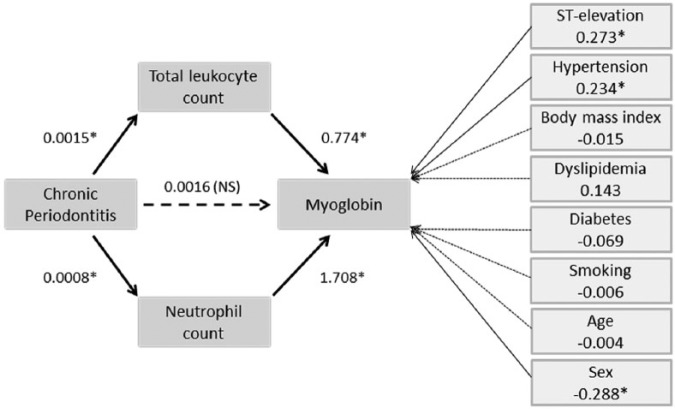
Myo serum levels were also indirectly related to CP extent (estimated indirect effect of 0.0025, BCa 95% CI: 0.011, 0.0045). Mediated regression results indicated that the total neutrophil count rather than the Lk pathway significantly mediated the CP-Myo relationship (0.0014, BCa 95% CI: 0.0003, 0.0030; 0.001, BCa 95% CI: 0.0000, 0.0032, respectively). The whole mediated model accounted for 36.95% of the Myo variability (19.32% if no account is taken of ST elevation or remaining covariates; Figure 3).
Figure 3.
Mediated regression model for the chronic periodontitis–myoglobin link.
Discussion
Three main findings were obtained in this study of AMI patients. First, cTnI and Myo levels were positively associated with indicators of the extent and severity of CP. Second, increased Lk and neutrophil counts were significantly associated with the extent of CP but only marginally associated with its severity. Finally, only the indirect pathway from CP to cTnI/Myo proved significant when these 2 general inflammatory markers were introduced as mediators in a mediated regression analysis, indicating a potential influence of CP extent on AMI size through inflammation.
The mediated regression analysis results showed that the Arbes CP extent and PISI CP severity significantly predicted cTnI levels (infarct size). Although Myo is less specific than troponin as a biomarker of myocardial necrosis, both were used in the present study because the elevation of both markers offers a better prediction of patients with NSTEMI and is associated with a higher final troponin peak, indicating an infarct of larger size (Sanchis et al., 2003). cTnI is also a useful marker for estimating infarct size in patients with STEMI (Vasile et al., 2008). Thus, its determination within 96 hr was positively correlated with infarct size, as estimated with imaging techniques such as positron-emission tomography and contrast-enhanced magnetic resonance imaging, allowing a faster assessment of the prognosis of these patients (Giannitsis et al., 2008; Steen et al., 2006).
The relationships between CP and cTnI and between CP and Myo were mediated by the total Lk count after taking ST elevation and classic AMI factors into account. Mediated linear regression analysis (MacKinnon et al., 2007) reveals whether an observed relationship between 2 variables (e.g., CP and cTnI) can be accounted for, by assuming that it depends on a third variable—that is, a mediator (e.g., total Lk or neutrophil counts). As in ordinary linear regression, when causality conditions are met, variations in the potential causal agent cause some degree of the observed variability in the outcome. Given that CP develops over years whereas cTnI and Myo increase early after AMI onset, the observed relationship cannot be in the opposite direction—that is, an increase or decrease in cTnI or Myo can be caused by direct or indirect effects of the degree of CP on the outcome variable but not vice versa. Thus, the above results indicate that CP-cTnI and CP-Myo relationships may be mediated by total Lk and neutrophil counts and that ST elevation and classic risk factors exert an influence on the outcomes (cTnI or Myo) but not, in our sample, on the mediators (Preacher and Hayes, 2008).
Total Lk and neutrophil counts are strongly associated with infarct size. Lks are attracted to necrotic foci (Chia et al., 2009), and Friedman et al. (1974) and Núñez et al. (2005) demonstrated the predictive value of Lks in AMI. Other authors described the Lk count as a simple, reproducible, and low-cost procedure offering valuable prognostic information for CVD patients (Giugliano et al., 2010). The Lk count was found to be higher with a greater degree of CP, while high endothelial venules in inflamed gingiva, not seen in healthy gingiva, were proposed as sites for Lk migration and recirculation in inflamed periodontal tissues (Wynne et al., 1988). Finally, a large predominance of neutrophils over lymphocytes in transendothelial migration has been demonstrated in patients with CP (Kasprzak et al., 2013).
