Abstract
The precise regulation of odontoblast differentiation and osteoclastogenic cytokine expression in human dental pulp cells (HDPCs) is crucial for the pathology of bacteria-related pulpitis. Although the up-regulation of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) has been reported in inflamed human dental pulps, the role of NOD2 in the differentiation of HDPCs remains unclear. Here, we show the involvement of NOD2 in odontoblast differentiation together with osteoclastogenic cytokine expression in HDPCs. Treatment with muramyl dipeptide (MDP), a known NOD2-agonist, significantly inhibited odontoblast differentiation of HDPCs, as revealed by reduced ALP activity, osteoblast/odontoblast marker expression, and mineralized nodule formation. Importantly, the forced down-regulation of NOD2 by small interfering RNA (siRNA) recovered MDP-down-regulated odontoblast differentiation. MDP-elicited suppression of odontoblast differentiation resulted from the increased expression of MKP-1 protein and the subsequent decline of MAPKs phosphorylation, which is a prerequisite for odontoblast differentiation. Furthermore, we found that MDP treatment elevated the expression of osteoclastogenic cytokines in HDPCs, which was also reversed by NOD2 silencing. Analysis of these data, taken together, suggests that the regulation of NOD2 expression upon MDP challenge might serve as an intrinsic mechanism that underlies the hindered dentin formation and accelerated dentin resorption in bacterial infection-mediated pulpitis.
Abbreviations: HDPCs, human dental pulp cells; NOD2, nucleotide-binding oligomerization domain-containing protein 2; MDP, muramyl dipeptide; siRNA, small interfering RNA; MAPKs, mitogen-activated protein kinases; MKP-1, MAPK phosphatase-1; BMMs, bone-marrow-derived macrophages; and CM, conditioned medium.
Keywords: pulpitis, dental pulp, muramyl dipeptide, osteoclasts, osteoblasts, Map kinase phosphatase 1
Introduction
Pulpitis is characterized as the immune response that is primarily triggered by the invasion of caries-related bacteria into dentin tissue and pulp (Adachi et al., 2007). With pulpitis development, increased expression of pro-inflammatory mediators is found in inflamed pulp, especially in human dental pulp cells (HDPCs) (Nakanishi et al., 1995). Pro-inflammatory cytokines are produced by the immune system in response to the recognition of pathogen-related molecular patterns (PAMPs), such as lipopolysaccharide (LPS), peptidoglycan, and nucleic acid variants, by the immune system.
Recently, it has been reported that HDPCs express TLR2 and TLR4 and therefore retain the ability to participate in the innate immune response (Mutoh et al., 2007). Similar to TLRs, nucleotide-binding oligomerization domain-containing protein 1 (NOD1) and NOD2 are intracellular pattern-recognition receptors, which recognize a specific molecular structure called muramyl dipeptide (MDP) in Gram-positive/negative bacteria (Girardin et al., 2003). Stimulation of NODs induces the expression of inflammatory cytokines via the activation of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling (Inohara and Nuñez, 2003). Among the 2 isoforms, NOD2 is expressed in healthy dental pulp (Hirao et al., 2009) and is known to be up-regulated in inflamed pulps challenged by cariogenic bacteria (Keller et al., 2011).
Osteoblasts express NOD1 and NOD2 and therefore participate in the release of inflammatory cytokines and support osteoclastogenesis by providing macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) (Marriott et al., 2005). Escherichia coli LPS has been shown to interact with TLR4 on the surfaces of osteoblasts and up-regulates the expression of RANKL and down-regulates that of osteoprotegerin (OPG), a decoy receptor for RANKL (Suda et al., 2004). More importantly, NOD2-mediated signals are involved in MDP-induced RANKL expression in osteoblasts (Yang et al., 2005).
Recently, it was reported that the expression of cyclo-oxygenase-2 (COX2) and prostaglandin E2 (PGE2), which are potent activators of osteoclasts, is enhanced by co-stimulation with a NOD1 or NOD2 ligand and a TLR2 or TLR4 ligand in HDPCs (Marriott et al., 2005). Moreover, the production of osteoclastogenic cytokines, including IL-1β, IL-6, and IL-8, is accelerated by co-stimulation with NOD1 or NOD2 and TLR2 or TLR4 ligands via increased expression of TNF receptor-associated factor 6 (TRAF6) in periodontal ligament cells (Tang et al., 2011). In addition, MDP synergistically enhances LPS-mediated osteoclast differentiation through increased RANKL expression in osteoblasts (Yang et al., 2005). These findings suggest that MDP might play an important role in pulpal immune responses, destruction of pulpal architecture, and, ultimately, periapical bone destruction. However, the mechanisms involved in dental pulp destruction under pro-inflammatory stimuli are not fully understood. Here we show that NOD2 functions as a key mediator of MDP signals, which inhibits odontoblast differentiation and promotes osteoclastogenic cytokine expression. Our results indicate a novel role for NOD2 in dental pulp cell regulation that might be applied to the treatment of destructive pulp disease.
