Abstract
Recently, it was demonstrated that nitric oxide (NO) and cGMP are involved in the auxin response during the adventitious rooting process in cucumber (Cucumis sativus; Pagnussat et al., 2002, 2003). However, not much is known about the complex molecular network operating during the cell proliferation and morphogenesis triggered by auxins and NO in that process. Anatomical studies showed that formation of adventitious root primordia was clearly detected in indole acetic acid (IAA)- and NO-treated cucumber explants, while neither cell proliferation nor differentiation into root primordia could be observed in control explants 3 d after primary root was removed. In order to go further with signal transduction mechanisms that operate during IAA- and NO-induced adventitious root formation, experiments were designed to test the involvement of a mitogen-activated protein kinase (MAPK) cascade in that process. Cucumber explants were treated with the NO-donor sodium nitroprusside (SNP) or with SNP plus the specific NO-scavenger cPTIO. Protein extracts from those explants were assayed for protein kinase (PK) activity by using myelin basic protein (MBP) as substrate in both in vitro and in-gel assays. The activation of a PK of approximately 48 kD could be detected 1 d after NO treatment with a maximal activation after 3 d of treatment. In control explants, a PK activity was detected only after 4 d of treatment. The MBP-kinase activity was also detected in extracts from IAA-treated explants, while no signal was observed in IAA + cPTIO treatments. The PK activity could be inhibited by the cell-permeable MAPK kinase inhibitor PD098059, suggesting that the NO-dependent MBP-kinase activity is a MAPK. Furthermore, when PD098059 was administered to explants treated with SNP or IAA, it produced a delay in root emergence and a dose-dependent reduction in root number. Altogether, our results suggest that a MAPK signaling cascade is activated during the adventitious rooting process induced by IAA in a NO-mediated but cGMP-independent pathway. The activation of MAPKs is discussed in relation to the cell responses modulating mitotic process.
Auxins play a central role in numerous developmental processes functioning as a signal for cell division, elongation and differentiation. Among auxin actions, an indole acetic acid (IAA) dependence of root formation, apical dominance, and tropic responses have been described (Theologis, 1986; Estelle, 1992; Davies, 1995). The molecular basis of auxin action is an area of intense study. Genetic approaches to dissect how this hormone triggers a variety of effects in plants rely on the analysis of mutants modified in their response to auxin (Walden and Lubenow, 1996). The available mutants display an array of phenotypes that indicates changes in auxin action or response including altered growth, changes in root length and apical dominance, and reduced gravitropism (Lincoln et al., 1990; Pickett et al., 1990; Wilson et al., 1990; Hobbie and Estelle, 1995; Leyser et al., 1996).
Adventitious root formation (ARF) involves the development of a meristematic tissue after removal of the primary root system. The auxin IAA promotes this process through the regulation of cell dedifferentiation to reestablish the new apical meristem. Although a variety of components of auxin transport and signal transduction have been identified, the molecular mechanisms and intermediates underlying the signal transduction of auxin-promoted root formation remains a major goal for a large number of biotechnological procedures (Walker and Estelle, 1998).
The mitogen-activated protein kinase (MAPK) transduction cascades are important mediators in signal transmission, connecting the perception of external stimuli to cellular responses. MAPKs are involved in signaling various biotic and abiotic stresses, and have been implicated in the regulation of cell cycle and developmental processes. Convincing evidence on the requirement of a MAPK cascade for plant cytokinesis comes from experiments carried out in tobacco (Nicotiana tabacum) and Arabidopsis. It has been shown that a tobacco MAPK kinase kinase (MAPKKK), called nucleus and phragmoplast-localized protein kinase (NPK1), accumulates in the equatorial region of the phragmoplast (Nishihama et al., 2001). It was demonstrated that NPK1 expression is essential for phragmoplast expansion and its absence results in the formation of multinucleate cells. In addition, Krysan et al. (2002) provided evidence that MAPKKKs play a role in the control of plant cell division using a reverse-genetic approach. In that report, Arabidopsis plants carrying knockout alleles of the MAPKKK genes ANP1, ANP2, and ANP3 were isolated. The molecular and structural phenotypes displayed by the mutants support a model in which MAPKKKs positively regulate cell division and growth.
On the one hand, the role of MAPK cascades in auxin signaling has been recently discussed (Morris, 2001). The observation that extracts from auxin-treated tobacco cells activated MAPKs (Mizoguchi et al., 1994) was contradicted by Tena and Renaudin (1998) who have shown that low doses of auxin would not induce a myelin basic protein (MBP)-kinase activity in the same tobacco cell lines, but weak acids would, suggesting that high levels of auxins induce a MBP-kinase activity, probably as a result of cytosolic acidification. Later, and working with whole plants, Mockaitis and Howell (2000) reported an increase in MBP-kinase activity in response to low levels of auxins. Although this finding represents a great advance in the knowledge of auxin signal transduction pathway, the cell components that act as signal molecules from IAA stimulus to MAPK activation and the beginning of the mitotic process remain to be identified.
