Abstract
Vitamin D deficiency and oral diseases (periodontitis, caries, and tooth loss) are highly prevalent in Germany. Previous studies suggested that vitamin D might be a modifiable and protective factor for periodontitis, caries, and tooth loss. However, prospective studies investigating such associations are limited. We explored the association between the concentration of serum 25-hydroxy vitamin D (25OHD) and incidence of tooth loss, progression of clinical attachment loss (CAL) ≥ 3 mm, and progression of restorative and caries status in a population-based longitudinal study. We analyzed data from 1,904 participants from the Study of Health in Pomerania with a five-year follow-up. Generalized estimating equation models were applied to evaluate tooth-specific associations between serum 25OHD and incidence of tooth loss, progression of CAL ≥ 3 mm, and progression of restorative and caries status. Age, sex, education, smoking status, alcohol drinking, waist circumference, dental visit frequency, reasons of dental visit, vitamin D or calcium supplements, and season of blood draw were considered as confounders. Serum 25OHD was inversely associated with incidence of tooth loss. A significant dose-response relationship (p = .0022) was observed across the quintiles of serum 25OHD. After adjusting for multiple confounders, each 10-µg/L increase of serum 25OHD was associated with a 13% decreased risk of tooth loss (risk ratio: 0.87; 95% confidence interval: 0.79, 0.96). The association was attenuated for changes of CAL ≥ 3 mm when adjusting for multiple confounders. No significant association was found between serum 25OHD and caries progression. Vitamin D might be a protective factor for tooth loss. The effect might partially be mediated by its effect on periodontitis.
Keywords: periodontitis, caries, clinical attachments loss, probing depth, cohort study, Study of Health in Pomerania
Introduction
Periodontitis is highly prevalent in Germany (Holtfreter et al., 2009) and throughout the world (Eke et al., 2012). It is a chronic inflammatory disease characterized by periodontal attachment loss. Dental caries is another very common oral disease with a high prevalence in the elderly (Liu et al., 2013). Both periodontitis and caries are primary causes of tooth loss and account for 70% of tooth loss in Germany, while trauma, orthodontics, prosthodontics, and wisdom teeth account for the other 30% of tooth loss (Glockmann et al., 2011). Periodontitis, caries, and tooth loss are closely associated with poor quality of life (Al-Harthi et al., 2013), incidence of systemic diseases (Dietrich et al., 2013), and cancer (Zeng et al., 2013).
Recent studies suggested that elevated serum 25-hydroxy vitamin D (25OHD) might decrease the risk of gingival inflammation (Dietrich et al., 2005), periodontitis (Millen et al., 2013), caries (Grant, 2011), and tooth loss (Jimenez et al., 2014). Likewise, vitamin D intake was reported to protect against periodontal disease progression (Alshouibi et al., 2013). Although previous studies suggested relationships between vitamin D and periodontitis, tooth loss, or caries, they were hardly suitable to assess the causality of these relations. Prospective population-based studies with reasonable sample size are needed to further examine these associations.
In the present study, we explored the association between serum 25OHD and incident tooth loss, progression of periodontal diseases, and progression of the restorative and carious status, using five-year follow-up data from the Study of Health in Pomerania (SHIP).
Materials & Methods
Study Participants
SHIP is a population-based prospective study in northeast Germany. Details of the study design were published previously (Volzke et al., 2011). In brief, baseline examinations (SHIP-0) were conducted between 1997 and 2001: 7,008 inhabitants were sampled from the source population via a 2-stage cluster sampling design. Because of death, migration, or medical problems, 746 participants were excluded. Of the remaining 6,262 participants, 68.8% responded, resulting in 4,308 participants. The five-year follow-up (SHIP-1) was conducted between 2002 and 2006 and comprised 3,300 participants. The 11-year follow-up (SHIP-2) was conducted between 2008 and 2012 and included 2,333 participants.