Materials & Methods
Chemicals and Reagents
α-modified Eagle medium (α-MEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Gibco BRL Co. (Grand Island, NY, USA). L-ascorbic acid, β-glycerophosphate, and muramyl dipeptide (MDP) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Lipofectamine 2000 was obtained from Invitrogen Life Technology (Grand Island, NY, USA). The small interfering RNAs (siRNA) against NOD2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibody against NOD2 and Triptolide (PG490) were purchased from Santa Cruz Biotechnology.
Cell Culture
Immortalized HDPCs, established by stable transfection of human telomerase catalytic component (hTERT), were kindly provided by Prof. Takashi Takata (Hiroshima University, Japan). A detailed characterization of this cell line concerning odontoblast differentiation was thoroughly described previously (Kitagawa et al., 2007). For odontoblast differentiation, HDPCs were cultured in osteogenic medium (OM, 50 µg/mL ascorbic acid and 10 mM β-glycerophosphate, and 0.1 μM dexamethasone) for 14 days as previously described (Wei et al., 2007).
Alkaline Phosphatase Assay
ALP activity was measured with p-nitrophenyl phosphate (3 mM final concentration) as the substrate in 0.7 M 2-amino-methyl-1-propanol, pH 10.3, and 6.7 mM MγCl2. Absorbance was measured at 410 nm by means of an enzyme-linked immunosorbent assay reader (Beckman Coulter, Fullerton, CA, USA).
Alizarin Red Staining
After 14 days of culture, HDPCs were fixed in 70% ice-cold ethanol for 1 hr and rinsed with distilled water. Cells were then stained with 40 mmol/L alizarin red S (pH = 4.2) for 10 min under conditions of gentle agitation. The images of alizarin red S staining were photographed with a digital camera, and the intensity of staining was quantified with the ImageJ program (ver. 1.40; available on the National Institutes of Health [USA] Website).
NOD2 siRNA Transfection
Small interfering RNAs (siRNAs) were used for transient gene knockdown studies. The sequences of the sense and antisense strands of the human NOD2 siRNA were as follows: 5′-GAUGAAGUUGACCUCCUCA-3′ (sense) and 5′-UGAGG AGGUCAACUUCAUC-3′ (antisense). The control-siRNA (Catalog No. SN-1003) was purchased from Bioneer (Bioneer Corporation, Daejeon, South Korea). HDPCs were transfected with siRNAs duplexes (100 nmol/mL) for 24 hr with Lipofectamine 2000 (Gibco; Invitrogen Ltd, Paisley, UK) according to the manufacturer’s instructions.
RNA Isolation and Reverse-transcriptase-Polymerase Chain-reaction (RT-PCR)
The total RNA of cells was extracted with Trizol reagent (Life Technologies, Gaithersburg, MD, USA) according to the manufacturer’s instructions. A 1-µg quantity of isolated RNAs from each culture was reverse-transcribed with oligo (dT)15 primers with AccuPower RT PreMix (Bioneer). Next, the generated cDNAs were amplified with AccuPower PCR PreMix (Bioneer). Primer sequences are listed in the Appendix Table.
Western Blot Analysis
Cells were solubilized in ice-cold 1% Triton X-100 lysis buffer. Proteins (30 µg) were mixed with an equal volume of 2 × sodium dodecyl sulphate (SDS) sample buffer, boiled for 5 min, and then resolved by SDS-polyacrylamide gel electrophoresis (12% acrylamide) and transferred to nitrocellulose membranes. The protein bands were visualized by the enhanced chemiluminescence (ECL) system according to the manufacturer’s instructions and exposed to x-ray film.
Preparation of Conditioned Medium
HDPCs (2 × 106) were seeded on 100-mm culture dishes and cultured for 14 days in OM to allow for odontoblast differentiation. To obtain conditioned medium (CM) from these cells, we incubated the cultured HDPCs with fresh α-MEM (without MDP) for the last 24 hr of culture. To normalize the differences between cell densities due to proliferation during the culture period, we collected cells from each plate and determined total DNA content/plate (spectrophotometric absorbance, 260 nm). The CM was then normalized for DNA content between samples by the addition of α-MEM medium.