Nitric oxide (NO) is a diffusible chemical second messenger first described in mammals, where it plays variable functions ranging from dilation of blood vessels to neurotransmission and defense during immune response (Gow and Ischiropoulos, 2001). In human cells, it was reported that the NO-induced cell proliferation occurs via the activation of MAPK cascades (Chou et al., 2002). Even though NO research in plants is not as advanced as in animals, recent discoveries have extensively shown its presence and functionality in the plant kingdom. Novel roles have been attributed to this gas in many physiological processes such as growth, pathogen defense, programmed cell death, and stress tolerance (Gouvêa et al., 1997; Laxalt et al., 1997; Delledonne et al., 1998; Durner et al., 1998; Beligni and Lamattina, 2000; Lamattina et al., 2003). Previous studies have revealed that NO is involved in the activation of MAPK activity during plant defense responses against pathogen infections in tobacco (Kumar and Klessig, 2000) and in Arabidopsis cells (Clarke et al., 2000). More recently, it was demonstrated that NO is involved in the auxin response during ARF in cucumber (Pagnussat et al., 2002) and a subsequent report showed that an NO-mediated cGMP-dependent pathway is operating in that process (Pagnussat et al., 2003). In this study, we describe the involvement of a MAPK signal cascade in ARF induced by IAA and NO, suggesting that convergent and complex cGMP-dependent and -independent signaling pathways are probably orchestrating the formation of a new root system when primary root is removed.
RESULTS
Anatomical Study
Previous results showed that NO mediates the IAA-induced ARF in cucumber explants. Within 3 d after removal of the primary root system, emergence of adventitious roots was detected in explants treated with IAA, or with the NO-donors sodium nitroprusside (SNP) and S-nitroso, N-acetyl penicillamine (SNAP), while at that time no roots emerged in water-treated explants (Pagnussat et al., 2002).
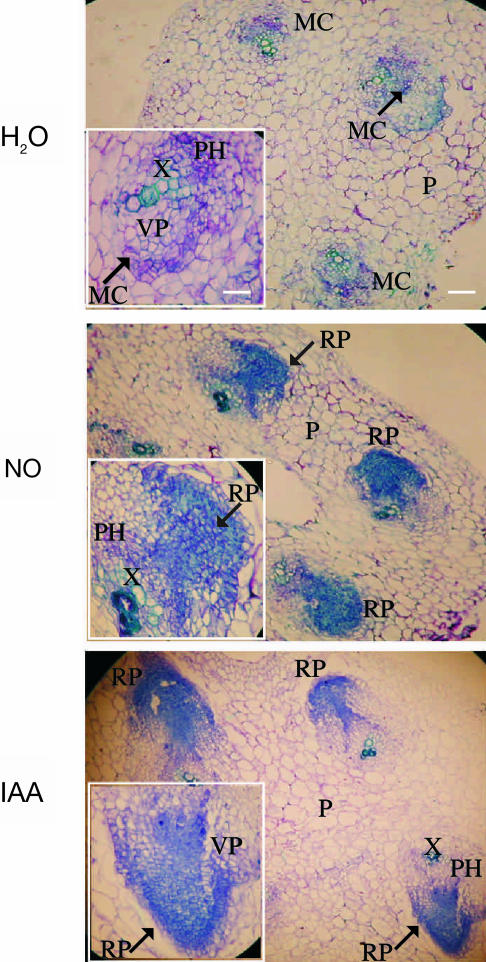
To observe the primordia formation during the first stages of ARF, an anatomical study was performed. We analyzed transverse sections of cucumber hypocotyls treated with water (control) or with SNP or IAA (Fig. 1). After 2 d of NO or IAA treatment, cells with meristematic characteristics such as small size, strongly toluidine blue stained, and condensed cytoplasm were detected at the vascular parenchyma located centrifugal to primary xylem poles (not shown). Thus, some cells of xylematic or phloematic parenchyma dedifferentiate and acquire meristematic activity resulting in the formation of adventitious root primordia (RP). Figure 1 shows that, upon 3 d, cell proliferation and differentiation into RP were clearly detected both in NO- and IAA-treated explants. At the same time, cell proliferation was only barely detected and no RP could be observed in control treatment (Fig. 1).
Figure 1.
Primordia formation during adventitious root development in cucumber explants. Primary roots were removed and the explants were treated with water or 10 μm of the NO-donor SNP or 10 μm IAA for 3 d. Hypocotyl transverse sections were stained with toluidine blue and photographed using a digital camera attached to the microscope. For photographs and insets, magnifications of ×100 (bar indicates 0.2 mm) and ×400 (bar indicates 0.05 mm) were respectively used. MC, meristematic cells; P, parenchyma; PH, phloem; RP, root primordium; VP, vascular parenchyma; X, xylem. Arrows indicate the MC or RP magnified in the insets.
