Abstract
Whole body vibration (WBV) stimulation has a beneficial effect on the recovery of osteoporotic bone. We aimed to investigate the immediate effect of WBV on lipopolysaccharide (LPS)–mediated inflammatory bone loss by varying the exposure timing. Balb/C mice were divided into the following groups: control, LPS (L), and LPS with vibration (LV). The L and LV groups received LPS (5 mg/kg) by 2 intraperitoneal injections on days 0 and 4. The LV group was exposed to WBV (0.4 g, 45 Hz) either during LPS treatment (LV1) or after cessation of LPS injection (LV2) and then continued WBV treatment for 10 min/d for 3 d. Evaluation based on micro–computed tomography was performed 7 d after the first injection, when the L group showed a significant decrease in bone volume (−25.8%) and bone mineral density (−33.5%) compared with the control group. The LV2 group recovered bone volume (35%) and bone mineral density (19.9%) compared with the L group, whereas the LV1 group showed no improvement. This vibratory signal showed a suppressive effect on the LPS-mediated induction of inflammatory cytokines such as IL-1β or TNF-α in human mesenchymal stem cells in vitro. These findings suggest that immediate exposure to WBV after the conclusion of LPS treatment efficiently reduces trabecular bone loss, but WBV might be less effective during the course of treatment with inflammatory factor.
Keywords: cytokines, inflammation, lipopolysaccharides, tibia, resorption, recovery
Introduction
Lipopolysaccharide (LPS), a bacterial constituent in the cell wall of Gram-negative bacteria, has been reported to potently stimulate bone resorption both in vitro and in vivo (Orcel et al., 1993; Itoh et al., 2003). LPS is a main pathogenic factor of periodontal disease, and it has been shown to induce increased expression of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and prostaglandin E2 (PGE2) from osteoblasts as well as macrophages (Aznar et al., 1990; Nair et al., 1996; Itoh et al., 2003). LPS-injected mice show bone loss in alveolar bone and in the femur, with a rapid increase in the number of osteoclasts and the surface area of erosion (Droke et al., 2007; Tomomatsu et al., 2009). Thus, this small animal model with controlled LPS injection provides an efficient tool for the induction of inflammatory bone loss.
Because bone loss has many severe clinical implications, methods by which bone loss might be mitigated or reversed are the focus of many studies. For example, whole body vibration (WBV) at a low magnitude and high frequency delivered via oscillatory platforms is being explored to improve bone quality (Liu et al., 2011). In addition, many studies on WBV treatment have shown successful outcomes that involve the prevention of postmenopausal bone loss (Rubin et al., 2004) and anabolic effect in trabecular bone in those with diseases such as cerebral palsy (Ward et al., 2004), highlighting its potential as an option for a non-drug intervention. A possible mechanism by which WBV affects bone involves activation of mechanotransduction by increasing fluid flow in bone and stimulation of osteogenesis, similar to the effect of physical activity (Shafrir and Forgacs, 2002; Totosy de Zepetnek et al., 2009). Another suggestion is that WBV might indirectly influence the regulation of bone remodeling via an acute change in testosterone and growth hormone levels (Bosco et al., 2000). Despite their different etiology, bone loss induced by an inflammatory response shares common properties with osteoporotic bone loss induced by estrogen deficiency in that both processes involve severe bone loss in the femur and the induction of inflammatory cytokines, such as IL-1, IL-6, and PGE2 (Kobayashi et al., 2012). Thus, it is highly possible that WBV stimulation exerts a protective effect against inflammatory bone loss, as seen in osteoporosis. Because there is little evidence of an effect of WBV on bone loss resulting from inflammatory response, in this study we tested the effect of varying the exposure timing of WBV using an LPS-injected mouse model that shows acute bone loss.
The current study was performed in vivo and in vitro. The in vivo study involved the characterization of an acute bone loss murine model induced by intraperitoneal injection of LPS. Evaluation was based on micro–computed tomography to first determine the suitable timing and skeletal site to observe the LPS effect and then to investigate the effect of low-magnitude and high-frequency WBV on LPS-mediated bone loss and its dependence on the timing of the vibratory stimulation (either during or after the LPS treatment). This study also included an in vitro investigation into the effect of vibration on the LPS-mediated expression of inflammatory cytokines or osteoblast differentiation-related markers using human mesenchymal stromal cells (hMSCs) to suggest the relevance of this study at human cells.