Osteoclast Differentiation Assays
Mouse bone-marrow-derived macrophages (BMMs) obtained from five-week-old female ICR mice (outbred CD-1® mice; Charles River Laboratories, Seoul, South Korea) were used as osteoclast precursor cells for in vitro osteoclastogenesis experiments. Briefly, mouse whole bone marrow cells, isolated by flushing the marrow space of tibiae, were incubated overnight on culture dishes in α-MEM supplemented with 10% FBS. After attached cells were discarded, floating cells were further cultured for 3 days in the presence of M-CSF (30 ng/mL) on Petri dishes. After 3 days’ culture, BMMs became adherent and were used as osteoclast precursor cells. Upon two-day treatment of BMMs with 30 ng/mL M-CSF and 100 ng/mL RANKL, > 80% of the cells became mononuclear TRAP-positive cells (pre-osteoclasts). To evaluate the osteoclastogenic activity of CM from HDPCs, we treated the CM mixture (60% CM plus 40% fresh α-MEM without M-CSF & RANKL) with pre-osteoclast-stage cells and further cultured it for up to 4 days to achieve mature osteoclast differentiation. After 4 days of culture, cells were stained for TRAP activity with a leukocyte acid phosphatase kit (Sigma). Cells with 3 or more nuclei were counted as multinucleated mature osteoclasts.
Statistical Analysis
The data are expressed as mean ± SD of at least 3 independent experiments. Statistical significance was evaluated by one-way analysis of variance with the SPSS (Version 11.0; SPSS, Chicago, IL, USA) computer program.
Results
MDP, an NOD2 Agonist, Negatively Regulates the Odontoblastic Differentiation of HDPCs
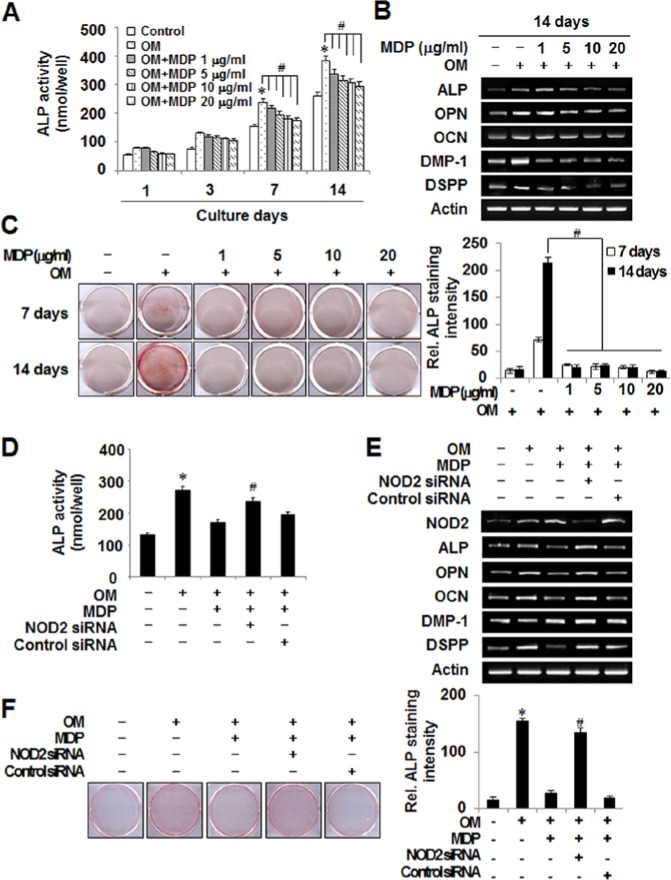
To determine whether the NOD2 ligand MDP affects the odontoblast differentiation of HDPCs, we assessed ALP activity, mRNA expression of odontoblast differentiation markers, and mineralized nodule formation after MDP treatment. As shown in Figs. 1A and 1B, MDP reduced ALP activity and odontoblast (e.g., DMP-1 and DSPP)/osteoblast (e.g., ALP, OPN, and OCN) gene mRNA expression in a dose-dependent manner. The maximal down-regulation of ALP activity and odontoblast/osteoblast marker gene expression was achieved at 10 or 20 μg/mL of MDP; however, the mineralized nodule formation was almost completely inhibited from 1 μg/mL MDP treatment (Fig. 1C). Interestingly, the MDP treatment profoundly increased expression of NOD2, TLR2, and TLR4, which are known to be expressed as pattern-recognition receptors in HDPCs (Appendix Fig.). Next, we tested whether the inhibitory effect of MDP was mediated by NOD2 by introducing NOD2-targeting siRNA into HDPCs. The ALP activity, odontoblast/osteoblast mRNA expression, and mineralized nodule formation recovered in the NOD2-siRNA transfected HDPCs in the presence of MDP (Figs. 1D-1F). Analysis of these data indicates that NOD2 mediates the inhibitory effect of MDP on odontoblast differentiation.