For the present study, data of SHIP-1 and SHIP-2 were used, as serum 25OHD concentrations were measured only in SHIP-1. During the follow-up period, 249 participants died and 14 migrated. After exclusion of those who were lost for follow-up (n = 814), with self-reported liver disorder, kidney disorder, estimated glomerular filtration rate < 30 mL/mim/1.73m2, or pregnancy (n = 121); no teeth in SHIP-1 (n = 154); missing dental examination (n = 18); missing serum 25OHD (n = 24); and missing data on other covariates (n = 2), 1,904 participants were included in the present analyses (Figure 1).
Figure 1.
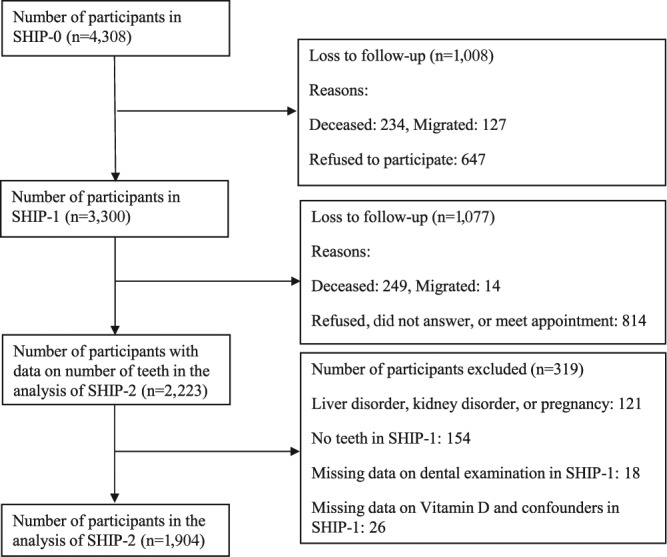
Flowchart of Study of Health in Pomerania (SHIP)
The present study was in accordance with the ethical standards of the responsible institutional or regional committee on human experimentation or in accordance with the Helsinki Declaration of 1975 as revised in 1983. The present study also complied with STROBE checklists. The study protocol was approved on December 12, 2001, by the local ethics committee of the University of Greifswald (registration number: III UV 73/01), and all participants gave informed written consent.
Assessment of Serum 25OHD
Nonfasting blood samples were taken from the cubital vein of participants in the supine position between 8:00 am and 8:00 pm. Serum aliquots were stored at −80°C. Serum 25OHD was measured on the IDS-iSYS Multi-Discipline Automated Analyser (Immunodiagnostic Systems Limited, Frankfurt am Main, Germany) with the IDS-iSYS 25-Hydroxy Vitamin D assay. During the course of the study, the coefficients of variation were 16.8%, 13.9%, and 12.0% at low, medium, and high concentrations of control material, respectively.
Dental Examinations
Dental examinations were conducted by 6 calibrated and licensed dentists in both studies (3 were identical in both studies). Tooth status (present/absent) was assessed, and the number of present teeth was counted, excluding the third molars (maximum number, 28 teeth; full mouth). In both surveys, periodontal examinations were done with a periodontal probe (SHIP-1: PCP-2; SHIP-2: PCP-11, Hu-Friedy, Chicago, IL, USA) according to the half-mouth method, alternating on the left or right side, excluding third molars. Measurements were made at 4 sites per tooth (mesiobuccal, midbuccal, distobuccal, and midlingual/midpalatal). For recording, measurements were mathematically rounded to the nearest whole millimeter. Coronal caries was assessed on a half-mouth basis. Carious defects, missing, and filled surfaces were diagnosed, and the sum of decayed and filled surfaces per tooth was calculated.
Covariates Ascertainment
Waist circumference was measured to the nearest 0.5 cm at the midpoint between the lower ribs and the iliac crest, with the participant wearing light clothes. Smoking status was defined as never, former, and current smoking. Education level was classified into < 10, 10, and > 10 yr. Dental visit frequency was recorded via “How often have you been to the dentist during the last year?” and classified into 0, 1, and ≥ 2. Alcohol drinking was classified as no drinking, 1 or 2 drinks/d, and ≥ 3 drinks/d. Reasons for last dental visit were collected via “Do you only consult a dentist because of pain or complaints or also for regular check-ups?” and the presence of loose teeth was recorded as “Do you have loose teeth?” Date of blood draw was recorded on scene and classified into 4 quarters of a year.