Nitric Oxide Is Required for an IAA-Induced MBP-Kinase Activity during Adventitious Root Development
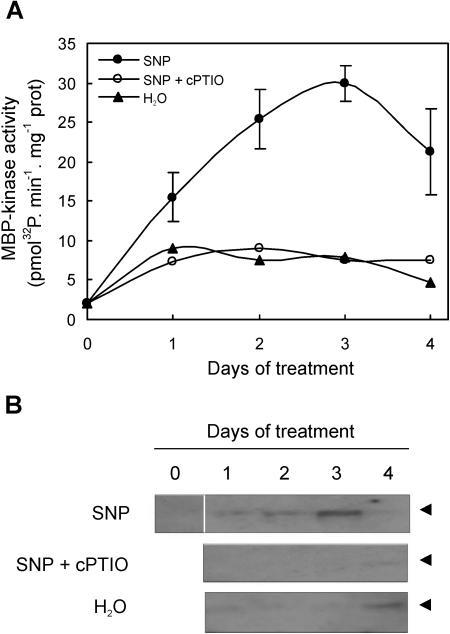
Since the MAPK phosphorylation cascades play a key role in regulating many aspects of growth and development, we analyzed whether NO affects the MAPK activity during ARF. Protein extracts from hypocotyls of explants treated with water or the NO-donor SNP or with SNP plus the specific NO-scavenger cPTIO were assayed for PK activity by in vitro and in-gel analysis using MBP as substrate. After 1 d of SNP treatment, an increase in soluble MBP-phosphorylating activity was observed, reaching a maximum after 2 d of treatment (Fig. 2A). In concordance, a PK activity of approximately 48 kD that phosphorylates MBP was detected in in-gel assays with a maximal activation after 3 d of treatment (Fig. 2B). The activity of this PK decreased after 4 d of treatment. In the case of SNP plus cPTIO-treated explants, the 48 kD activity was only barely detected after 4 d of treatment (Fig. 2B). In water-treated explants, a weak PK activity was measured during the first 3 d of treatment, which increased at the fourth d (Fig. 2B).
Figure 2.
MBP-kinase activity during adventitious root development induced by NO. A, Cucumber explants were incubated for different times with water or 10 μm of the NO-donor SNP or with SNP plus 200 μm of the specific NO-scavenger cPTIO. In vitro MBP-kinase activity was measured in total soluble extracts from those explants. Data are means of two independent experiments with three repetitions for each point. The sd of water and SNP plus cPTIO-treatments are not shown for the sake of clarity of the figure. B, In-gel protein kinase assays. The arrow heads indicate an apparent molecular mass of approximately 48 kD.
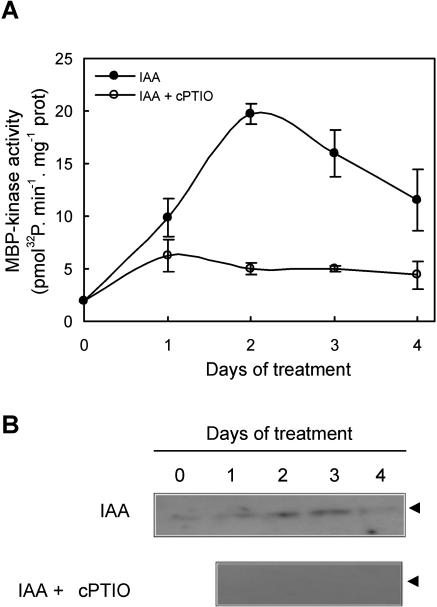
PK activity was also assayed in hypocotyls of explants treated either with the auxin IAA or with IAA plus the NO-scavenger cPTIO. MBP-kinase activity could be detected in IAA-treated explants using both in vitro and in-gel assays, with a maximal activation around the second and third d of treatment, respectively (Fig. 3, A and B). Again, a MBP-phosphorylating activity of 48 kD was observed in extracts from IAA-treated explants (Fig. 3B). MBP-kinase activity was weakly detected in explants treated with IAA in the presence of cPTIO in in vitro assays (Fig. 3A), while no signal was obtained in in-gel assays (Fig. 3B).
Figure 3.
MBP-kinase activity during the IAA-induced adventitious root development requires endogenous NO. A, Cucumber explants were incubated for different times either with 10 μm IAA or with IAA plus 200 μm of the specific NO-scavenger cPTIO. In vitro MBP-kinase activity was measured in total soluble extracts from those explants. Values are expressed as mean ± sd from two independent experiments with three repetitions for each point. B, Protein kinase activity was measured by in-gel assays as described. The arrow heads indicate an apparent molecular mass of approximately 48 kD.
In all in-gel experiments, we found only one activity band corresponding to a PK with an apparent molecular mass of approximately 48 kD. This MBP-kinase activity was highly active in the absence of calcium (the kinase assays were performed in the presence of 2 mm EGTA) and exhibited a strong preference for MBP among substrates tested (histone proteins and casein, not shown). Moreover, when protein extracts were assayed for kinase activity in the absence of vanadate (a general protein Tyr phosphatase inhibitor), the 48-kD PK activity was lost (not shown). Even though these data strongly suggest that the MBP-kinase activity detected by in-gel assays can be ascribed to a MAPK, some caution would be advisable. Indeed, it has been also reported that MBP can be phosphorylated by other protein-Ser/Thr kinases, such as tobacco NPK15 and calcium-dependent protein kinases (Roberts and Harmon, 1992; Ito et al., 1994).