Figure 1.
MDP treatment negatively regulates odontoblast differentiation through NOD2 in HDPCs. (A) HDPCs were cultured with MDP at concentrations ranging from 0 to 20 µg/mL for the indicated days in osteogenic media (10 mM β-glycerophosphate, 10−7 M dexamethasone, and 50 µg/mL L-ascorbic acid). The unstimulated (Control) and OM-alone-treated cells (OM) were used as negative and positive controls, respectively. ALP activity was determined. (B) The mRNA expression of osteoblast/odontoblast differentiation markers was evaluated by RT-PCR. (C) The mineralized nodule formation was analyzed by alizarin red staining. The intensity of alizarin red staining was quantified as described in Materials & Methods. (D) Control siRNA- or NOD2 siRNA-transfected cells were incubated for 24 hr and further cultured with MDP (10 µg/mL) for 7 days in OM. At the end of culture, ALP activity was assessed. (E) Control siRNA- or NOD2 siRNA-transfected HDPCs were cultured in the presence of MDP (10 µg/mL) for 7 days in osteogenic media (OM). At the end of culture, the mRNA expression of osteoblast/odontoblast markers was examined by RT-PCR. (F) Mineralized nodule formation was analyzed by alizarin red staining of the cells treated as in (E). The bar graph indicates intensity of staining. *Statistically significant difference vs. control, p < .05. #Statistically significant difference in OM alone, p < .05. Similar data were obtained from 3 independent experiments. This figure is available in color online at http://jdr.sagepub.com.
MDP Reduced Phosphorylation of MAPKs through the Induction of MKP-1
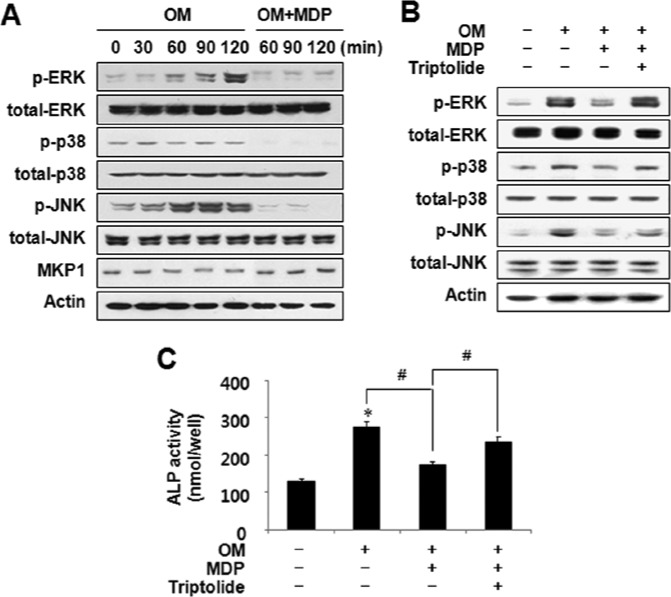
For odontoblast differentiation, the activation of mitogen-activated protein kinases (MAPKs) is essential (Jaiswal et al., 2000; Su et al., 2010). NOD2 is known to induce MAPK signaling (Inohara and Nuñez, 2003). To further delineate the mechanism for the suppression of odontoblast differentiation by MDP, we investigated the phosphorylation of MAPKs with MDP treatment. Notably, the MDP treatment prominently decreased phosphorylation of ERK, p38, and JNK (Fig. 2A). Since these 3 major MAPKs are negatively regulated by a common dual-specificity phosphatase known as MAPK phosphatase 1 (MKP-1) (Owens and Keyse, 2007; Huang and Tan, 2012), we hypothesized that MKP-1 expression might be up-regulated by MDP. As expected, MKP-1 protein expression increased in the MDP-treated cultures (Fig. 2A), and, consistent with this result, treatment with an MKP-1 inhibitor, Triptolide, restored MAPK phosphorylation in the presence of MDP (Fig. 2B). In addition, the reduced ALP activity in the MDP-treated HDPCs was rescued by MKP-1 inhibition (Fig. 2C). Therefore, we suggest that the MDP suppression of odontoblast differentiation was mediated by MKP-1 and the subsequent dephosphorylation of MAPKs.
Figure 2.