The mean Lk count in the present patients (10.73 ± 3.9 [×1,000 µL]) was higher than reported by other authors (Renvert et al., 2010), and the neutrophil count was 71%, above the normal range of 42% to 52%. The relationship between white cell count and myocardial infarction in periodontal disease was first demonstrated in 1993 (Kweider et al., 1993) and has been reported in subsequent studies (Loos et al., 2000; Monteiro et al., 2009). The association between the Lk count in periodontitis and greater cardiovascular risk has been attributed to the higher activity in periodontal patients of platelets and Lks, forming platelet-Lk complexes that may contribute to increasing atherothrombotic activity (Nicu et al., 2009). In the present study, CP extent (Arbes) and severity (PISI) indices were positively correlated with peripheral blood Lk and neutrophil counts. Neutrophil counts have been related to the extent of atherosclerosis (Kostis et al., 1984) and to the destabilization of atheromatous plaque (Massberg et al., 2010). A wide study in humans demonstrated a positive correlation between the number of circulating neutrophils and the risk of a cardiovascular event (Guasti et al., 2011).
The contribution of periodontitis-associated systemic inflammation to other classic CVD risk factors is not fully known. Thus, no published study has addressed the relationship between the extent or severity of CP and AMI size. In this paper, we compared 2 clinical indices of CP with biomarkers of cardiac necrosis; however, further studies of crevicular fluid markers related to CP intensity would be needed to verify these novel findings. It is also necessary to follow up periodontal patients who have suffered an AMI to determine whether they have a worse clinical evolution (e.g., new coronary event, heart failure, death). If this proves to be the case, it might be possible to consider periodontitis as a predictor of AMI development and to propose its inclusion in risk stratification scores (TIMI and GRACE risk scores).
Study limitations include the observational design and relatively small sample size. Although statistically significant, the variability of the studied markers was low, and the results should be interpreted with caution. It would also have been useful to determine other markers of inflammation and cell activation to complement the present results. Further studies are required to verify our hypothesis that periodontal disease may be a mortality risk factor and may play an important role in the prognosis of AMI.
Conclusion
To our knowledge, this study contributes the first research data showing that the extent and severity of periodontitis is positively associated with AMI size as measured by serum troponin I and Myo levels.
Footnotes
This study was funded by research group CTS-583 of the Junta de Andalucía (Andalusian Regional Government).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ainamo J, Bay I. (1975). Problems and proposals for recording gingivitis and plaque. Int Dent J 25:229-235. [PubMed] [Google Scholar]
- Albandar JM, Brunelle JA, Kingman A. (1999). Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol 70:13-29. [DOI] [PubMed] [Google Scholar]
- Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, et al. (1996). Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 335:1342-1349. [DOI] [PubMed] [Google Scholar]
- Arbes SJ, Jr, Slade GD, Beck JD. (1999). Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res 78:1777-1782. [DOI] [PubMed] [Google Scholar]
- Bahekar AA, Singh S, Saha S, Molnar J, Arora R. (2007). The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J 154:830-837. [DOI] [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. (1996). Periodontal disease and cardiovascular disease. J Periodontol 67(10 Suppl):1123-1137. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Mantyla P, Paju S, Peltola JS, Nieminen MS, Sinisalo J, et al. (2011). Periodontitis is associated with angiographically verified coronary artery disease. J Clin Periodontol 38:1007-1014. [DOI] [PubMed] [Google Scholar]
- Cueto A, Mesa F, Bravo M, Ocana-Riola R. (2005). Periodontitis as risk factor for acute myocardial infarction: a case control study of Spanish adults. J Periodontal Res 40:36-42. [DOI] [PubMed] [Google Scholar]
- Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, et al. (2009). Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 103:333-337. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washington) (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914-920. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Klatsky AL, Siegelaub AB. (1974). The leukocyte count as a predictor of myocardial infarction. N Engl J Med 290:1275-1278. [DOI] [PubMed] [Google Scholar]
- Gaziano TA, Gaziano JM. (2012). Epidemiology of cardiovascular disease. In: Harrison’s Principles of Internal Medicine, 18th ed. AS Fauci AS, Braunwald E, editors. New York, NY: McGraw-Hill. [Google Scholar]