Materials & Methods
Experimental Design in vivo
Six-week-old Balb/C mice (total n = 38) were used in this study. The experimental protocol was approved by the Animal Care and Use Committee of Seoul National University. The mice were divided into a control group (n = 13), an LPS-treated group (L; n = 13), and an LPS + WBV group (LV; n = 12). The control group was injected with vehicle (sterile water), and the L group was intraperitoneally injected on days 0 and 4 with LPS (5 mg/kg; Escherichia coli 0111:B4, Sigma-Aldrich) according to previously described methods with a slight modification (Miyaura et al., 2003). In the LV group, mice were exposed to WBV on the day after the first LPS injection (LV1; both legs of 6 mice) or on the day after the second injection (LV2; both legs of 6 mice) for 10 min/d. Both groups received WBV (0.4 g, 45 Hz) for 3 consecutive days through an adjustable vibratory platform (Turbosonic Korea, Seoul, Korea) adapted to a special cage (Appendix Figure). WBV exposure was performed in the same room with the non-vibrated animals, which were left unstimulated in the cages with a distance of 2 m from the vibration machine. A placebo treatment for the non-WBV group was not done. Animals were sacrificed at 7 or 14 d after the first injection, and further protocols for evaluation of bone structure are described in detail in the Appendix.
Vibratory Effect on LPS-treated hMSCs in vitro
Primary hMSCs were isolated from the bone marrow of a healthy donor (a 25-year-old man) as previously described (Caterson et al., 2002). Detailed culture methods, identification of stem cell markers, and multipotency of hMSCs are described in the Appendix. hMSCs were treated with LPS at doses of 10, 100, and 1000 ng/mL in DMEM supplemented with ascorbic acid (50 µg/mL) and β-glycerophosphate (10 mM). As a control, another sample of hMSCs was treated without or with the same regimen of LPS but was not exposed to vibration. Beginning the day after plating, experimental groups received WBV (0.4 g, 45 Hz) daily for 10 min for 2 d, as described in our previous study (Kim et al., 2012). Non-vibrated control plates were placed on a clean bench during vibration exposure of the experimental group. After vibratory stimulation, gene expression of hMSCs was evaluated using real-time reverse transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA). Detailed methods and oligonucleotide primers used for real-time RT-PCR and methods for ELISA are described in the Appendix.
Statistical Analysis
All data are presented as mean ± standard error of the mean or standard deviation. Statistical analyses were performed with SPSS 20 (IBM Co., Armonk, NY, USA). Data between the 2 groups were evaluated with a 2-tailed Student’s t test, and the comparison of data in more than 2 groups was done through a 1-way analysis of variance in the animal studies according to the Tukey method for the post hoc test. In vitro results were evaluated with 2-way analysis of variance post hoc via the Bonferroni method for the comparison of the 4 LPS dosages with or without vibration. Differences with p < .05 were considered significant.
Results
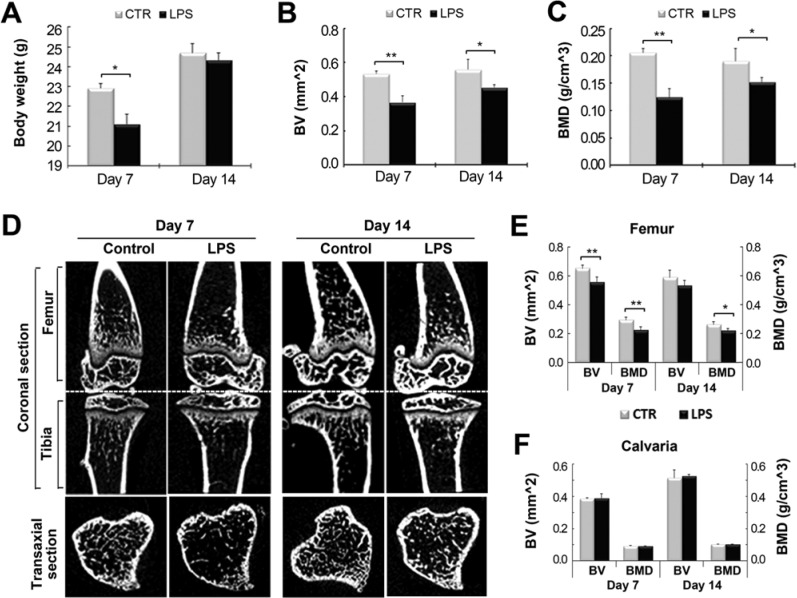