Statistical Analysis
Baseline characteristics were presented as mean ± standard deviation for continuous variables and numbers (percentage) for categorical variables by quintiles of serum 25OHD. Chi-square tests were used to test proportions, and univariate analyses of variance were applied to test means. The tooth-specific incidence of tooth loss, progression of clinical attachment loss (CAL) ≥ 3mm (at least 1 of the 4 measured sites of a tooth with increase of CAL ≥ 3 mm), and progression of restorative or caries status (at least 1 additional surface with a new filling or new primary or secondary carious lesion) were based on 10,000 tooth-years.
Analyses on tooth level (a tooth as the analyzing unit) were conducted to explore the associations between serum 25OHD and incident tooth loss, progression of CAL ≥ 3 mm, and progression of restorative or caries status. To account for the dependence of teeth within participants, generalized estimating equations with log link function, exchangeable working matrix, and log-transformed follow-up years as offset were used.
We chose to parameterize serum 25OHD as follows: quintiles (see Appendix), dichotomous with a cutoff value below 20 µg/L as vitamin D deficiency (Holick et al., 2011), and increase of serum 25OHD by 10 µg/L. Risk ratios (RRs) and 95% confidence intervals (CIs) were reported. Models were adjusted for age, sex, waist circumference, smoking, alcohol drinking, education, dental visit frequency, reasons for dental visit, vitamin D (Anatomical Therapeutic Chemical code: A11CC) and calcium supplements (Anatomical Therapeutic Chemical code: A12A), and season of blood draw. Testing for statistical interactions between serum 25OHD and confounders revealed no significant interactions. To examine whether mean CAL or number of filled or carious surfaces per tooth or loose teeth at baseline were mediators of the effect of serum 25OHD on tooth loss, a fourth model was fitted by entering mean CAL, number of filled or carious surfaces per tooth, or loose teeth separately into the models using data based on half-mouth recordings only (CAL and caries were only half-mouth recorded as opposed to tooth status). We used inverse probability weighting according to variables (serum 25OHD, age, sex, education, smoking, alcohol drinking, dental visit frequency, and season of blood draw) to take into account the loss to follow-up for sensitivity analysis. Statistical tests were 2-tailed, and the threshold for statistical significance was set at p < .05. All analyses were performed in Stata/MP 12.1 (StataCorp 2011, Stata Statistical Software: Release 12, College Station, TX, USA).
Results
Baseline characteristics of the study participants by quintiles of serum 25OHD are presented in Table 1. In total, 1,143 (60.0%) participants were vitamin D deficient. The number of present teeth and number of teeth with caries at the baseline did not differ significantly across the quintiles (p = .052 and .078), while mean CAL showed a significant difference (p = .030). Distributions of age, waist circumference, sex, alcohol drinking, and season of blood draw were different (p < .05), whereas those of education, smoking, dental visit frequency, reasons of dental visit, and loose teeth were comparable across the quintiles (p > .05).
Table 1.