The MBP-Kinase Activity Is Inhibited by the MAPK Kinase Inhibitor PD098059
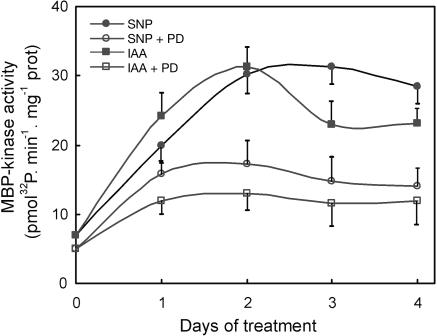
To further confirm that the MBP-kinase activity detected in cucumber explants is a MAPK activity, the PK activity was assayed by in vitro and in-gel analysis in explants treated with the MAPK kinase (MAPKK) inhibitor PD098059. This inhibitor prevents the activation of MAPKK, the direct activator of MAPK, by binding to its inactive dephosphorylated form (Alessi et al., 1995). Figure 4 shows that in the presence of 50 μm PD098059, the increase in MBP-kinase activity observed after SNP or IAA treatments was reduced2- to 3-fold. When in-gel experiments were performed, no MBP-kinase activity was detected in PD098059-treated explants (not shown).
Figure 4.
MBP-kinase activity induced by IAA and NO is inhibited by the MAPKK inhibitor PD098059. In vitro MBP-kinase activity was measured in total soluble extracts from cucumber explants treated with 10 μm SNP or 10 μm IAA, or with SNP or IAA in the presence of 50 μm of PD098059 for different times as indicated. Values are expressed as mean ± sd from two independent experiments with three repetitions for each point.
MAPKK Inhibition Prevents Adventitious Root Formation Induced by Either IAA or NO Treatments
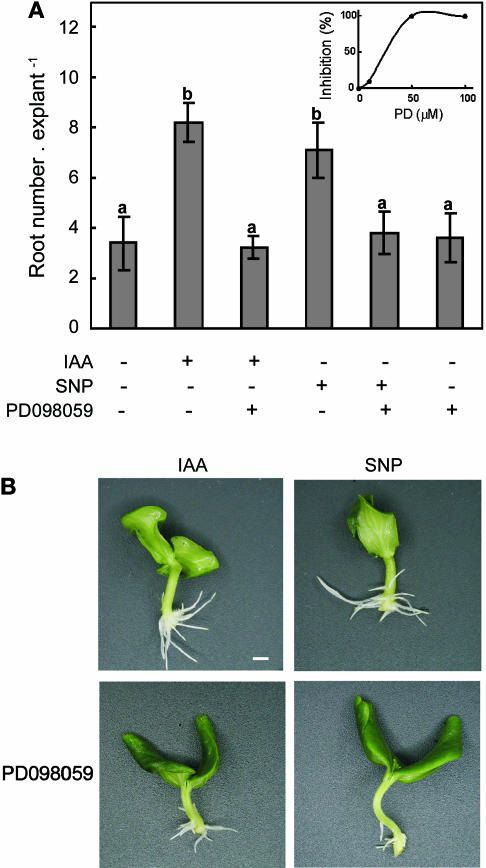
In order to test the involvement of the activation of a MAPK pathway in ARF, the MAPKK inhibitor PD098059 was assayed for its capacity to affect the promotion of either the IAA- or NO-induced root development. Results of experiments performed with SNP in the presence of different PD098059 concentrations showed that the effect of the inhibitor was dose-dependent, with a maximal biological response at 50 μm (Fig. 5A, inset). Cucumber explants were treated with water, IAA, or the NO-donor SNP either in the presence or the absence of 50 μm PD098059 or with 50 μm PD098059 alone. Explant treatments with SNP plus PD098059 produced a delay in root emergence and a significant reduction in both root length (not shown) and root number (Fig. 5, A and B). In addition, PD098059 also produced a significant decrease in the root number of the IAA-treated explants (Fig. 5, A and B). When PD098059 was administered alone, adventitious root number was similar to the control untreated explants (Fig. 5A).
Figure 5.
MAPK activity is required for adventitious root formation induced by IAA and NO. A, Cucumber explants were treated for 5 d as indicated. Root number values are expressed as mean ± se (n = 10 explants from at least 3 independent experiments). Bars with different letters are significantly different with P < 0.05 (t test). The cell-permeable MAPKK inhibitor PD098059 was used at 50 μm and SNP and IAA were used at 10 μm. The inset shows the effect of different concentrations of PD098059 on the NO-induced adventitious root formation. Cucumber explants were treated with water (control) or 10 μm SNP in the presence or absence of 10 μm, 50 μm, or 100 μm of the MAPKK inhibitor PD098059 for 4 d. Inhibition values are expressed as %, where 100% means root number values per explant similar to those obtained in control explants. B, Photographs were taken after 4 d of treatment. Bar indicates 5 mm.