We evaluated the change in bone structure at different skeletal sites to determine the effect of LPS without WBV by evaluation based on micro–computed tomography, with the trabecular bone being evaluated 1 mm under the cartilage growth plate in the metaphyseal area (region 1) of the tibia and femur. The calvariae were analyzed in a square area (5 × 5 mm) with the central point located at the intersection of the coronal suture and the sagittal suture on the superior middle portion of the calvaria. Seven days after the first injection, the mean weight, mean bone volume (BV), and mean bone mineral density (BMD) in tibia and femur of the L group were significantly lower than those of the vehicle-treated control group. Body weight was reduced by 7.9% (p < .05) on day 7 but had recovered on day 14 almost to the value of the control group (Figure 1A). Bone loss in the tibia was most severe on day 7, accompanied by a decrease in BV (−31.2%, p < .01) and BMD (−39.6%, p < .01) (Figure 1B, C). This trend of decreased BV and BMD was maintained until 14 d post-LPS injection, with a 18.5% decrease (p < .05) in BV and 20.1% decrease (p < .05) in BMD. The change of BV and BMD of the femur in the L group followed a similar trend of that of tibia in the L group with a decrease of BV (−14.9%) and BMD (−23.4%) (both, p < .01) on day 7, compared with the control group (Figure 1E). However, the decrease of BV (−10.1%) of femur in L group was not significant on day 14, although BMD of femur was still decreased by 16.1% (p < .05). In addition, there was no change in BV and BMD in the calvaria during the observation period (Figure 1F). On the basis of these results, we chose day 7 as the time point to observe the effect of WBV on trabecular bone loss of the tibia and femur induced by LPS injection in subsequent experiments.
Figure 1.
Characterization of lipopolysaccharide (LPS)–induced bone loss model. LPS (5 mg/kg) was administered to Balb/C mice by 2 intraperitoneal injections on days 0 and 4. Body weight and micro–computed tomography (micro-CT) analysis of trabecular bone of tibia, femur, and calvaria were assessed 7 and 14 d after the first injection of LPS. (A) The effect of LPS on body weight. * p < .05 indicates a significant difference from the non-injected control group (CTR). (B, C) Bone volume (BV) and bone mineral density (BMD) of a 1-mm-long region under the growth plate of the metaphyseal area of the tibial trabecular bone were determined by micro-CT analysis. * p < .05 and ** p < .01 indicate significant differences from the control group. (D) Micro-CT images of the coronal sections of tibia and femur and the transaxial section of tibia. The effect of LPS injections on bone loss in trabecular bone of the femur (E) and calvaria (F) was evaluated by assessing BV and BMD. ** p < .01 indicates significant difference from the control group.
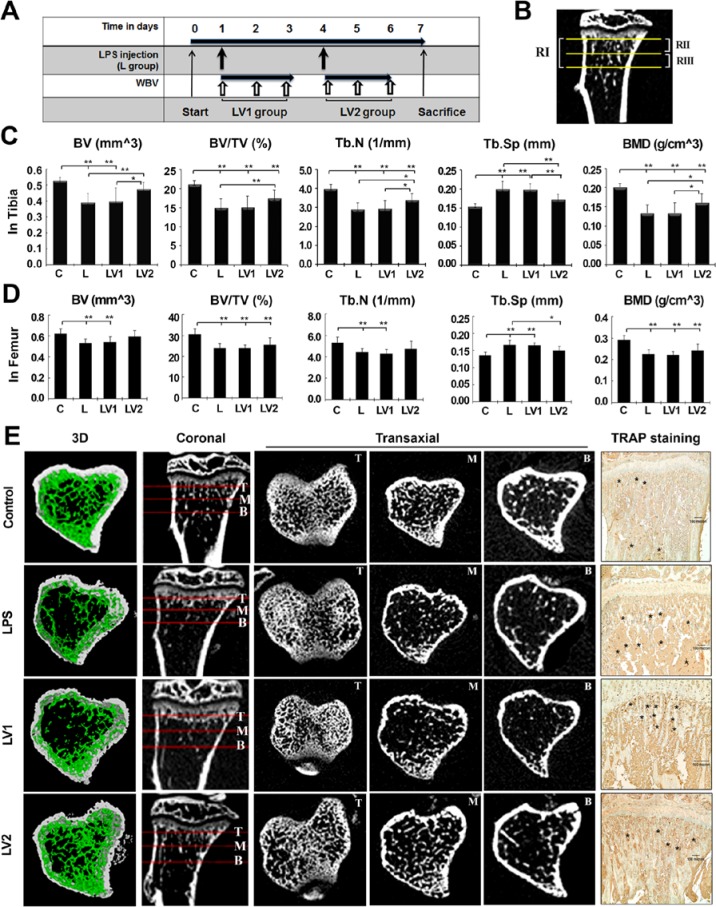