MDP treatment reduced phosphorylation of MAPKs via the induction of MAPK phosphatase (MKP)-1 in HDPCs. (A) HDPCs were stimulated with MDP at 10 µg/mL for 120 min in osteogenic media (OM) with 10 mM β-glycerophosphate, 10−7 M dexamethasone, and 50 µg/mL L-ascorbic acid. The phosphorylation of MAPKs and the expression of MKP-1 were determined by Western blotting. (B) HDPCs were pre-treated with MKP-1 inhibitor (Triptolide, TP 0.5 mM) for 4 hr, and stimulated with MDP for 120 min. The phosphorylation of MAPKs was examined by Western blotting. (C) HDPCs were cultured in the presence of MDP or Triptolide for 14 days. At the end of culture, ALP activity was examined. *Statistically significant difference vs. control, p < .05. #Statistically significant difference in OM alone, p < .05. Similar data were obtained from 3 independent experiments.
MDP Augments Osteoclastogenic Cytokine Expression in HDPCs
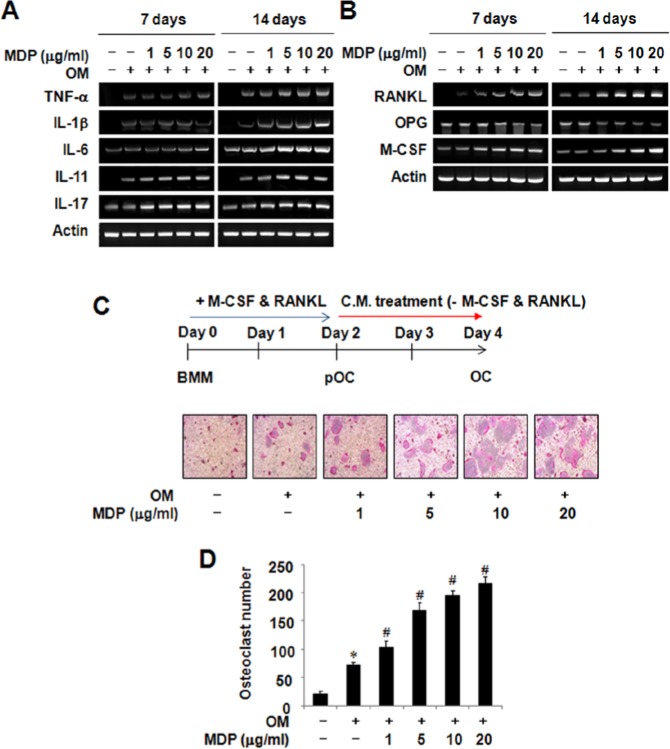
Since MDP is one of the peptidoglycan constituents of Gram-positive/-negative bacteria (Girardin et al., 2003), and since bacterial infection-triggered pulpitis goes with osteoclastic bone resorption (Adachi et al., 2007; Silva et al., 2012), we investigated the effect of MDP on osteoclastogenic cytokine expression in HDPCs. MDP treatment enhanced the expression of osteoclastogenic cytokines, which are known to support osteoclast differentiation (Fig. 3A). Importantly, RANKL and M-CSF, the two key factors essential for osteoclast formation, were also increased in the presence of MDP (Fig. 3B). On the contrary, the mRNA expression of a soluble decoy receptor for RANKL, known as osteoprotegerin (OPG), was substantially reduced upon MDP treatment. Thus, it seems that the presence of MDP provided favorable conditions for osteoclastogenesis through HDPCs. To verify the enhanced osteoclastogenic ability of MDP-treated HDPCs, pre-osteoclasts were allowed to differentiate into mature osteoclasts with the conditioned medium (CM), prepared from MDP-treated HDPCs. According to the RT-PCR results, MDP-treated CM increased osteoclast formation in an MDP dose-dependent manner (Figs. 3C, 3D). Analysis of these data suggests that MDP could enhance osteoclastogenesis by altering the state of supporting HDPCs.
Figure 3.
The treatment of MDP potentiates osteoclastogenic cytokine expression in human dental pulp cells. (A, B) The effects of MDP on the mRNA expression of osteoclastogenic cytokine in HDPCs were examined by RT-PCR analysis as described in Materials & Methods. (C) The osteoclastogenic effects of conditioned medium (CM) from MDP-treated HDPCs were evaluated by an in vitro osteoclast differentiation system. Human dental pulp cells were cultured in the presence of indicated concentrations of MDP for 14 days, and CM was prepared as described in Materials & Methods. At the end of culture, cells were stained for TRAP activity. (D) The numbers of osteoclasts/well were counted. Similar data were obtained from 3 independent experiments. *Statistically significant difference vs. control, p < .05.