The MBP-Kinase Activity Is Induced by NO in a cGMP-Independent Pathway
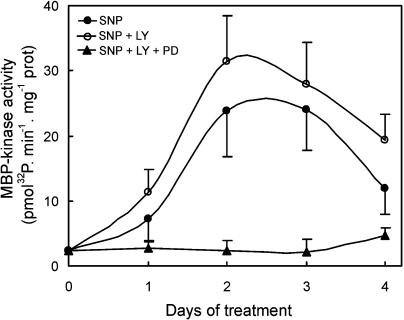
As was previously reported, a cGMP-dependent pathway is operating during the NO-mediated ARF in cucumber (Pagnussat et al., 2003). In order to study whether the MAPK signaling cascade is activated by NO in a cGMP-dependent pathway, we measured the MBP-kinase activity in explants treated with the guanylate cyclase inhibitor LY83583. At a concentration of 50 μm, LY83583 was reported to significantly reduce root development induced either by IAA or NO in cucumber explants (Pagnussat et al., 2003). As shown in Figure 6, the in vitro MBP-kinase activity was induced by NO and displayed a similar response in both SNP and SNP plus LY83583 treatments. When 50 μm of the MAPKK inhibitor PD098059 was administered to cucumber explants together with SNP and LY83583, the MBP-kinase activity, as expected, dramatically decreased (Fig. 6).
Figure 6.
The MBP-kinase activity is induced by NO in a cGMP-independent pathway. Cucumber explants were incubated for different times with 10 μm of the NO-donor SNP or with SNP plus 50 μm of the guanylate cyclase inhibitor LY83583 in the presence or absence of 50 μm of the MAPKK inhibitor PD098059. In vitro MBP-kinase activity was measured in total soluble extracts from those explants. Data are expressed as mean ± sd from two independent experiments with three repetitions for each point.
DISCUSSION
In this study, we present evidence that a MAPK signaling cascade is activated during the adventitious rooting process induced by IAA in a NO-mediated and cGMP-independent pathway. Auxin is the hormone responsible for the initiation of ARF, a process that involves cell division and root primordia formation. Auxin induces dedifferentiation of parenchyma cells and entrance to cell division to form the root meristem (De Klerk et al., 1995; Fujita and Syôno, 1996). More recently, it was demonstrated that NO and cGMP mediate the auxin response during ARF in cucumber (Pagnussat et al., 2002, 2003). In a broader sense, NO was already involved in the regulation of different plant growth processes in a large diversity of biological systems (Gouvêa et al., 1997; Leshem et al., 1998; Beligni and Lamattina, 2001).
Here we report an increase in in vitro MBP-kinase activity in cucumber explants after 1 d of exposure to the NO-donor SNP or to the auxin IAA (Figs. 2A and 3A). Maximal activation of a PK of 48 kD was obtained after 3 d of these treatments (Figs. 2B and 3B). Interestingly, the specific NO-scavenger cPTIO prevented the activation of the PK activity in both SNP- and IAA-treated explants (Figs. 2 and 3), suggesting that the48-kD PK detected by in-gel assays is the same and requires the presence of endogenous NO. We do not yet know whether the activation of the 48-kD PK is accompanied by a parallel increase in protein level. Cucumber explants treated with SNP or IAA in the presence of the MAPKK inhibitor PD098059 showed only a basal MBP-kinase activity measured by in vitro assays (Fig. 4), while the 48 kD MBP-kinase could not be detected by in-gel assays (not shown). Interestingly, the cell-permeable MAPKK inhibitor PD098059 was also able to prevent the IAA- and NO-induced ARF (Fig. 5). These results support that the MBP-kinase activated by SNP- or IAA-treatments during ARF is a MAPK.
The increase in MAPK activity could be related to the changes observed at the cellular level in transverse sections of hypocotyls treated with NO or IAA. The maximal MAPK activity (Figs. 2 and 3) was detected simultaneously with cell proliferation and adventitious root primordia formation in both NO- and IAA-treated explants (Fig. 1). Compelling evidence on the requirement of a MAPK cascade for plant cell division comes from studies published during recent years. Mutations in a MAPKKK disrupt cytokinesis in tobacco (Nishihama et al., 2001) and a MAPK was detected at the phragmoplast in alfalfa cells (Bögre et al., 1999). More recently, Krysan et al. (2002) demonstrated a direct link between mutation of a group of MAPKKK encoding genes (ANP genes) and the disruption of cell division. Here we show that treatment of cucumber explants with SNP or IAA plus the MAPKK inhibitor PD098059 produced a dose-dependent reduction in ARF (Fig. 5, inset), indicating that a MAPK cascade could be involved in this auxin-induced process. Knockout mutants of MAPKKK genes in Arabidopsis presented incomplete cell walls and binucleate cells, indicative of failed cell division (Krysan et al., 2002). Coincidently, binucleate cells were also found in transverse sections of IAA- and NO-treated cucumber explants when assayed with the MAPKK inhibitor (not shown). Together, these results indicate that the MAPK activity could be involved in the regulation of cell division rather than in adventitious root primordia formation.