The effect of WBV was investigated by varying the timing of exposure to 0.4 g and 45 Hz, conditions that promoted bone regeneration of the rat calvarial defect in our previous study (Hwang et al., 2009). For analysis of region 1 of tibia, all parameters of L group showed significant differences from control group. LV2 group showed a 21.9% (p < .01) and 16.5% (p < .01) increase in BV and BV/tissue volume (TV), respectively, compared with the L group, whereas there was no change in the BV and BV/TV of the LV1 group. Measurements of BMD of the LV2 group followed a similar trend to BV, with a 20.0% increase (p < .05) (Figure 2C). Consistent with these findings, the trabecular number of the LV2 group increased by 17.5% (p < .05) compared with the L group, whereas trabecular separation decreased by 13.7% (p < .01), suggesting that WBV promoted a more dense structure of trabecular bone. We further evaluated bone parameters by splitting region 1 of tibia into the 0.5-mm section most proximal to the growth plate (region 2) and the 0.5-mm section most distal to the growth plate (region 3) to find the region most sensitive to LPS treatment (Figure 2B). BV/TV of the L group was decreased by 28.7%, 24.5%, and 37.1% in regions 1, 2, and 3, respectively, (all p < .01), indicating that LPS-mediated bone loss was greatest at region 3. The same trend was seen for the WBV effect in the LV2 group for the sequence of region 3, region 1, and region 2 with BV increasing by 32.4%, 21.9%, and 17.6%; with BV/TV increasing by 25.3%, 16.5%, and 13.3%; and with BMD increasing by 36.7%, 20.0%, and 15.4%, respectively, compared with the L group (region 1 and 3, p < .01; region 2, p < .05). The detailed values of histomorphometric parameters for all groups are described in the Table. However, WBV failed to rescue bone loss in the analysis of region 1 of femur (Figure 2D) and body weight (Appendix). TRAP expression as an osteoclast marker was apparent in the L group and LV1 group, compared with the control or LV2 group (Figure 2E).
Figure 2.
Immediate exposure to whole body vibration (WBV) reduces lipopolysaccharide (LPS)–mediated trabecular bone loss of the tibia. (A) Schematic representation of experimental protocols. The mice were divided into a control group (C), LPS-treated group (L; 5 mg/kg), and LPS + WBV group (LV). Mice of the LV group were exposed to WBV (45 Hz, 0.4 g) the day after the first LPS injection (LV1) or the day after the second injection (LV2) for 10 min/d for 3 d. All animals were sacrificed 7 d after the first injection of LPS. (B) According to micro–computed tomography (micro-CT) analysis, the 1-mm-long area under the growth plate of the metaphyseal area (region 1) of each group was divided into the proximal 0.5-mm portion (region 2) and the distal 0.5-mm portion (region 3) to identify the most sensitive region to LPS treatment. RI, RII, and RIII indicate regions 1, 2, and 3, respectively. (C) Histomorphometric parameters obtained at the end of the experiment include bone volume/tissue volume (BV/TV), trabecular number (Tb.N), trabecular separation (Tb.Sp), and bone mineral density (BMD) of region 1. Values are expressed as mean ± SD. * p < .05 and ** p < .01 indicate significant differences between the 2 groups. (D) BV and BMD of the femur in RI (defined as for the tibia). (E) Micro-CT images of coronal sections and transaxial sections are presented at the top, middle, and bottom portions of RI. Three-dimensional reconstructions of RIII support quantifiable changes in both density and architecture of the bone. Representative images of immunohistochemical staining for TRAP expression are presented to detect osteoclasts. Marked positions with asterisks correspond to cells. Scale bar indicates 100 µm.