Basic Characteristics of Study Participants by Quintiles of 25-hydroxy Vitamin D in SHIP-1
| Q1: 5 µg/L- |
Q2: 12.1 µg/L- |
Q3: 16.1 µg/L- |
Q4: 20.9 µg/L- |
Q5: 27.1 µg/L- |
||
|---|---|---|---|---|---|---|
| n = 374 | n = 380 | n = 380 | n = 387 | n = 383 | p a | |
| Age, yr | 49.7 ± 13.4 | 50.5 ± 13.5 | 50.8 ± 13.0 | 52.0 ± 13.2 | 50.3 ± 13.0 | .002 |
| Waist circumference, cm | 92.1 ± 12.9 | 92.4 ± 13.9 | 91.8 ± 13.7 | 90.2 ± 13.8 | 87.2 ± 12.2 | < .001 |
| Mean clinical attachment loss, mm | 2.3 ± 1.7 | 2.4 ± 1.8 | 2.4 ± 1.8 | 2.3 ± 1.6 | 2.1 ± 1.5 | .030 |
| No. of teeth | 20.6 ± 7.2 | 20.6 ± 7.0 | 20.6 ± 6.8 | 20.6 ± 7.2 | 21.8 ± 6.5 | .052 |
| No. of teeth with caries/fillings | 5.5 ± 2.6 | 6.0 ± 2.5 | 5.9 ± 2.6 | 5.9 ± 2.7 | 6.2 ± 2.6 | .078 |
| Sex | .015 | |||||
| Men | 162 (43.3) | 197 (51.8) | 184 (48.4) | 203 (52.5) | 165 (43.1) | |
| Women | 212 (56.7) | 183 (48.2) | 196 (51.6) | 184 (47.5) | 218 (56.9) | |
| Education, yr | .189 | |||||
| < 10 | 106 (28.3) | 111 (29.2) | 126 (33.2) | 133 (34.4) | 99 (25.9) | |
| 10 | 202 (54.0) | 208 (54.7) | 192 (50.5) | 183 (47.3) | 209 (54.6) | |
| > 10 | 66 (17.7) | 61 (16.1) | 62 (16.3) | 71 (18.4) | 75 (19.6) | |
| Smoking | .147 | |||||
| Never | 173 (46.3) | 149 (39.2) | 167 (44.0) | 174 (45.0) | 185 (48.3) | |
| Former | 83 (22.2) | 128 (33.7) | 118 (31.1) | 137 (35.4) | 113 (29.5) | |
| Current | 118 (31.6) | 103 (27.1) | 95 (25.0) | 76 (19.6) | 85 (22.2) | |
| Alcohol drinking | .023 | |||||
| No | 30 (8.0) | 18 (4.7) | 22 (5.8) | 15 (3.9) | 9 (2.3) | |
| 1-2 drinks/d | 269 (71.9) | 278 (73.2) | 260 (68.4) | 281 (72.6) | 283 (73.9) | |
| ≥3 drinks/d | 75 (20.1) | 84 (22.1) | 98 (25.8) | 91 (23.5) | 91 (23.8) | |
| Dental visit frequency in the past year | .587 | |||||
| 0 | 28 (7.5) | 20 (5.2) | 19 (5.0) | 23 (5.9) | 19 (5.0) | |
| 1 | 110 (29.4) | 104 (27.4) | 100 (26.3) | 121 (31.3) | 108 (28.2) | |
| ≥ 2 | 236 (63.1) | 256 (67.4) | 261 (68.7) | 243 (62.8) | 256 (66.8) | |
| Reasons for past dental visit | .294 | |||||
| Pain or complaints | 333 (96.2) | 349 (96.9) | 347 (96.1) | 352 (96.7) | 359 (98.6) | |
| Others | 13 (3.8) | 11 (3.1) | 14 (3.9) | 12 (3.3) | 5 (1.4) | |
| Presence of loose teeth | .854 | |||||
| Yes | 39 (10.4) | 46 (12.1) | 37 (9.7) | 39 (10.1) | 10 (10.4) | |
| No | 335 (89.6) | 334 (87.9) | 343 (90.3) | 348 (89.9) | 343 (89.6) | |
| Season | < .001 | |||||
| Jan-Mar | 195 (52.2) | 152 (40.0) | 93 (24.5) | 93 (24.0) | 45 (11.8) | |
| Apr-Jun | 94 (25.1) | 107 (28.1) | 117 (30.8) | 102 (26.4) | 90 (23.5) | |
| Jul-Sep | 15 (4.0) | 30 (7.9) | 59 (15.5) | 86 (22.2) | 155 (40.5) | |
| Oct-Dec | 70 (18.7) | 91 (24.0) | 111 (29.2) | 106 (27.4) | 93 (24.2) |
Data are presented as mean ± standard deviation for continuous variables and n (percentage) for categorical variables.
SHIP-1, Study of Health in Pomerania, five-year follow-up.
Analysis of variance for continuous variables and chi-square test for categorical variables.
The mean follow-up time was 5.9 yr (range: 3.8-9.7 yr). Incidence rates of tooth loss on tooth level significantly increased with decreasing serum 25OHD concentrations (p < .001; Appendix Table 1). Likewise, incidence rates for changes of CAL ≥ 3 mm were also increasing across quintiles (p < .001), whereas the incidence rates for caries were comparable across quintiles of serum 25OHD (p = .158).