Collectively, these results contribute to unraveling part of the molecular events that take place downstream of IAA to trigger ARF. One pathway would be the activation of a MAPK cascade mediated by NO. Our results fit with those obtained by Mizoguchi et al. (1994) and by Mockaitis and Howell (2000), who demonstrated the activation of a MAPK cascade in response to auxins. However, Mockaitis and Howell (2000) reported a rapid (peak in 5 min auxin treatment) and transient increase in MAPK activity in Arabidopsis seedling roots. Furthermore, the hyperactivation of the auxin-stimulated MAPK pathway was more than doubled in the presence of the MAPKK inhibitors PD098059 and U0126 (Mockaitis and Howell, 2000). Taken together with our results, these inhibitors appear to exert different effects on different MAPK signal transduction pathways. These pathways seem to vary in the kinetics of MAPK activation, upstream activator components, or the type and number of MAPK isoforms present in each system (Mockaitis and Howell, 2000). Given this scenario, the specificity of the MAPK signaling cascades could be supplied by the cellular type, the environment surrounding the cell, the previous history of the cell, the plant specie, or a combination of these aspects.
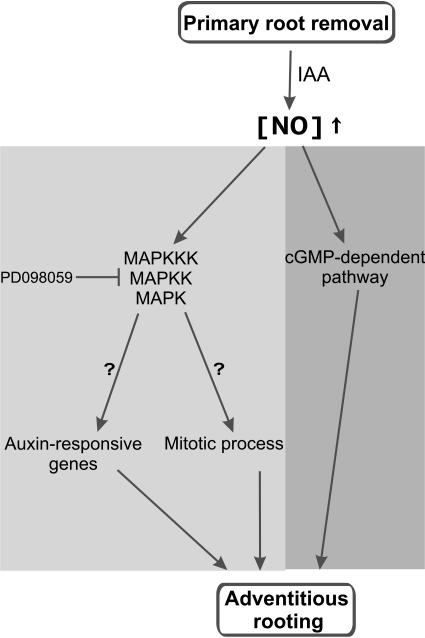
Figure 7 illustrates a representative scheme integrating the pathways and molecules involved in ARF in cucumber, which were described to date and the still unknown steps to be deciphered. A MAPK pathway could mediate adventitious root development in response to IAA and NO not only by the activation of mitotic process, but also by regulating the expression of some auxin-responsive genes (Fig. 7; Mockaitis and Howell, 2000). The MAPK signaling cascade involved in ARF in cucumber explants seems to be cGMP-independent, since NO-induced in vitro MBP-kinase activity was not affected by the guanylate cyclase inhibitor LY83583 (Fig. 6). Here, an interesting point should be highlighted. The inhibitor LY83583 does not affect the in vitro MAPK activity induced by NO (Fig. 6) but it is able to prevent the ARF induced by NO (Pagnussat et al., 2003). Thus, activation of the MAPK pathway alone does not seem sufficient to assure ARF. As a consequence, it can be suggested that NO activates at least two different pathways during the induction of ARF: cGMP-dependent and cGMP-independent that involves a MAPK signaling cascade (Fig. 7). The activation of both pathways seems to be required for the development of adventitious roots in cucumber since if one of them is blocked no ARF develops. This finding is consistent with earlier reports suggesting an NO action via a cGMP-independent pathway, activating phosphatases, and protein kinases including MAPKs (for review, see Lamattina et al., 2003; Neill et al., 2003).
Figure 7.
Schematic illustration of the signaling networks involving IAA, NO, and cellular messengers that regulate ARF in cucumber. The auxin IAA triggers a transient NO accumulation (Pagnussat et al., 2002). In turn, NO regulates the activation of a MAPK signaling cascade. This pathway could be mediating both mitotic process and the expression of auxin-responsive genes (Mockaitis and Howell, 2000). In parallel, a cGMP-dependent pathway is also present leading to ARF (Pagnussat et al., 2003). NO-mediated signaling pathways that regulate ARF in cucumber: light gray, cGMP-independent; dark gray, cGMP-dependent.
Altogether, these data suggest that the components of animal NO signaling are also functional in plants and reveal the remarkable complexity of the cellular responses regulated by NO, which may be mediated by phosphorylation-, nitrosylation-, or cGMP-controlled mechanisms. In particular, ARF seems to be regulated by a complex set of cellular messengers, among them MAPK and cGMP appear to be activated by upstream components involving IAA and NO.
MATERIALS AND METHODS
Plant Material
Cucumber seeds (Cucumis sativus L. cv Poinsett 76) were germinated into petri dishes on filter papers imbibed in distilled water and maintained at 25°C for 5 d with a 14-h photoperiod (photosynthetically active radiation = 200 μmol s−1 m−2). Primary roots of 5-d-old seedlings were removed and cucumber explants were then maintained under the same conditions of temperature and photoperiod for up to 5 d in the presence of different media as indicated below.