Table.
Histomorphometric Parameters of Micro–computed Tomography Obtained at the End of the Experiment
| Volume |
Trabecular |
||||||
|---|---|---|---|---|---|---|---|
| Group | Bone, mm3 | Tissue, mm3 | Bone/Tissue, % | Number, 1/mm | Separation, mm | Thickness, mm | BMD, g/cm3 |
| Region 1 | |||||||
| C | 0.52 ± 0.02 | 2.47 ± 0.06 | 21.17 ± 0.96 | 3.96 ± 0.23 | 0.15 ± 0.00 | 0.05 ± 0.00 | 0.20 ± 0.00 |
| L | 0.39 ± 0.05 ** | 2.58 ± 0.12* | 15.10 ± 2.16** | 2.88 ± 0.34** | 0.19 ± 0.02** | 0.05 ± 0.00 | 0.13 ± 0.02** |
| LV1 | 0.39 ± 0.08 ** | 2.60 ± 0.17* | 15.17 ± 2.83** | 2.92 ± 0.43** | 0.19 ± 0.01** | 0.05 ± 0.00 | 0.13 ± 0.03** |
| LV2 | 0.47 ± 0.04 †† | 2.71 ± 0.20 **† | 17.58 ± 2.02 **†† | 3.39 ± 0.35 **†† | 0.17 ± 0.01 **†† | 0.05 ± 0.00 | 0.16 ± 0.02 **†† |
| Region 2 | |||||||
| C | 0.34 ± 0.01 | 1.38 ± 0.04 | 25.10 ± 0.92 | 4.84 ± 0.25 | 0.12 ± 0.00 | 0.05 ± 0.00 | 0.24 ± 0.02 |
| L | 0.27 ± 0.05** | 1.46 ± 0.10* | 18.95 ± 3.00** | 3.67 ± 0.47** | 0.16 ± 0.01** | 0.05 ± 0.00 | 0.17 ± 0.03** |
| LV1 | 0.29 ± 0.07* | 1.45 ± 0.12 | 19.84 ± 4.25** | 3.84 ± 0.64** | 0.15 ± 0.01** | 0.05 ± 0.00 | 0.18 ± 0.04** |
| LV2 | 0.32 ± 0.03 †† | 1.52 ± 0.04 ** | 21.46 ± 1.95 **† | 4.19 ± 0.35 **†† | 0.13 ± 0.01 **†† | 0.05 ± 0.00 | 0.20 ± 0.02 **† |
| Region 3 | |||||||
| C | 0.17 ± 0.01 | 1.10 ± 0.03 | 15.92 ± 1.42 | 2.88 ± 0.28 | 0.18 ± 0.00 | 0.06 ± 0.01 | 0.15 ± 0.01 |
| L | 0.11 ± 0.02** | 1.12 ± 0.04 | 10.02 ± 1.98** | 1.91 ± 0.33** | 0.22 ± 0.02** | 0.05 ± 0.01 | 0.08 ± 0.03** |
| LV1 | 0.10 ± 0.02** | 1.14 ± 0.06 | 9.21 ± 1.63** | 1.8 ± 0.27** | 0.22 ± 0.01** | 0.05 ± 0.00 | 0.07 ± 0.02** |
| LV2 | 0.14 ± 0.02 **†† | 1.18 ± 0.07 **†† | 12.55 ± 2.53 **†† | 2.41 ± 0.43 **†† | 0.19 ± 0.01 †† | 0.05 ± 0.00 | 0.11 ± 0.03 **†† |
Values are expressed as mean ± SD. Balb/C mice were divided into control (C), lipopolysaccharide (L), and lipopolysaccharide with vibration (LV). The L and LV groups received lipopolysaccharide (5 mg/kg) by 2 intraperitoneal injections on days 0 and 4. The LV group was exposed to whole body vibration (0.4 g, 45 Hz) either during lipopolysaccharide treatment (LV1) or after stop of lipopolysaccharide injection (LV2), then proceeding to 10 min/d for 3 d. After sacrifice at day 7 after first injection, bone parameters were evaluated by splitting the 1-mm-long volume under the growth plate (region 1) into the 0.5 mm most proximal to the growth plate (region 2) and the 0.5 mm most distal to the growth plate (region 3).