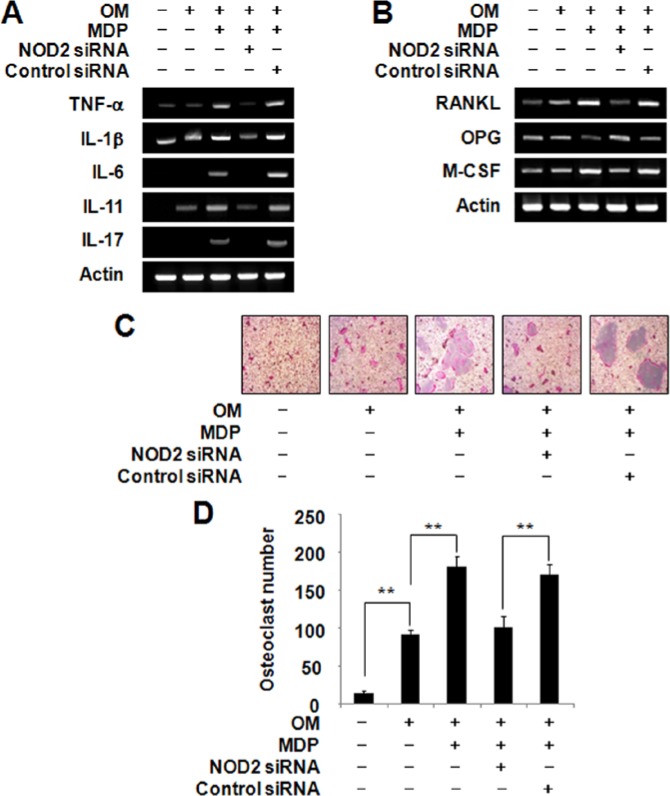
NOD2 Silencing Diminishes the MDP-mediated Osteoclastogenic Cytokine Expression in HDPCs
Since the expression of NOD2 was significantly increased upon MDP treatment and the inhibitory effect of MDP on odontoblast differentiation was exerted by NOD2, the role of NOD2 for the osteoclastogenic cytokine expression in HDPCs was tested by the introduction of NOD2 siRNA into HDPCs. Introduction of siRNA specific for NOD2 reduced osteoclastogenesis-supporting cytokine expression compared with control siRNA-transfected HDPCs (Fig. 4A). More importantly, increased expression of RANKL and M-CSF in the presence of MDP was markedly down-regulated upon knockdown of NOD2 (Fig. 4B), suggesting that NOD2 functions downstream of MDP. To examine the function of NOD2 in osteoclastogenic cytokine expression, we evaluated osteoclastogenic activity of CM from NOD2-silenced HDPCs under an osteoclast differentiation system. Although the addition of MDP resulted in increased osteoclast formation, this effect was abolished by knockdown of NOD2 (Figs. 4C, 4D). Therefore, we conclude that MDP caused elevated osteoclastogenic cytokine expression in HSPCs by mediating NOD2.
Figure 4.
The knockdown of NOD2 reversed MDP-mediated osteoclastogenic cytokine expression in HDPCs. (A, B) Control siRNA- or NOD2 siRNA-transfected HDPCs were cultured in the presence of MDP (10 µg/mL) for 7 days in osteogenic media (OM). The mRNA levels of osteoclastogenic cytokines were evaluated by RT-PCR analysis. (C) The osteoclastogenic activity of conditioned medium (CM) from NOD2-silenced HDPCs was assessed by a mouse osteoclast differentiation system. HDPCs were transfected with either control siRNA or NOD2 siRNA and subsequently treated with MDP (10 µg/mL) for 14 days. The CM was collected as described in Materials & Methods and treated on pre-osteoclast cells. After 2 days, the cultures were stained for TRAP activity. (D) The numbers of osteoclasts/well were counted. *Statistically significant difference vs. control, p < .05. #Statistically significant difference in OM alone, p < .05. Similar data were obtained from 3 independent experiments.
Discussion
The pathogenesis of pulpal and periapical lesions is largely attributed to host inflammatory responses caused by the bacterial infection of the pulp cavity and root canals (Kakehashi et al., 1965). Periapical bone loss reflects enhanced osteoclast activity and suppressed osteoblast activity during the host response to bacteria. Therefore, we hypothesized that MDP, a peptidoglycan constituent of both Gram-positive/-negative bacteria (Girardin et al., 2003), might be involved in negative odontoblast differentiation as well as up-regulation of osteoclastogenic cytokine expression in HDPCs; the culmination of this process is periapical alveolar bone destruction or internal pulp resorption.