As shown in Table 2 and Appendix Table 2, higher serum 25OHD concentrations were associated with a lower risk of tooth loss in all models. When participants were classified into vitamin D deficient and nondeficient, the latter had a lower risk of tooth loss (RR: 0.86; 95% CI: 0.73, 1.00). With serum 25OHD as a continuous variable, each 10-µg/L increase was associated with a 13% lower risk of tooth loss after multivariable adjustment. Compared with participants in the first quintile, those in the fifth quintile exhibited a 30% lower risk of tooth loss (RR: 0.70; 95% CI: 0.54, 0.91) after adjustment for age and sex in model 1. When additionally adjusted for multiple confounders, the association was slightly attenuated in model 2 (RR: 0.77; 95% CI: 0.60, 0.99).
Table 2.
Association of 25-hydroxy Vitamin D and Tooth-specific Tooth Loss from SHIP-1 to SHIP-2 Based on Generalized Estimating Equations
| Risk Ratios (95% Confidence Intervals) |
||
|---|---|---|
| Cutoff | Model 1 | Model 2 |
| 0-20 µg/L | 1 (ref.) | 1 (ref.) |
| ≥ 21 µg/L | 0.79 (0.67, 0.94) | 0.86 (0.73, 1.00) |
| Increase | ||
| 10 µg/L | 0.84 (0.77, 0.92) | 0.87 (0.79, 0.96) |
SHIP-1, Study of Health in Pomerania, five-year follow-up; SHIP-2, 11-year follow-up.
Model 1: 25-hydroxy vitamin D.
Model 2: Model 1 plus age, sex, waist circumference, smoking, alcohol drinking, education, dental visit frequency, reasons for last dental visit, vitamin D or calcium supplements, and season of blood draw.
Associations of serum 25OHD and tooth-specific incidence of changes of CAL ≥ 3 mm are presented in Table 3 and Appendix Table 3. No significant association between serum 25OHD and incidence of changes of CAL ≥ 3 mm was observed. When further adjusting for multiple confounders, the RR was 0.92 (95% CI: 0.73, 1.17) for each 10-µg/L increase in serum 25OHD.
Table 3.
Association of 25-hydroxy Vitamin D and Tooth-specific Incidence of Changes of Clinical Attachment Loss ≥ 3 mm from SHIP-1 to SHIP-2 Based on Generalized Estimating Equations
| Risk Ratios (95% Confidence Intervals) |
||
|---|---|---|
| Cutoff | Model 1 | Model 2 |
| 0-20 µg/L | 1 (ref.) | 1 (ref.) |
| ≥ 21 µg/L | 0.87 (0.78, 0.97) | 0.91 (0.78, 1.07) |
| Increase | ||
| 10 µg/L | 0.93 (0.88, 0.98) | 0.95 (0.87, 1.03) |
SHIP-1, Study of Health in Pomerania, five-year follow-up; SHIP-2, 11-year follow-up.
Model 1: 25-hydroxy vitamin D.
Model 2: Model 2 plus age, sex, waist circumference, smoking, alcohol drinking, education, dental visit frequency, reason s for last dental visit, vitamin D or calcium supplements, and season of blood draw.
We additionally evaluated the association between serum 25OHD and incident caries on tooth level (Table 4 and Appendix Table 4). The RR was 0.96 (95% CI: 0.83, 1.10) for each 10-µg/L increase in serum 25OHD when adjusting for other covariates. No linear trend was observed across the quintiles of serum 25OHD in neither model (p > .10).
Table 4.
Association of 25-hydroxy Vitamin D and Tooth-specific Incident Teeth with Caries from SHIP-1 to SHIP-2 Based on Generalized Estimating Equations
| Risk Ratios (95% Confidence Intervals) |
||
|---|---|---|
| Cutoff | Model 1 | Model 2 |
| 0-20 µg/L | 1 (ref.) | 1 (ref.) |
| ≥ 21 µg/L | 0.95 (0.87, 1.03) | 0.96 (0.87, 1.05) |
| Increase | ||
| 10 µg/L | 0.98 (0.94, 1.03) | 0.99 (0.94, 1.03) |
Teeth with caries: at least 1 additional surface with a new filling or new primary or secondary carious lesion.