Explant Treatments
Cucumber explants were placed into petri dishes containing filter papers imbibed in water (control), or 10 μm of the auxin IAA (Fluka, Buch, Switzerland), or 10 μm of the NO-donor SNP (Merck, Darmstadt, Germany) and kept at 25°C for different periods according to the experiment. As a control, 200 μm of the specific NO-scavenger 2-(4-carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide, potassium salt (cPTIO, Molecular Probes, Eugene, OR) was added together with SNP or IAA. Where indicated, 10 μm, 50 μm, or 100 μm of the MAPK kinase inhibitor 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one (PD098059, Sigma-Aldrich, St. Louis) and/or 50 μm of the guanylate cyclase inhibitor 6-anilino-5, 8-quinilinedione (LY83583, Sigma-Aldrich) were administered to explants.
Microscopy
Cucumber explants were treated as indicated above. Hypocotyls from explants treated for 3 d with water, SNP, or IAA were transversally cut into3-mm segments and fixed in FAA solution (ethanol to distilled water to formaldehyde to glacial acetic acid [10:7:2:1, v/v] for 1 d. Segments were included in a paraffin matrix (Hystoplast) at 60°C and cut into 5- to 10-μm sections using a rotary microtome. Sections were stained with toluidine blue and deparaffined with xylene, slowly rehydrated, and sequentially washed with water and TBS buffer. Sections were then examined by bright field microscopy in a Nikon Eclipse E 200 microscope (Tokyo). Images were captured at ×100 and ×400 amplifications using a digital camera attached to the microscope.
Preparation of Protein Extracts
All the extraction procedures were performed on ice or at 4°C. To prepare extracts from treated explants, hypocotyls were ground in a mortar with liquid nitrogen and extracted with an equal volume (1 mL/1 g fresh weight of tissue) of extraction buffer (100 mm Tris, pH 7.5, 5 mm EDTA, 5 mm EGTA, 10 mm dithiothreitol (DTT), 10 mm Na3VO4, 10 mm NaF, 50 mm β-glycerophosphate, 1 mm phenylmethylsulfonyl fluoride, 5 μg/mL aprotinin, and 5 μg/mL leupeptin). After centrifugation at 20,000g for 15 min at 4°C, supernatants were transferred into clean tubes and immediately used for analysis.
Protein Determination
Quantification of proteins was performed according to Bradford (1976) using bovine serum albumin as standard.
In Vitro Protein Kinase Activity Assay
The kinase activity was determined in vitro by measuring phosphate incorporation into myelin basic protein (MBP, Sigma-Aldrich). The reaction was carried out in a final volume of 15 μL. Twelve microliters of sample was assayed in a reaction mixture containing 0.25 mg/mL of MBP, 0.5 μCi γ-32P-ATP (6000 Ci/mmol), 25 mm Tris pH 7.5, 5 mm MgCl2, 1 mm EGTA, and 1 mm DTT. After incubating at room temperature for 10 min, 10 μL of the mixture were spotted onto P81 phosphocellulose squares (Whatman, Springfield Mill, Maidstone, UK) and the reaction was stopped by immersion in 150 mm H3PO4. After washing extensively with 150 mm H3PO4, the paper squares were dried and counted as described (Ulloa et al., 1991).
In-gel Protein Kinase Activity Assay
In-gel kinase activity assays were performed as described previously (Zhang and Klessig, 1997). Extracts containing 10 μg of protein were electrophoresed on 10% SDS-polyacrylamide gels imbibed in 0.25 mg/mL of MBP in the separating gel as substrate for the kinases. After electrophoresis, SDS was removed by washing the gel with washing buffer (25 mm Tris, pH 7.5, 0.5 mm DTT, 0.1 mm Na3VO4, 5 mm NaF, 0.5 mg/mL BSA, and 0.1% TritonX-100 [v/v]) three times for 30 min each at room temperature. The kinases were allowed to renature in 25 mm Tris, pH 7.5, 1 mm DTT, 0.1 mm Na3VO4, and 5 mm NaF at 4°C overnight with three changes of buffer. The gel was then incubated at room temperature in 30 mL of reaction buffer (25 mm Tris, pH 7.5, 2 mm EGTA, 12 mm MgCl2, 1 mm DTT, and 0.1 mm Na3VO4) with 200 nm cold ATP plus 50 μCi γ-32P-ATP (6000 Ci/mmol) for 60 min. The reaction was stopped by transferring the gel into 5% trichloroacetic acid (w/v)/1% NaPPi (w/v). The unincorporated γ-32P-ATP was removed by washing with the same solution for at least 6 h with five changes. The gel was dried onto Whatman 3MM paper and exposed to Kodak XAR-5 film (Rochester, NY). Prestained size markers (Bio-Rad, Hercules, CA) were used to calculate the size of the kinases.
Acknowledgments
We thank Dr. Arjen ten Have for helpful discussions and critical reading of the manuscript. L.L is a career member, M.C.L. is a technical assistant and G.C.P. is a research postdoctoral fellow from CONICET, Argentina. M.L.L. is a student fellow from UNMdP, Argentina.