HDPCs are the major constituent of pulp tissue and are capable of playing a pivotal role in wound-healing processes, pathological alterations, and the production of various inflammatory cytokines. In addition, HDPCs are considered odontoblast progenitor cells that are capable of proliferating and differentiating into odontoblast-like cells that conduct dentin formation (Fitzgerald et al., 1990). Our results show that treatment with the NOD2 ligand MDP decreased ALP activity, mineralized nodule formation, and the expression of odontoblast markers in HDPCs. These results are similar to those of previous reports in mouse osteoblasts (Pelt et al., 2002) and rat bone marrow cells (Andreou et al., 2004) treated with LPS, a bacterial component.
Recently, it has been reported that HDPCs express stimulatory (RANKL, M-CSF) and inhibitory (OPG) factors (Sundqvist et al., 1995), which means that the key molecules in osteoclast progenitor cell expansion and differentiation are both expressed in pulp tissue. Evidence demonstrates that RANKL is expressed in dental pulp tissue in periapical lesions (Zhang and Peng, 2005) and during root resorption (Shapiro et al., 2002). Since RANKL expression could be up-regulated by bone-resorbing factors such as IL-1, IL-6, IL-11, IL-17, PGE2, and parathyroid hormone in osteoblasts (Blair and Athanasou, 2004), we examined the expression of RANKL in HDPCs upon MDP treatment. Interestingly, MDP increased pro-inflammatory cytokine expression (i.e., TNF-α, IL-1β, IL-6, IL-11, and IL-17) as well as RANKL expression in HDPCs (Fig. 3). Although the question of whether MDP up-regulated RANKL expression directly or indirectly through the induction of pro-inflammatory mediators could not be clearly answered by these results, a noticeable increase of osteoclast formation was observed upon treatment with CM prepared from MDP-treated HDPCs (Fig. 3). MDP alone could not induce osteoclast formation in the osteoclast differentiation culture (data not shown), but enhanced osteoclast formation was evident with CM from MDP-treated HDPCs. More importantly, the MDP-mediated osteoclastogenic cytokine expression in HDPCs was reduced by NOD2 knockdown (Fig. 4). To our knowledge, this study is the first demonstration that the MDP-NOD2 pathway is directly involved in osteoclastogenic cytokine expression in HDPCs.
Inohara et al. (2005) reported that NOD1 is ubiquitously expressed in numerous cell types, whereas NOD2 is mainly expressed in immune cells. NOD2 regulates the coordination of signaling pathways downstream of bacterial infection by inducing activation of MAPKs, which usually initiates the production of pro-inflammatory cytokines (Inohara and Nuñez, 2003). In this study, we demonstrated that treatment with MDP induced dephosphorylation of p38, ERK, and JNK (Fig. 2) by inducing MKP-1 expression. Since the activation of MAPKs is essential for odontoblast differentiation (Jaiswal et al., 2000; Su et al., 2010), it is likely that the observed reduction of odontoblast differentiation induced by MDP was mainly caused by the direct inhibition of MAPKs. It should be noted that the suppression of odontoblast differentiation by MDP vanished in NOD2-silenced HDPCs (Fig. 1). Thus, analysis of these data adds another line of evidence that the MDP contained in bacteria negatively regulates odontoblast differentiation via NOD2.