SHIP-1, Study of Health in Pomerania, five-year follow-up; SHIP-2, 11-year follow-up.
Model 1: 25-hydroxy vitamin D.
Model 2: Model 2 plus age, sex, waist circumference, smoking, alcohol drinking, education, dental visit frequency, reasons for last dental visit, vitamin D or calcium supplements, and season of blood draw.
To test whether the effect of serum 25OHD on tooth loss might be mediated by CAL, caries, or fillings, we performed additional analyses using data recorded on a half-mouth basis. The association between serum 25OHD and tooth loss was attenuated slightly from 0.87 to 0.92 for a 10-µg/L increase in serum 25OHD when mean CAL was included (Appendix Table 5). Adjusting for the number of carious surfaces per tooth, number of filled surfaces, and loose teeth did not change the association relevantly.
The results hardly changed in the sensitivity analysis, when loss of follow-up was taken into account using inverse probability weighting.
Discussion
In this community-based prospective study, we found an inverse association between serum 25OHD concentration and tooth loss. Compared with participants in the lowest quintile of 25OHD, those in the highest quintile had a 23% lower risk of tooth loss. The association exhibited a linear trend and was independent of age, sex, education, dental visit frequency, reasons of dental visit, waist circumference, smoking, alcohol drinking, vitamin D and calcium supplements, and season of blood draw. No significant interactions were observed between serum 25OHD and the other variables. Regarding the association between serum 25OHD and incidence of changes of CAL ≥ 3 mm on tooth level, the linear trend test across the quintiles was not significant. The association was not independent of other confounders. Regarding the progression of caries, there were no significant findings.
Increasing evidence linking vitamin D and oral health status has been emerging during the past decades. The Health Professionals Follow-up Study, the largest study with the longest follow-up, reported that participants with a predicted 25OHD score in the highest quintile had a 14% lower risk of self-reported tooth loss than those in the lowest quintile (Jimenez et al., 2014). A case-control study also linked serum 25OHD with periodontal disease in pregnant women (Boggess et al., 2011). In line, a recent cross-sectional study asserted that women with 25OHD ≥ 50 nmol/L (25 µg/L) had 33% lower odds of periodontal disease compared with those having 25OHD concentrations < 50 nmol/L (Millen et al., 2013). Previous reports also found periodontitis and gingival inflammation to be more prevalent in those with low serum 25OHD (Dietrich et al., 2005). In addition to the observational studies, studies assessing vitamin D intake or supplementations also reported benefits on oral health. A prospective study found that vitamin D intake ≥ 800 IU/d (equals 20 µg/d) was associated with 33% lower risk of severe periodontal disease compared with an intake of < 400 IU/d (equals 10 µg/d). Vitamin D supplements were also found to have beneficial effects on tooth loss (Krall et al., 2001) and chronic periodontitis prevention (Garcia et al., 2011). A meta-analysis concluded that vitamin D supplements might lower the risk of caries in children (Hujoel, 2013). Furthermore individuals with sufficient serum 25OHD at the time of periodontal surgery benefited more than those with deficient serum 25OHD, but vitamin D supplementation at the time of surgery failed to prevent negative clinical outcomes, and it might be advisable to ensure adequate 25OHD levels to attain the best results in advance of periodontal surgery (Bashutski et al., 2011). Our findings were consistent with these results in the establishment of the association between serum 25OHD and tooth loss. The association between serum 25OHD and incidence of changes of CAL ≥ 3 mm in our study was also similar to the result reported in the Health Professionals Follow-up Study.
Several biological mechanisms might be proposed to explain the statistical links between serum 25OHD and tooth loss or periodontal disease. In the first place, the primary function of vitamin D has long been recognized as regulating calcium maintenance, which plays a key role in bone metabolism. Several animal studies found that vitamin D and calcium status might control the formation of dental alveolar bone and prevent the periodontal ligament from mineralization (Chen et al., 2012). Periodontitis, periodontal ligament, alveolar bone loss, and tooth loss are closely related. In the second place, anti-inflammatory or antimicrobial effects of vitamin D were discussed in a number of studies and might partially explain the observed associations (Zanetti et al., 2014). Interleukin and tumor necrosis factor α were suggested to be involved in bone loss (Graves, 2008). Vitamin D was also reported to be able to inhibit the periodontal inflammation by decreasing interleukin 6, interleukin 8, and tumor necrosis factor α expression (Tang et al., 2013). These results may suggest a protective effect of vitamin D on periodontal health.