BMD, bone mineral density.
p < .05. ** p < .01. Significantly different from control group.
p < .05. †† p < .01. Significantly different from lipopolysaccharide group.
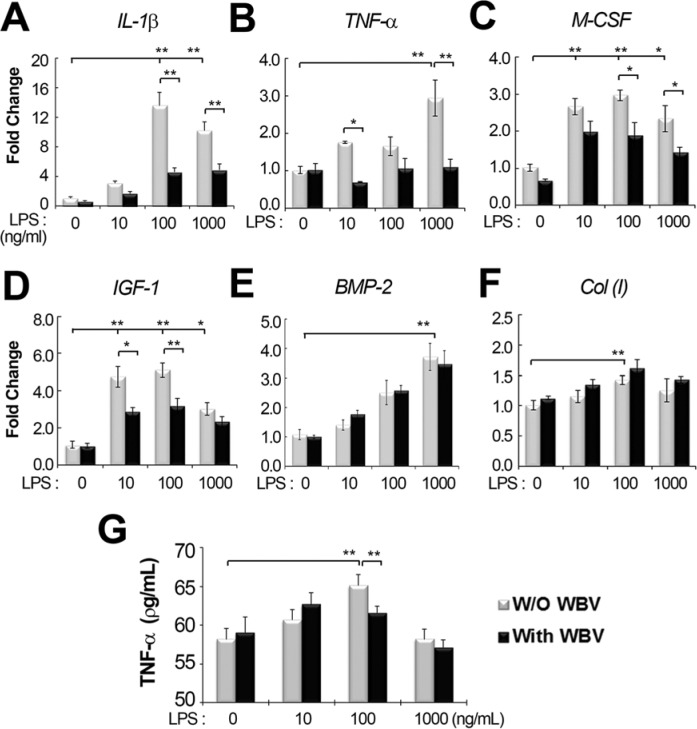
The effect of vibration on hMSCs was investigated in vitro. Over a 2-d observation period, the effect of increasing doses of LPS (10, 100, and 1000 ng/mL) and the effect of vibration on gene expression were investigated via real-time RT-PCR (Figure 3A-F). The IL1B, TNFA, and PGE2 genes are known to be induced by LPS treatment (Itoh et al., 2003). L group showed increased expression of IL-1β and TNF-α, compared with the no-LPS, non-vibrated control group. However, the amplification of these genes from cDNA occurred close to the cycle of blank control, which was RT-PCR mixes without cDNA, indicating a minor transcriptional expression. Additionally, there was little expression of PGE2. The L group also showed upregulation of bone formation markers such as type I collagen (ColI), BMP-2, IGF-1, and another resorption-related cytokine, M-CSF, compared with the control group. The induction of IL1B by LPS was dose dependent between 10 and 100 ng/mL of LPS, whereas the expression of other genes was independent of LPS concentration. The LV group exhibited reduced expression of IGF-1 and the resorption-related cytokines IL-1β, TNF-α, and M-CSF, compared with the L group, but did not have a suppressive effect on the LPS-mediated increase in ColI and BMP-2 expression. The amount of secreted TNF-α was very low at the range of 50 to 70 ρg/mL over all in ELISA. A LPS-mediated increase was detected just at 100 ng/mL of LPS, where the combined treatment of WBV resulted in a minor decrease by 5.5 % (p < .01) compared to the treatment of LPS alone (Figure 3G).
Figure 3.
Effect of whole body vibration (WBV) on lipopolysaccharide (LPS)–induced gene expression. Human mesenchymal stromal cells (hMSCs) were treated with increasing doses of LPS (0, 10, 100, and 1000 ng/mL) and stimulated without (W/O) or with WBV (0.4 g, 45 Hz) for 10 min/d for 2 d. Real-time reverse transcription polymerase chain reaction was performed to assess the transcript levels of (A) IL1B, (B) TNFA, (C) M-CSF, (D) IGF-1, (E) BMP-2, and (F) Col (I). (G) Extracellular release of TNF-α was measured with ELISA. * p < .05 and ** p < .01 indicate significant differences between the 2 groups.