This work was supported by grants to L.L from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica), Fundación Antorchas, and UNMdP (Universidad Nacional de Mar del Plata), Argentina.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.038554.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR (1995) PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270: 27489–27494 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (2000) Nitric oxide induces seed germination and de-etiolation, and inhibits hypocotyls elongation, three light-inducible responses in plants. Planta 210: 215–221 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (2001) Nitric oxide: a non-traditional regulator of plant growth. Trends Plant Sci 6: 508–509 [DOI] [PubMed] [Google Scholar]
- Bögre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S, Jonak C, Pollaschek CH, Barker P, Huskisson S, et al. (1999) A MAP kinase is activated late in mitosis and becomes localized to the plane of cell division. Plant Cell 11: 101–114 [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chou FP, Tseng TH, Chen JH, Wang HC, Wang CJ (2002) Induced proliferation of human MRC-5 cells by nitrogen oxides via direct and indirect activation of MEKK1, JNK, and p38 signals. Toxicol Appl Pharmacol 181: 203–208 [DOI] [PubMed] [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24: 667–677 [DOI] [PubMed] [Google Scholar]
- Davies PJ (1995) The plant hormones: their nature, ocurrence and functions. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, the Netherlands, pp 1–12
- Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant defense resistance. Nature 394: 585–588 [DOI] [PubMed] [Google Scholar]
- De Klerk G-J, Keppel M, Ter Brugge J, Meekes H (1995) Timing of the phases in adventitious root formation in apple microcuttings. J Exp Bot 46: 965–972 [Google Scholar]
- Durner J, Wendehenne D, Klessig D (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle M (1992) The plant hormone auxin: insight in sight. Bioessays 14: 439–444 [DOI] [PubMed] [Google Scholar]
- Fujita H, Syôno K (1996) Genetic analysis of the effects of polar auxin transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol 37: 1094–1101 [DOI] [PubMed] [Google Scholar]
- Gouvêa CM, Souza CP, Magalhaes CAN, Martin IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21: 183–187 [Google Scholar]
- Gow AJ, Ischiropoulos HJ (2001) Nitric oxide chemistry and cellular signaling. J Cell Physiol 187: 277–282 [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr 4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7: 211–220 [DOI] [PubMed] [Google Scholar]
- Ito Y, Banno H, Moribe T, Hinata K, Machida Y (1994) NKP15, a tobacco protein-serine/threonine kinase with a single hydrophobic region near the amino-terminus. Mol Gen Genet 245: 1–10 [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Jester PJ, Gottwald JR, Sussman MR (2002) An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell 14: 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Klessig DF (2000) Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene and jasmonic acid. Mol Plant Microbe Interact 13: 347–351 [DOI] [PubMed] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat GC (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Physiol Plant Mol Biol 54: 109–136 [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Beligni MV, Lamattina L (1997) Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur J Plant Pathol 73: 643–651 [Google Scholar]
- Leshem YY, Wills RBH, Ku VV (1998) Evidence for the function of the free radical gas -nitric oxide (NO)- as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36: 825–833 [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M (1996) Mutations in the axr 3 gene of Arabidopsis results in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J 10: 403–413 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the aur 1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Gotoh Y, Nishida E, Yamaguchi-Shinozaki K, Hayashida N, Iwasaki T, Kamada H, Shinozaki K (1994) Characterization of two cDNAs that encode MAP kinase homologues in Arabidopsis thaliana and analysis of the possible role of auxin in activating such kinase activities in cultured cells. Plant J 5: 111–122 [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Howell SH (2000) Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J 24: 785–796 [DOI] [PubMed] [Google Scholar]
- Morris PC (2001) MAP kinase signal transduction pathway in plants. New Phytol 151: 67–89 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159: 11–35 [DOI] [PubMed] [Google Scholar]
- Nishihama R, Ishikawa M, Araki S, Soyano T, Asada T, Machida Y (2001) The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev 15: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129: 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the IAA-induced adventitious rooting process. Plant Physiol 132: 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M (1990) The aux 1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Harmon AC (1992) Calcium-modulated proteins: targets of intracellular calcium signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol 43: 375–414 [Google Scholar]
- Tena G, Renaudin JP (1998) Cytosolic acidification but not auxin at physiological concentration is an activator of MAP kinases in tobacco cells. Plant J 16: 173–182 [DOI] [PubMed] [Google Scholar]
- Theologis A (1986) Rapid gene regulation by auxin. Annu Rev Plant Physiol 37: 407–438 [Google Scholar]
- Ulloa RM, Torres HN, Ochatt CM, Téllez-Iñon MT (1991) Ca2+ calmodulin-dependent protein kinase activity in the ascomycetes Neurospora crassa. Mol Cell Biochem 102: 155–163 [DOI] [PubMed] [Google Scholar]
- Walden R, Lubenow H (1996) Genetic dissection of auxin action: more questions than answers? Trends Plant Sci 1: 27–32 [Google Scholar]
- Walker L, Estelle M (1998) Molecular mechanisms of auxin action. Curr Opin Plant Biol 1: 434–439 [DOI] [PubMed] [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M (1990) A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet 222: 377–383 [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1997) Salicylic acid activates a 48 kDa MAP kinase in tobacco. Plant Cell 9: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]