In the present study, we hypothesized that vitamin D might influence tooth loss through periodontal status, the progression of caries/fillings, and loose tooth. We conducted additional analyses on a half-mouth basis to test this hypothesis. Considering the results from Tables 3 and 4, we conclude that the observed association between serum 25OHD and tooth loss might be partially explained by changes in the periodontal status. This hypothesized biological pathway should be further tested in future studies with full-mouth periodontal examinations. Apart from that, the observed associations might also be confounded by socioeconomic status or systemic disease (Autier et al., 2014). Serum 25OHD concentration was lower in people with disadvantaged socioeconomic position (Navarro Mdel et al., 2013), inflammation (Querfeld, 2013), and cardiovascular diseases (Norman and Powell, 2014), to which tooth loss and periodontal status were also related (Dietrich et al., 2013). Thus, residual confounding of socioeconomic position or systemic disease might explain this association and should be examined in the future.
The strength of the present study is its longitudinal design in a community-based sample. To the best of our knowledge, this is the first study investigating associations of serum 25OHD with tooth loss, periodontal progression, and caries prospectively. Apart from that, periodontal data were collected by clinical examination, and serum 25OHD was assessed rather than predicted from questionnaire. Additionally, we tested whether the effects of serum 25OHD on tooth loss was through periodontitis or caries. Further studies are still warranted to clarify the biological pathway.
The major limitation of the present study was the half-mouth examination of CAL and caries. Analyses based on half-mouth examinations have a decreased power and information loss and might shift the association toward the null effect because of misclassification (Beck et al., 2006). As a second limitation, serum 25OHD was measured only in SHIP-1 but not in the follow-up, SHIP-2. Thus, information on whether the serum 25OHD concentrations had changed during the follow-up period was not available. Another limitation is that serum 25OHD was not measured in the same season in all participants. Vitamin D has a strong seasonal variation in Germany, which affects the diagnosis of vitamin D deficiency (Bolland et al., 2007). To overcome this, we adjusted for season of blood draw in the analysis. We also acknowledged loss of follow-up in the present study; however, we used inverse probability weighting in the analysis, which showed that the results hardly changed. Taken together, the aforementioned limitations might result in a nondifferential bias toward the null.
Conclusions
In conclusion, the present results suggest that higher serum 25OHD concentrations are independently associated with a lower risk of tooth loss. Our findings might have important public health and clinical implications for the prevention or treatment of periodontal disease and tooth loss.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the German Federal Ministry of Education and Research (BMBF; grant 01ZZ96030, 01ZZ0701), the Ministry of Education, Research, and Cultural Affairs, as well as the Ministry of Social Affairs of the Federal State of Mecklenburg-West Pomerania. Y.Z. was supported by RAPID (Rheumatoid Arthritis and Periodontal Inflammatory Disease; grant 290246), Seventh Framework Programme of the European Union. B.H. was supported by an unlimited grant from GABA, Switzerland. Furthermore, we received an independent research grant for determination of vitamin D in serum samples from Immunodiagnostic Systems.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Al-Harthi LS, Cullinan MP, Leichter JW, Thomson WM. (2013). The impact of periodontitis on oral health-related quality of life: a review of the evidence from observational studies. Aust Dent J 58:274-277. [DOI] [PubMed] [Google Scholar]
- Alshouibi EN, Kaye EK, Cabral HJ, Leone CW, Garcia RI. (2013). Vitamin D and periodontal health in older men. J Dent Res 92:689-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Boniol M, Pizot C, Mullie P. (2014). Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2:76-89. [DOI] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. (2011). The impact of vitamin D status on periodontal surgery outcomes. J Dent Res 90:1007-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JD, Caplan DJ, Preisser JS, Moss K. (2006). Reducing the bias of probing depth and attachment level estimates using random partial-mouth recording. Community Dent Oral Epidemiol 34:1-10. [DOI] [PubMed] [Google Scholar]