Discussion
This study investigated the effect of WBV on inflammatory bone loss using LPS injection, which leads to acute bone loss in a small animal model (Inada et al., 2006). Assessment of the pattern of acute bone loss pattern after 2 intraperitoneal injections of LPS at 3-d intervals showed that the tibia was the most sensitive skeletal site and that peak timing of bone loss was approximately 7 d after the first injection, consistent with a previous study (Chiang et al., 1999). The murine LPS model used in this study failed to show bone loss in the calvarial region, explaining why LPS is locally injected into the outside of the mouse lower gingiva for the periodontitis model (Rogers et al., 2007; Kobayashi et al., 2012). Nonetheless, the LPS model is a suitable experimental method for the induction of short-term bone loss, showing a pattern typical of osteoporotic bone, and is appropriate for use as a screening tool to identify new anticatabolic agents.
Exposure to WBV to improve bone quality that is affected by osteoporotic conditions has been usually applied to animals during drug treatment or while under systemic pressure for bone resorption, such as an ovariectomized state (Judex et al., 2007; de Oliveira et al., 2010). Our data indicate that a switch in the treatment strategy of WBV would enhance its efficacy. Although our experimental design has some limitations regarding the generalization of the WBV effect, the current findings indicate that the effect of WBV differs depending on skeletal sites or treatment timing. Although LPS induced bone loss in the tibia and femur, WBV rescued bone loss only in the tibia during this short-term observation, probably because of the distance of the femur from the oscillating platform. However, in another study, the WBV signal did stimulate bone formation of the femur in long-term observations of over 1 yr (Rubin et al., 2002). Interestingly, the effect of WBV differed according to its timing relative to exposure to inflammatory factor. WBV stimulated an increase in the quantity and quality of bone if it was given immediately after the LPS treatment ended, but it had no significant effect if given during the LPS treatment time. Lack of improvement in the LV1 group is presumably attributed to the experimental design, in which WBV was stopped at the time of the second LPS injection and therefore could not prevent the second round of bone loss. The condition of the LV1 group is presumably related to the fact that the bone is being exposed to inflammatory factors. In contrast, in the LV2 group WBV effectively attenuated bone loss in the absence of an additional strong signal of bone loss; given the in vitro gene expression results, this might be partly mediated by modulation of LPS signaling. One recent study reported the healing effect of low-magnitude and high-frequency vibration on bone loss induced by glucocorticoids, which are an important cause of secondary osteoporosis in humans (de Oliveira et al., 2010). In this case, WBV stimulation during glucocorticoid treatment did not improve BV or BMD but did improve trabecular number and separation in the nine-week long-term observation period. With our findings, these results indicate that WBV therapy might be less effective when a signal-inducing inflammatory factor is present.
The in vitro response of hMSCs to LPS was consistent with our findings in macrophages of rodents: LPS upregulated the expression of IL1B, TNFA, and M-CSF genes (Aznar et al., 1990), which are known to stimulate osteoclast activation. However, LPS also increased the expression of ColI, BMP-2, and IGF-1 in hMSCs, which is consistent with previous reports of increased production of IGF-I, FGF-2, and VEGF from MSCs incubated with LPS (200 ng/mL) (Crisostomo et al., 2008). These results indicate that LPS plays dual roles in osteogenesis by contributing to both anabolic and catabolic processes, as seen with other proinflammatory cytokines (Mountziaris and Mikos, 2008). In a search for the molecular effects of WBV on LPS signaling, we found that vibratory signals modulated the LPS-induced expression of several of these genes. For example, vibratory signals significantly inhibited the LPS-mediated upregulation of TNFA, IL1B, M-CSF, and IGF-1, whereas the upregulation of ColI and BMP-2 genes was not affected by vibration. Taken together, these results indicate that LPS signaling in hMSCs has a similar pattern in the expression of inflammatory cytokines between MSCs and macrophages, which was selectively suppressed by WBV. However, further study is necessary for the WBV effect on LPS signaling at other cellular levels, such as macrophages, to confirm that the effect of WBV on efficient reduction in inflammatory bone loss in vivo is linked to its suppression on TNFA, IL1B, or M-CSF expression, given that the LPS-mediated expression of TNFA or IL1B is detected at a minor level in hMSCs.