To summarize, we propose dual roles for NOD2 during odontoblast differentiation and osteoclastogenic cytokine expression in HDPCs. The increased NOD2 expression in MDP-challenged HDPCs results in decreased odontoblast differentiation by increasing MKP-1 protein levels and the subsequent down-regulation of MAPKs. NOD2 also mediates osteoclastogenic cytokine expression in HDPCs in the presence of MDP. Therefore, during the invasion of caries-related bacteria into dentin tissue, MDP results in disastrous bone loss, reflecting enhanced osteoclastogenesis and suppressed odontoblastogenesis. The mechanism described in this study might improve our understanding about the pathogenesis of pulpal and periapical lesions.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NO, 2012R1A5A2051384) and by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A111412).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adachi T, Nakanishi T, Yumoto H, Hirao K, Takahashi K, Mukai K, et al. (2007). Caries-related bacteria and cytokines induce CXCL10 in dental pulp. J Dent Res 86:1217-1222. [DOI] [PubMed] [Google Scholar]
- Andreou V, D’Addario M, Zohar R, Sukhu B, Casper RF, Ellen RP, et al. (2004). Inhibition of osteogenesis in vitro by a cigarette smoke-associated hydrocarbon combined with Porphyromonas gingivalis lipopolysaccharide: reversal by resveratrol. J Periodontol 75:939-948. [DOI] [PubMed] [Google Scholar]
- Blair HC, Athanasou NA. (2004). Recent advances in osteoclast biology and pathological bone resorption. Histol Histopathol 19:189-199. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Chiego DJ, Jr, Heys DR. (1990). Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 35:707-715. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. (2003). Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- Hirao K, Yumoto H, Takahashi K, Mukai K, Nakanishi T, Matsuo T. (2009). Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J Dent Res 88:762-767. [DOI] [PubMed] [Google Scholar]
- Huang CY, Tan TH. (2012). DUSPs, to MAP kinases and beyond. Cell Biosci 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Nuñez G. (2003). NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 3:371-382. [DOI] [PubMed] [Google Scholar]
- Inohara N, Chamaillard M, McDonald C, Nuñez G. (2005). NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355-383. [DOI] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. (2000). Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem 275:9645-9652. [DOI] [PubMed] [Google Scholar]
- Kakehashi S, Stanley HR, Fitzgerald RJ. (1965). The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 20:340-349. [DOI] [PubMed] [Google Scholar]
- Keller JF, Carrouel F, Staquet MJ, Kufer TA, Baudouin C, Msika P, et al. (2011). Expression of NOD2 is increased in inflamed human dental pulps and lipoteichoic acid-stimulated odontoblast-like cells. Innate Immun 17:29-34. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Ueda H, Iizuka S, Sakamoto K, Oka H, Kudo Y, et al. (2007). Immortalization and characterization of human dental pulp cells with odontoblastic differentiation. Arch Oral Biol 52:727-731. [DOI] [PubMed] [Google Scholar]
- Marriott I, Rati DM, McCall SH, Tranguch SL. (2005). Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun 73:2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N, Tani-Ishii N, Tsukinoki K, Chieda K, Watanabe K. (2007). Expression of Toll-like receptor 2 and 4 in dental pulp. J Endod 33:1183-1186. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Matsuo T, Ebisu S. (1995). Quantitative analysis of immunoglobulins and inflammatory factors in human pulpal blood from exposed pulps. J Endod 21:131-136. [DOI] [PubMed] [Google Scholar]
- Owens DM, Keyse SM. (2007). Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26:3203-3213. [DOI] [PubMed] [Google Scholar]
- Pelt P, Zimmermann B, Ulbrich N, Bernimoulin JP. (2002). Effects of lipopolysaccharide extracted from Prevotella intermedia on bone formation and on the release of osteolytic mediators by fetal mouse osteoblasts in vitro. Arch Oral Biol 47:859-866. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Giertsen E, Guggenheim B. (2002). An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res 36:93-100. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Kajiya M, AlShwaimi E, Sasaki H, Hong J, Ok P, et al. (2012). Bacteria-reactive immune response may induce RANKL-expressing T cells in the mouse periapical bone loss lesion. J Endod 38:346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JL, Chiou J, Tang CH, Zhao M, Tsai CH, Chen PS, et al. (2010). CYR61 regulates BMP-2-dependent osteoblast differentiation through the {alpha}v{beta}3 integrin/integrin-linked kinase/ERK pathway. J Biol Chem 285:31325-31336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda K, Udagawa N, Sato N, Takami M, Itoh K, Woo JT, et al. (2004). Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J Immunol 172:2504-2510. [DOI] [PubMed] [Google Scholar]
- Sundqvist G, Rosenquist JB, Lerner UH. (1995). Effects of bradykinin and thrombin on prostaglandin formation, cell proliferation and collagen biosynthesis in human dental-pulp fibroblasts. Arch Oral Biol 3:247-256. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhou XD, Wang Q, Zhang L, Wang Y, Li XY, et al. (2011). Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch Oral Biol 56:1064-1072. [DOI] [PubMed] [Google Scholar]
- Wei X, Ling J, Wu L, Liu L, Xiao Y. (2007). Expression of mineralization markers in dental pulp cells. J Endod 33:703-708. [DOI] [PubMed] [Google Scholar]
- Yang S, Takahashi N, Yamashita T, Sato N, Takahashi M, Mogi M, et al. (2005). Muramyl dipeptide enhances osteoclast formation induced by lipopolysaccharide, IL-1 alpha, and TNF-alpha through nucleotide-binding oligomerization domain 2-mediated signaling in osteoblasts. J Immunol 175:1956-1964. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peng B. (2005). Immunolocalization of receptor activator of NF kappa B ligand in rat periapical lesions. J Endod 31:574-577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.