- Boggess KA, Espinola JA, Moss K, Beck J, Offenbacher S, Camargo CA., Jr (2011). Vitamin D status and periodontal disease among pregnant women. J Periodontol 82:195-200. [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, et al. (2007). The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr 86:959-964. [DOI] [PubMed] [Google Scholar]
- Chen YC, Ninomiya T, Hosoya A, Hiraga T, Miyazawa H, Nakamura H. (2012). 1α,25-Dihydroxyvitamin D3 inhibits osteoblastic differentiation of mouse periodontal fibroblasts. Arch Oral Biol 57:453-459. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. (2005). Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr 82:575-580. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Sharma P, Walter C, Weston P, Beck J. (2013). The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol 40(4):S70-S84. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914-920. [DOI] [PubMed] [Google Scholar]
- Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL, et al. (2011). One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 82:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockmann E, Panzner K-D, Huhn P, Sigusch B, Glockmann K. (2011). Ursachen des Zahnverlustes in Deutschland—Dokumentation einer bundesweiten Erhebung (2007) [Reasons for tooth loss in Germany—documentation of a nationwide survey (2007)]. URL accessed on 2014-04-18 at: http://www.bzaek.de/fileadmin/PDFs/idz/IDZ_0211_web.pdf. (in German)
- Grant WB. (2011). A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinol 3:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. (2008). Cytokines that promote periodontal tissue destruction. J Periodontol 79(8):1585S-1591S. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911-1930. [DOI] [PubMed] [Google Scholar]
- Holtfreter B, Schwahn C, Biffar R, Kocher T. (2009). Epidemiology of periodontal diseases in the Study of Health in Pomerania. J Clin Periodontol 36:114-123. [DOI] [PubMed] [Google Scholar]
- Hujoel PP. (2013). Vitamin D and dental caries in controlled clinical trials: systematic review and meta-analysis. Nutr Rev 71:88-97. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Giovannucci E, Krall Kaye E, Joshipura KJ, Dietrich T. (2014). Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr 17:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. (2001). Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med 111:452-456. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Wu W, Cheng M, Li Y, Cheng R. (2013). Prevalence and correlates of dental caries in an elderly population in northeast China. PLoS One 8:e78723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen AE, Hovey KM, LaMonte MJ, Swanson M, Andrews CA, Kluczynski MA, et al. (2013). Plasma 25-hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. J Periodontol 84:1243-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro Mdel C1, Saavedra P, Jódar E, Gómez de Tejada MJ, Mirallave A, Sosa M. (2013). Osteoporosis and metabolic syndrome according to socio-economic status, contribution of PTH, vitamin D and body weight: the Canarian Osteoporosis Poverty Study (COPS). Clin Endocrinol (Oxf) 78:681-686. [DOI] [PubMed] [Google Scholar]
- Norman PE, Powell JT. (2014). Vitamin D and cardiovascular disease. Circ Res 114:379-393. [DOI] [PubMed] [Google Scholar]
- Querfeld U. (2013). Vitamin D and inflammation. Pediatr Nephrol 28:605-610. [DOI] [PubMed] [Google Scholar]
- Tang X, Pan Y, Zhao Y. (2013). Vitamin D inhibits the expression of interleukin-8 in human periodontal ligament cells stimulated with Porphyromonas gingivalis . Arch Oral Biol 58:397-407. [DOI] [PubMed] [Google Scholar]
- Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, et al. (2011). Cohort profile: the study of health in Pomerania. Int J Epidemiol 40:294-307. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Harris SS, Dawson-Hughes B. (2014). Ability of vitamin D to reduce inflammation in adults without acute illness. Nutr Rev 72:95-98. [DOI] [PubMed] [Google Scholar]
- Zeng XT, Luo W, Huang W, Wang Q, Guo Y, Leng WD. (2013). Tooth loss and head and neck cancer: a meta-analysis of observational studies. PLoS One 8:e79074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