In conclusion, this study showed that WBV attenuates inflammatory bone loss induced by LPS depending on exposure timing. WBV rescued trabecular bone loss of the tibia if it was applied immediately after LPS drug treatment, but it was less efficient if given during the drug treatment period. This WBV effect might be partly mediated by the suppression on LPS-mediated upregulation of inflammatory cytokines such as TNF-α, IL-1β, or M-CSF.
Supplementary Material
Acknowledgments
We thank J.J. Han, T.H. Cho, and J.H. Oh for their assistance in preparation of the manuscript.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2011-0026313).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aznar C, Fitting C, Cavaillon JM. (1990). Lipopolysaccharide-induced production of cytokines by bone marrow-derived macrophages: dissociation between intracellular interleukin 1 production and interleukin 1 release. Cytokine 2:259-265. [DOI] [PubMed] [Google Scholar]
- Bosco C, Iacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, et al. (2000). Hormonal responses to whole-body vibration in men. Eur J Appl Physiol 81:449-454. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. (2002). Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol 20:245-256. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Kyritsis G, Graves DT, Amar S. (1999). Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun 67:4231-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. (2008). Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294:C675-682. [DOI] [PubMed] [Google Scholar]
- de Oliveira ML, Bergamaschi CT, Silva OL, Nonaka KO, Wang CC, Carvalho AB, et al. (2010). Mechanical vibration preserves bone structure in rats treated with glucocorticoids. Bone 46:1516-1521. [DOI] [PubMed] [Google Scholar]
- Droke EA, Hager KA, Lerner MR, Lightfoot SA, Stoecker BJ, Brackett DJ, et al. (2007). Soy isoflavones avert chronic inflammation-induced bone loss and vascular disease. J Inflamm (Lond) 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Lublinsky S, Seo YK, Kim IS, Judex S. (2009). Extremely small-magnitude accelerations enhance bone regeneration: a preliminary study. Clin Orthop Relat Res 467:1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. (2006). Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J Immunol 177:1879-1885. [DOI] [PubMed] [Google Scholar]
- Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, et al. (2003). Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J Immunol 170:3688-3695. [DOI] [PubMed] [Google Scholar]
- Judex S, Lei X, Han D, Rubin C. (2007). Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40:1333-1339. [DOI] [PubMed] [Google Scholar]
- Kim IS, Song YM, Lee B, Hwang SJ. (2012). Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J Dent Res 91:1135-1140. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matsumoto C, Hirata M, Tominari T, Inada M, Miyaura C. (2012). The correlation between postmenopausal osteoporosis and inflammatory periodontitis regarding bone loss in experimental models. Exp Anim 61:183-187. [DOI] [PubMed] [Google Scholar]
- Liu PY, Brummel-Smith K, Ilich JZ. (2011). Aerobic exercise and whole-body vibration in offsetting bone loss in older adults. J Aging Res 2011:379674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura C, Inada M, Matsumoto C, Ohshiba T, Uozumi N, Shimizu T, et al. (2003). An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med 197:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountziaris PM, Mikos AG. (2008). Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev 14:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. (1996). Bacterially induced bone destruction:mechanisms and misconceptions. Infect Immun 64:2371-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcel P, Feuga M, Bielakoff J, De Vernejoul MC. (1993). Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol 264(3, pt 1):E391-E397. [DOI] [PubMed] [Google Scholar]
- Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. (2007). Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol 78:550-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, et al. (2002). Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res 17:349-357. [DOI] [PubMed] [Google Scholar]
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. (2004). Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19:343-351. [DOI] [PubMed] [Google Scholar]
- Shafrir Y, Forgacs G. (2002). Mechanotransduction through the cytoskeleton. Am J Physiol Cell Physiol 282:C479-C486. [DOI] [PubMed] [Google Scholar]
- Tomomatsu N, Aoki K, Alles N, Soysa NS, Hussain A, Nakachi H, et al. (2009). LPS-induced inhibition of osteogenesis is TNF-alpha dependent in a murine tooth extraction model. J Bone Miner Res 24:1770-1781. [DOI] [PubMed] [Google Scholar]
- Totosy de Zepetnek JO, Giangregorio LM, Craven BC. (2009). Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J Rehabil Res Dev 46:529-542. [DOI] [PubMed] [Google Scholar]
- Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. (2004). Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 19:360-369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.