- Geerts SO, Legrand V, Charpentier J, Albert A, Rompen EH. (2004). Further evidence of the association between periodontal conditions and coronary artery disease. J Periodontol 75:1274-1280. [DOI] [PubMed] [Google Scholar]
- Giannitsis E, Steen H, Kurz K, Ivandic B, Simon AC, Futterer S, et al. (2008). Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J Am Coll Cardiol 51:307-314. [DOI] [PubMed] [Google Scholar]
- Giugliano G, Brevetti G, Lanero S, Schiano V, Laurenzano E, Chiariello M. (2010). Leukocyte count in peripheral arterial disease: a simple, reliable, inexpensive approach to cardiovascular risk prediction. Atherosclerosis 210:288-293. [DOI] [PubMed] [Google Scholar]
- Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, et al. (2011). Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation: a systematic review on more than 34,000 subjects. Thromb Haemost 106:591-599. [DOI] [PubMed] [Google Scholar]
- Kasprzak A, Surdacka A, Tomczak M, Konkol M. (2013). Role of high endothelial postcapillary venules and selected adhesion molecules in periodontal diseases: a review. J Periodontal Res 48:1-21. [DOI] [PubMed] [Google Scholar]
- Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. (2009). Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 361:868-877. [DOI] [PubMed] [Google Scholar]
- Kostis JB, Turkevich D, Sharp J. (1984). Association between leukocyte count and the presence and extent of coronary atherosclerosis as determined by coronary arteriography. Am J Cardiol 53:997-999. [DOI] [PubMed] [Google Scholar]
- Kweider M, Lowe GD, Murray GD, Kinane DF, McGowan DA. (1993). Dental disease, fibrinogen and white cell count: links with myocardial infarction? Scott Med J 38:73-74. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. (2002). Inflammation and atherosclerosis. Circulation 105:1135-1143. [DOI] [PubMed] [Google Scholar]
- Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. (2000). Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 71:1528-1534. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. (2007). Mediation analysis. Annu Rev Psychol 58:593-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyla P, Buduneli E, Emingil G, Tervahartiala T, Pussinen PJ, Baris N, et al. (2012). Acute myocardial infarction elevates serine protease activity in saliva of patients with periodontitis. J Periodontal Res 47:345-353. [DOI] [PubMed] [Google Scholar]
- Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. (2010). Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 16:887-896. [DOI] [PubMed] [Google Scholar]
- Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesaniemi YA, Syrjala SL, et al. (1989). Association between dental health and acute myocardial infarction. BMJ 298:779-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro AM, Jardini MA, Alves S, Giampaoli V, Aubin EC, Figueiredo Neto AM, et al. (2009). Cardiovascular disease parameters in periodontitis. J Periodontol 80:378-388. [DOI] [PubMed] [Google Scholar]
- Nicu EA, Van der Velden U, Nieuwland R, Everts V, Loos BG. (2009). Elevated platelet and leukocyte response to oral bacteria in periodontitis. J Thromb Haemost 7:162-170. [DOI] [PubMed] [Google Scholar]
- Núñez J, Fácila L, Llàcer A, Sanchís J, Bodí V, Bertomeu V, et al. (2005). [Prognostic value of white blood cell count in acute myocardial infarction: long-term mortality]. Rev Esp Cardiol 58:631-639. [PubMed] [Google Scholar]
- O’Leary TJ, Drake RB, Naylor JE. (1972). The plaque control record. J Periodontol 43:38. [DOI] [PubMed] [Google Scholar]
- Papapanagiotou D, Nicu EA, Bizzarro S, Gerdes VE, Meijers JC, Nieuwland R, et al. (2009). Periodontitis is associated with platelet activation. Atherosclerosis 202:605-611. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40:879-891. [DOI] [PubMed] [Google Scholar]
- Renvert S, Ohlsson O, Pettersson T, Persson GR. (2010). Periodontitis: a future risk of acute coronary syndrome? A follow-up study over 3 years. J Periodontol 81:992-1000. [DOI] [PubMed] [Google Scholar]
- Sanchis J, Bodí V, Llácer A, Facila L, Núñez J, Bertomeu V, et al. (2003). Usefulness of concomitant myoglobin and troponin elevation as a biochemical marker of mortality in non-ST-segment elevation acute coronary syndromes. Am J Cardiol 91:448-451. [DOI] [PubMed] [Google Scholar]
- Scott DA, Krauss J. (2012). Neutrophils in periodontal inflammation. Front Oral Biol 15:56-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. (2013). Periodontology: past, present, perspectives. Periodontol 2000 62: 7-19. [DOI] [PubMed] [Google Scholar]
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. (2006). Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 48:2192-2194. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. (2010). Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 31:2197-2204. [DOI] [PubMed] [Google Scholar]
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. (2008). Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 54:617-619. [DOI] [PubMed] [Google Scholar]
- Wynne SE, Walsh LJ, Seymour GJ. (1988). Specialized postcapillary venules in human gingival tissue. J Periodontol 59:328-331. [DOI] [PubMed] [Google Scholar]